Introduction
Breast cancer is the most common type of cancer in
women (1). Although the prognosis of
human breast cancer can be significantly improved by surgical
therapy, radiotherapy, chemotherapy, endocrine therapy and
molecular targeted therapy, breast cancer incidence has increased
in recent decades (2–5). Breast cancer comprises a group of
heterogeneous diseases, whose prognoses differ due to variation in
molecular and pathological characteristics (6). Treatment of breast cancer is based on
various clinicopathological parameters, including the status of
lymph node metastasis and hormone receptor expression (7,8). Invasion
of the lymph nodes implicates that tumor cells may spread, and
combined therapies are required (9).
Furthermore, the expression of estrogen receptor (ER) provides a
basis for endocrine therapy (10).
Numerous oncogenes are involved in the occurrence
and progression of breast cancer. PARP is a family of proteins
involved in cellular processes, including DNA repair and apoptosis
(11). The most well-established PARP
protein is PARP1, which can be cleaved and inactivated by caspase
during apoptosis (12). Notably,
PARP9 lacks PARP activity, despite having similar carboxyl-terminal
amino acid sequences to other members of the PARP family (13). Previous studies suggest that PARP9 may
promote metastasis in a variety of tumors. PARP9 is overexpressed
in lymphoma and prostate cancer and has been demonstrated to
positively correlate with metastasis, recurrence and chemotherapy
resistance (14,15). However, the expression of PARP9 and
its implication in breast cancer remain elusive. In the present
study, the expression of PARP9 in 57 pairs of breast cancer and
normal breast tissues was investigated, as well as the association
between PARP9 and clinicopathological parameters. Furthermore, the
effects of PARP9 on breast cancer-cell migration were detected.
Materials and methods
Tissues samples
A total of 57 pairs of breast cancer and normal
breast tissues were obtained from the tissue bank at West China
Hospital (Chengdu, China). All patients were female, who underwent
surgery between February 2010 and May 2015. The diagnosis of breast
cancer was confirmed by two patologists at West China Hospital,
following biopsy. The expression of human epithelial growth factor
receptor-2 (Her-2) was defined as positive by immunohistochemistry
(3+) or fluoresence in situ hybridisation examination (+).
Ki-67 levels were considered high if the percentage was ≥14%
detected by immunohistochemistry.
No radiotherapy or chemotherapy had been received
prior to operation. The age of patients ranged from 25–81 years,
with a median age of 52 years. There were 54 cases of invasive
ductal carcinoma, 2 cases of invasive lobular carcinoma and only 1
case of mucinous carcinoma.
Reagents
The polyclonal rabbit anti-human PARP9 antibody
(cat. no. 17535-1-AP) was purchased from Proteintech, Inc. (Wuhan,
China). The monoclonal mouse GAPDH (cat. no. 200306) and actin
(cat. no. 250132) antibodies, and horseradish peroxidase-conjugated
goat anti-mouse IgG (cat. no. 511103) and goat anti-rabbit IgG
(cat. no. 511203) secondary antibodies were purchased from Zen
Bioscience Co., Ltd. (Chengdu, China).
Western blot analysis
Tissues were lysed with ice-cold lysis buffer
containing 1% Triton X-100, 40 mM Hepes pH 7.5, 120 mM NaCl, 1 mM
EDTA, 10 mM pyrophosphate, 10 mM glycerophosphate, 50 mM NaF, 0.5
mM orthovanadate, PMSF and aprotinin (Roche Diagnostics, Basel,
Switzerland). Tissue lysates were ground for 30 sec/time (4 times)
until the tissues were totally triturated, then the mixture were
incubated on ice for 30 min and centrifuged for 15 min at 14,000 ×
g to remove cell debris. The supernatants were harvested and
protein concentration was using a BCA assay (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The proteins were heated at
100°C in 4× loading buffer [containing 200 nM Tri-HCl, 40%
glycerol, 10% SDS (w/v), 400 nM DTT, 0.04% Bromphenil blue (w/v)]
for 10 min, samples containing 20 µg protein were resolved by 10%
SDS-PAGE, then transferred to a polyvinylidene difluoride membrane
(Millipore; Merck KGaA, Darmstadt, Germany). Each membrane was
blocked with 5% non-fat dry milk in Tris buffered saline with
Tween-20 (TBST) for 1 h at room temperature. The membranes were
then incubated with PARP9 (1:1,500), actin (1:1,000) and GAPDH
(1:1,000) primary antibodies overnight at 4°C and the appropriate
secondary antibodies (1:1,500) for 1 h at room temperature.
