Introduction
Colorectal cancer is one of the leading causes of
cancer-related deaths among men and women, causing approximately
1.4 million new cases and more than 0.5 million deaths per year
worldwide (1). Its onset is
insidious, often without any obvious clinical manifestations
(2). Several factors were responsible
for the development of colon cancer (3,4). The
accumulation of mutations in certain protooncogenes and tumor
suppressor genes might result in cacer initiation (5). Dietary factors as well as lifestyle have
been considered to play a major role in its incidence (6,7). Diet with
high fat and low carbohydrate can increase the possibility of
occurrence of colon cancer. In particular, consumption with red
meat and processed meats, highly refined grains and starches, and
sugars is related to rising risk of colon cancer (8).
Lactic acid bacteria (LAB) are commonly known
as a kind of probiotics which are facultative anaerobes and widely
exist in human intestine and have been reported to possess many
useful properties, including immune-modulatory, anti-inflammatory,
anti-oxidant and anti-proliferative activity (9). Although recent studies have revealed
that desirable biological activity of LAB can be achieved by using
living or dead bacteria (10,11), exopolysaccharides (EPS) produced by
LAB have particularly received intensive interests for their
potential as valuable compounds and in health applications
(12). EPS is generally related to
all forms of polysaccharides found outside the microbial cell wall.
Moreover, EPS represents one of the most important functional
components of LAB metabolic products (13), which have been reported to exerted
several physiological functions such as immunoregulatory effects
(14), anti-oxidant activities
(15), anti-hypertensive effects
(16) and antitumor activities
(17). Among them, antitumor activity
has particularly received intensive interest due to the growing
number and the high mortality of patients suffered from cancer.
Though the antitumor agents used currently in chemotherapy practice
possess strong activity, many doubts have raised about their safety
and side effects (18) that the
public attention has transferred to identification of antitumor
agents from natural sources (19).
Whether EPS from LAB could be served as an ideal substitute to the
synthetic antitumor agents from the safe natural sources has been
investigated by a large number of studies (14,17,20–22).
However, most of them focused on the EPS produced only by a single
LAB strain. Lactobacillus is one of the most notable strains
of the LAB group and also commonly used in dairy product
fermentation and as probiotic (23,24). The
primary aim of this study was to investigate the effects of EPS
from different Lactobacillus strains, which were facultative
anaerobes and showed activity in large intestine (25–27), on a
mostly used colon cancer cell line called HT-29. The potential
application as an anticancer agent was further discussed.
Materials and methods
Strains and culture conditions
Nine Lactobacillus strains with high bio-activity,
namely L. casei ×11, L. casei ×12, L. casei
K11, L. casei J5, L. rhamnosus J10, L. casei
M5, L. casei M23, L. rhamnosus IN4125, and L.
casei SB27 were choosen based on previous researches (25,26,28). Among
them, Lactobacillus IN4125 was isolated from infant faeces;
Lactobacillus J5 and J10 were isolated from fermented foods
in Gansu Province; Lactobacillus M5 and M23 were isolated
from Koumiss in Sinkiang; Lactobacillus ×11 and ×12 were
isolated from Cheese in Sinkiang; Lactobacillus K11 was
isolated from Kefir in Tibet.
L. rhamnosus GG (LGG, ATCC53103) was used as
reference strain, which is a probiotic of human origin and
commercially exploited to help maintain a ‘good balance’ of
bacteria in the human intestines by preventing the growth of
harmful bacteria (29,30). Stock culture of all
Lactobacillus strains was maintained at −80°C in
freeze-dried 12.5% skim milk containing 2.5% glycerol. All strains
were subcultured twice at 37°C for 24 h prior to use in the
experiments in 12.5% sterilized skim milk medium under anaerobic
conditions.
Colon cancer cell culture
The human colon cancer cell line, HT-29, was
obtained from the Cancer Institute of the Chinese Academy of
Medical Science (Beijing, China). HT-29 cells were grown in
RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.). The
cells were incubated in 25 cm2 flasks at 37°C in a
humidified atmosphere of 95% filtered air and 5% CO2 in
a CO2 incubator (HEPA class 100; Thermo Fisher
Scientific, Inc.). The medium was changed daily to maintain
exponential growth of the cells. Cell counts were assessed by
standard procedures of cell counting using a hemacytometer.
Preparation of extracellular polymeric
substances and MTT assay
Two hundred mini-liter inoculum was prepared as
described by Ai et al with some modifications (31). After an approximately 36 h incubation
period, pH values of the fermentation broth were decreased to 4.5
and the final fermentation was then boiled for 10 min at 100°C to
coagulate the protein and inhibit enzyme activity. After cooling,
coagulated proteins and heat-treated bacterial cells were separated
by centrifugation (12,000 × g for 15 min at 4°C). Supernatants of
the 10 Lactobacillus strains (9 experimental strains and 1
control strain) were filtered by 0.22 µm membrane and their pH was
adjusted to 7.4 with 10 M NaOH to obtain the extracellular
polymeric substances as the previous description (32).
MTT [3-(4,
5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was
used to test the anti-proliferative effects of the EPS which had
little effects on noncancerous cells (33–35).
Briefly, HT-29 colon cells at a density of 1×105 cells
per ml were seeded into 96-well plates. After 12 h of incubation,
cells were treated with 100 µl extracellular polymeric substances
and then incubated for 48 h. Negative controls were treated with
equal volume of RPMI-1640 medium (35,36).
Positive controls were treated with 5 µg/ml daunorubicin (37,38). At
the end of each treatment, 10 µl (5 mg/ml) of MTT was added and the
tumor cells were inoculated for another 4 h. The liquid was then
removed and 100 µl DMSO was added to the well. After dissolving of
the formed crystal formazan, the absorbance was measured at 570 nm
with an enzyme-linked immunosorbent assay plate reader (BioTek-Eon,
Gene Company Limited, USA). Results were displayed as the
inhibition rates. All results were transformed into percentages
based on their separate controls. Calculation was performed by
using the following formula: Inhibition rate={1-(absorbance in test
well)/(absorbance in control well)} ×100%.
Extract and purification of EPS
Starter culture was prepared as previously described
of inoculum, then batch fermentation was performed in a 5.0L
capacity fermentor (Biotech-2002; Bao Xing Bio-Engineering
Equipment Co., Ltd, Shanghai, China) at 37°C with an inoculum
concentration of 3.0% to obtain the fermented broth (15). Extracellular polymeric substances were
obtained as described previously (34). The crude EPS fractions were separated
and purified according to procedures described by Lin et al
with slight modifications (39). The
obtained crude EPS fractions were then lyophilized in a
freeze-drier (FD-1C-50; Boyikang, Beijing, China). The acidic EPS
was purified by using anion exchange chromatography on a DEAE
Sepharose Fast-Flow (GE Healthcare, Chicago, IL, USA) column
(1.6×20 cm) with the NaCl gradient (0~1M) as the elution buffer at
a flow rate of 1.0 ml/min. The purified acidic EPS was collected by
using a fraction collector with 5 ml per tube. The eluent was
assayed for carbohydrate contents by the phenol-sulfuric acid
method described by DuBois et al (40). The peak fractions containing
polysaccharides were pooled and dialyzed with deionized water every
6 h for 48 h and freeze-dried.
