Introduction
Lung cancer is the leading cause of cancer
mortality, and there were ~1,800,000 incident lung cancer cases in
2012 globally, representing ~13% of the total global cancer
incidence (1). Lung cancer primarily
consists of two histological types: Small cell lung cancer and
non-small-cell lung cancer (NSCLC), which account for 15 and 85% of
total cases of lung cancer, respectively (2). Lung adenocarcinoma (LAC), which is the
most widespread histological type of NSCLC, causes >500,000
mortalities every year worldwide (3).
Therefore, it is important to reveal the molecular mechanisms of
LAC progression and development, and to develop novel therapeutic
targets for patients with LAC.
Epithelial-mesenchymal transition (EMT) is an
important process that is characterized by the loss of cell
polarity and cell-cell contact, and is activated in tumor
metastasis and resistance to chemoradiotherapy (4,5).
Activation of EMT is associated with the altered expression of
numerous genes, including the downregulation of epithelial markers
such as occluden-1 and E-cadherin, and the upregulation of
mesenchymal markers including N-cadherin and vimentin (6). A number of studies have demonstrated
that EMT is a critical process in tumor invasion and metastasis in
NSCLC (7–9).
Manganese-12 acetate (Mn12Ac) is a magnetically
bistable molecule exhibiting a combination of strong magnetic
anisotropy and high spin properties (10,11).
However, there have been no studies concerning the association
between Mn12Ac and disease therapy, particularly in cancer. In the
present study, the biological function of Mn12Ac in lung cancer
treatment was investigated.
Materials and methods
Cell culture
The human lung cancer A549 cell line (American Type
Culture Collection, Manassas, VA, USA) was cultured in RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 10% fetal bovine serum (GE Healthcare, Chicago, IL, USA),
100 U/ml penicillin and 100 g/ml streptomycin at 37°C in a
humidified incubator containing 5% CO2. Cells were
treated with 100 nM Mn12Ac (dissolved in H2O, and
H2O was used as control) and cultured at 37°C for 48 h,
and subsequent experiments were performed.
Total RNA extraction and quantitative
polymerase chain reaction (qPCR) assay
Total RNA was isolated from cancer cells using the
RNeasy Mini kit according to the protocol of the manufacturer
(Qiagen GmbH, Hilden, Germany) and used for the qPCR assay. Three
independent experimental repeats of qPCR were performed.
qPCR was used to detect the mRNA expression of
E-cadherin, N-cadherin, vimentin, zinc finger protein SNAI1
(Snail), zinc finger protein SNAI2 (Slug), twist-related protein 1
(Twist1), zinc finger E-box-binding homeobox 1 (ZEB1) and
programmed death-ligand 1 (PD-L1). The PCR reactions were performed
in a total volume of 20 µl, including 10 µl 2× Power
SYBR® Green PCR Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 2 µl cDNA (5 ng/µl) and
1 µl primer mix (10 µM each). PCR amplification and detection were
performed in a LightCycler 480 II (Roche Applied Science, Penzberg,
Germany) as follows: Initial denaturation at 95°C for 10 min, then
40 cycles of denaturation at 95°C for 15 sec and annealing and
elongation at 60°C for 1 min. The relative gene expression was
calculated using the comparative Cq method. The gene expression of
the target gene was normalized to an endogenous reference gene
(GAPDH), and data relative to the calibrator were calculated using
the formula 2−ΔΔCq. ΔCq was calculated by subtracting
the average GAPDH Cq value from the average Cq value of the gene of
interest (12). The ratio defined the
level of relative expression of the target gene to that of GAPDH.
The primers were as follows: E-cadherin forward primer,
5′-GAACGCATTGCCACATACAC-3′; reverse primer,
5′-GAATTCGGGCTTGTTGTCAT-3′; N-cadherin forward primer,
5′-GTGCCATTAGCCAAGGGAATTCAGC-3′, reverse primer,
5′-GCGTTCCTGTTCCACTCATAGGAGG-3′; vimentin forward primer,
5′-TGAGTACCGGAGACAGGTCGAG-3′, reverse primer,
5′-TAGCAGCTTCAACGCAAAGTTC-3′; Snail forward primer,
5′-ACCACTATGCCGCGCTCTT-3′, reverse primer,
5′-GGTCGTAGGGCTGCTGGAA-3′; Slug forward primer,
5′-GCGCATGCTCCATTGTCTTAC-3′, reverse primer,
5′-AGGCACTTGGAAGGGGTATTG-3′; Twist1 forward primer,
5′-AGAAGTCTGCGGGCTGTGGCG-3′, reverse primer,
5′-GAGGGCAGCGTGGGGATGATC-3′; ZEB1 forward primer,
5′-CTACTCAACTACGGTCAGCCC-3′, reverse primer,
5′-TTGGGCGGTGTAGAATCAGAG-3′; programmed death ligand 1 (PD-L1)
forward primer, 5′-GAACTACCTCTGGCACATCCT-3′, reverse primer,
5′-CACATCCATCATTCTCCCTTT-3′; GAPDH forward primer,
5′-AAATCCCATCACCATCTTCCAG-3′ and reverse primer,
5′-GAGTCCTTCCACGATACCAAAGTTG-3′.
