Introduction
Gastric cancer (GC) is the third leading cause of
cancer-associated mortality worldwide (1–3). Due to
the lack of effective techniques for early diagnosis, the majority
the patients with GC are diagnosed at late stages of GC. Despite
advances in the diagnosis and treatment of GC, the 5-year overall
survival rate of patients with GC remains low (4). Chemotherapy is the primary treatment for
GC. However, chemoresistance remains to be a major obstacle for the
clinical treatment of the disease. Therefore, investigating the
molecular mechanism underlying chemoresistance is essential for
effective treatments in patients with GC.
Long noncoding RNAs (lncRNAs, >200 nucleotides in
length) are dysregulated in various human diseases and disorders,
including cancer (5–13). LncRNA metastasis-associated lung
adenocarcinoma transcript 1 promotes the development of
hepatocellular carcinoma by upregulating serine/arginine-rich
splicing factor 1 and activating mammalian target of rapamycin
(14). LncRNA FEZF1 antisense RNA1
(AS1) may repress the expression of p21 and promote the
proliferation of GC cells through lysine-specific demethylase
1-mediated H3K4 dimethylation (15).
LncRNA SPRY4-intronic transcript 1 may lead to
microRNA-101-3p-mediated proliferation and metastasis of bladder
cancer cells through upregulating enhancer of zeste homolog 2
(16). These studies suggest that
lncRNAs may be involved in tumor development and progression.
P73 antisense RNA 1T also known as TP73-AS1 or PDAM,
is a long noncoding RNA which may regulate apoptosis via
p53-dependent anti-apoptotic genes, and may be deregulated in
cancer (17,18). To the best of our knowledge, the
biological function of TP73-AS1 in patients with GC has not been
examined. Additionally, the function of TP73-AS1 in cisplatin
resistance of GC remains unclear.
Reverse-transcription quantitative polymerase chain
reaction (RT-qPCR) analysis was conducted to detect the expression
levels of TP73-AS1 in GC tissues and cell lines. Following
transfection, loss-of function assays were conducted in GC cells,
to measure the effects of silenced TP73-AS1 on cell growth and the
chemosensitivity of GC cells. Mechanism experiments were performed
to examine the functional mechanism underlying TP73-AS1 and
mobility group 1 (HMGB1)/receptor for advanced glycation
endproducts (RAGE) signaling pathway in GC; therefore, the study
investigated the function and mechanism underlying TP73-AS1 in
GC.
Materials and methods
Clinical tissues
A total of 58 patients with GC underwent surgery at
the Department of Gastrointestinal Surgery, the First Affiliated
Hospital of Sun Yat-Sen University (Guangdong, China) and were
enrolled in the present study. In total, 58 pairs of GC tissues and
adjacent non-tumor tissues were collected between September 2008
and September 2011 and stored at −80°C. Patients who were not
diagnosed with gastric cancer were excluded from the present study.
Patients who received previous treatment were excluded from this
study. The clinicopathological characteristics of patients with GC
are presented in Table I. Tumor
differentiation was defined based on the cellular differentiation
degree, which may be divided into three grades including well
differentiation, moderate differentiation and poor differentiation
(19). The present study was approved
by the Department of Gastrointestinal Surgery, the First Affiliated
Hospital of Sun Yat-Sen University (Guangdong, China). Written
informed consent was obtained from all participants.
 | Table I.Association between the expression of
lncRNA-TP73-AS1 and clinical features in gastric cancer. |
Table I.
Association between the expression of
lncRNA-TP73-AS1 and clinical features in gastric cancer.
|
| LncRNA-TP73-AS1
expression, n |
|
|---|
|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Sex |
|
| 0.113 |
|
Male | 20 | 11 |
|
|
Female | 11 | 16 |
|
| Age, years |
|
| 0.428 |
|
<60 | 12 | 14 |
|
|
≥60 | 19 | 13 |
|
| T stage |
|
| 0.001 |
|
T1-T2 | 22 | 7 |
|
|
T3-T4 | 9 | 20 |
|
| Lymph node
metastasis |
|
| 0.008 |
| No | 21 | 8 |
|
|
Yes | 10 | 19 |
|
| Distant
metastasis |
|
| 0.034 |
| No | 17 | 7 |
|
|
Yes | 14 | 20 |
|
| Tumor size, cm |
|
| 0.124 |
|
<5 | 18 | 10 |
|
| ≥5 | 13 | 17 |
|
Cell culture
GC cell lines, including AGS, SGC-7901, BGC-823 and
MGC-803 and a normal gastric epithelial cell line (GES-1) were
obtained from the Institute of Biochemistry and Cell Biology of the
Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) medium supplemented with
10% of fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in a
humidified atmosphere containing 5% CO2.
Plasmid construction and
transfection
The full-length TP73-AS1 sequence was synthesized
and then sub-cloned into pcDNA3.1 vector (Thermo Fisher Scientific,
Inc.) to construct the pcDNA3.1-TP73-AS1 vector. The blank vector
was obtained from the Thermo Fisher Scientific, Inc.. Subsequently,
the pcDNA3.1-TP73-AS1 vector (2 µg) or the empty vector (2 µg) was
transfected into the cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) in Hybridoma
serum-free medium (Gibco, Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions.
The primers (Thermo Fisher Scientific, Inc.) were as
follows: TP73-AS1, 5′-TCAGGTTCGTAACGGTGCGTT-3′ (forward) and
5′-TCGTATCTCGCGACTCTTCC-3′ (reverse). The empty pcDNA3.1 vector was
used as a negative control. The small interfering RNA (siRNA)
sequence for TP73-AS1 was as follows:
5′-CCTGCTGCCTCTCCAAGAGACTGCTATTA-3′. The plasmid of pcDNA/TP73-AS1
was transfected into GES-1 cells (90%) at a density of
0.8×106 cells at a final concentration of 2 µg/ml using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.), and were
incubated for 48 h.