Measurement of anti-proliferative
effects of EPS
The anti-proliferation activity of crude and acidic
EPS on HT-29 cells was measured by using MTT assay as described
above. After 12 h of incubation, cells were treated with EPS at a
range of concentrations of 10, 20, 100, 200, and 500 µg/ml
(41) and then incubated for 48 h.
The ultimate treatment concentration was determined as 500 µg/ml in
consideration of the solubility of EPS in RPMI-1640 medium being
less than 600 µg/ml. The cells treated only with RPMI-1640 medium
were used as the control. All samples were subjected to
polysaccharide concentration test before MTT assay. All
concentrations of crude and acidic EPS that tested for their
inhibition effect on HT-29 colon cell were compared with the
control to calculate the inhibition ratio.
Cell cycle analysis
Measurements of HT-29 cell cycles were performed as
described by Liu et al by flow cytometry (FACS Calibur; BD
Biosciences, Franklin Lakes, NJ, USA) (42). Like the apoptosis determination
procedure, HT-29 cells were seeded onto 6-well plates and treated
after 24 h of incubation with crude or acidic EPS fractions for 48
h. HT-29 cells were trypsinized and washed twice with pro-cooling
PBS and fixed in 70% ethanol at 20°C for 1 h. Fixed cells were then
washed twice with PBS and re-suspended in 500 µl of 0.5% Triton
X-100/PBS at 37°C for 30 min with 1 mg/ml of RNase A
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Cells were stained
with 10 µl of propidium iodide (PI) solution (BD Biosciences) in
the dark and analyzed using a FACS Calibur flow cytometer installed
with Cell Quest software (both BD Biosciences) (43,44).
Hoechst 33258 staining
Morphological changes in the nuclear chromatin of
HT-29 cells treated with acidic EPS fractions were detected by
Hoechst 33258 staining. Cells were cultured in RPMI 1640 medium
containing 10% FBS and seeded onto 6-well plates with the
concentration of 2×106 cells/well in 2 ml medium. After
24 h of incubation, cells were treated with 500 µg/ml acidic EPS
fractions for 48 h. Subsequently, the EPS-treated cells were
harvested and washed twice with PBS. Then cells were fixed with 4%
paraformaldehyde at 37°C for 20 min. After washed twice with PBS,
the cells were incubated with 50 µg/ml Hoechst 33258 staining
solution for 15 min at room temperature in the dark followed by
wash with PBS for 5 min and repeated twice. Cells were examined and
photographed using the inverted fluorescence microscope (Axio Vert.
A1; Zeiss AG, Oberkochen, Germany).
Cell apoptosis by flow cytometry
Measurements of cell apoptosis and necrosis were
performed as previously described by Sharma et al (45). Briefly, HT-29 cells were seeded onto
6-well plates 24 h and then treated with 500 µg/ml of crude or
acidic EPS fractions for 48 h. Then cells were harvested, washed
twice with pro-cooling PBS (pH 7.4) and resuspended in 100 µl
binding buffer (BD Biosciences). Annexin V-FITC (5 µl Fluorescein
isothiocyanate; Ex 488 nm, Em 515 nm) and 5 µl of PI (Ex 633 nm)
were added to the solution at room temperature (25°C) in the dark.
The cells were supplemented with 200 µl of binding buffer and
further analyzed by flow cytometry using FACS Calibur (BD
Biosciences). Data were analyzed by Cell Quest software that a
region with less cell debris to calculate the percentages of four
quadrants and apoptosis rate%=(Q2+Q4)/(Q2+Q4) ×100% (46,47).
Measurement of caspase-3 activity
The commercially available caspase-3 colorimetric
assay kit (Keygen Biotech, Nanjing, China) was employed to
determine the activity of caspase-3. Lysates of HT-29 cells were
prepared after treatment with 500 µg/ml acidic EPS fractions
(47). Assays were performed by
incubating 150 µl of cell lysate per sample with 50 µl of reaction
buffers (including 0.5 µl DTT) on 96-well microtiter plates. Then 5
µl of caspase-3 substrate was added into the samples and incubated
at 37°C for 4 h. The absorbance was measured at 405 nm to determine
the caspase-3 activity of the tested samples using a microplate
reader. Detailed data analyses process was performed according to
the manufacturer's recommendation.
Statistical analysis
All data were expressed as mean ± standard deviation
(SD) of three replicates (x ± SD, n=3). Tests of significant
differences were determined by one-way ANOVA by SPSS 20.0 (IBM
Corp., Armonk, NY, USA). Duncan's and LSD's multiple range tests
were used to determine differences among groups. P<0.05 was
considered to indicate a statistically significantly
differenence.
Results
Screening of Lactobacillus strains for
anti-proliferation effects by MTT assay
A comparative analysis of proliferation inhibition
on HT-29 cells by extracellular polymeric substances, which were
produced by 9 Lactobacillus strains and one reference strain
LGG, respectively, was presented in Table
I. The results indicated that 3 Lactobacillus strains,
L. casei M5, L. casei SB27 and L. casei ×12,
exerted the most robust anti-proliferation activity, which were
significantly higher than LGG reference strain (P<0.01). The
extracellular polymeric substances from L. casei K11 also
showed significant higher anti-proliferation activities compared
with that of the LGG strain (P<0.05). The extracellular
polymeric substances produced by other 5 strains, however,
presented moderate anti-proliferation activity on HT-29 cells.
Thus, we identified 4 of 9 tested strains presented a significant
higher inhibitory effect upon HT-29 cellular proliferation.
 | Table I.Anti-proliferation activity of
extracellular polymeric substances isolated from
Lactobacillus strains. |
Table I.
Anti-proliferation activity of
extracellular polymeric substances isolated from
Lactobacillus strains.
| Strain | Origina | Species | Inhibitory rate
(%) |
|---|
| X11 | Traditional cheese;
Sinkiang, China | L.
casei |
16.22±0.40c |
| X12 | Traditional cheese;
Sinkiang, China | L.
casei |
21.10±0.98c |
| K11 | Traditional Tibetan
kefir; Tibet, China | L.
casei | 20.11±0.36 |
| J5 | Traditional
fermented vegetable juice; Gansu Province, China | L.
casei |
5.07±1.39c |
| J10 | Traditional
fermented vegetable juice; Gansu Province, China | L.
rhamnosus |
11.07±1.07c |
| M5 | Traditional
koumiss; Sinkiang, China | L.
casei |
25.75±0.63c |
| M23 | Traditional
koumiss; Sinkiang, China | L.
casei |
10.04±0.50c |
| IN4125 | Infant feces; W/21
months | L.
rhamnosus |
9.66±0.48c |
| SB27 | Traditional
fermented yaks' milk food; Gansu Province, China | L.
casei |
35.98±0.47c |
| LGGb | ATCC53103 | L. rhamnosus
GG | 18.82±1.01 |
In vitro anti-proliferation activity
of crude and acidic EPS on HT-29 cells
The anti-proliferation effect of crude and acidic
EPS produced by L. casei K11, L. casei M5, L.
