Introduction
Lung cancer is the leading cause of
cancer-associated mortality globally, and is characterized by
uncontrolled proliferation and metastasis of lung cancer cells
(1,2).
Although progress has been made in lung cancer research, the
outcome of patients with lung cancer remains poor, and only 17% of
patients survive >5 years after diagnosis (1,2).
Proliferation and metastasis are the primary causes of mortality in
patients with lung cancer (3), which
emphasizes the requirement for an improved understanding of lung
cancer carcinogenesis.
Long non-coding RNAs (lncRNAs) are >200
nucleotides in length and regulate gene expression at
transcriptional or post-transcriptional levels in various
biological processes, including cancer development. Liu et
al (4) reported that lncRNA
regulator of reprogramming (ROR) shares the binding site of miR-145
with POU class 5 homeobox 1 (OCT4) mRNA. Suppression of lncRNA ROR
expression in prostate cancer cells may lead to increased
expression of microRNA (miR)-145, thus preventing cell
proliferation by decreasing OCT4 expression. Wang et al
(5) demonstrated that lncRNA
urothelial cancer associated 1 (UCA1) increased the metastatic
ability of gastric cancer cells by promoting G protein-coupled
receptor kinase 2 ubiquitination and degradation, suggesting that
lncRNA UCA1 may function as a mediator of protein ubiquitination
and may be a target for gastric cancer therapy. Chen et al
(6) reported that lncRNA X inactive
specific transcript (XIST) was able to bind miR-200-3p, promoting
zinc finger E-box binding homeobox (ZEB)1 expression, a tumor
suppressor gene, suggesting that lncRNA XIST may be used as a
prognostic biomarker for patients with CRC. Wang et al
(7) demonstrated that lncRNA HOXD
cluster antisense RNA 1 was highly expressed in hepatocellular
carcinoma, competitively binding to miR-130a-3p to increase the
expression of SRY-box 4, and subsequently promoting metastasis of
hepatocellular carcinoma. Numerous reports have indicated lncRNAs
participate in tumor genesis and progression, including that of
lung cancer (8,9). However, the detailed molecular
mechanisms of lncRNA activity in lung cancer require further
study.
LncRNA activated by TGF-β (ATB) was first identified
in hepatocellular carcinoma, in which it upregulated ZEB1 and ZEB2
expression by competitively binding the miR-200 family, thus
inducing epithelial-mesenchymal-transition (EMT) and invasion of
cancer cells (10). Recently, it has
been demonstrated that ATB promotes malignancy in other types of
cancer, including breast cancer (11), glioma (12) and colon cancer (13). However, the function of ATB in lung
cancer remains unclear.
The present study aimed to investigate the role of
ATB in lung cancer. It was demonstrated that ATB was highly
expressed in lung cancer tissue, and that knockdown of ATB in lung
cancer cell lines inhibited the proliferation and metastasis of
lung cancer cells. Finally, the potential mechanism of ATB in lung
cancer was investigated.
Materials and methods
Clinical sample collection and
preparation
A total of 32 lung cancer tissue specimens and
adjacent non-cancerous tissue specimens were collected from
patients (21 male and 11 female; the age ranged from 47 to 73
years, with a mean age of 63.3 years) from Southwest Hospital in
(Chongqing, China) between January 2015 and December 2016. None of
these patients had received radiotherapy or chemotherapy prior to
tissue collection. All of the tissues were washed with the
RNase-free PBS and frozen in liquid nitrogen at −196°C. Written
informed consent was obtained from all patients and the
experimental protocol was approved by the Institutional Board of
the Southwest Hospital.
Cell lines and cell culture
The human lung cancer cell lines, H1299, H358,
HCC827 and A549, and the normal human bronchial epithelial cell
line, HBE-1, were obtained from the Institute of Biochemistry and
Cell Biology at the Chinese Academy of Sciences (Shanghai, China).
H358, HCC827, H1299 and A549 cells were maintained and grown in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and HBE cells were maintained in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare,
Chicago, IL, USA), 100 U/ml penicillin and 100 µg/ml streptomycin
in an atmosphere of 5% CO2 at 37°C.
Cell Counting Kit-8 (CCK-8) assay
The target cells were seeded in 96-well plates at a
density of 2×103 cells/well in a 200-µl suspension. A
total of 10 µl Cell Counting kit-8 (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan) in 100 µl RPMI-1640 medium was added to each
well, and cultured for 2 h at 37°C. The absorbance was measured at
450 nm. A total of five repeats/group were performed, three times
independently.