RNA extraction and RT-qPCR
Total RNA was isolated from cells and tissues using
TRIzol reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. According to the manufacturer's protocols,
PrimeScript™ RTMaster Mix (Takara Biotechnology Co., Ltd., Dalian,
China) was used to reverse transcribe RNA to cDNA. RT-qPCR was
performed using random primers from Augct DNA-Syn Biotechnology
Co., Ltd. (Beijing, China). Real time PCR conditions were: 1 cycle
of 2 min at 50°C; 1 cycle of 10 min at 95°C; and 40 cycles of 15
sec at 95°C and 1 min at 60°C. RT-qPCR was performed using ABI 7300
Real-time PCR system and Power SYBR- Green PCR master mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The primers were as follows: TP73-AS1,
5′-CCGGTTTTCCAGTTCTTGCAC-3′ (forward) and
5′-GCCTCACAGGGAAACTTCATGC-3′ (reverse); GAPDH,
5′-GTCAACGGATTTGGTCTGTATT-3′ (forward) and
5′-AGTCTTCTGGGTGGCAGTGAT-3′ (reverse). Relative expression levels
were determined using the 2−ΔΔCq method (20). StepOne™ Software Version 2.1 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was utilized to analyze
Cq value. GAPDH was used as the internal reference. All experiments
were performed in triplicate.
Cell viability
GC cells (3–6×103) were incubated at 37°C
in a 96-well plate in DMEM (200 µl/well) in a humidified atmosphere
containing 5% CO2 for 24–72 h and 20 µl MTT solution (5
mg/ml; Merck KGaA, Darmstadt, Germany) was added into each well. At
4 h, the culture medium was discarded and dimethyl sulfoxide (DMSO;
150 µl) (Merck KGaA) was added into each well and mixed for 10 min
to dissolve crystallization. Absorbance values were determined
using a microplate reader at a wavelength of 570 nm at indicated
time points (12, 24, 48, 72 and 96 h). All experiments were
performed in triplicate. The chemosensitivity was determined using
an MTT assay (5 mg/ml; Merck KGaA, Darmstadt, Germany). Cells were
cultured in 96-well plates and were treated with cisplatin (0, 5,
10, 15 and 20 µm/ml; BioVision, Inc., Milpitas, CA, USA). At 48 h
post-treatment, MTT solution was added into each well. At 4 h, the
medium was removed and 100 µl DMSO was added into each well.
Absorbance values were determined using a microplate reader at a
wavelength of 560 nm. All experiments were performed in
triplicate.
Colony formation assay
Cells (500 cells/well) were plated in 6-well plates
and incubated in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% of FBS at 37°C for 2 weeks. Following
incubation, cells were fixed with 4% methanol for 15 min at room
temperature and stained with 0.1% of crystal violet at room
temperature for 30 min. The number of visible colonies was counted
manually using an Olympus optical microscope (DSX100; Olympus
Corporation, Tokyo, Japan).
Flow cytometric analysis of
apoptosis
Cells were transfected with indicated plasmids
(pcDNA/TP73-AS1) or negative control for 48 h as aforementioned.
Cells were stained using an Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) kit (BD Biosciences, San Jose, CA,
USA), according to the manufacturer's protocol. Cells were analyzed
using a flow cytometer and CellQuest software version 0.9.3.1 (BD
Biosciences). All experiments were performed in triplicate.
Flow cytometric analysis of cell cycle
distribution
Cells were collected at 48 h post-transfection,
washed with ice-cold phosphate-buffered saline (PBS). Following
this, the cells were fixed with 70% ethanol at 4°C for 2 h. Fixed
cells were rehydrated in PBS for 10 min and then were incubated in
RNase A (1 mg/ml) for 30 min at 37°C, and stained with PI/RNase (1
ml) at 4°C overnight in a dark place. Cells were analyzed using a
flow cytometer (BD Biosciences). All experiments were performed in
triplicate.
Western blot analysis
Total protein was isolated from cells using
radioimmunoprecipitation assay buffer (Merck KGaA) with Complete
Protease Inhibitor Cocktail (Roche Diagnostics GmbH, Mannheim,
Germany) and stored at −20°C. Protein concentration was evaluated
with the BCA protein assay kit (Thermo Fisher Scientific, Inc.).