casei SB27 and L. casei ×12 on HT-29 cells was
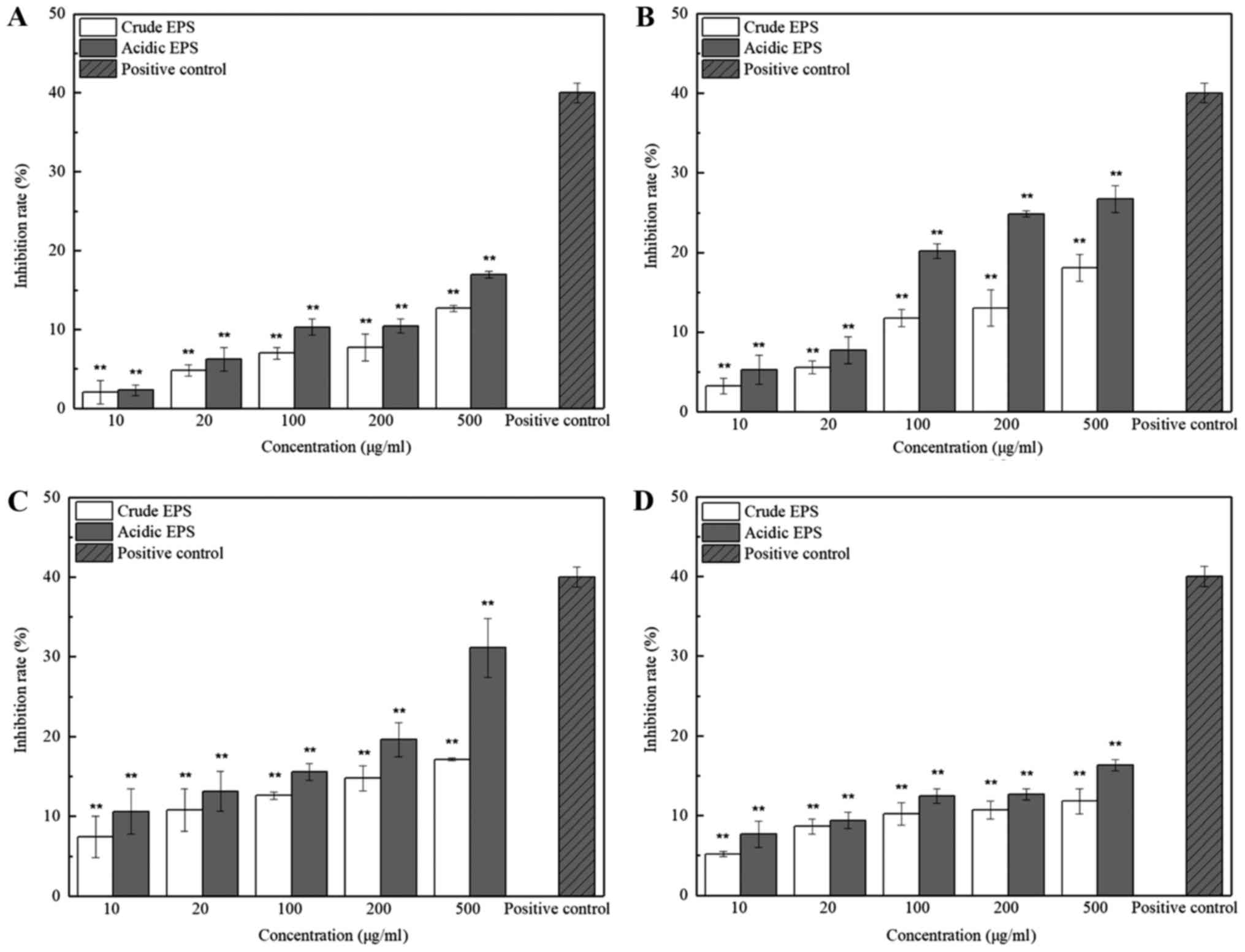
determined by MTT assay under 5 different concentrations. As shown
in Fig. 1(c), the inhibition effect
of both crude and acidic EPS produced by L. casei SB27 on
HT-29 cells significantly increased along with the increased
concentrations (P<0.01). Inhibition of the crude and acidic EPS
produced by other 3 strains showed same trend but with less effect.
These results indicated the dose-dependent inhibition effects of
EPS on HT-29 cells. In addition, the anti-proliferation activities
of the acidic EPS group was higher than that of the crude EPS
group, especially at higher concentration. Since 500 µg/ml EPS
showed the highest inhibition rate on HT-29 cells, especially in
that of L. casei SB27, subsequent experiments were conducted
with EPS at this concentration further tested. The inhibition rate
of 500 µg/ml acidic EPS from K11 M5, SB27, and ×12 strain in Vero
cell line was 1.02±0.71, 4.20±0.77, 2.38±1.37, and 4.76±0.93%,
respectively, which implied that the EPS was non-toxic for normal
cells. This concentration was accordingly selected for subsequent
experiments.
Effects of crude and acidic EPS on
cell division cycle
Excessive proliferation is well known as one of the
most salient characteristics of cancer cells. Therefore, any agent
by which cancer cell cycles can be arrested represents an effective
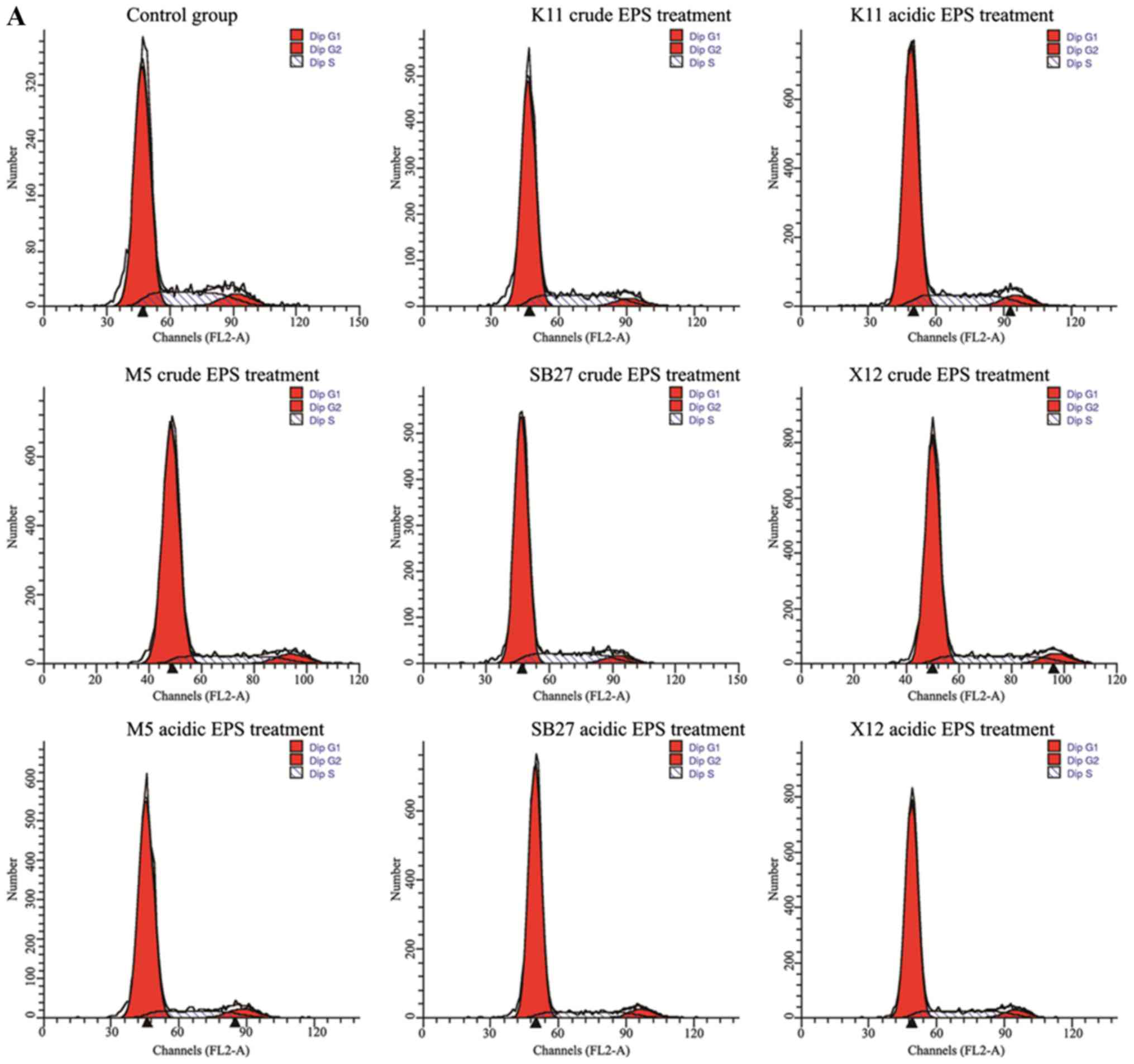
anticancer substance. The effects of crude and acidic EPS from 4
L. casei strains on the HT-29 cell cycle phase distribution
was examined by flow cytometry (Fig.