Western blotting
The cells were lysed in the lysis buffer (20 mM
Tris, 2 mM EDTA, 50 mM 2-mecaptoethanol, 10% glycerol, pH 7.4). The
homogenates were placed on ice for 30 min and centrifuged at 12,000
× g for 15 min at 4°C. Subsequently, the protein concentration of
the lysates was determined using a Protein Assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Equal amounts (15 µg) of
total proteins were loaded onto a 10% PAGE, electrophoretically
transferred to polyvinylidene difluoride membrane, and then blocked
with 10% non-fat milk for 2 h at room temperature. The membranes
were incubated with specific primary antibodies overnight at 4°C
and probed with corresponding secondary antibodies (goat
anti-rabbit IgG-HRP, sc-2004, 1:5,000 and goat anti-mouse IgG-HRP,
sc-2005, 1:5,000, Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 1 h at room temperature. The protein bands were visualized
using Enhanced Chemiluminescence Blotting Detection reagents
(Applygen Technologies, Inc., Beijing, China). The primary
antibodies used are as follows: E-cadherin (1:1,000; sc-71009;
Santa Cruz Biotechnology, Inc.), N-cadherin (1:1,000; sc-59987;
Santa Cruz Biotechnology, Inc.), vimentin (1:1,000; sc-80975; Santa
Cruz Biotechnology, Inc.), Wnt1 (1:1,000; sc-6266; Santa Cruz
Biotechnology, Inc.), β-catenin (1:1,000; sc-65480; Santa Cruz
Biotechnology, Inc.), phosphorylated-phosphoinositide 3-kinase
(p-PI3K; 1:1,000; 4228; Cell Signaling Technology, Inc., Danvers,
MA, USA), PI3K (1:1,000; sc-376112; Santa Cruz Biotechnology,