Detection was performed using a chemiluminescence kit (BeyoECL
plus; cat. no. P0018; Beyotime Biotechnology, Shanghai, China).
Images were collected by Alpha Innotech's FluorChem imaging system
(Alpha Innotech, San Leandro, CA, USA). GAPDH was used as an
internal control. Western blots were subjected to densitometric
analysis using Quantity One software (version 4.6.2; Bio-RAD,
Hercules, CA, USA). The PARP9/GAPDH ratio in all breast cancer
samples was calculated. The level of PARP9 expression was
considered high if the PARP9/GAPDH ratio exceeded the median value
in these samples.
Cell culture
The breast cancer cell line, HCC1806, was a gift
from Professor Ceshi Chen (Kunming Institute of Zoology, Chinese
Academy of Sciences, Shanghai, China). The cells were maintained in
Dulbecco's minimal essential medium (Life Technologies, Grand
Island, NY, USA) containing 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.). Cells were incubated at 37°C in a humidified
atmosphere with 5% CO2.
RNA interference
All small interfering RNAs (siRNAs) were synthesized
by GenePharma, Co, Ltd. (Shanghai, China). The target sequence used
for knockdown of PARP9 was 5′-GGACAGAGTTAGAGATTGAAAC-3′, and the
sequence of si-Control was 5′-ACGUGACACGUUCGGAGAATT-3′. siRNA (10
µM) were transfected into HCC1806 cells (~7,000 cells per well) by
Lipofectamine RNAimax reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Cells were cultured for
48 h prior to subsequent western blotting.
Wound-healing assay
Cells were seeded in 6-well plates at 60,000
cells/well. The cells were cultured in DMEM medium containing 5%
fetal bovine serum for 24 h. To inhibit cell proliferation, the
cells were treated with 2 µg/ml mitomycin C at 37°C for 96 h. 24 h
after the transfection of si-PARP9 or si-Control, a 1-mm scratch
was made in the confluent cultures with a pipette tip, and washed
twice with PBS to remove debris. The area of the scratch was
measured by taking images under a phase-contrast microscope. To
ensure the consistency of observation, specific observing points