Each sample (40 mg/lane) was isolated by 10% SDS-PAGE
electrophoresis and transferred to polyvinylidene fluoride
membranes (Sangon Biotech Co., Ltd.). The membranes were blocked
with 5% skimmed milk at 37°C for 1 h. The membranes were incubated
with the following primary antibodies: Anti-HMGB1 (1:1,000;
ab79823), anti-RAGE (1:1,000: ab3611), nuclear factor (NF)-κB
(1:1,000; ab222497), anti-p21 (1:1,000; ab109520),
anti-cyclin-dependent kinases (CDK)2 (1:1,000; ab208697), anti-CDK4
(1:1,000; ab199728), anti-CDK6 (1:1,000; ab151247) and anti-GAPDH
(1:1,000; ab9485) at 4°C overnight and with horseradish
peroxidase-conjugated goat anti-mouse IgG H&L (1:2,000; ab6789)
at 37°C for 1 h. All antibodies used in this experiment were
obtained from Abcam (Cambridge, UK). The molecular weight of
candidate proteins was referred to the Pre-stained SeeBlue Rainbow
marker (Thermo Fisher Scientific, Inc.) loaded in parallel. The
blots were visualized using the enhanced chemiluminescence
detection reagent (Thermo Fisher Scientific, Inc.). The results
were analyzed with Quantity One software (V4.4.0; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data were analyzed using SPSS software (version
19.0; IBM Corp., Armonk, NY, USA). The relevant data are expressed
as the mean ± standard deviation (SD). The Chi-square test was used
to assess the association between TP73-AS1 expression and
clinicopathological factors. Differences between two groups were
analyzed using Student's t-test. One-way analysis of variance
(Least-Significance-Difference post-hoc test) was performed when
multiple comparisons were performed. Survival analysis was
performed using the Kaplan-Meier method and the log-rank test. Cox
proportional hazards regression model was generated to identify
factors associated with overall survival through a multivariate
survival analysis. Correlation among the expression levels of
TP73-AS1, HMGB1 and RAGE in 58 cases of GC s were analyzed using
Spearman's correlation analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
TP73-AS1 is upregulated in human GC
tissues and is associated with poor prognosis
To explore the biological function of TP73-AS1 in
GC, the expression level of TP73-AS1 was examined in 58 GC tissues
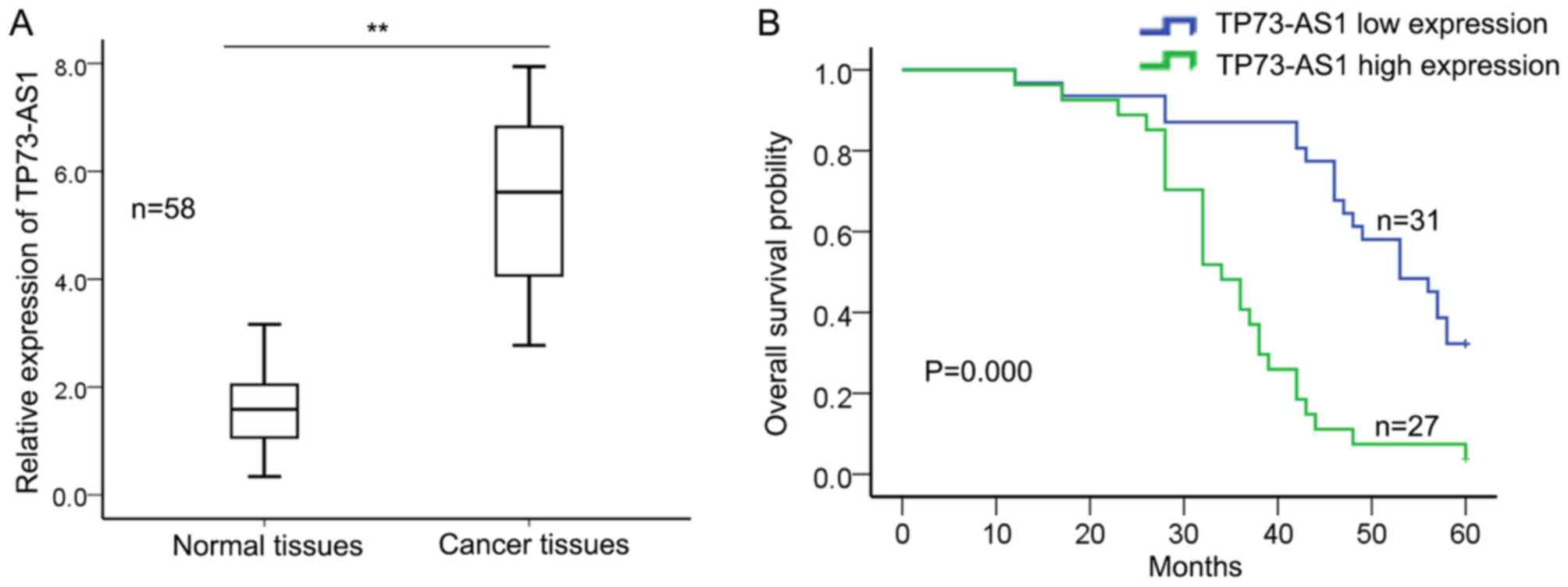
and adjacent normal tissues using RT-qPCR. Fig. 1A demonstrated that the relative
expression of TP73-AS1 was significantly increased in GC tissues
compared with that in adjacent normal tissues. Then, the
association between the expression level of TP73-AS1 and the
clinicopathological parameters of 58 patients with GC was
evaluated. The mean value of TP73-AS1 in GC tissues was used as a
cutoff value and patients with GC were divided into two groups
(high expression group, n=27; low expression group, n=31). Table I demonstrated that increased
expression level of TP73-AS1 was significantly associated with
tumor stage (P=0.001), lymph node metastasis (P=0.008), distant
metastasis (P=0.034) and differentiation (P=0.017), but was not
significantly associated with age, sex and tumor size (P>0.05).
Furthermore, Kaplan-Meier method analysis and log-rank test was
performed to determine the association between TP73-AS1 expression
and overall survival of patients with GC. Fig. 1B demonstrates that patients with
increased expression of TP73-AS1 exhibited a significantly shorter
overall survival compared with those with low expression level of
TP73-AS1 (P=0.000). Cox's proportional hazards analysis revealed
that the increased expression level of TP73-AS1 (P=0.012; Table II) may be a prognostic factor in GC.
These results suggest that TP73-AS1 may act as an oncogene in GC
and may be considered as a specific biomarker for poor prognosis in
GC.
 | Table II.Multivariate analysis of prognostic
parameters in patients with gastric cancer by Cox's proportional
hazard model analysis. |
Table II.
Multivariate analysis of prognostic
parameters in patients with gastric cancer by Cox's proportional
hazard model analysis.