Inc.), p-AKT (1:1,000; sc-271964; Santa Cruz Biotechnology, Inc.),
AKT (1:1,000; sc-1619; Santa Cruz Biotechnology, Inc.), PD-L1
(1:1,000; 13684; Cell Signaling Technology, Inc.) and β-actin
(1:5,000; sc-517582; Santa Cruz Biotechnology, Inc.).
Transwell assay
For the Transwell invasion assay, 60 µl
Matrigel® was diluted with precooled serum-free medium
in a ratio of 1:4, and was added to the bottom of the Transwell
chamber and incubated for 1 h at 37°C. A total of 2×105
cells (Mn12Ac treated cells and the H2O treated control
cells) were seeded into the upper chambers (24-well insert; pore
size 8 µm), while medium supplemented with 10% FBS (600 µl) was
placed in the lower chamber. Following incubation at 37°C for 24 h,
the cells on the upper chamber side of the inserts were removed
gently with a cotton swab. The inserts were then fixed with 4%
methanol for 15 min at room temperature and stained with 0.1%
crystal violet for 15 min at room temperature. The mean number of
migratory cells was calculated by counting five randomly selected
fields each time under the light microscope at ×200 magnification.
The experiments were repeated three times.
For the Transwell migration assay, the remaining
protocol was the same as the Transwell invasion assay, except that
the inserts were not pre-coated with Matrigel®.
Statistical analysis
All data were analyzed by unpaired Student's t-test
or one-way analysis of variance with post hoc comparisons with
Tukey's honest significant difference test for multiple group