Cell transfection
The cells (A549 and HCC827) were plated in a 6-well
plate. Serum-free culture DMEM medium was added 2 h before
transfection at 70–90% confluency. The suppression plasmid
(pGPU6/GFP/Neo) of lncRNA ATB was synthesized by Sangon Biotech
Co., Ltd. (Shanghai, China). The short hairpin RNA (shRNA)
sequences in the plasmid vectors were as follows: ATB shRNA,
TATGGCCTAGATTACCTTTCCATT; negative control (NC),
UUCUCCGAACGUGUCACGUTT. Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.) was used to transfect cells according to
the manufacturer's protocol. A total of 1×105 cells were
seed in each well; with a transfection time of 6 h, then the
supernatant was discarded and DMEM medium containing 10% serum was
added into the well.
Wound-healing assay
The cells (A549 and HCC827) were seeded into 6-well
plates at a density of 4×105 cells/well, and cultured to
80% confluence. The cell monolayer was scratched with a 10 µl
pipette tip to in a straight line and then washed with PBS. Then,
the cells were cultured in serum-free RPMI-1640 medium for 24 h at
37°C. The wound width was then inspected and imaged to analyze the
ability of cell migration. The experiment was repeated three times.
The migration ability was measured by as the migration rate (MR):
(D0-D1)/D0. D0 being the width of the wound at 0 h and D1 being the
width of the wound at 24 h. The MR was measured at five different
points of each wound.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from cultured cells and clinical tissues
was extracted using RNAsio Plus (Takara Biotechnology Co., Ltd.,
Dalian, China), according to the manufacturer's protocol. The RNA
was reverse transcribed into cDNA using PrimeScript™ RT reagent kit
with gDNA Eraser (Takara Biotechnology Co., Ltd.), according to the
manufacturer's protocol. The quantitative analysis of lncRNA ATB
expression was performed using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.) using 2 µl cDNA, 1 µl forward primer, 1 µl
reverse primer, 0.4 µl ROX (from SYBR Premix Ex Taq), 5.6 µl
RNase-free water and 10 µl SYBR mix. The thermocycling conditions
were as follows: 10 min at 95°C; and then 40 cycles of the
following three steps: 10 sec at 95°C, 30 sec at 60°C, and 20 sec
at 72°C. GAPDH was used as an internal control to quantify
lncRNA-ATB expression. The primer sequences were as follows:
lncRNA-ATB forward, 5′-CTTCACCAGCACCCAGAGA-3′ and reverse,
5′-AAGACAGAAAAACAGTTCCGAGTC-3′; GADPH forward,
5′-GGTGAAGGTCGGAGTCAACG-3′ and reverse,
5′-CAAAGTTGTCATGGATGHACC-3′). Expression was quantified using the
2−ΔΔCq method (14).
Western blotting
The target cells were washed three times with PBS,
and total protein was extracted using radioimmunoprecipitation
assay buffer containing the protein inhibitor, phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology, Haimen, China). The
concentration of protein was assessed using a BCA assay. A total of
40 µg protein was loaded for 10% SDS-PAGE, then transferred onto
Immobilon-P polyvinylidene fluoride membranes (Merck KGaA,
Darmstadt, Germany). The membranes were then blocked with 5%
non-fat milk for 1 h at room temperature. This was followed by
incubation of the following primary antibodies at 4°C overnight:
Anti-E-cadherin (dilution, 1:1,000; cat. no. 14472; anti-mouse),
anti-p38 (dilution, 1:1,000; cat. no. 9212; anti-rabbit),
anti-N-cadherin (dilution, 1:1,000; cat. no. 14215; anti-mouse)
(all from Cell Signaling Technology, Inc., Danvers, MA, USA). The
membranes were washed using Tris-buffered saline with 0.1% Tween 20
for 10 min at room temperature, then incubated with secondary
antibodies [Goat anti-Mouse IgG (H+L) Cross-Adsorbed secondary
antibody, dilution, 1:1,000; cat. no. A1607; goat anti-mouse; and
Goat anti-Rabbit IgG (H+L) Cross-Adsorbed secondary antibody,
dilution 1:1,000; cat. no. A16104; goat anti-rabbit; both from
Thermo Fisher Scientific, Inc.] for 2 h at room temperature. The
protein blots were visualized using an ECL chemiluminescent
detection system (Thermo Fisher Scientific, Inc.) and the protein
bands were quantified with Quantity One software (version 4.62;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation, and all experiments were repeated ≥3 times. The
difference between two groups was analyzed by the Student's t-test,
and one-way analysis of variance (ANOVA) followed by post-hoc
Brown-Forsythe test, to validate ANOVA, was used to analyzed data
from >2 groups. Survival analysis was performed using the
Kaplan-Meier method, and the log-rank test was used to assess the
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
LncRNA ATB is highly expressed in lung
cancer tissue and cancer cell lines
To investigate the role of ATB in lung cancer, the
expression of ATB in lung cancer tissue (n=32) was analyzed by
RT-qPCR, which revealed that ATB was significantly increased in the
lung cancer tissue compared non-cancerous adjacent tissues
(Fig. 1A). The expression of ATB was
also analyzed in lung cancer cell lines, H358, HCC827, H1299 and
A549, and the normal lung epithelial cell line, HBE-1. It was
demonstrated that ATB was also highly expressed in lung cancer cell
lines compared with HBE-1 cells (Fig.