2). The percentage of G0/G1 phase
increased significantly (P<0.01) when HT-29 cells were incubated
with crude and acidic for 48 h, while the percentage of cells at
G2 and S phases decreased. L. casei K11 crude EPS
group were the exception, whose cell percentage at the S phase
increased compared with control but cell percentage of the
G2 phase was with a sharp decrease (P<0.01). In
addition, acidic EPS group gained more percentage of cells at the
G0/G1 phase compared with crude EPS group.
Specially, maximal increase (from 71.93 to 81.93%) of HT-29 cell
percentage at the G0/G1 phase was observed in
L. casei SB27 acidic EPS group.
Effects of crude and acidic EPS on
HT-29 cell apoptosis by flow cytometry
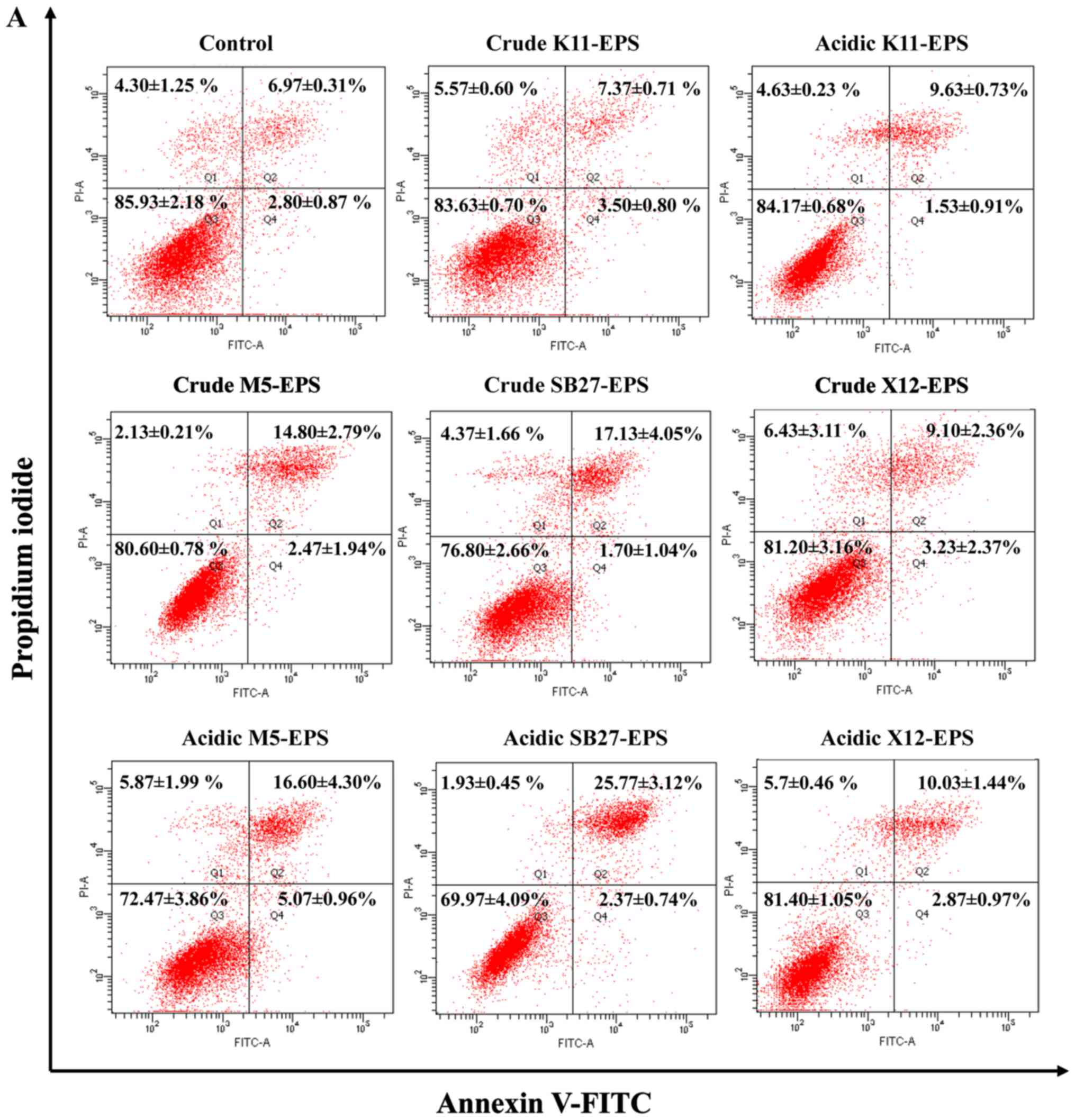
Annexin V-FITC and PI staining method was employed
and apoptosis analyses were performed to evaluate the apoptosis
induction effect of EPS on HT-29 cells. As illustrated in Fig. 3, the four L. casei strains
induced HT-29 cell apoptosis to different levels compared to the
untreated control cells. Acidic EPS induced a higher apoptosis rate
on HT-29 cells than crude EPS, which was consistent with the effect
on anti-proliferation. Significant induction of apoptosis was
observed in HT-29 cells after treatment with EPS either from L.
casei SB27 or L. casei M5, whereas only mild apoptosis
was triggered by L. casei ×12. For L. casei K11, its
acidic EPS induced a moderate apoptosis on HT-29 cells while crude
EPS did not show significant effects on induction of apoptosis.