comparisons using SPSS version 20.0 (IBM Corp., Armonk, NY, USA)
and GraphPad Prism (version 5.0; GraphPad, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
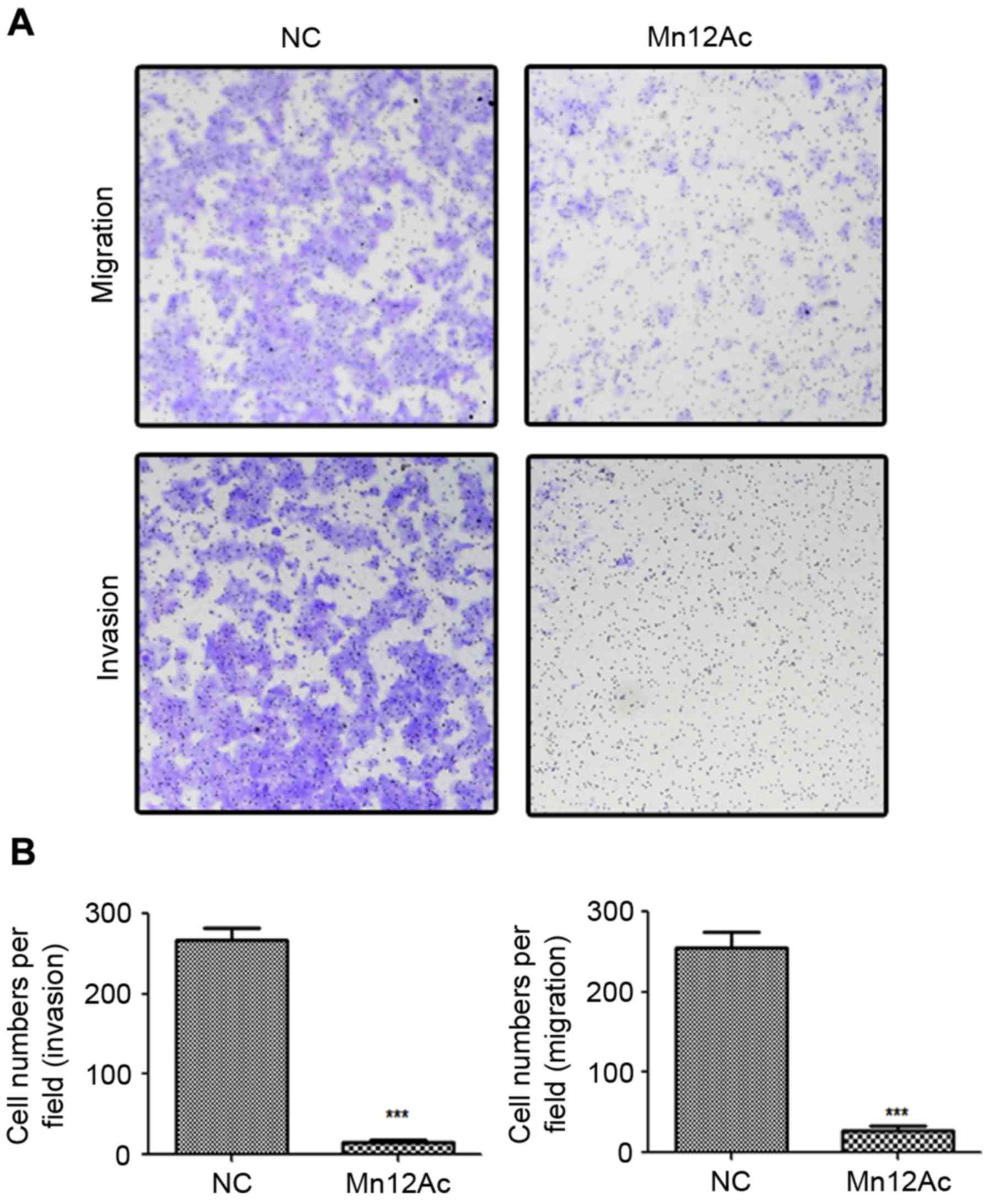
Mn12Ac is able to significantly
inhibit the migration and invasion of A549 lung cancer cells
In order to investigate the anti-tumor activity of
Mn12Ac, Transwell migration and invasion assays were performed to
analyze the effects of Mn12Ac on lung cancer cell metastasis. The
results demonstrated that Mn12Ac was able to significantly inhibit
the migration and invasion of A549 cells compared with the negative
control (Fig. 1A and B).
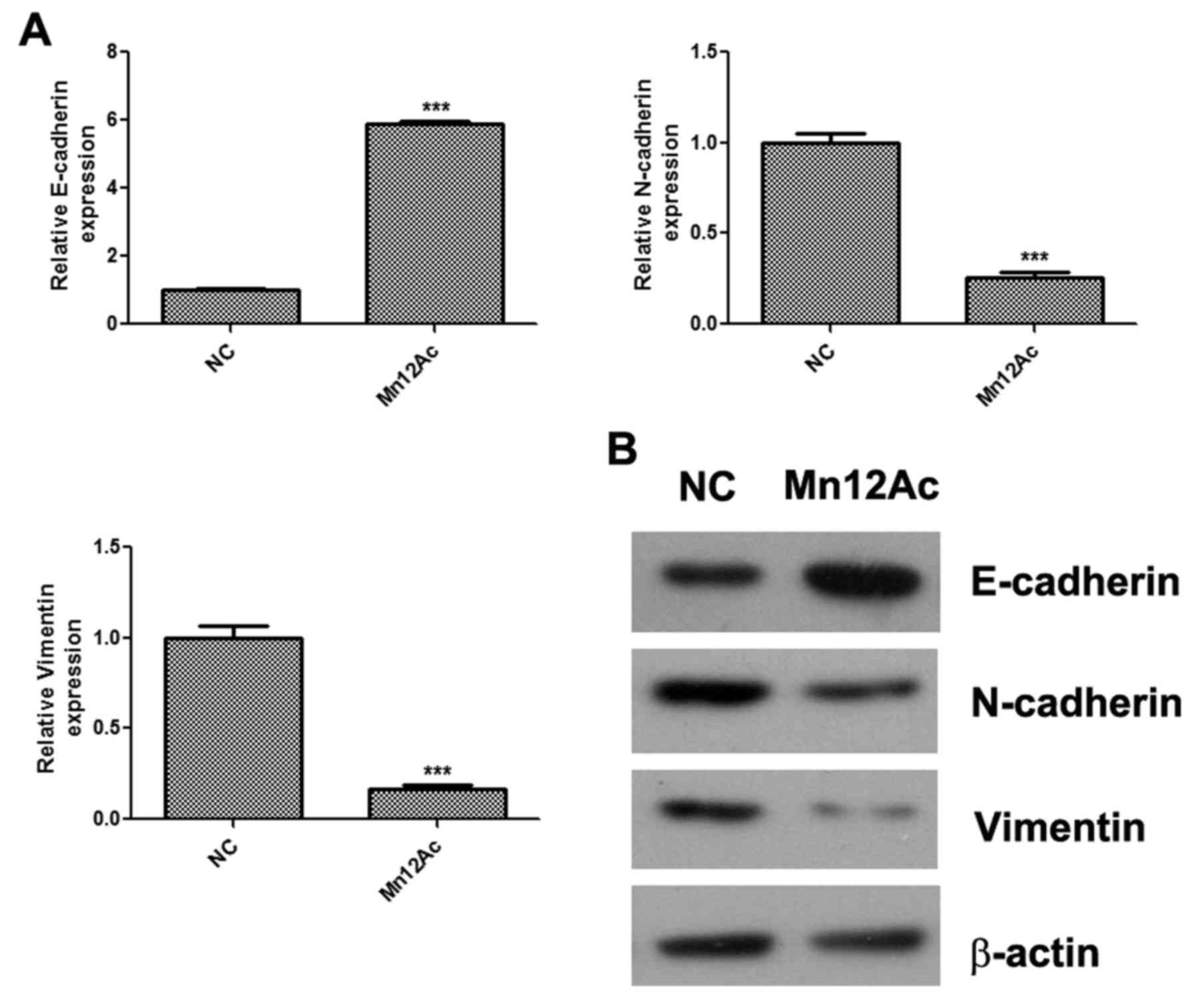
Mn12Ac inhibits EMT in A549 lung
cancer cells
EMT is activated during tumor metastasis and serves
an important role in the process (13); therefore whether Mn12Ac affected the
EMT process in A549 lung cancer cells was investigated. The qPCR
assay results indicated that Mn12Ac was able to significantly
upregulate the epithelial marker E-cadherin, and downregulate the
mesenchymal markers N-cadherin and vimentin in A549 cells (Fig. 2A). In addition, it was indicated by
western blotting that Mn12Ac was also able to markedly upregulate
the protein expression of E-cadherin and reduce the protein
expression levels of N-cadherin and vimentin (Fig. 2B).
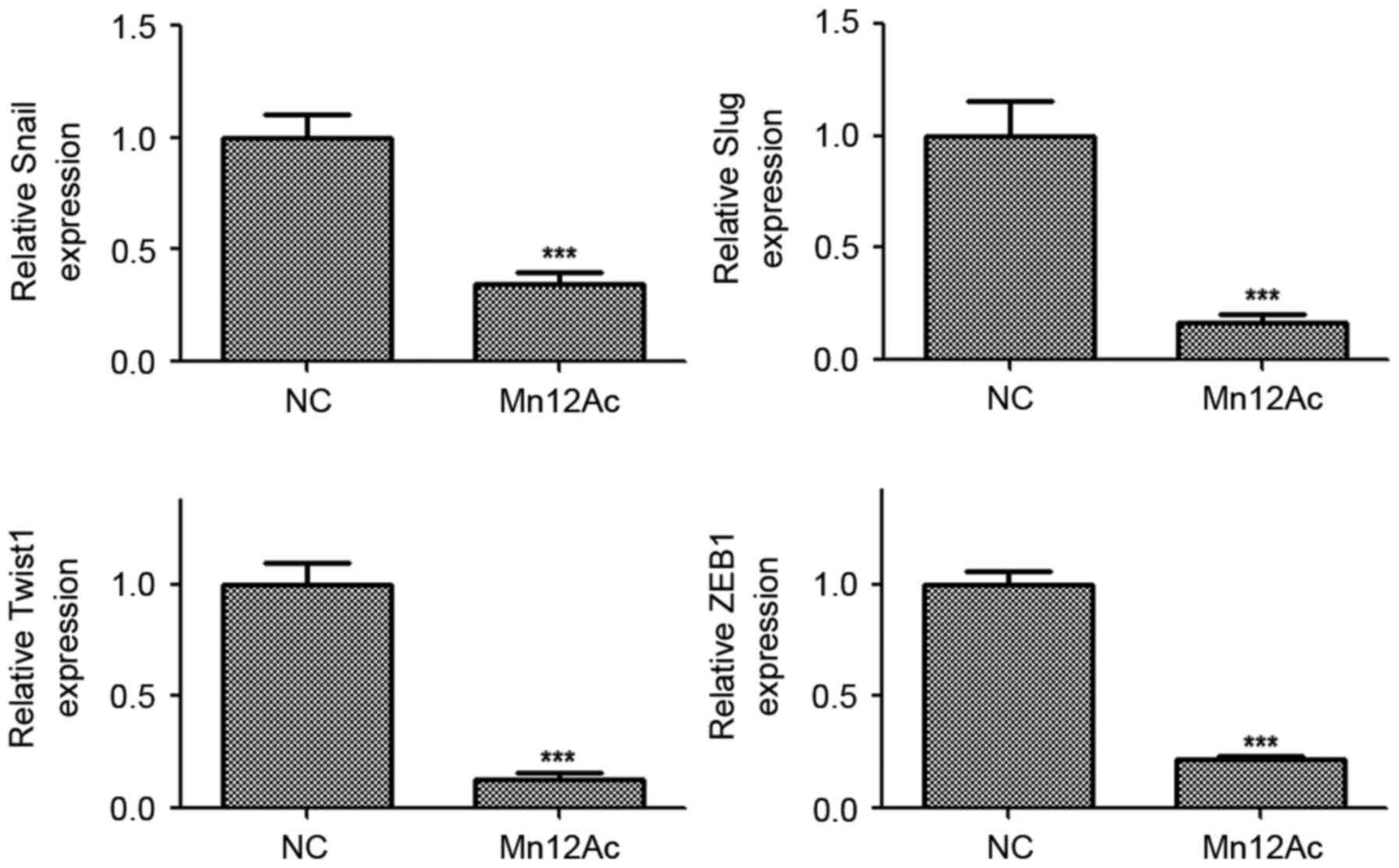
Mn12Ac significantly inhibits the
expressions of EMT-associated transcription factors in A549 lung
cancer cells
Snail, slug, twist1 and ZEB1 are EMT-associated
transcription factors in cancer (14–16).