1B). Among the four lung cancer cell lines, ATB was most highly
expressed in A549 and HCC827, which were, therefore, selected for
use in the following experiments. These results indicated that ATB
was highly expressed in lung cancer, but its function remained
unclear.
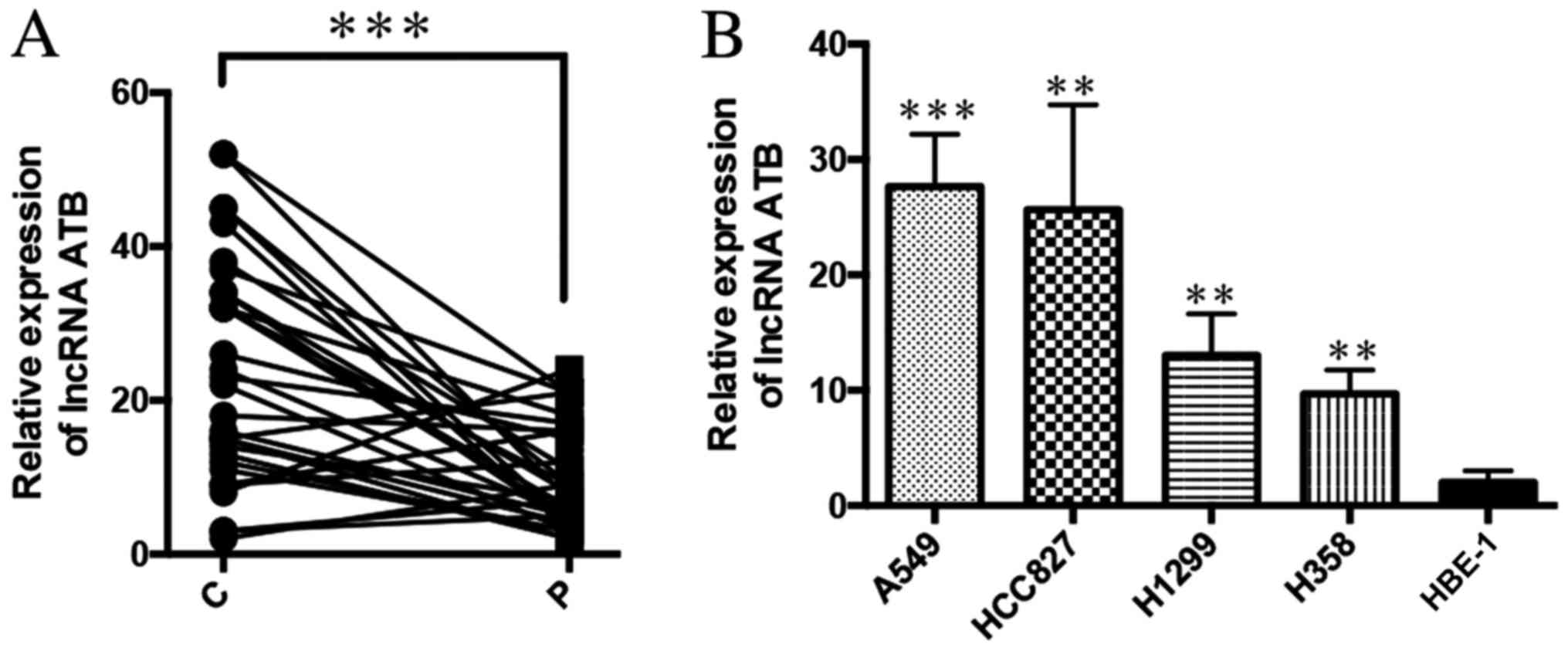 | Figure 1.LncRNA ATB is highly expressed in lung
cancer tissues and cancer cell lines. (A) The expression of ATB in
lung cancer tissue and paracancerous tissue was detected by
RT-qPCR. ***P<0.0001; n=32. (B) RT-qPCR was used to detect the
expression of ATB in lung cancer cell line and normal lung
epithelial cell line HBE-1, from left to right, P=0.0007, P=0.003,
P=0.007, P=0.0045. **P<0.001, ***P<0.0001 vs. HBE-1. LncRNA,