| Variable | P-value |
|---|
| Sex | 0.459 |
|
Male |
|
|
Female |
|
| Age, years | 0.494 |
|
<60 |
|
|
≥60 |
|
| T stage | 0.897 |
|
T1-T2 |
|
|
T3-T4 |
|
| Lymph node
metastasis | 0.652 |
| No |
|
|
Yes |
|
| Distant
metastasis | 0.257 |
| No |
|
|
Yes |
|
|
Differentiation | 0.002 |
|
Well/moderate |
|
|
Poor |
|
| Tumor size, cm | 0.602 |
|
<5 |
|
| ≥5 |
|
| TP73-AS1
expression | 0.012 |
|
Low |
|
|
High |
|
Knockdown of TP73-AS1 suppresses cell
proliferation and increases the sensitivity of GC cells to
cisplatin
To determine the biological function of TP73-AS1 in
GC, the expression level of TP73-AS1 was evaluated in GC cell lines
(AGS, SGC-7901, BGC-823 MGC-803) and a normal gastric epithelial
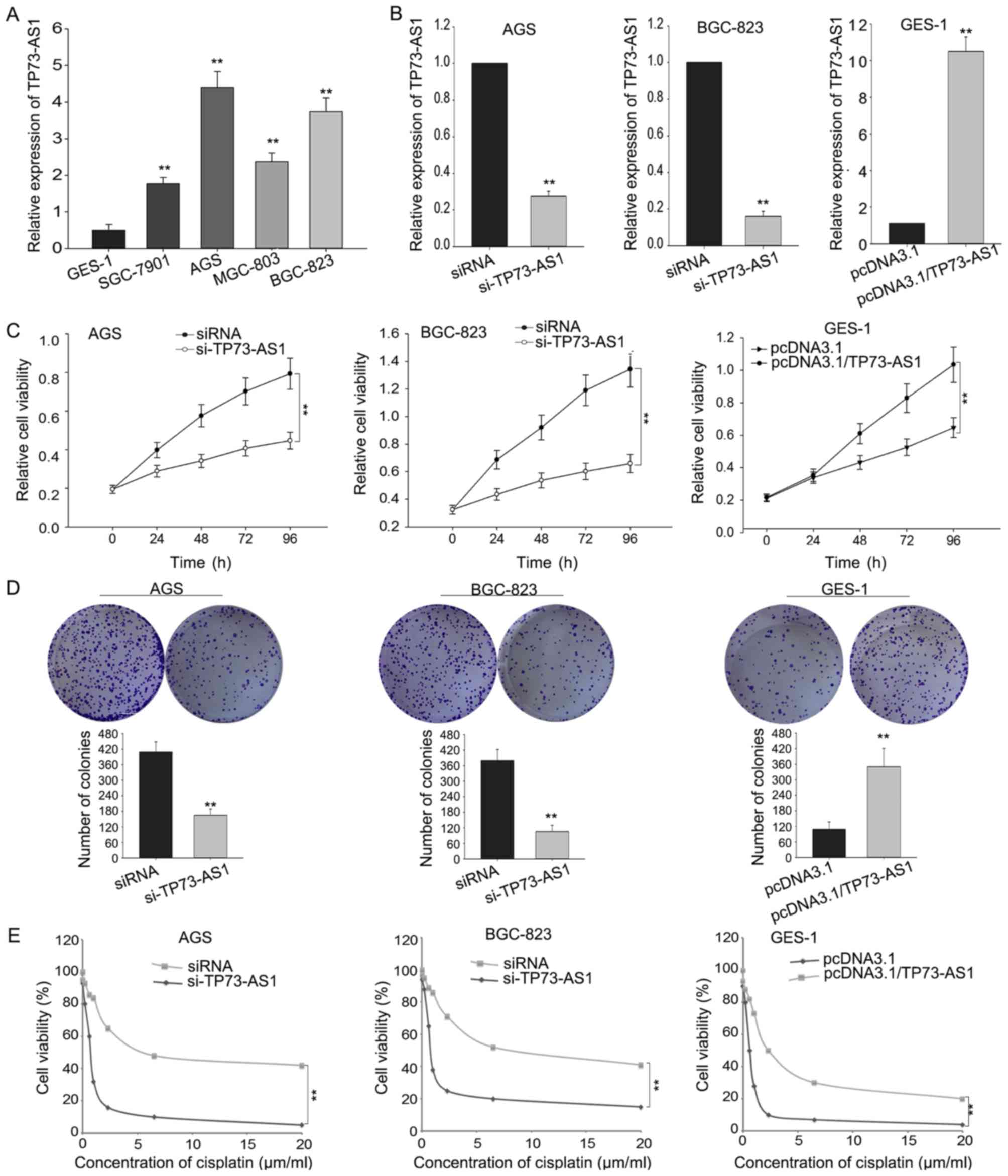
cell line (GES-1). Fig. 2A
demonstrates that the expression level of TP73-AS1 in GC cells was
significantly increased compared with that in the normal gastric
epithelial cell line. Among the four GC cells, the expression of
TP73-AS1 was increased in AGS and BGC-823 cells compared with that
in the remaining cell lines. Therefore, AGS and BGC-823 cells were
selected for subsequent experiments.
AGS and BGC-823 cells were transfected with TP73-AS1
specific siRNA in order to downregulate the endogenous level of
TP73-AS1. GES-1 cells were transfected with TP73-AS1 expression
vector (pcDNA3.1/TP73-AS1) to enhance the expression level of
TP73-AS1. The results demonstrated that the expression of TP73-AS1
was downregulated in AGS and BGC-823 cells following transfection
with si-TP73-AS1 compared with that in the negative control
(Fig. 2B). Additionally, the
expression level of TP73-AS1 in pcDNA3.1/TP73-AS1-transfected GES-1
cells was increased compared with that in the negative control
(pcDNA3.1) (Fig. 2B).
MTT and colony formation assays were also performed.
The results demonstrated that the cell proliferation was impaired
in AGS and BGC-823 cells transfected with siRNA compared with that
in the negative control (Fig. 2C).
Additionally, overexpression of TP73-AS1 in GES-1 cells promoted
cellular proliferative ability compared with that in the negative
control (Fig. 2C). The colony
formation ability of AGS and BGC-823 cells transfected with siRNA
was decreased compared with that in the negative control (Fig. 2D), whereas an increased colony
formation ability was observed in GES-1 cells transfected with
pcDNA3.1/TP73-AS1 (Fig. 2D).
Additionally, downregulation of TP73-AS1 increased the sensitivity
of AGS and BGC-823 cells to cisplatin compared with
control-transfected cells, whereas overexpressed TP73-AS1
significantly decreased the sensitivity of GES-1 cells to cisplatin
(Fig. 2E). These results indicated
that TP73-AS1 may be involved in the progression of GC.