Therefore, whether Mn12Ac regulated EMT via affecting these
transcription factors was examined. Using qPCR, it was revealed
that Mn12Ac was able to significantly decrease the mRNA expression
levels of Snail, Slug, Twist1 and ZEB1 in A549 cancer cells
(Fig. 3). These results suggested
that Mn12Ac inhibited EMT in lung cancer cells by downregulating
EMT-inducing transcription factors.
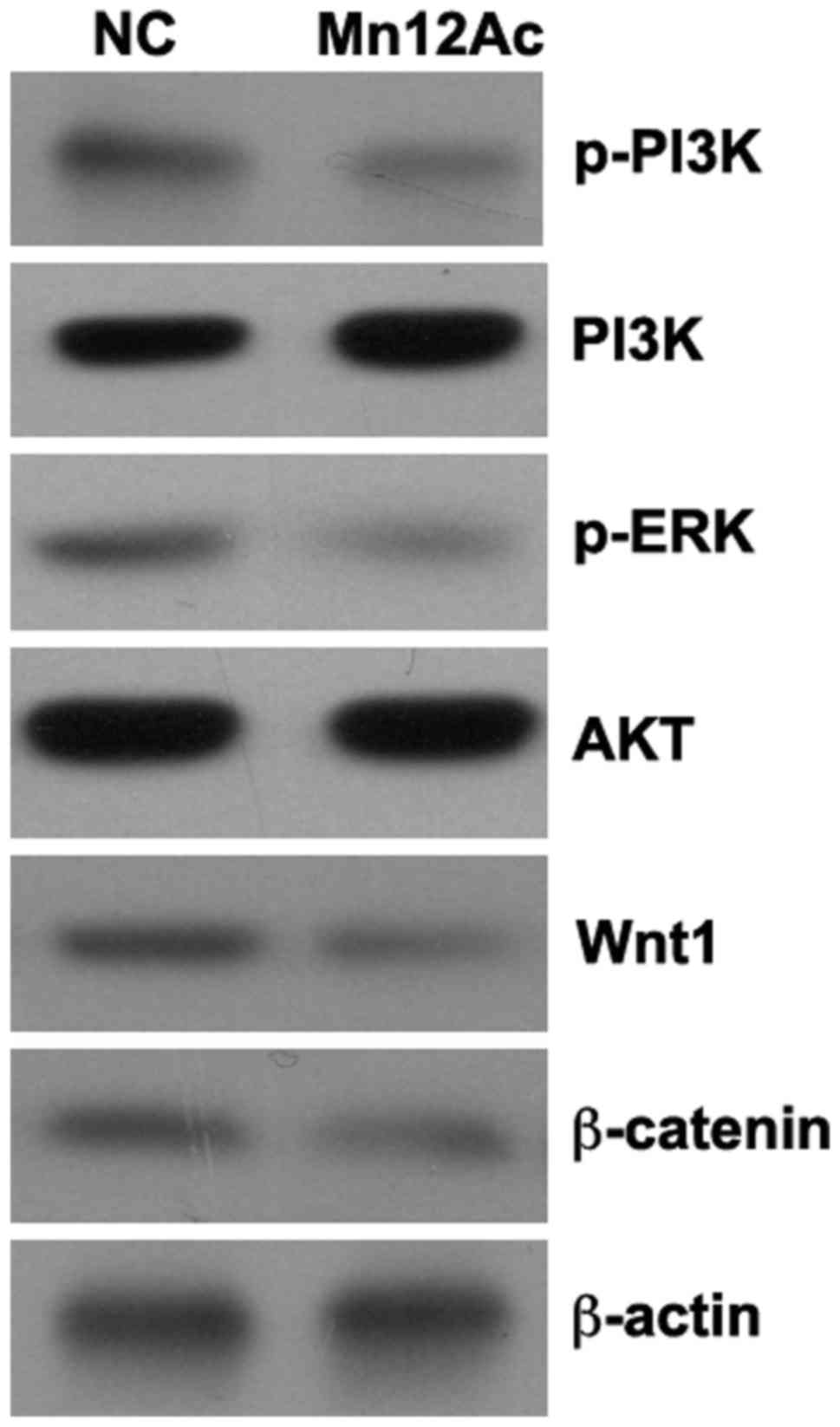
Mn12Ac inhibits the Wnt/β-catenin and
PI3K/AKT signaling pathways
The Wnt/β-catenin and PI3K/AKT signaling pathways
serve important roles in the regulation of EMT (17–19), and
it was hypothesized that Mn12Ac may inhibit EMT in lung cancer
cells by regulating these two pathways. Using western blotting
assay, it was identified that Mn12Ac was able to markedly reduce
the expression levels of Wnt1 and β-catenin and inhibit the
phosphorylation of PI3K and AKT in A549 lung cancer cells (Fig. 4).