long non-coding RNA; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; C, lung cancer tissue; P, paracancerous
tissue. |
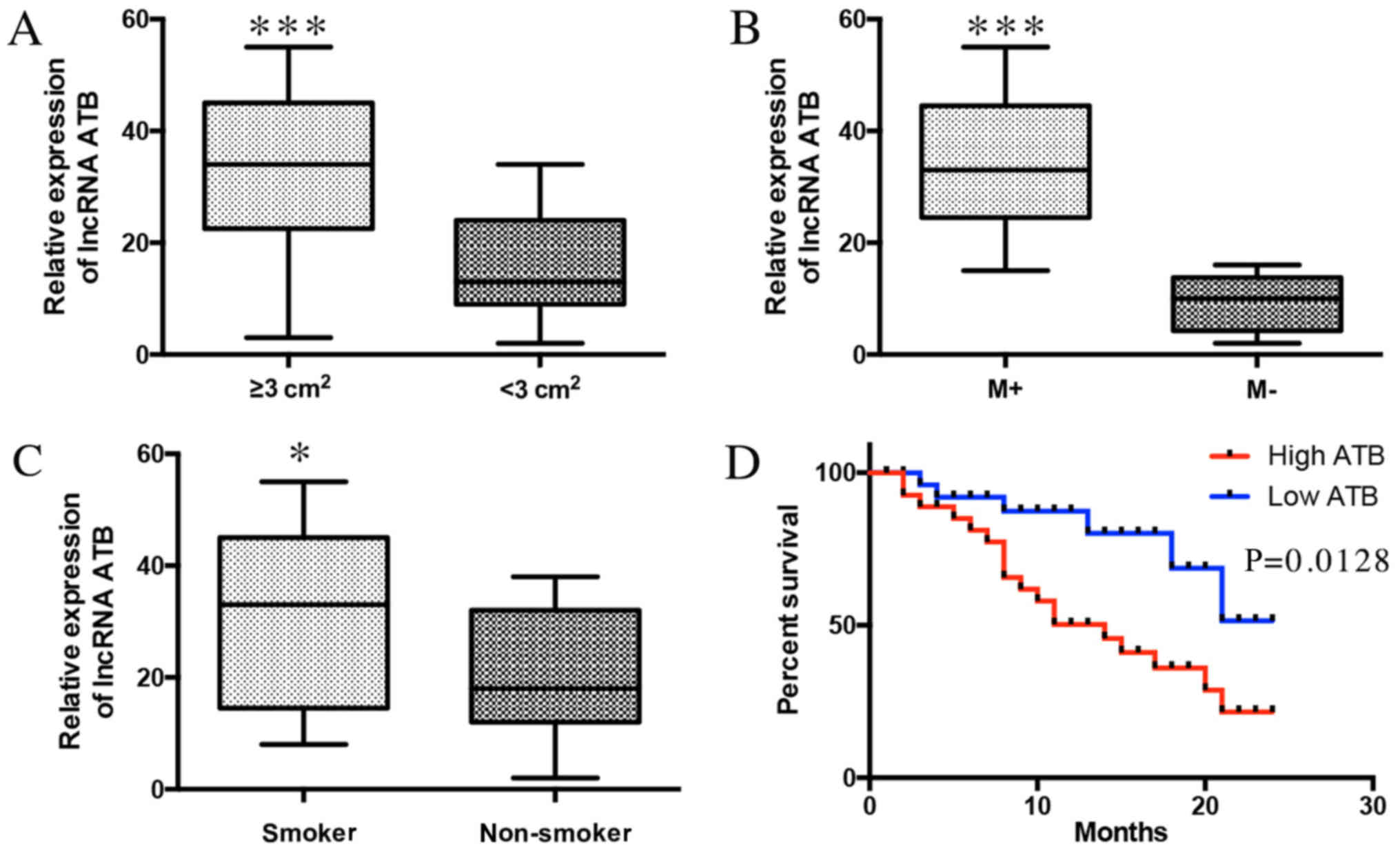
ATB expression indicates a poor
prognosis of patients with lung cancer
To evaluate the clinical significance of ATB in lung
cancer, the associations between ATB and clinicopathological
characteristics were investigated. ATB was positively associated
with tumor size (Fig. 2A) and lymph
node metastasis (Fig. 2B). ATB was
also associated with smoking history (Fig. 2C). Additionally, it was demonstrated
that high expression of ATB was negatively associated with the
survival time of lung cancer patients (Fig. 2D). These results indicate that the
expression of ATB was associated with poor prognosis of patients
with lung cancer, and positively associated with lung cancer
progression.