along the wound were labeled, and the same fields were observed at
multiple time points.
Statistical analysis
Statistical analysis was conducted using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA) and Graphpad
Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA) was used to produce graphs. Fisher's exact test was applied to
determine statistical significance on the relationship between
PARP9 and clinicopathological parameters. Student's t-test was used
to determine the statistical difference in migration rate between
siControl- and siPARP9-transfected cells. P<0.05 was considered
to indicate a statistically significant difference.
Results
PARP9 is frequently overexpressed in
human breast cancer
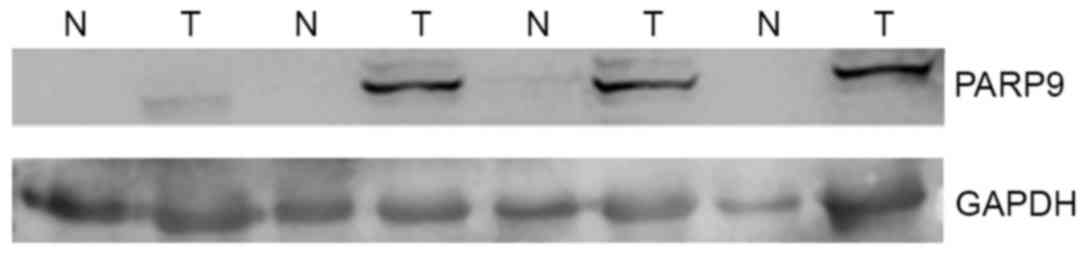
PARP9 expression was not detected in 43 normal
breast tissues (75.4%), whereas it was detected in all 57 breast
cancer tissues. Low levels of PARP9 (PARP9/GAPDH ratio <10% of
the median value in cancer tissues) were detected in 13 cases
(22.8%), and modest levels of PARP9 (PARP9/GAPDH ratio ~1:1) were
detected in only 1 case (1.7%). In all 57 cases, the levels of
PARP9 in breast cancer tissues were higher than that in paired
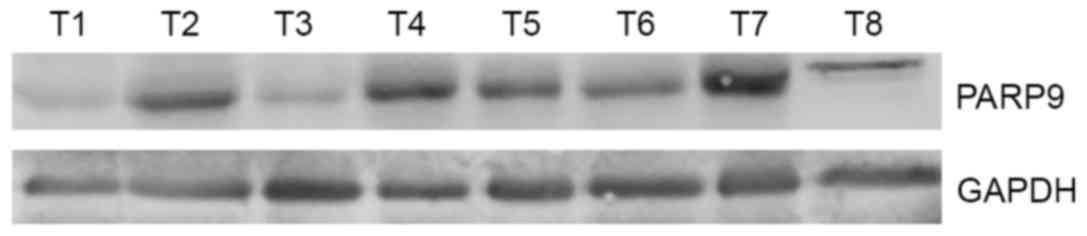
normal breast tissues (Fig. 1). In 57
cases of breast cancer tissues, high levels of PARP9 were detected
in 25 cases (43.8%), while low levels of PARP9 were detected in 32
cases (56.1%) (Fig. 2).
PARP9 expression is associated with
lymph node metastasis
In 57 breast cancer tissues, 35 cases were
ERα-positive, while 22 cases were ERα-negative. Overexpression of
PARP9 was negatively associated with ERα expression (Table I). Axillary lymph nodes metastasis was
detected in 31 cases (54.4%), and high levels of PARP9 were
detected in 25 cases (43.8%) of breast cancer. Overexpression of
PARP9 was positively associated with axillary lymph nodes
metastasis (P<0.01; Table I).
PARP9 expression was not associated with age, Her-2 expression,
Ki-67 or tumor size (P>0.05; Table
I).
 | Table I.The association between PARP9