Mn12Ac decreases the mRNA and protein
expression levels of PD-L1
PD-1/PD-L1-targeted immunotherapy has emerged as a
promising therapeutic strategy for lung cancer, and PD-L1
expression was associated with EMT transition in lung cancer
(20). Therefore, the present study
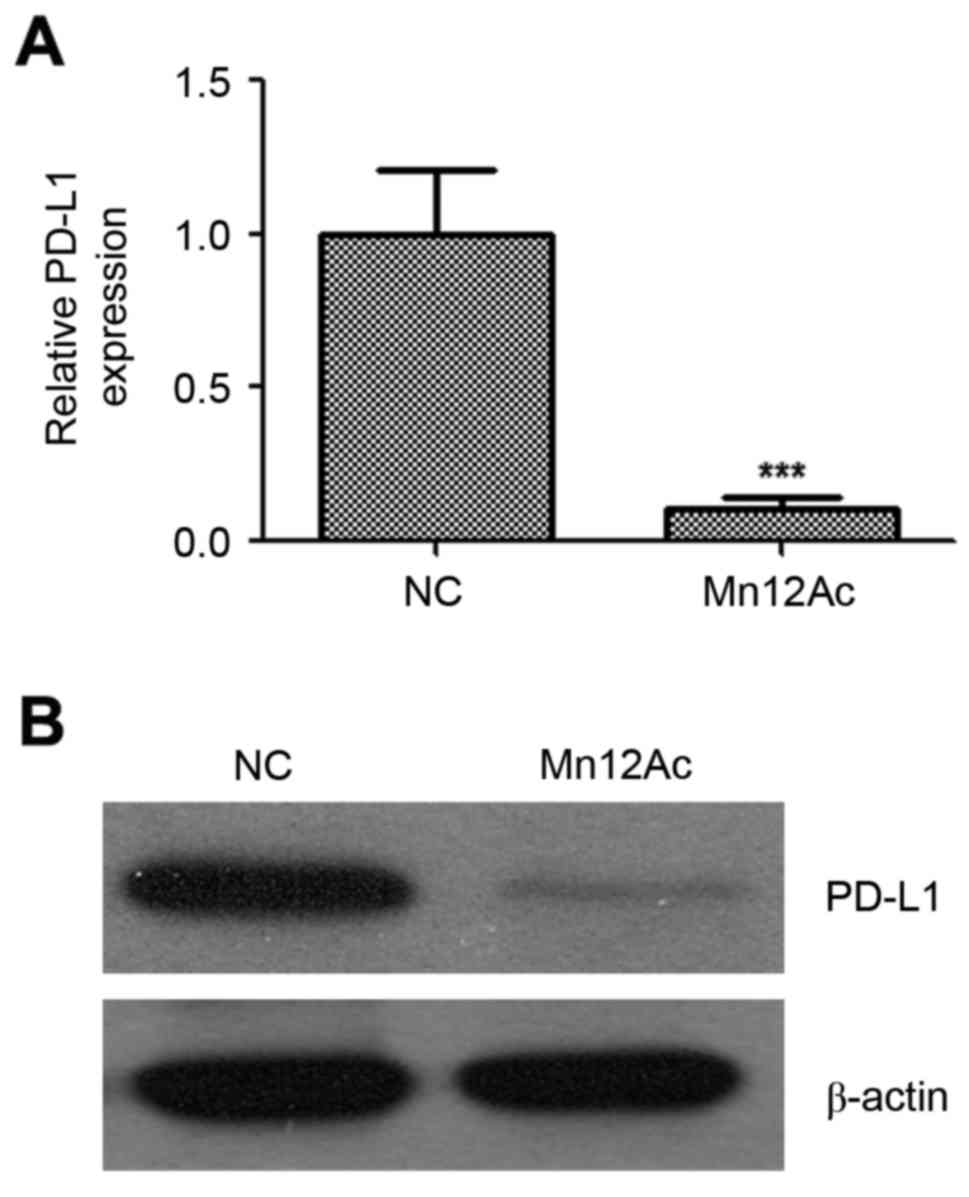
investigated whether Mn12Ac affected PD-L1 in lung cancer. Notably,
the results indicated that Mn12Ac was able to reduce the mRNA and
protein expression levels of PD-L1 in A549 lung cancer cells by
qPCR and western blotting assay, respectively (Fig. 5).
Discussion
Human lung cancer is the most common cause of global
cancer-associated mortality (21).
Lung cancer develops through a multistep process with oncogenic
mutations in lung epithelial cells (22). It is important to reveal the molecular
mechanisms of LAC progression and development, and to develop novel
therapeutic targets for patients with LAC.