Knockdown of ATB inhibits
proliferation and metastasis of lung cancer cell in vitro
To further investigate the biological function of
ATB in lung cancer cell lines, ATB knockdown was performed using
shRNA (Fig. 3A). It was demonstrated
that suppression of ATB expression significantly inhibited the
proliferation of A549 and H358 cells compared with the respective
NC groups (Fig. 3B), suggesting that
ATB promotes cell growth. A wound healing assay was performed to
analyze the effect of ATB on the migration of lung cancer cells. It
was demonstrated that silencing ATB significantly inhibited the
migratory ability of the two lung cancer cell lines compared with
their controls (Fig. 3C and D). These
results indicate that knockdown of ATB may inhibit the
proliferation and metastasis of lung cancer cells.
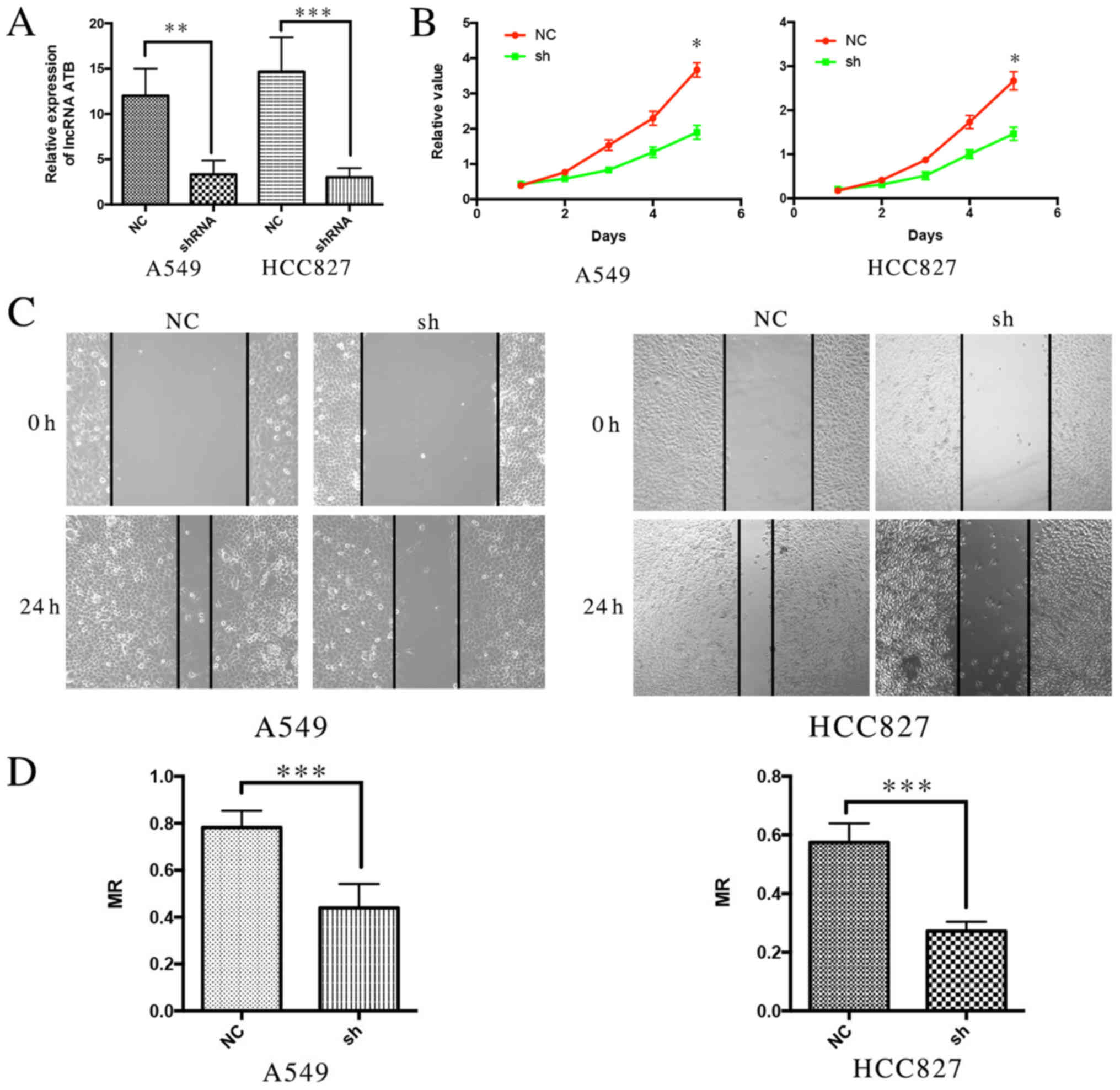 | Figure 3.Knockdown of lncRNA ATB inhibits
proliferation and metastasis of lung cancer cells in vitro.