expression and clinicopathological parameters. |
Table I.
The association between PARP9
expression and clinicopathological parameters.
| Clinicopathological
parameters | PARP9high
(n=25) | PARP9low
(n=32) | P-value |
|---|
| Age (years) |
|
| 0.108 |
| ≤45 | 8 | 18 |
|
|
>45 | 17 | 14 |
|
| ER status |
|
| 0.017 |
|
Positive | 11 | 24 |
|
|
Negative | 14 | 8 |
|
| PR status |
|
| 0.117 |
|
Positive | 14 | 11 |
|
|
Negative | 11 | 21 |
|
| Her-2 status |
|
| 0.592 |
|
Positive | 12 | 14 |
|
|
Negative | 11 | 18 |
|
| Axillary lymph node
metastasis |
|
| 0.007 |
|
Positive | 19 | 12 |
|
|
Negative | 6 | 20 |
|
| Ki-67 index (%) |
|
| 0.135 |
| ≤14 | 4 | 11 |
|
|
>14 | 21 | 20 |
|
| Tumor size (cm) |
|
| 0.513 |
| ≤2 | 4 | 6 |
|
|
>2 | 21 | 25 |
|
| Stage |
|
| 0.575 |
| I/II | 16 | 23 |
|
|
III/IV | 9 | 9 |
|
| Molecular type |
|
| 0.087 |
| Luminal
A | 2 | 8 |
|
| Luminal
B | 9 | 13 |
|
|
Her-2-enriched | 7 | 2 |
|
|
Basal-like | 7 | 7 |
|
PARP9-knockdown inhibits breast
cancer-cell migration
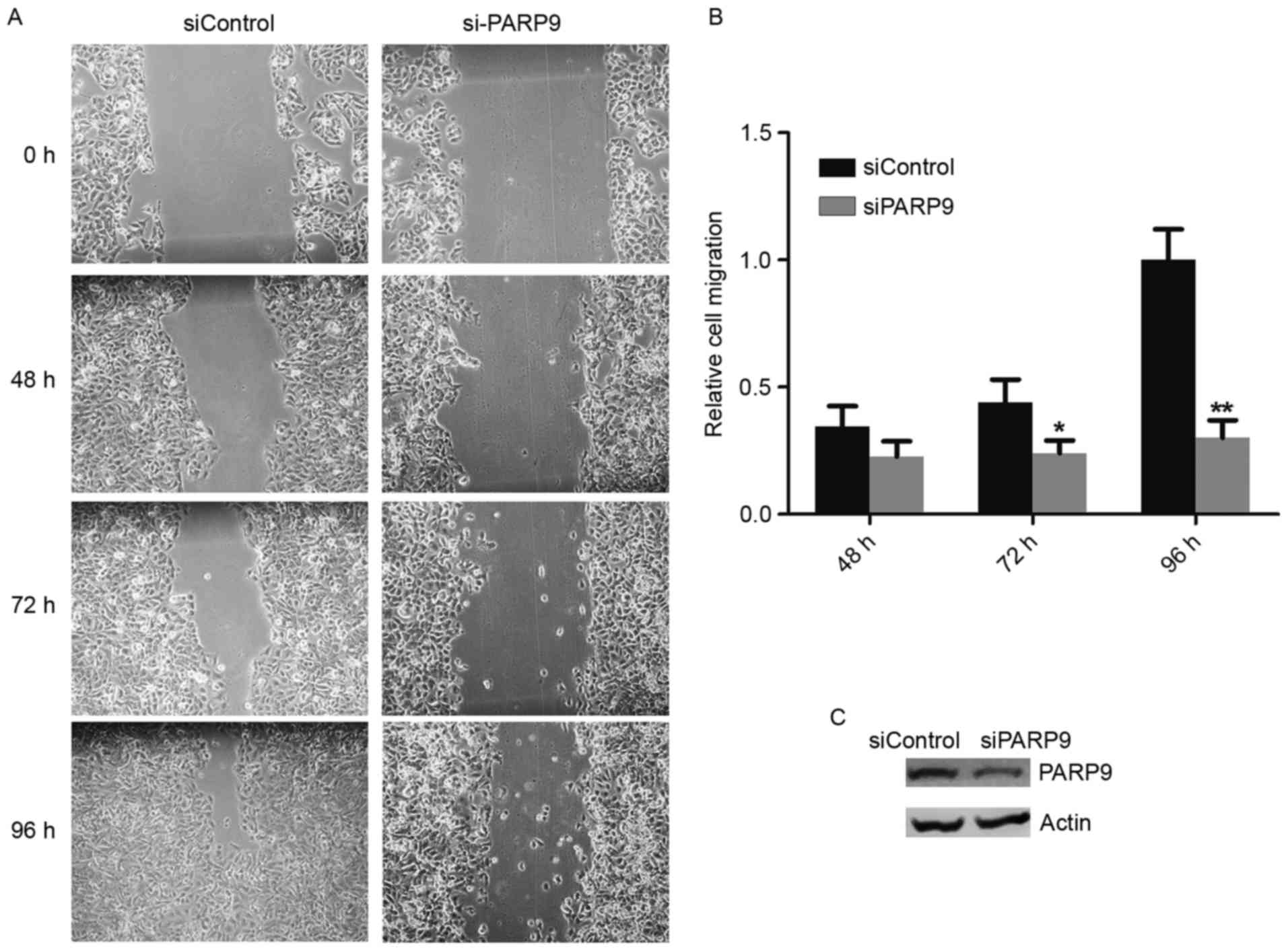
Since overexpression of PARP9 was positively
associated with lymph node metastasis, the effects of PARP9 on
breast cancer-cell migration were investigated. PARP9 siRNA was
transfected into HCC1806 cells, followed by a wound-healing assay.
The efficiency of PARP-knockdown was confirmed by western blotting,
and it was demonstrated that PARP9-knockdown significantly
inhibited HCC1806 cell migration (Fig.
3).
Discussion
The progression of breast cancer involves aberrant
expression of hormone receptors, growth factor receptors and other
oncogenes, including mechanistic target of rapamycin kinase
(16,17). In the present study, it was
demonstrated that PARP9 was overexpressed in 43.8% of human breast
cancer cases. Overexpression of PARP9 was negatively associated
with ER expression, but positively associated with axillary lymph
node metastasis. PARP9-knockdown was indicated to inhibit breast
cancer cell migration. These data suggest that PARP9 may promote
breast cancer progression.