Silencing of TP73-AS1 inhibits cell
proliferation and increases chemosensitivity through regulating
cell cycle and apoptosis
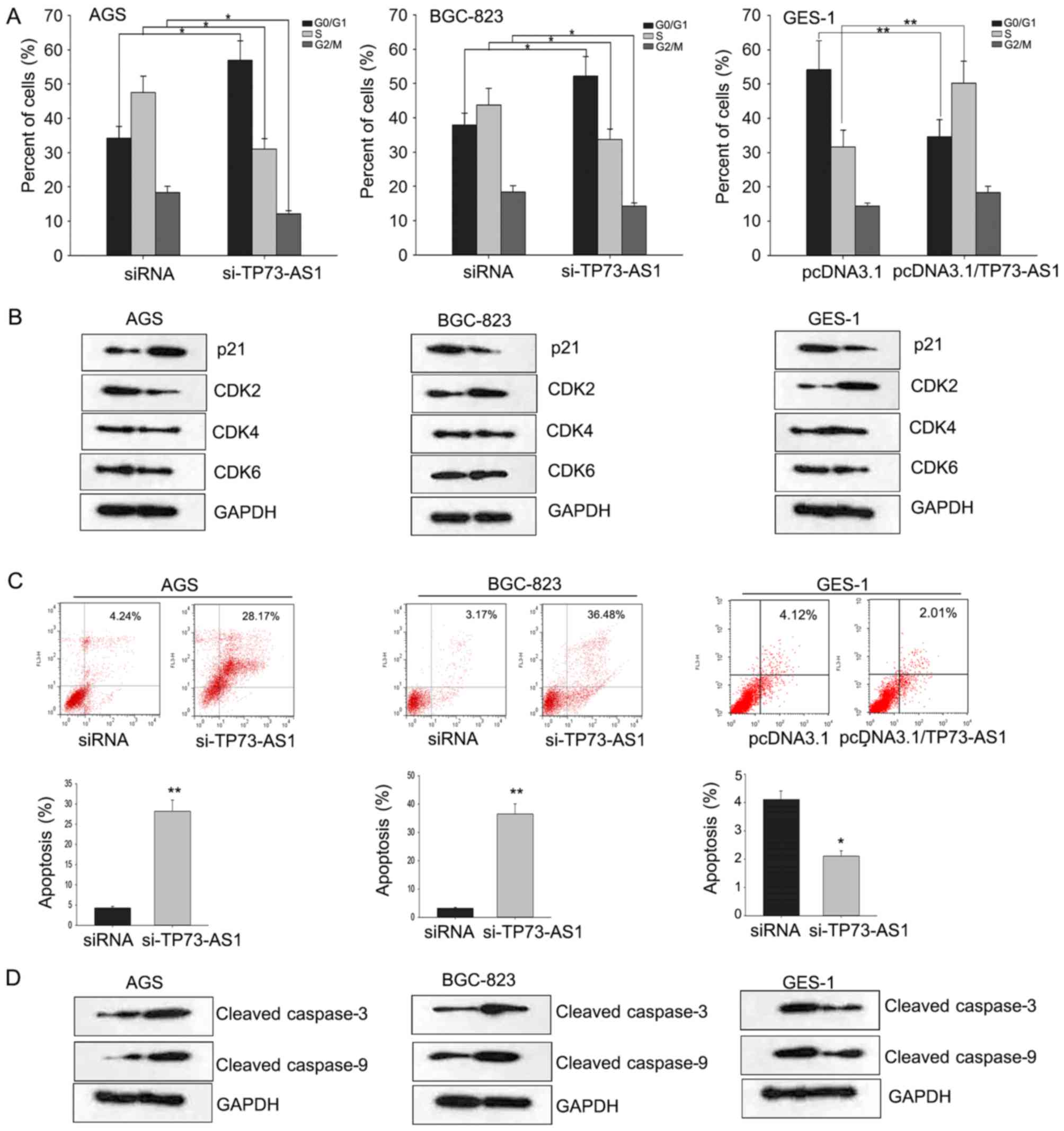
Flow cytometric analyses were conducted to
investigate the effects of dysregulated TP73-AS1 on cell apoptosis
and cell cycle in GC. As demonstrated in Fig. 3A, knockdown of TP73-AS1 in AGS and
BGC-823 cells induced cell cycle arrest at G1 phase, whereas
overexpressed TP73-AS1 promoted cell cycle progression. CDKs are
key factors in G1/S phase transition. The dysregulation of the cell
cycle may be mediated by deregulation of CDKs (21). To determine the mechanism by which
TP73-AS1 may regulate cell cycle, the expression level of CDKs
(CDK2, 4 and 6) was determined. The results demonstrated that CDK2
may be positively regulated by TP73-AS1 (Fig. 3B).
CDK2 may serve a crucial function in cell cycle
progression and apoptosis and its activity may be regulated by the
CDK inhibitor p21 (22). Therefore,
the expression level of p21 was evaluated in
TP73-AS1-downregulated/overexpressed GC cells. The results
demonstrated that p21 may be negatively regulated by TP73-AS1
(Fig. 3B). These results indicated
that TP73-AS1 may affect the cell cycle through targeting p21 in
GC. Additionally, downregulation of TP73-AS1 significantly
increased the apoptosis rate of AGS and BGC-823 cells (Fig. 3C). The levels of apoptosis-associated
proteins (cleaved caspase-3 and −9) were examined in indicated GC
cells (Fig. 3D). These results
indicated that downregulation of TP73-AS1 inhibited cell
proliferation and increased chemosensitivity, which may be mediated
through the regulation of cell cycle and apoptosis.