Metastasis is the primary cause of cancer-associated
mortality, and the epithelial EMT process is critical for
epithelial cell cancer progression. The results of the present
study demonstrated that Mn12Ac was able to significantly inhibit
the migration and invasion of A549 lung cancer cells. These results
suggested that Mn12Ac suppressed the EMT process in lung cancer
cells. EMT is a morphological change in cells from an epithelial
form to a fibroblast-like mesenchymal form. In addition to these
morphological changes, key biomarkers that involved in the steps of
the process include cell adhesion molecules such as E-cadherin,
N-cadherin, and transcription factors Snail, Slug, Twist1 and ZEB1
(5,23). The expression levels of these EMT
molecules were demonstrated to be associated with drug resistance,
and the inhibition of EMT may interfere with tumor progression and
drug resistance (24). The results of
the present study suggested that Mn12Ac may inhibit the EMT process
by downregulating the transcription factors Snail, Slug, Twist1 and
ZEB1. In addition, the results of the present study suggest that
Mn12Ac may promote drug sensitivity in cancer therapy.
The PI3K/AKT signaling pathway serves an important
role in the EMT process and tumor metastasis. Shao et al
(25) suggested that irisin may
inhibit EMT and the invasion of lung cancer cells by regulating the
PI3K/AKT/Snail signaling pathway. Tumor protein D52 inhibited the
growth and metastasis of renal cell carcinoma cells by
downregulating the levels of p-PI3K and p-Akt (26). Curcumin was also reported to suppress
EMT in renal tubular epithelial cells through the inhibition of the
Akt/mTOR signaling pathway (19). The
Wnt/β-catenin signaling pathway was also associated with the EMT
process. CDGSH iron sulfur domain 2 may promote proliferation and
EMT in pancreatic cancer cells by upregulating the Wnt/β-catenin
signaling pathway (27). Wang et
al (28) reported that calpain
small subunit 1 promoted EMT in human melanoma cells through the
activation of the Wnt/β-catenin signaling pathway (28). Zuo et al (29) observed that long non-coding RNA
protein SPRY4 intronic transcript 1 was able to modulate
trophoblast cell invasion, migration and EMT processes by
regulating the Wnt/β-catenin signaling pathway (29). The results of the present study
revealed that Mn12Ac may inhibit the Wnt/β-catenin and PI3K/AKT
signaling pathways in lung cancer cells.
PD-L1 in tumor cells is known to promote immune
escape of cancer cells by interacting with programmed cell death
protein 1 in tumor-infiltrating immune cells (30). Immunotherapy targeting these molecules
is emerging as a novel strategy for the treatment of different
types of cancer. Notably, the results of the present study
indicated that Mn12Ac was able to markedly reduce the expression of
PD-L1 at the level of mRNA and protein in lung cancer cells.
Taken together, the results of the present study
revealed that Mn12Ac may significantly inhibit migration, invasion
and EMT in lung cancer cells by suppressing the Wnt/β-catenin and
PI3K/AKT signaling pathways, and Mn12Ac may also reduce the
expression of PD-L1. In the future, additional studies should focus
on the potential clinical applications of Mn12Ac.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Project
of Kunming Technology Program (grant no. 2015-1-S-00877,
2015-1-S-00576).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author.
Author's contributions
CZ, WQ and JH designed the work that led to the
submission. HJ and XX performed RT-qPCR analysis. LP, ZW, TZ, JX,
LL and LD conducted western blot analysis. CZ and JH drafted the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Heymach JV and Lippman SM: Lung
cancer. N Engl J Med. 359:1367–1380. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Imielinski M, Berger AH, Hammerman PS,
Hernandez B, Pugh TJ, Hodis E, Cho J, Suh J, Capelletti M,
Sivachenko A, et al: Mapping the hallmarks of lung adenocarcinoma
with massively parallel sequencing. Cell. 150:1107–1120. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:pii: E17. 2016.
View Article : Google Scholar
|
|
5
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seton-Rogers S: Epithelial-mesenchymal
transition: Untangling EMT's functions. Nat Rev Cancer. 16:12016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang DX, Zou YJ, Zhuang XB, Chen SX, Lin
Y, Li WL, Lin JJ and Lin ZQ: Sulforaphane suppresses EMT and
metastasis in human lung cancer through miR-616-5p-mediated
GSK3β/β-catenin signaling pathways. Acta Pharmacol Sin. 38:241–251.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye Z, Yin S, Su Z, Bai M, Zhang H, Hei Z
and Cai S: Downregulation of miR-101 contributes to
epithelial-mesenchymal transition in cisplatin resistance of NSCLC
cells by targeting ROCK2. Oncotarget. 7:37524–37535. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen S, Jiao S, Jia Y and Li Y: Effects of
targeted silencing of FOXC1 gene on proliferation and in vitro
migration of human non-small-cell lung carcinoma cells. Am J Transl
Res. 8:3309–3318. 2016.PubMed/NCBI
|
|
10
|
Rakvin B, Zilić D, North JM and Dalal NS:
Probing magnetic fields on crystals of the nanomagnet Mn12-acetate
by electron paramagnetic resonance. J Magn Reson. 165:260–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morello A, Bakharev ON, Brom HB, Sessoli R
and de Jongh LJ: Nuclear spin dynamics in the quantum regime of a
single-molecule magnet. Phys Rev Lett. 93:1972022004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo C, Zhao D, Zhang Q, Liu S and Sun MZ:
miR-429 suppresses tumor migration and invasion by targeting CRKL
in hepatocellular carcinoma via inhibiting Raf/MEK/ERK pathway and
epithelial-mesenchymal transition. Sci Rep. 8:23752018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amoroso MR, Matassa DS, Agliarulo I,
Avolio R, Lu H, Sisinni L, Lettini G, Gabra H, Landriscina M and
Esposito F: TRAP1 downregulation in human ovarian cancer enhances
invasion and epithelial-mesenchymal transition. Cell Death Dis.