Acidic EPS induced nuclei
morphological changes in HT-29 cell
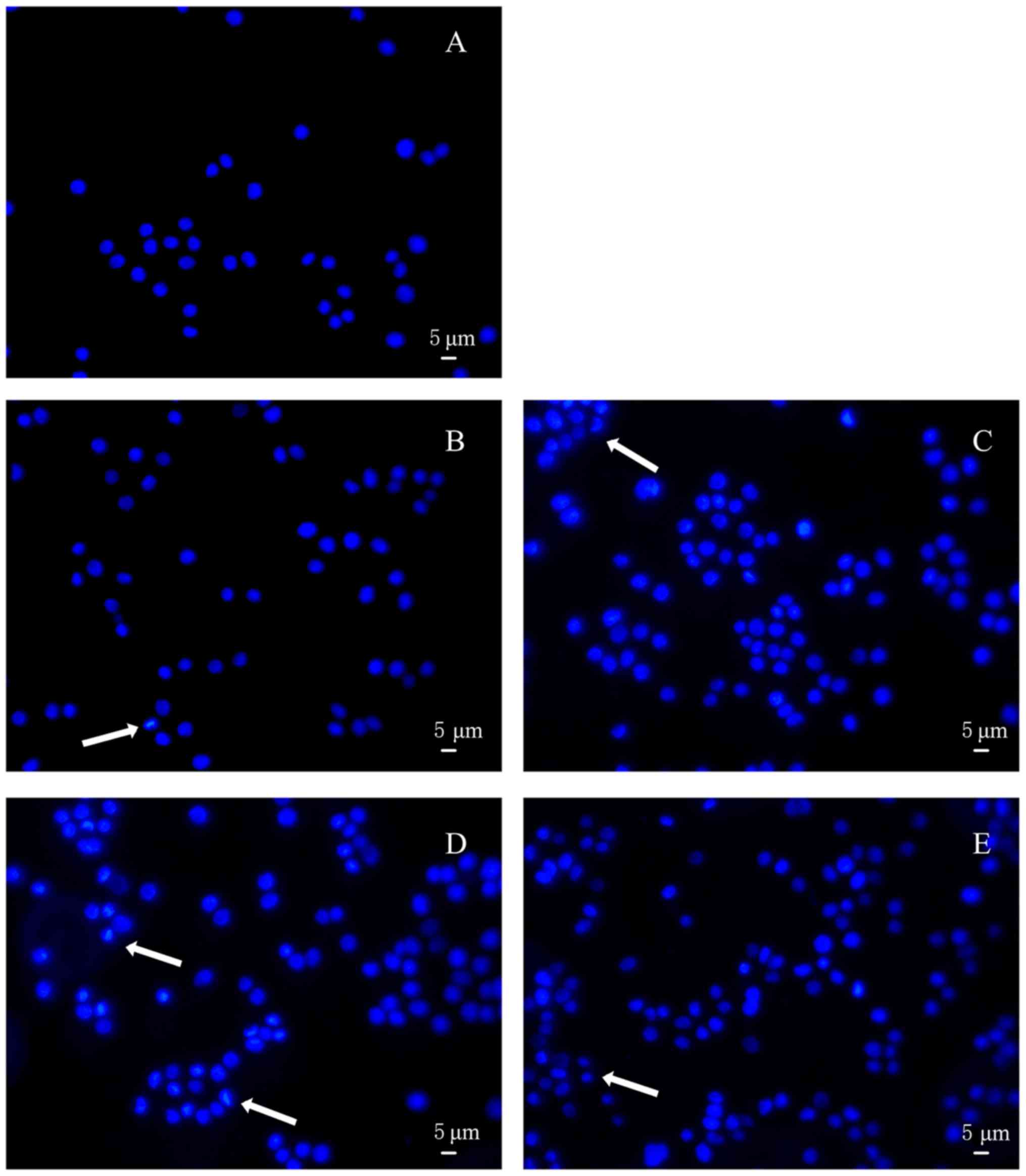
Hoechst staining was conducted to further appraise
the apoptosis of HT-29 cells treated with acidic EPS. As shown in
Fig. 4A, EPS-untreated cells appeared
circular or elliptical, with no condensation of the nucleus being
presented. In contrast, cells treated with acidic EPS produced by 4
kinds of L. casei strains showed different degrees of
morphological changes within nucleus and markedly condensed dots
known as apoptotic bodies. Furthermore, cells treated with acidic
L. casei SB27 EPS exhibited the most obvious morphological
changes when subjected to Hoechst 33258 staining (Fig. 4D). Overall, based on the results of
nuclei morphological changes, acid EPS group indeed induced HT-29
colon cancer cell apoptosis.
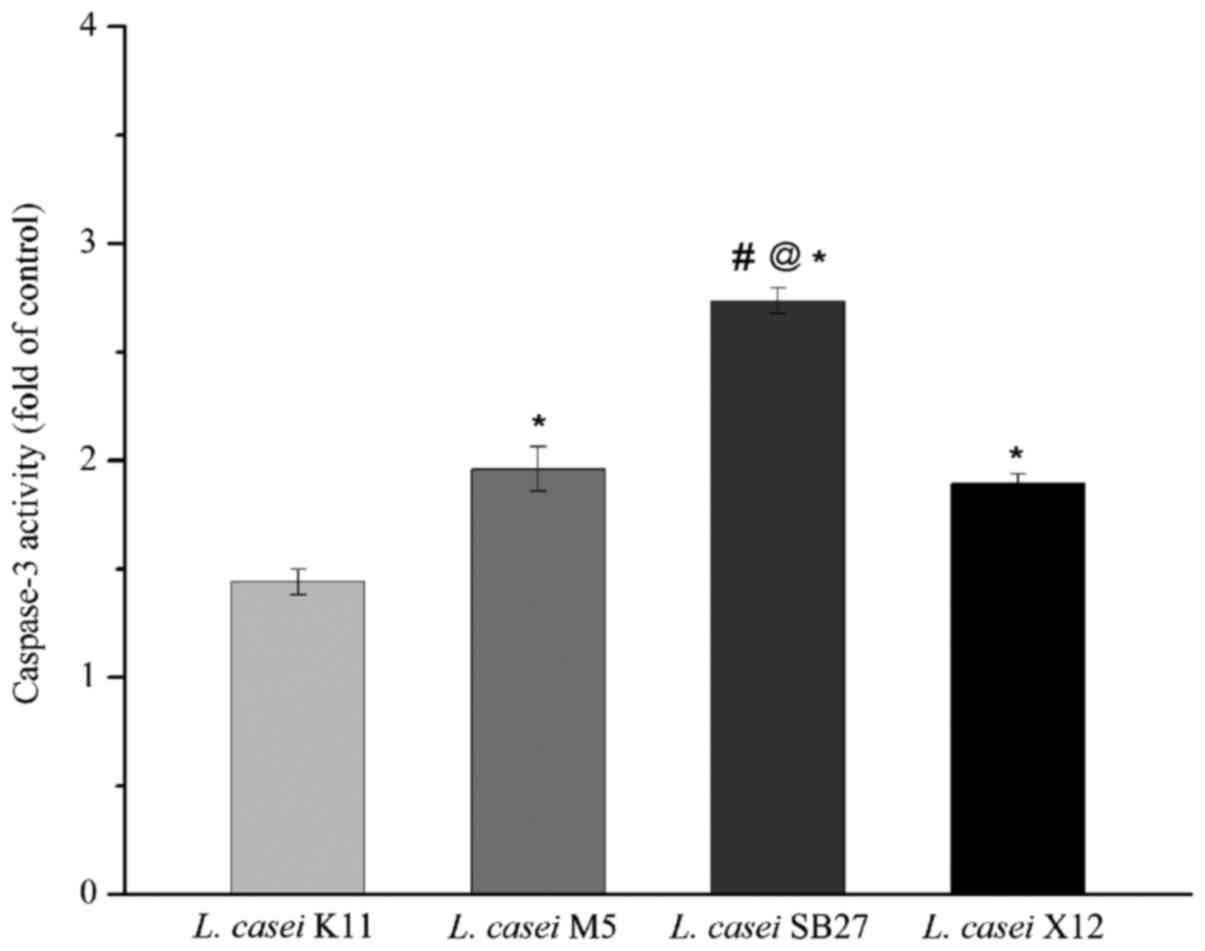
Effects of acidic EPS treatment on
activity of caspase-3
HT-29 cells treated with acidic EPS were subjected
to caspase-3 activity assay. A statistically increase in the ratio
of absorbance over that of controls was obtained (Fig. 5), which revealed that all 4 kinds of
tested EPS were able to activate caspase-3. The maximal increase of
caspase-3 activation was observed in HT-29 colon cancer cells
treated with L. casei SB27 acidic EPS, while the minimal
increase of caspase-3 activation was observed in L. casei
K11 acidic EPS group. The results indicated that L. casei
SB27 acidic EPS was more effective in activating caspase-3, which
was consistent with its effect on inducing apoptosis.