Downregulation of TP73-AS1 increased
the sensitivity of GC cells to cisplatin through targeting the
HMGB1 signaling pathway
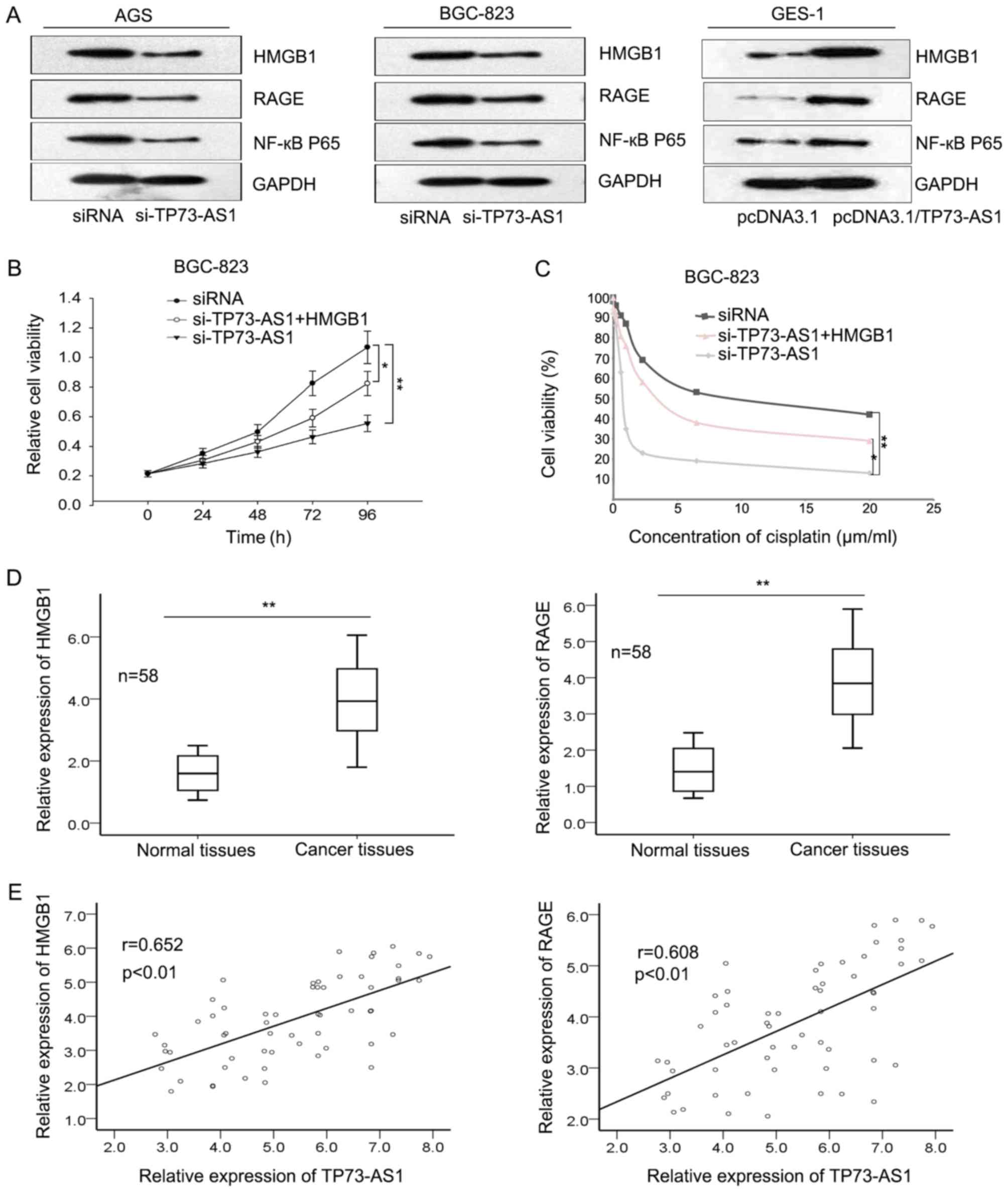
HMGB1 is an evolutionarily ancient and critical
regulator for cell death and survival. It has been revealed that
HMGB1 may activate the RAGE signaling pathway and induce the
activation of NF-κB to promote cellular processes (23). Previous studies demonstrated that the
HMGB1/RAGE signaling pathway may be involved in the biological
function of TP73-AS1 in hepatocellular carcinoma and glioma
(17,24). To determine whether the HMGB1/RAGE
signaling pathway was involved in TP73-AS1-mediated effects in GC,
the levels of HMGB1, RAGE and NF-κB were evaluated in response to
downregulation or upregulation of TP73-AS1. Fig. 4A demonstrates that the protein levels
of HMGB1, RAGE and NF-κB were significantly decreased in AGS and
BGC-823 cells following knockdown of TP73-AS1 (achieved by
si-TP73-AS1) whereas their protein levels were increased in GES-1
cells with an overexpression of TP73-AS1. Rescue assays were
performed to confirm the association between TP73-AS1 and HMGB1.
Fig. 4B and C demonstrate that
co-transfection with HMGB1 overexpression vector may restore the
proliferative ability and the sensitivity to cisplatin mediated by
si-TP73-AS1 in BGC-823 cells. Additionally, the expression levels
of HMGB1 and RAGE were evaluated in GC tissues. The results
demonstrated that the expression of HMGB1 and RAGE was upregulated
in GC tissues (Fig. 4D), and were
positively correlated with TP73-AS1 (Fig.
4E). Collectively, the results revealed that TP73-AS1 regulated
the sensitivity of GC cells to cisplatin through the HMGB1/RAGE
signaling pathway.
Discussion
GC is a common malignancy in humans and is
associated with an increased incidence in China (25). Several studies have investigated
strategies for improving the diagnostic methods in GC. Dakal et
al (26) revealed that the
deregulation of IL-8 may be an important prognostic marker for
patients with GC. Due to the lack of effective techniques for early
diagnosis, the majority the patients with GC are diagnosed at late
stages of GC. Chemotherapy is the primary treatment for GC and is
used in patients at advanced stage of GC. However, chemoresistance
remains to be a major obstacle for clinical treatment of GC. The
molecular mechanism underlying chemoresistance is complex and
involves a deregulation of various biological processes involved in
drug metabolism and transport, apoptosis and DNA repair (27–32).
Despite several advances, the molecular mechanisms underlying
chemoresistance remain unclear. Therefore, further investigation on
the molecular mechanism underlying the chemoresistance in GC is
required.