(A) The expression of ATB in lung cancer cell lines following ATB
was knocked down, P=0.0086, P=0.0067. (B) Cell Counting kit-8
assays demonstrated that the proliferation ability of lung cancer
cell lines was reduced following ATB knockdown, P=0.022, P=0.013.
(C) Wound Healing assays demonstrated that the metastasis ability
of lung cancer cell lines was reduced following ATB knockdown. (D)
The MR indicated the migration ability of lung cancer cells
following ATB knockdown. P=0.003, P=0.002. *P<0.05,
**P<0.001, ***P<0.0001. LncRNA, long non-coding RNA; NC,
negative control; sh/shRNA, short hairpin RNA; MR, migration
rate. |
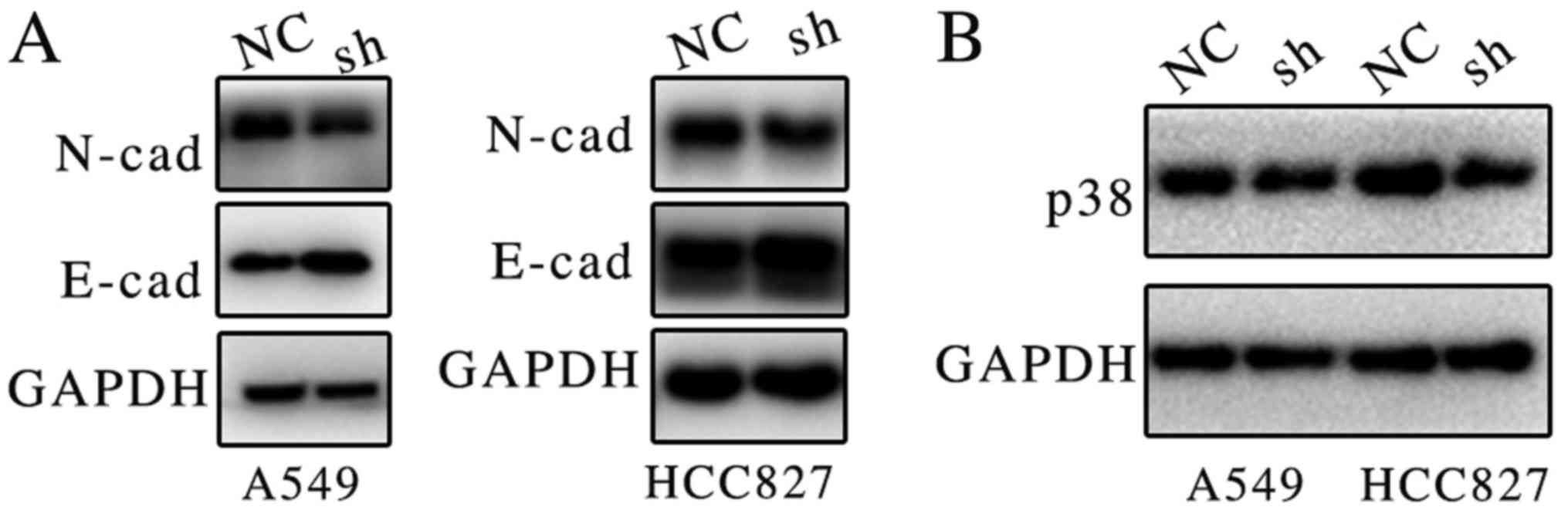
Suppression of ATB downregulates p38
and N-cadherin protein expression
Previous results suggested that ATB may be
associated with proliferation and metastasis (12,13);
however, the molecular mechanism remained unclear. Previous studies
have indicated that ATB may bind members of the miR-200 family, and
induce EMT and invasion in different types of cancer (10,11). It
was demonstrated in the present study that the suppression of ATB
expression markedly increased E-cadherin and decreased N-cadherin
protein expression in both lung cancer cell lines (Fig. 4A).