A previous study demonstrated that PARP9 is involved
in the interferon (IFN)-γ-signal transducer and activator of
transcription 1 (STAT1) pathway. PARP9 interacts with STAT1α and
STAT1β, upregulates the expression of interferon regulatory factor
(IRF)2 and downregulates that of IRF1, thereby promoting cell
proliferation, survival and migration (18). Also, PARP9 has been demonstrated to
promote recurrence, metastasis and chemotherapy-resistance in
prostate cancer and lymphoma (14,15). The
expression of PARP9 has been demonstrated to be more abundant in
the androgen-resistant prostate cell lines, PC3 and DU145, compared
with the AR-dependent cell line, LNCap, and normal prostate
epithelial cell line, SUDHL7 (15).
PARP9 has been indicated to enhance tumor cell survival and
doxorubicin-resistance via the E3 ubiquitin ligase, DTX3L, which
selectively ubiquitinates histone H4 and protects cells against
DNA-damaging agents following binding to PARP in
chemotherapy-resistant diffuse large B cell lymphoma (19).
Although STAT1 has been established to have
anti-tumor effects, increasing the expression of STAT1 and
IFN-γ-induced genes contributed to metastatic potential by
enhancing the ability of malignant cells to invade the stroma and
migrate to distant sites in node-positive triple negative breast
tumors (20). PARP9 has also been
demonstrated to form complex with STAT1 and DTX3L, which may affect
the nuclear activities of STAT1 by antagonistically regulating the
tyrosine phosphorylation of STAT1 on Y701 (21). In consistence with previous studies on
PARP9 in prostate cancer, the present study demonstrates that PARP9
promotes breast cancer cell migration. Thus, overexpression of
PARP9 may promote the progression of human breast cancer.
Acknowledgements
Not applicable.
Funding
No finding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XT, JJ and YJ conceived the study and wrote the
manuscript. XT and HZ performed the experiments. YL and HH assisted
in collecting and analyzing the patient data regarding the
clinicopathological parameters of breast cancer. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the ethics committee of
West China Medical Center, Sichuan University and informed consent
to participate was signed by the patients and/or guardians.
Patient consent for publication
Informed consent was signed by the patients and/or
guardians.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xiang M, Su H, Shu G, Wan D, He F, Loaec
M, Ding Y, Li J, Dovat S, Yang G and Song C: Amplexicaule A exerts
anti-tumor effects by inducing apoptosis in human breast cancer.
Oncotarget. 7:18521–18530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Peto R, Davies C, Godwin J, Gray R,
Pan HC, Clarke M, Cutter D, Darby S, McGale P, et al: Comparisons
between different polychemotherapy regimens for early breast
cancer: Meta-analyses of long-term outcome among 100,000 women in
123 randomised trials. Lancet. 379:432–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zeng H, Zheng R, Zhang S, Zou X and Chen
W: Female breast cancer statistics of 2010 in China: Estimates
based on data from 145 population-based cancer registries. J Thorac
Dis. 6:466–470. 2014.PubMed/NCBI
|
|
4
|
Azim HA Jr, de Azambuja E, Colozza M,
Bines J and Piccart MJ: Long-term toxic effects of adjuvant
chemotherapy in breast cancer. Ann Oncol. 22:1939–1947. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
EBCTCG (Early Breast Cancer Trialists'
Collaborative Group), . McGale P, Taylor C, Correa C, Cutter D,
Duane F, Ewertz M, Gray R, Mannu G, Peto R, et al: Effect of
radiotherapy after mastectomy and axillary surgery on 10-year
recurrence and 20-year breast cancer mortality: Meta-analysis of
individual patient data for 8135 women in 22 randomised trials.
Lancet. 383:2127–2135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang XL, Tao L, Zhou XL, Wei H and Sun JW:
Initial experience of automated breast volume scanning (ABVS) and
ultrasound elastography in predicting breast cancer subtypes and
staging. Breast. 30:130–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Selli C, Dixon JM and Sims AH: Accurate
prediction of response to endocrine therapy in breast cancer
patients: Current and future biomarkers. Breast Cancer Res.