Accumulating evidence suggest that lncRNAs are
associated with various biological processes (32–37). The
prognostic potential of lncRNAs has been demonstrated in several
types of cancer, including GC. Wu et al (38) demonstrated that increased expression
of long noncoding RNA colon cancer-associated transcript 2
indicated poor prognosis of GC. Tan et al (39) revealed that plasma lncRNA-gastric
cancer associated transcript 2 may be a valuable marker for the
screening of GC. Moreover, Liu and Shangguan (40) demonstrated that the upregulation of
lncRNA CARLo-5 was associated with poor prognosis in patients with
GC. However, whether additional lncRNAs may be associated with
chemoresistance remains to be investigated. TP73-AS1, a lncRNA
transcribed from chromosome 1p36, has been reported to be
associated with cell proliferation and tumor progress (17,18).
Previous studies predicted that TP73-AS1 may be upregulated in
glioma and esophageal squamous cell carcinoma and was associated
with the progression and prognosis of cancer (17,18).
However, its biological function in GC still remains unclear.
The results of the present study demonstrated that
TP73-AS1 was differentially expressed in the GC tissues and cell
lines compared with those of controls, and increased expression
level of TP73-AS1 was associated with poor prognosis of GC. Cox's
proportional hazards analysis revealed that increased expression of
TP73-AS1 may be considered as a specific biomarker for the poor
prognosis of GC. Furthermore, cellular transfection experiments
revealed that knockdown of TP73-AS1 significantly suppressed the
proliferative ability and increased the sensitivity to cisplatin of
GC cells. Flow cytometric analysis revealed that downregulation of
TP73-AS1 may induce cell cycle arrest and promote cell apoptosis.
The results demonstrated that the HMGB1/RAGE signaling pathway was
involved in TP73-AS1-mediated function in GC. Taken together, the
results of the present study investigated the lncRNA-mediated
regulation of chemoresistance in GC and provide a potential
candidate for novel therapeutic strategies in GC.
Acknowledgements
The author would like to thank the laboratory
members, who assisted the author to finish the experiments.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JP wrote the present manuscript. All experiments
were designed and conducted by JP. Data were collected by JP.
Ethics approval and consent to
participate
The present study was approved by the Department of
Gastrointestinal Surgery, the First Affiliated Hospital of Sun
Yat-Sen University (Guangdong, China). Written informed consent was
obtained from all participants.
Patient consent for publication
All patients have provided written informed consent
for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Niccolai E, Taddei A, Prisco D and Amedei
A: Gastric cancer and the epoch of immunotherapy approaches. World
J Gastroenterol. 21:5778–5793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forman D and Burley VJ: Gastric cancer:
Global pattern of the disease and an overview of environmental risk
factors. Best Pract Res Clin Gastroenterol. 20:633–649. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terry MB, Gaudet MM and Gammon MD: The
epidemiology of gastric cancer. Semin Radiat Oncol. 12:111–127.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin Y, Cui Z, Li X, Jin X and Peng J:
Upregulation of long non-coding RNA PlncRNA-1 promotes
proliferation and induces epithelial-mesenchymal transition in
prostate cancer. Oncotarget. 8:26090–26099. 2017.PubMed/NCBI
|
|
6
|
Conte F, Fiscon G, Chiara M, Colombo T,
Farina L and Paci P: Role of the long non-coding RNA PVT1 in the
dysregulation of the ceRNA-ceRNA network in human breast cancer.
PLoS One. 12:e01716612017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Sun L, Xuan L, Pan Z, Li K, Liu
S, Huang Y, Zhao X, Huang L, Wang Z, et al: Reciprocal changes of
circulating long non-coding RNAs ZFAS1 and CDR1AS predict acute
myocardial infarction. Sci Rep. 6:223842016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei X, Wang C, Ma C, Sun W, Li H and Cai
Z: Long noncoding RNA ANRIL is activated by hypoxia-inducible
factor-1α and promotes osteosarcoma cell invasion and suppresses
cell apoptosis upon hypoxia. Cancer Cell Int. 16:732016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng W, Wang Z and Fan H: LncRNA NEAT1
impacts cell proliferation and apoptosis of colorectal cancer via
regulation of Akt signaling. Pathol Oncol Res. 23:651–656. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tao H, Cao W, Yang JJ, Shi KH, Zhou X, Liu
LP and Li J: Long noncoding RNA H19 controls DUSP5/ERK1/2 axis in
cardiac fibroblast proliferation and fibrosis. Cardiovasc Pathol.
25:381–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizrahi I, Mazeh H, Grinbaum R, Beglaibter
N, Wilschanski M, Pavlov V, Adileh M, Stojadinovic A, Avital I,
Gure AO, et al: Colon cancer associated transcript-1 (CCAT1)
expression in adenocarcinoma of the stomach. J Cancer. 6:105–110.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao Y, Guo Q, Chen J, Hu J, Wang S and
Sun Y: Role of long non-coding RNA HULC in cell proliferation,
apoptosis and tumor metastasis of gastric cancer: A clinical and in
vitro investigation. Oncol Rep. 31:358–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kallen AN, Zhou XB, Xu J, Qiao C, Ma J,
Yan L, Lu L, Liu C, Yi JS, Zhang H, et al: The imprinted H19 lncRNA
antagonizes let-7 microRNAs. Mol Cell. 52:101–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Malakar P, Shilo A, Mogilevsky A, Stein I,
Pikarsky E, Nevo Y, Benyamini H, Elgavish S, Zong X, Prasanth KV
and Karni R: Long noncoding RNA MALAT1 promotes hepatocellular
carcinoma development by SRSF1 upregulation and mTOR activation.
Cancer Res. 77:1155–1167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YW, Xia R, Lu K, Xie M, Yang F, Sun M,
De W, Wang C and Ji G: LincRNAFEZF1-AS1 represses p21 expression to
promote gastric cancer proliferation through LSD1-Mediated H3K4me2
demethylation. Mol Cancer. 16:392017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X,
Wang M, Huang C, Wang L, Zeng F and Jiang G: LncRNA SPRY4-IT1
sponges miR-101-3p to promote proliferation and metastasis of
bladder cancer cells through up-regulating EZH2. Cancer Lett.