A recent study demonstrated that miR-200 may inhibit
p38 mitogen-activated protein kinase (MAPK) signaling pathway via
MAPK14, thus promoting cell death (14). The present study indicates that ATB
may promote the expression of p38. It was demonstrated that
suppression of ATB markedly inhibited the expression of p38
(Fig. 4B). Activation of p38 MAPK
signaling may promote proliferation in lung cancer (15). It is hypothesized that ATB may promote
proliferation and metastasis of lung cancer via regulation of
E-cadherin and p38 expression.
Discussion
Previous research has suggested that lncRNAs
regulate the progression of different types of cancer (11–13). Ke
et al (16) demonstrated that
lncRNA ATB was highly expressed in lung cancer tissue, and that
downregulation of ATB was able to promote cell apoptosis,
viability, migration and invasion. However, the molecular mechanism
of ATB in lung cancer has remained unclear. Therefore, the present
study aimed to the influence of ATB expression on the proliferation
and metastasis of lung cancer, and its underlying mechanism. The
results demonstrate that ATB expression was significantly increased
in lung cancer tissues and cell lines, compared with normal
controls. The expression of ATB was also significantly associated
with poor prognosis of patients with lung cancer. Suppression of
ATB expression may inhibit the proliferation and metastasis of lung
cancer cells. It was also indicated that this function may be
executed via E-cadherin and p38.
ATB was reported to be activated by transforming
growth factor-β (TGF-β) in liver cancer (10). In the present study, it was
demonstrated that ATB was highly expressed in 32 lung cancer
tissues, and significantly associated with tumor size and lymph
node metastasis. These results indicated that ATB was highly
expressed in lung cancer. It is well established that smoking is a
key risk factor in lung cancer (17).
Checa et al (18) demonstrated
that cigarette smoke enhanced the expression of TGF-β in alveolar
epithelial cells, and Islas-Vazquez et al (19) demonstrated that cigarette smoke
increased the expression of TGF-β in lung adenocarcinoma. The
present study suggests that high expression of ATB may be
associated with smoking history in patients with lung cancer.
Previous studies have suggested that ATB may target
the miR-200 family to promote cancer development (10,12,20). A
previous study demonstrated that miR-200 inhibits p38 expression in
inducing cell death (21). The
present study demonstrated that suppression of ATB expression
markedly downregulated the protein expression level of p38. It has
been reported that p38 MAPK signaling is important in regulating
proliferation of lung cancer cells (22). Taken together, these results suggest
that ATB may affect proliferation of lung cancer via p38.
It has been previously suggested that ATB may affect
the migration of cancer by regulating EMT (11,13). Yue
et al (13) demonstrated that
ATB suppressed E-cadherin expression, thus promoting EMT in colon
cancer progression. In addition, Shi et al (11) reported that ATB promoted trastuzumab
resistance and EMT in breast cancer. Similarly, in the present
study, it was demonstrated that suppression of ATB increased the
expression of E-cadherin, while decreasing that of N-cadherin,
suggesting that ATB may influence the migratory ability of lung
cancer via EMT.
Overall, it was demonstrated that ATB was highly
expressed in lung cancer tissues and cells, and was associated with
tumor size, lymph node metastasis and smoking history, indicating
that lncRNA ATB could be used as a potential treatment target for
lung cancer. In vitro studies demonstrated that suppression
of ATB causes significant inhibition of proliferation and migration
of lung cancer cells, which may occur via p38 and EMT regulation;
however, the mechanism underlying lncRNA ATB regulating p38 and EMT
requires further study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LW performed data analyses and wrote the manuscript.
TW and PH contributed significantly in data analyses. JLZ and WW
conceived and designed the study. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and the experimental protocol was approved by the
Institutional Board of the Southwest Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hao Y, Yang X, Zhang D, Luo J and Chen R:
Long noncoding RNA LINC01186, regulated by TGF-β/SMAD3, inhibits
migration and invasion through Epithelial-Mesenchymal-Transition in
lung cancer. Gene. 608:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sang H, Liu H, Xiong P and Zhu M: Long
non-coding RNA functions in lung cancer. Tumour Biol. 36:4027–4037.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Chi H, Chen J, Chen C, Huang Y, Xi
H, Xue J and Si Y: Curcumin suppresses proliferation and in vitro
invasion of human prostate cancer stem cells by ceRNA effect of
miR-145 and lncRNA-ROR. Gene. 631:29–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang ZQ, He CY, Hu L, Shi HP, Li JF, Gu
QL, Su LP, Liu BY, Li C and Zhu Z: Long noncoding RNA UCA1 promotes
tumour metastasis by inducing GRK2 degradation in gastric cancer.