Discussion
Colon cancer is one of the most notorious malignant
tumors with high incidence and mortality. Chemotherapy is one of
the most commonly used therapeutic modalities for the treatment of
cancer, but most anticancer drugs currently used in chemotherapy
are cytotoxic to normal cells, resulting in multiple-organ toxicity
such as hemopoetic suppression and immunotoxicity (18). Polysaccharides from natural sources
are recently found as effective, relatively nontoxic substance with
a wide range of biological activities and accordingly have
attracted lots of attention (48). It
suggested that the polysaccharides with antitumor property can be
used as one ideal substitute for tumor therapy. The present study
evaluated the effects of EPS from nine previously reported
Lactobacillus strains with high degree of bio-activity on
HT-29 cells which were used in several studies of antitumor
activity of EPS (36,38).
We identified 4 L. casei strains, including
L. casei M5, L. casei SB27, L. casei ×12, and
L. casei K11, having a significant inhibitory effect on
HT-29 cell proliferation, whereas the rest 5 strains presented poor
anti-proliferation effects. Subsequent MTT assay of purified EPS
from these four strains further verified their anti-proliferation
effects on HT-29 cells but nontoxic to Vero cells, a normal cell.
Since the inhibition rate of EPS in cancer cells was less than that
of clinical drugs, the inhibition was considered to be effective
when the rate was >20% by using 100 µg/ml EPS (17,36). While
the inhibition effect of EPS produced by L. casei SB27 on
HT-29 cells significantly increased along with the increased
concentrations (P<0.01), acidic EPS produced by L. casei
M5 showed a inhibition rate close to 20% at a concentration of 100
µg/ml. The inhibition effect of produced EPS on HT-29 cells varied
from strain to strain, although these nine Lactobacillus
strains belong to the same genus. Our results were consistent with
previous report that EPS isolated from different strains showed
clear differences in their characteristics and biological
activities (49), which may be
attributed to the genetic differences of the strains.
Cell proliferation is tightly regulated by the cell
cycle: S phase for DNA synthesis, M phase for mitosis, G0/G1 and G2
phase. The G1/S transition is a vital checkpoint in the progress of
cell cycle and responsible for the initiation and completion of DNA
replication (50). By using FACS
analyses we observed that the inhibitory effect of crude and acidic
EPS on HT-29 cell proliferation was related to the prevention of G1
to S transition.
Cell shrinkage, nuclear fragmentation, and chromatin
condensation are included in a series of typical morphological
features of apoptosis (51). Both
flow cytometry and hoechst staining showed the apoptotic evidence
of EPS on HT-29 cells. Caspase-3 is initially formed as a 32 kDa
zymogen and cleaved into 17 and 12 kDa subunits when it was
activated in the apoptotic cell both by extrinsic (death ligand)
and intrinsic (mitochondrial) pathways. Activation of caspase-3 is
often the signal to ensure that the cellular components are
degraded in a controlled manner, carrying out cell death with
minimal effect on surrounding tissues (52). The increased caspase-3 activity
indicated the apoptosis induced in HT-29 colon cancer cells by
acidic ESPs were, in part, mediated by a caspase-dependent pathway.
In the near future, it would be interesting to further study the
molecular events of cell apoptosis under the treatment of EPS.
An interesting finding in the present study is that
acidic EPS has stronger effect of anti-proliferation and apoptosis
on HT-29 cell than crude EPS. Coincidentally, Zhang et al
compared the antitumor activity of neutral vs. acidic
polysaccharides isolated and purified from the dried bulbs of
Allium macrostemon Bunge against human gastric carcinoma
cells BGC-823 and found that acidic polysaccharides showed
significantly higher inhibition rate than neutral polysaccharides
(53). This phenomenon might be
attributed to differences between crude and acidic EPS in
composition and structure characteristics. In this study, the
obtained crude EPS were mixtures of acidic polysaccharides and
proteins, while the acidic EPS primarily consisted of acidic
polysaccharides with trace proteins. In our previous study, two
high molecular weight fractions (LW1 and LW2) were identified in
EPS from L casei SB27 and showed a sheet-like appearance
with a folded surface and a compact structure (54). We will continue to determine the
structural characteristics of the acidic EPS to further understand
the key factors affecting its activity.
There were some limitations of our study. The effect
of 500 ug/ml EPS was only tested on HT-29 cells. It is unclear
whether the EPS identified in present study has effects on other
cancer cell lines. Meanwhile, this concentration is relatively high
and expected to be toxic when used in human. The toxicity of EPS
was tested on Vero cell line in consideration of its extensive
usage in researches of various types of biological pharmaceuticals
(55) and little effect on its
proliferation was found, which was consistent to reports from other
group (56). However, in order to
evaluate its safety, it is necessary to test the toxicity of EPS on
normal human colon cells and human in vivo. We are planning
to do this after elucidating the mechanism of antitumor activity of
EPS. Furthermore, previous reports showed the immunologic reaction
elicited by the EPS, such as increased expression of TNF-α, IL-10,
and IL-1β (57–59). Whether the EPS from the 9
Lactobacillus strains has immunomodulatory activity on cells
and human being is unknown.
In conclusion, the results of present study suggest
that EPS from L. casei M5, L. casei SB27, L.
casei ×12, and L. casei K11, especially acidic EPS
produced by L. casei SB27, exerted significant
anti-proliferation effect on HT-29 cells via the induction of G0/G1
cell cycle arrest and caspase-3-dependent apoptosis. In the future,
it is necessary to detect whether the EPS has broad spectrum
antitumor activity on other cancer cell lines and immunomodulatory
activity in order to help us to evaluate the clinical implications
and safety of EPS.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
program of Harbin outstanding academic leaders (grant no.
2014RFXXJ026), the National Natural Science Foundation of China
(grant no. 31271906/C200204) and the National Natural Science
Foundation of China (grant no. 31301515).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WD performed the experiments and wrote the
manuscript. LZ and XH contributed to the conception of the study
and revised the manuscript. HY performed the data analyses and
revised the manuscript. XH reviewed and polished the manuscript. YZ
helped perform the analysis. LX performed the data analyses and all
authors approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Conflict of interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fotiadis CI, Stoidis CN, Spyropoulos BG
and Zografos ED: Role of probiotics, prebiotics and synbiotics in
chemoprevention for colorectal cancer. World J Gastroenterol.