18:1182016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andree C, Schmidt VJ, Munder BIJ,
Seidenstücker K, Behrendt P, Witzel C, Horch RE, Andrews BT and
Richrath P: Detecting of breast cancer metastasis by means of
regional lymph node sampling during autologous breast
reconstruction-a screening of 519 consecutive patients. Med Sci
Monit. 18:CR605–CR610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicolini A, Ferrari P and Duffy MJ:
Prognostic and predictive biomarkers in breast cnacer: Past,
present and future. Semin Cancer Biol pii. 2017.(Epub ahead of
print). View Article : Google Scholar
|
|
10
|
Thomas C and Gustafsson JA: The different
roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer.
11:597–608. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lehmann M, Pirinen E, Mirsaidi A, Kunze
FA, Richards PJ, Auwerx J and Hottiger MO: ARTD1-induced
poly-ADP-ribose formation enhances PPARγ ligand binding and
co-factor exchange. Nucleic Acids Res. 43:129–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin WD, Liu GL, Wang J, Wang H, Zhang JN,
Zhang F, Ma Y, Ji XY, Li C and Zhang MX: Poly(ADP-ribose)
polymerase 1 inhibition protects cardiomyocytes from inflammation
and apoptosis in diabetic cardiomyopathy. Oncotarget.
7:35618–35631. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwata H, Goettsch C, Sharma A, Ricchiuto
P, Goh WW, Halu A, Yamada I, Yoshida H, Hara T, Wei M, et al: PARP9
and PARP14 cross-regulate macrophage activation via STAT1
ADP-ribosylation. Nat Commun. 7:128492016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Juszczynski P, Kutok JL, Li C, Mitra J,
Aguiar RC and Shipp MA: BAL1 and BBAP are regulated by a gamma
interferon-responsive bidirectional promoter and are overexpressed
in diffuse large B-cell lymphomas with a prominent inflammatory
infiltrate. Mol Cell Biol. 26:5348–5359. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bachmann SB, Frommel SC, Camicia R,
Winkler HC, Santoro R and Hassa PO: DTX3L and ARTD9 inhibit IRF1
expression and mediate in cooperation with ARTD8 survival and
proliferation of metastatic prostate cancer cells. Mol Cancer.
13:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Tan M, Hawthorne VS, Klos KS, Lan
KH, Yang Y, Yang W, Smith TL, Shi D and Yu D: Activation of the
Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2
overexpression predicts tumor progression in breast cancers. Cancer
Res. 10:6779–6788. 2004.
|
|
17
|
Yin Y, Hua H, Li M, Liu S, Kong Q, Shao T,
Wang Y, Luo Q, Wang J, Luo T and Jiang Y: mTORC2 promotes type I
insulin-like growth factor receptor and insulin receptor activation
through the tyrosine kinase activity of mTOR. Cell Res. 26:46–65.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Camicia R, Bachmann SB, Winkler HC, Beer
M, Tinguely M, Haralambieva E and Hassa PO: BAL1/ARTD9 represses
the anti-proliferative and pro-apoptotic IFNgamma-STAT1-IRF1-p53
axis in diffuse large B-cell lymphoma. J Cell Sci. 126:1969–1980.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Mao D, Roswit WT, Jin X, Patel
AC, Patel DA, Agapov E, Wang Z, Tidwell RM, Atkinson JJ, et al:
PARP9-DTX3L ubiquitin ligase targets host histone H2BJ and viral 3C
protease to enhance interferon signaling and control viral
infection. Nat Immunol. 16:1215–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Greenwood C, Metodieva G, Al-Janabi K,
Lausen B, Alldridge L, Leng L, Bucala R, Fernandez N and Metodiev
MV: Stat1 and CD74 overexpression is co-dependent and linked to
increased invasion and lymph node metastasis in triple-negative
breast cancer. J Proteomics. 75:3031–3040. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan Q, Xu R, Zhu L, Cheng X, Wang Z, Manis
J and Shipp MA: BAL1 and its partner E3 ligase, BBAP, link
poly(ADP-ribose) activation, ubiquitylation, and double-strand DNA
repair independent of ATM, MDC1, and RNF8. Mol Cell Biol.
33:845–857. 2013. View Article : Google Scholar : PubMed/NCBI
|