Cancer Lett. 408:10–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen DL, Chen LZ, Lu YX, Zhang DS, Zeng
ZL, Pan ZZ, Huang P, Wang FH, Li YH, Ju HQ and Xu RH: Long
noncoding RNA XIST expedites metastasis and modulates
epithelial-mesenchymal transition in colorectal cancer. Cell Death
Dis. 8:e30112017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Huo X, Yang XR, He J, Cheng L,
Wang N, Deng X, Jin H, Wang N, Wang C, et al: STAT3-mediated
upregulation of lncRNA HOXD-AS1 as a ceRNA facilitates liver cancer
metastasis by regulating SOX4. Mol Cancer. 16:1362017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Z, Wang J, Wang S, Chang H, Zhang T
and Qu J: LncRNA CCAT2 promotes tumorigenesis by over-expressed
Pokemon in non-small cell lung cancer. Biomed Pharmacother.
87:692–697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roth A and Diederichs S: Long noncoding
RNAs in lung cancer. Curr Top Microbiol Immunol. 394:57–110.
2016.PubMed/NCBI
|
|
10
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi SJ, Wang LJ, Yu B, Li YH, Jin Y and
Bai XZ: LncRNA-ATB promotes trastuzumab resistance and
invasion-metastasis cascade in breast cancer. Oncotarget.
6:11652–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma CC, Xiong Z, Zhu GN, Wang C, Zong G,
Wang HL, Bian EB and Zhao B: Long non-coding RNA ATB promotes
glioma malignancy by negatively regulating miR-200a. J Exp Clin
Cancer Res. 35:902016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yue B, Qiu S, Zhao S, Liu C, Zhang D, Yu
F, Peng Z and Yan D: LncRNA-ATB mediated E-cadherin repression
promotes the progression of colon cancer and predicts poor
prognosis. J Gastroenterol Hepatol. 31:595–603. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Khan GJ, Gao Y, Gu M, Wang L, Khan S,
Naeem F, Yousef BA, Roy D, Semukunzi H, Yuan S and Sun L: TGF-β1
causes EMT by regulating N-Acetyl Glucosaminyl transferases via
downregulation of non muscle Myosin II-A through JNK/P38/PI3K
pathway in lung cancer. Curr Cancer Drug Targets. 18:209–219. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ke L, Xu SB, Wang J, Jiang XL and Xu MQ:
High expression of long non-coding RNA ATB indicates a poor
prognosis and regulates cell proliferation and metastasis in
non-small cell lung cancer. Clin Transl Oncol. 19:599–605. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van der Aalst CM, Ten Haaf K and de Koning
HJ: Lung cancer screening: Latest developments and unanswered
questions. Lancet Respir Med. 4:749–761. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Checa M, Hagood JS, Velazquez-Cruz R, Ruiz
V, Garcia-De-Alba C, Rangel-Escareno C, Urrea F, Becerril C,
Montano M, Garcia-Trejo S, et al: Cigarette smoke enhances the
expression of profibrotic molecules in alveolar epithelial cells.
PLoS One. 11:e01503832016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Islas-Vazquez L, Prado-Garcia H,
Aguilar-Cazares D, Meneses-Flores M, Galicia-Velasco M,
Romero-Garcia S, Camacho-Mendoza C and Lopez-Gonzalez JS: LAP
TGF-Beta subset of CD4(+)CD25(+)CD127(−) Treg cells is increased
and overexpresses LAP TGF-Beta in lung adenocarcinoma patients.
Biomed Res Int. 2015:4309432015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han F, Wang C, Wang Y and Zhang L: Long
noncoding RNA ATB promotes osteosarcoma cell proliferation,
migration and invasion by suppressing miR-200s. Am J Cancer Res.
7:770–783. 2017.PubMed/NCBI
|
|
21
|
Xiao Y, Yan W, Lu L, Wang Y, Lu W, Cao Y
and Cai W: p38/p53/miR-200a-3p feedback loop promotes oxidative
stress-mediated liver cell death. Cell Cycle. 14:1548–1558. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen T, Gong W, Tian H, Wang H, Chu S, Ma
J, Yang H, Cheng J, Liu M, Li X and Jiang C: Fibroblast growth
factor 18 promotes proliferation and migration of H460 cells via
the ERK and p38 signaling pathways. Oncol Rep. 37:1235–1242. 2017.
View Article : Google Scholar : PubMed/NCBI
|