14:6453–6457. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Commane D, Hughes R, Shortt C and Rowland
I: The potential mechanisms involved in the anti-carcinogenic
action of probiotics. Mutat Res. 591:276–289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong YN, Chang WC, Clapper M and Engstrom
PF: Chemoprevention of colorectal cancerSaltz L.B.: Colorectal
Cancer: Evidence-Based Chemotherapy Strategies. Humana Press Inc.;
Totowa, NJ: pp. 33–49. 2006
|
|
5
|
Wollowski I, Rechkemmer G and Pool-Zobel
BL: Protective role of probiotics and prebiotics in colon cancer.
Am J Clin Nutr. 73 2 Suppl:451S–455S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aachary AA, Gobinath D, Srinivasan K and
Prapulla SG: Protective effect of xylooligosaccharide from corncob
on 1,2-dimethylhydrazine induced colon cancer in rats. Bioact
Carbohydr Dietary Fibre. 5:146–152. 2015. View Article : Google Scholar
|
|
7
|
Huang L, Shan YJ, He CX, Ren MH, Tian PJ
and Song W: Effects of L. paracasei subp. paracasei ×12 on cell
cycle of colon cancer HT-29 cells and regulation of mTOR signalling
pathway. J Funct Foods. 21:431–439. 2016. View Article : Google Scholar
|
|
8
|
Chan AT and Giovannucci EL: Primary
prevention of colorectal cancer. Gastroenterology. 138:2029–2043.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
García-Ruiz A, Llano DGD,
Esteban-Fernández A, Requena T, Bartolomé B and Moreno-Arribas MV:
Assessment of probiotic properties in lactic acid bacteria isolated
from wine. Food Microbiol. 44:220–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonet AK, Strus M and Heczko PB: P1250
Influence of Lactobacilli probiotic strains on apoptosis of colon
cancer cells lines. Int J Antimicrob Agents. 29 2 Suppl:S343–S344.
2007. View Article : Google Scholar
|
|
11
|
Hu P, Song W, Shan Y, Du M, Huang M, Song
C and Zhang L: Lactobacillus paracasei subsp. paracasei M5L
induces cell cycle arrest and calreticulin translocation via the
generation of reactive oxygen species in HT-29 cell apoptosis. Food
Function. 6:2257–2265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laiño J, Villena J, Kanmani P and
Kitazawa HL: Immunoregulatory effects triggered by lactic acid
bacteria exopolysaccharides: New insights into molecular
interactions with host cells. Microorganisms. 4:E272016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruas-Madiedo P, Hugenholtz J and Zoon PL:
An overview of the functionality of exopolysaccharides produced by
lactic acid bacteria. Int Dairy J. 12:163–171. 2002. View Article : Google Scholar
|
|
14
|
Górska-Frączek S, Sandström C, Kenne L,
Paściak M, Brzozowska E, Strus M, Heczko P and Gamian A: The
structure and immunoreactivity of exopolysaccharide isolated from
Lactobacillus johnsonii strain 151. Carbohydr Res.
378:148–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Shi J, Yang X, Nan B, Liu Y and
Wang Z: Chemical and physical characteristics and antioxidant
activities of the exopolysaccharide produced by Tibetan kefir
grains during milk fermentation. Int Dairy J. 43:15–21. 2015.
View Article : Google Scholar
|
|
16
|
Ai LZ, Zhang H, Guo BH, Chen W, Wu ZJ and
Wu Y: Preparation, partial characterization and bioactivity of
exopolysaccharides from Lactobacillus casei LC2W. Carbohydr
Polym. 74:353–357. 2008. View Article : Google Scholar
|
|
17
|
Liu CT, Chu FJ, Chou CC and Yu RC:
Antiproliferative and anticytotoxic effects of cell fractions and
exopolysaccharides from Lactobacillus casei 01. Mutat Res.
721:157–162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ehrke MJ: Immunomodulation in cancer
therapeutics. Int Immunopharmacol. 3:1105–1119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Xu J, Fu Q, Fu X, Shu T, Bi Y and
Song B: Antitumor activity of a polysaccharide from Pleurotus
eryngii on mice bearing renal cancer. Carbohydr Polym. 95:615–620.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deepak V, Ramachandran S, Balahmar RM,
Pandian SR, Sivasubramaniam SD, Nellaiah H and Sundar K: In vitro
evaluation of anticancer properties of exopolysaccharides from
Lactobacillus acidophilus in colon cancer cell lines. In
Vitro Cell Dev Biol Anim. 52:163–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marta CL, Bernadeta N, Malgorzata S, Maria
W, Sabina GF, Andrzej G and Janusz M: Further studies on
immunomodulatory effects of exopolysaccharide isolated from
Lactobacillus rhamnosus KL37C. Cent Eur J Immunol.
38:1270–1271. 2013.
|
|
22
|
Li JY, Jin MM, Meng J, Gao SM and Lu RR:
Exopolysaccharide from Lactobacillus planterum LP6:
Antioxidation and the effect on oxidative stress. Carbohydr Polym.
98:1147–1152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Collins MD, Phillips BA and Zanoni P:
Deoxyribonucleic Acid Homology Studies of Lactobacillus casei,
Lactobacillus paracasei sp. nov., subsp. paracasei and subsp.
tolerans and Lactobacillus rhamnosus sp. nov., comb. nov.
Int J Syst Bacteriol. 39:105–108. 1989. View Article : Google Scholar
|
|
24
|
Ortu S, Felis GE, Marzotto M, Deriu A,
Molicotti P, Sechi LA, Dellaglio F and Zanetti S: Identification
and functional characterization of Lactobacillus strains
isolated from milk and Gioddu, a traditional Sardinian fermented
milk. Int Dairy J. 17:1312–1320. 2007. View Article : Google Scholar
|
|
25
|
Wang SM, Zhang LW, Fan RB, Han X, Yi HX,
Zhang LL, Xue CH, Li HB, Zhang YH and Shigwedha N: Induction of
HT-29 cells apoptosis by lactobacilli isolated from fermented
products. Res Microbiol. 165:202–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang YC, Zhang LW, Tuo YF, Guo CF, Yi HX,
Li JY, Han X and Du M: Inhibition of Shigella sonnei adherence to
HT-29 cells by lactobacilli from Chinese fermented food and
preliminary characterization of S-layer protein involvement. Res
Microbiol. 161:667–672. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang S, Han X, Zhang L, Zhang Y, Li H and
Jiao Y: Whole peptidoglycan extracts from the lactobacillus
paracasei subsp. Paracasei M5 strain exert anticancer activity
in vitro. Biomed Res Int. 2018:28717102018.PubMed/NCBI
|
|
28
|
Tuo YF, Zhang LW, Yi HX, Zhang YC, Zhang
WQ, Han X, Du M, Jiao YH and Wang SM: Short communication:
Antiproliferative effect of wild Lactobacillus strains
isolated from fermented foods on HT-29 cells. J Dairy Sci.
93:2362–2366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saxelin M: Lactobacillus GG-A human
probiotic strain with thorough clinical documentation. Food Rev
Int. 13:293e3131997. View Article : Google Scholar
|
|
30
|
Caro SD, Tao H, Grillo A, Elia C,
Gasbarrini G, Sepulveda AR and Gasbarrini A: Effects of
Lactobacillus GG on genes expression pattern in small bowel
mucosa. Dig Liver Dis. 37:320–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ai LZ, Zhang H, Guo BH, Chen W, Wu ZJ and
Tang J: Optimization of culture conditions for exopolysaccharide
production by Lactobacillus casei LC2W.