388:281–291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang R, Jin H and Lou F: The long
non-coding RNA TP73-AS1 interacted with miR-142 to modulate brain
glioma growth through HMGB1/RAGE pathway. J Cell Biochem.
119:3007–3016. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zang W, Wang T, Wang Y, Chen X, Du Y, Sun
Q, Li M, Dong Z and Zhao G: Knockdown of long non-coding RNA
TP73-AS1 inhibits cell proliferation and induces apoptosis in
esophageal squamous cell carcinoma. Oncotarget. 7:19960–19974.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng XC, Zeng Z, Huang YN, Deng YC and Fu
GH: Clinical significance of TM4SF1 as a tumor suppressor gene in
gastric cancer. Cancer Med. 7:2592–2600. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ansari SS, Sharma AK, Zepp M, Ivanova E,
Bergmann F, Konig R and Berger MR: Upregulation of cell cycle genes
in head and neck cancer patients may be antagonized by erufosine's
down regulation of cell cycle processes in OSCC cells. Oncotarget.
9:5797–5810. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang H, Regan KM, Lou Z, Chen J and
Tindall DJ: CDK2-dependent phosphorylation of FOXO1 as an apoptotic
response to DNA damage. Science. 314:294–297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen RC, Yi PP, Zhou RR, Xiao MF, Huang
ZB, Tang DL, Huang Y and Fan XG: The role of HMGB1-RAGE axis in
migration and invasion of hepatocellular carcinoma cell lines. Mol
Cell Biochem. 390:271–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Huang Y, Huang Y, Fu Y, Tang D, Kang
R, Zhou R and Fan XG: The long non-coding RNA TP73-AS1 modulates
HCC cell proliferation through miR-200a-dependent HMGB1/RAGE
regulation. J Exp Clin Cancer Res. 36:512017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coupland VH, Lagergren J, Lüchtenborg M,
Jack RH, Allum W, Holmberg L, Hanna GB, Pearce N and Møller H:
Hospital volume, proportion resected and mortality from oesophageal
and gastric cancer: A population-based study in England, 2004–2008.
Gut. 62:961–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dakal TC, Kala D, Dhiman G, Yadav V,
Krokhotin A and Dokholyan NV: Predicting the functional
consequences of non-synonymous single nucleotide polymorphisms in
IL8 gene. Sci Rep. 7:65252017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rassy EE, Assi T, Rizkallah J and Kattan
J: Diffuse edema suggestive of cytokine release syndrome in a
metastatic lung carcinoma patient treated with pembrolizumab.
Immunotherapy. 9:309–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jung KH, Choi IK, Lee HS, Yan HH, Son MK,
Ahn HM, Hong J, Yun CO and Hong SS: Oncolytic adenovirus expressing
relaxin (YDC002) enhances therapeutic efficacy of gemcitabine
against pancreatic cancer. Cancer Lett. 396:155–166. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh MJ, Lin CW, Yang SF, Sheu GT, Yu YY,
Chen MK and Chiou HL: A combination of pterostilbene with autophagy
inhibitors exerts efficient apoptotic characteristics in both
chemosensitive and chemoresistant lung cancer cells. Toxicol Sci.
156:5492017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fong Y, Wu CY, Chang KF, Chen BH, Chou WJ,
Tseng CH, Chen YC, Wang HD, Chen YL and Chiu CC: Dual roles of
extracellular signal-regulated kinase (ERK) in quinoline compound
BPIQ-induced apoptosis and anti-migration of human non-small cell
lung cancer cells. Cancer Cell Int. 17:372017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Elias KM, Harvey RA, Hasselblatt KT, Seckl
MJ and Berkowitz RS: Type I interferons modulate methotrexate
resistance in gestational trophoblastic neoplasia. Am J Reprod
Immunol. 77:e126662017. View Article : Google Scholar
|
|
32
|
Cai W, Chen G, Luo Q, Liu J, Guo X, Zhang
T, Ma F, Yuan L, Li B and Cai J: PMP22 regulates self-renewal and
chemoresistance of gastric cancer cells. Mol Cancer Ther.
16:1187–1198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu J, Cheng G, Zhang C, Zheng Y, Xu H,
Yang H and Hua L: Long noncoding RNA LINC01296 is associated with
poor prognosis in prostate cancer and promotes cancer-cell
proliferation and metastasis. Onco Targets Ther. 10:1843–1852.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rao AKDM, Rajkumar T and Mani S:
Perspectives of long non-coding RNAs in cancer. Mol Biol Rep.
44:203–218. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qian L, Xu F, Wang X, Jiang M, Wang J,
Song W, Wu D, Shen Z, Feng D, Ling B, et al: LncRNA expression
profile of ΔNp63α in cervical squamous cancers and its suppressive
effects on LIF expression. Cytokine. 96:114–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li S, Li B, Zheng Y, Li M, Shi L and Pu X:
Exploring functions of long noncoding RNAs across multiple cancers
through co-expression network. Sci Rep. 7:7542017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang S, Chen B, Wang X, Wu K and Sun Y:
Long non-coding RNA XIST regulates PTEN expression by sponging
miR-181a and promotes hepatocellular carcinoma progression. BMC
Cancer. 17:2482017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu SW, Hao YP, Qiu JH, Zhang DB, Yu CG and
Li WH: High expression of long non-coding RNA CCAT2 indicates poor
prognosis of gastric cancer and promotes cell proliferation and
invasion. Minerva Med. 108:317–323. 2017.PubMed/NCBI
|
|
39
|
Tan L, Yang Y, Shao Y, Zhang H and Guo J:
Plasma lncRNA-GACAT2 is a valuable marker for the screening of
gastric cancer. Oncol Lett. 12:4845–4849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu JN and Shangguan YM: Long non-coding
RNA CARLo-5 upregulation associates with poor prognosis in patients
suffering gastric cancer. Eur Rev Med Pharmacol Sci. 21:530–534.
2017. View Article : Google Scholar : PubMed/NCBI
|