7:e25222016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang W, Lai Y, Zhu M, Huang S, Feng W and
Gu X: Combretastatin A4 regulates proliferation, migration,
invasion, and apoptosis of thyroid cancer cells via PI3K/Akt
signaling pathway. Med Sci Monit. 22:4911–4917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hammers H, Fu C, Gerber S, Berg SVD,
Steenwinkel F, Moriarty W, Keizman D, Kachhap S and Carducci MA:
Epithelial-mesenchymal transition: A mechanism of resistance to
VEGF pathway inhibition in genitourinary cancers. J Clin Oncol. 30
5_suppl:S3902012. View Article : Google Scholar
|
|
17
|
Liu MH, Fu WJ, Cui YH, Guo QN and Zhou Y:
Downregulation of Semaphorin-3F is associated with poor prognostic
significance in osteosarcoma patients. Am J Cancer Res.
6:2252–2262. 2016.PubMed/NCBI
|
|
18
|
Liu B, Pan CF, He ZC, Wang J, Wang PL, Ma
T, Xia Y and Chen YJ: Long noncoding RNA-LET suppresses tumor
growth and EMT in lung adenocarcinoma. Biomed Res Int.
2016:46934712016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu FQ, Chen MJ, Zhu M, Zhao RS, Qiu W, Xu
X, Liu H, Zhao HW, Yu RJ, Wu XF, et al: Curcumin suppresses
epithelial-mesenchymal transition of renal tubular epithelial cells
through the inhibition of Akt/mTOR pathway. Biol Pharm Bull.
40:17–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim S, Koh J, Kim MY, Kwon D, Go H, Kim
YA, Jeon YK and Chung DH: PD-L1 expression is associated with
epithelial-to-mesenchymal transition in adenocarcinoma of the lung.
Hum Pathol. 58:7–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Larsen JE and Minna JD: Molecular biology
of lung cancer: Clinical implications. Clin Chest Med. 32:703–740.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arai K, Eguchi T, Rahman MM, Sakamoto R,
Masuda N, Nakatsura T, Calderwood SK, Kozaki K and Itoh M: A novel
high-throughput 3D screening system for EMT inhibitors: A pilot
screening discovered the EMT inhibitory activity of CDK2 inhibitor
SU9516. PLoS One. 11:e01623942016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dopeso H, Jiao HK, Cuesta AM, Henze AT,
Jurida L, Kracht M, Acker-Palmer A, Garvalov BK and Acker T: PHD3
controls lung cancer metastasis and resistance to EGFR inhibitors
through TGFα. Cancer Res. 78:1805–1819. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shao L, Li H, Chen J, Song H, Zhang Y, Wu
F, Wang W, Zhang W, Wang F, Li H and Tang D: Irisin suppresses the
migration, proliferation, and invasion of lung cancer cells via
inhibition of epithelial-to-mesenchymal transition. Biochem Biophys
Res Commun. 485:598–605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Z, Liu H, Hou J, Li T, Du X, Zhao X,
Xu W, Xu W and Chang J: Tumor protein D52 (TPD52) inhibits growth
and metastasis in renal cell carcinoma cells through the PI3K/Akt
signaling pathway. Oncol Res. 25:773–779. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang Y, Bai YS and Wang Q: CDGSH iron
sulfur domain 2 activates proliferation and EMT of pancreatic
cancer cells via Wnt/β-catenin pathway and has prognostic value in
human pancreatic cancer. Oncol Res. 25:605–615. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang E, Wang D, Li B, Ma H, Wang C, Guan
L, Zhang H, Yi L and Li S: Capn4 promotes epithelial-mesenchymal
transition in human melanoma cells through activation of the
Wnt/β-catenin pathway. Oncol Rep. 37:379–387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zuo Q, Huang S, Zou Y, Xu Y, Jiang Z, Zou
S, Xu H and Sun L: The Lnc RNA SPRY4-IT1 modulates trophoblast cell
invasion and migration by affecting the epithelial-mesenchymal
transition. Sci Rep. 6:371832016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chatterjee J, Dai W, Aziz NHA, Teo PY,
Wahba J, Phelps DL, Maine CJ, Whilding LM, Dina R, Trevisan G, et
al: Clinical use of programmed cell death-1 and its ligand
expression as discriminatory and predictive markers in ovarian
cancer. Clin Cancer Res. 23:3453–3460. 2017. View Article : Google Scholar : PubMed/NCBI
|