Milchwissenschaft-milk Sci Int. 61:374–377. 2006.
|
|
32
|
Ramos AN, Gobbato N, Rachid M, Gonzalez L,
Yantorno O and Valdez JC: Effect of Lactobacillus plantarum
and Pseudomonas aeruginosa culture supernatants on
polymorphonuclear damage and inflammatory response. Int
Immunopharmacol. 10:247–251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Choi SS, Kim Y, Han KS, You S, Oh S and
Kim SH: Effects of Lactobacillus strains on cancer cell
proliferation and oxidative stress in vitro. Lett Appl Microbiol.
42:452–458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Z, Zhang Z, Qiu L, Zhang F, Xu X, Wei
H and Tao X: Characterization and bioactivities of the
exopolysaccharide from a probiotic strain of Lactobacillus
plantarum WLPL04. J Dairy Sci. 100:6895–6905. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang K, Li W, Rui X, Chen X, Jiang M and
Dong M: Characterization of a novel exopolysaccharide with
antitumor activity from Lactobacillus plantarum 70810. Int J
Biol Macromol. 63:133–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Zhao X, Yang Y, Zhao A and Yang Z:
Characterization and bioactivities of an exopolysaccharide produced
by lactobacillus plantarum Yw32. Int J Biol Macromol.
74:119–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stefano DD, Tommonaro G, Simeon V, Poli A,
Nicolaus B and Carnuccio R: A polysaccharide from tomato
(Lycopersicon Esculentum) peels affects Nf-κb activation in
Lps-stimulated J774 macrophages. J Nat Prod. 70:1636–1639. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park S Y, Kim E J, Shin H K, Kwon DY, Kim
MS, Surh YJ and Park JH: Induction of apoptosis in Ht-29 colon
cancer cells by phloretin. J Med Food. 10:581–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin R, Liu H, Wu S, Pang L, Jia M, Fan K,
Jia S and Jia L: Production and in vitro antioxidant activity of
exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int J
Biol Macromol. 51:153–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
DuBois M, Gilles KA, Hamilton JK, Rebers
PA and Smith F: Colorimetric Method for Determination of Sugars and
Related Substances. Analytical Chemistry. 28:350–356. 1956.
View Article : Google Scholar
|
|
41
|
Wang X, Wang S, Li Y, Wang F, Yang X and
Yao J: Sulfated Astragalus polysaccharide can regulate the
inflammatory reaction induced by LPS in Caco2 cells. Int J Biol
Macromol. 60:248–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Xiao Y, Xiong C, Wei A and Ruan J:
Apoptosis induced by a new flavonoid in human hepatoma HepG2 cells
involves reactive oxygen species-mediated mitochondrial dysfunction
and MAPK activation. Eur J Pharmacol. 654:209–216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Choi HJ, Do Lim Y and Park JH: Induction
of G1 and G2/M cell cycle arrests by the dietary compound
3,3′-diindolylmethane in ht-29 human colon cancer cells. BMC
Gastroenterol. 9:1–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shang LH, Li CM, Yang ZY, Che DH, Cao JY
and Yu Y: Luffa echinata roxb. induces human colon cancer cell
(Ht-29) death by triggering the mitochondrial apoptosis pathway.
Molecules. 17:5780–5794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sharma S, Singh RL and Kakkar P:
Modulation of Bax/Bcl-2 and caspases by probiotics during
acetaminophen induced apoptosis in primary hepatocytes. Food
Chemical Toxicol. 49:770–779. 2011. View Article : Google Scholar
|
|
46
|
Huang T, Lin J, Cao J, Zhang P, Bai Y,
Chen G and Chen K: An exopolysaccharide from trichoderma
pseudokoningii and its apoptotic activity on human leukemia k562
cells. Carbohydr Polym. 89:701–708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Y, Zhang SP and Cai YQ: Cytoprotective
effects of selenium on cadmium-induced LLC-PK1 cells apoptosis by
activating JNK pathway. Toxicol In Vitro. 21:677–684. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ooi VE and Liu F: Immunomodulation and
anti-cancer activity of polysaccharide-protein complexes. Curr Med
Chem. 7:715–729. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kanmani P, kumar Satish R, Yuvaraj N,
Paari KA, Pattukumar V and Arul V: Production and purification of a
novel exopolysaccharide from lactic acid bacterium Streptococcus
phocae PI80 and its functional characteristics activity in vitro.
Bioresour Technol. 102:4827–4833. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nurse P: Ordering S phase and M phase in
the cell cycle. Cell. 79:547–550. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Saraste A and Pulkki K: Morphologic and
biochemical hallmarks of apoptosis. Cardiovasc Res. 45:528–537.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jung MY, Kwon SK and Moon A:
Chemopreventive allylthiopyridazine derivatives induce apoptosis in
SK-Hep-1 hepatocarcinoma cells through a caspase-3-dependent
mechanism. Eur J Cancer. 37:2104–2110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang Z, Wang F, Wang M, Ma L, Ye H and
Zeng XA: Comparative study of the neutral and acidic
polysaccharides from Allium macrostemon Bunge. Carbohydr
Polym. 117:980–987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Di W, Zhang L, Wang S, Yi H, Han X, Fan R
and Zhang Y: Physicochemical characterization and antitumour
activity of exopolysaccharides produced by Lactobacillus
casei SB27 from yak milk. Carbohydr Polym. 171:307–315. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Osada N, Kohara A, Yamaji T, Hirayama N,
Kasai F, Sekizuka T, Kuroda M and Hanada K: The genome landscape of
the african green monkey kidney-derived vero cell line. DNA Res.
21:673–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mukhopadhyay SK, Chatterjee S, Gauri SS,
Das SS, Mishra A, Patra M, Ghosh AK, Das AK, Singh SM and Dey S:
Isolation and characterization of extracellular polysaccharide
Thelebolan produced by a newly isolated psychrophilic Antarctic
fungus Thelebolus. Carbohydr Polym. 104:204–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
López P, Monteserín DC, Gueimonde M, de
los Reyes-Gavilán CG, Margolles A, Suárez A and Ruas-Madiedo P:
Exopolysaccharide-producing Bifidobacterium strains elicit
different in vitro responses upon interaction with human cells.
Food Res Int. 46:99–107. 2012. View Article : Google Scholar
|
|
58
|
Nikolic M, Lopez P, Strahinic I, Suarez A,
Kojic M, Fernandez-Garcia M, Topisirovic L, Golic N and
Ruas-Madiedo P: Characterisation of the exopolysaccharide
(EPS)-producing Lactobacillus paraplantarum BGCG11 and its
non-EPS producing derivative strains as potential probiotics. Int J
Food Microbiol. 158:155–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Patten DA, Leivers S, Chadha MJ, Maqsood
M, Humphreys PN, Laws AP and Collett A: The structure and
immunomodulatory activity on intestinal epithelial cells of the
EPSs isolated from Lactobacillus helveticus sp. Rosyjski and
Lactobacillus acidophilus sp. 5e2. Carbohydr Res.
384:119–127. 2014. View Article : Google Scholar : PubMed/NCBI
|