Introduction
Endometrial cancer is the most common reproductive
cancer in women (1). In China, the
incidence of endometrial cancer is 7.44 cases per 1,000,000
individuals (2), and in the USA, the
disease causes 8,590 mortalities every year (3).
Prostaglandin E2 (PGE2) is the most common
prostaglandin in humans and was first reported in 1936 (4). A previous study has revealed that PGE2
has a significant function in reproductive, neuronal, metabolic and
immune systems (5). In two previous
studies by Che et al (6) and
Zhu et al (6,7), certain cytokines, such as interleukin
(IL)-6 and oncostatin M, were revealed to promote proliferation and
invasion of endometrial cancer cells. Certain studies have also
reported that PGE2 serves a role in the development of ovarian,
breast and colorectal cancer (8–10).
However, to the best of our knowledge, no studies have investigated
the mechanism of the role served by PGE2 in endometrial cancer.
The signal transducer and activator of transcription
3 (STAT3) transcription factor was identified as a downstream
effecter of inflammatory mediators that modulates gene expression
and metabolism (11). STAT3 underwent
phosphorylation and activated the expression of numerous
STAT3-regulated genes (12). STAT3 is
usually highly expressed in cancer cells, and high levels of STAT3
prompt a poor outcome in ovarian cancer, glioblastoma, breast
cancer and prostate cancer (13–17).
The cytochrome P450 (CYP) superfamily of enzymes
mediates the catalytic conversion of drugs to reactive products
that bind to macromolecules, including proteins and DNA. CYP
enzymes account for ~75% of total drug metabolism (18). CYP17α hydroxylase (CYP17), an enzyme
involved in androgen synthesis, has been implicated in the
pathogenesis of numerous cancer types (19). A previous study has observed that
inhibiting CYP17 is beneficial in prostate carcinoma (20), suggesting that CYP17 may be an
important factor in hormone-associated cancer types.
In the present study, the PGE2 synthase-promoted
expression of a P450 enzyme in endometrial cancer cells was
analyzed, and the results identified CYP17 as the main enzyme
involved in this process. These factors resulted in enhanced
invasion in endometrial cancer cells.
Materials and methods
Reagents and antibodies
PGE2 (cat. no. 363-24-6) and androgen (cat. no.
1424-00-6) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). JSI-124 (cat. no. 2222-07-3) was obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Prostaglandin E
synthase 2 (PTGES2) antibody (cat. no. 10881-1-AP, dilution at
1:1,000) was purchased from Protein Tech Group, Inc. (Chicago, IL,
USA). Antibodies against CYP17 (cat. no. ab125022, dilution at
1:2,000), CYP19 (cat. no. ab35604, dilution at 1:1,000), IL-1 (cat.
no. ab8320, dilution at 1:2,000), pSTAT3 (phosphorylated Y705, cat.
no. ab76315, dilution at 1:20,000 and phosphorylated S727, cat. no.
ab32143, dilution at 1:2,000) were purchased from Abcam (Cambridge,
UK). The ELISA kits for estrogen (cat. no. CEA461Ge, 96T), androgen
(testosterone, cat. no. CEA458Ge, 96T) and progesterone (cat. no.
CEA459Ge, 96T) were purchased from Cloud-Clone Corp. (Houston, TX,
USA).
Patients and samples
Tissue samples for immunohistochemistry (IHC) were
obtained from 152 patients (all females aged between 34 and 79
years, with a mean age of 53.6 years) with endometrial cancer who
had undergone surgical resection at Anhui Provincial Hospital
(Hefei, China) between 2010 and 2016, and from 66 patients with
normal endometria during curettage of the uterus.
Clinicopathological characteristics including age, FIGO stage and
grade (21), myometrial invasion and
nodal metastasis were included. The project was approved by the
Human Investigation Ethics Committee of Anhui Provincial Hospital
and informed consent was obtained from all patients prior to the
study.
Cell lines and culture conditions
The human endometrial cancer Ishikawa cell line, the
293T cell line and primary endometrial (PE) cells were obtained
from Dr Feizhou Jiang (Department of Obstetrics and Gynecology, The
First Affiliated Hospital of Soochow University, Suzhou, China).
Ishikawa, PE and 293T cells were grown in Dulbecco's modified
Eagle's medium (DMEM)/F-12 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.). Ishikawa, PE and 293T cells were
incubated at 37°C in a humidified atmosphere containing 5%
CO2. All experiments were performed at the third passage
following thawing.
Total RNA extraction, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from Ishikawa cells using
TRIzol reagent (cat no. 15596-026; Invitrogen; Thermo Fisher
Scientific, Inc.) and cDNA was prepared using the reverse
transcriptase kit (Takara Biotechnology Co., Ltd., Dalian, China).
RT-qPCR was conducted using an ABI Prism 7500 sequence detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
performed with the SYBR Green PCR Master mix (Toyobo Life Science,
Osaka, Japan). According to supplier's protocol, the PCR conditions
comprised 95°C for 30 sec, followed by 40 cycles of amplification
(95°C for 3 sec and 60°C for 30 sec). The 2−∆∆Cq method
was used to analyze the relative changes in gene expression
(22). The results were expressed
relative to the internal reference gene GAPDH. Sequences of the
primer pairs used are listed in Table
I.
 | Table I.Primer sequences for quantitative
polymerase chain reaction analysis. |
Table I.
Primer sequences for quantitative
polymerase chain reaction analysis.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| PTGES2 |
CTTCCTTTTCCTGGGCTTCG |
GAAGACCAGGAAGTGCATCCA |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTTC |
| TNF-α |
TGGCCTCCCTCTCATCAGTT |
ATCGGCTGGCACCACTAGTT |
| CYP19 |
TGGAAATGCTGAACCCGATAC |
AATTCCCATGCAGTAGCCAGG |
| CYP17 |
AGAATTCTCTGGTCGGCC |
TTCTCCAGTTTCTGGCCA |
| IL-1 |
GCCCTAAACAGATGAAGTGCTC |
GAACCAGCATCTTCCTCAG |
| IL-10 |
GGCACCCAGTCTGAGAACAG |
ACTCTGCTGAAGGCATCTCG |
Western blot analysis
For western blot analysis, cells were lysed in cell
lysis buffer (Beyotime Institute of Biotechnology, Nantong, China)
for 30 min at 4°C. Total protein, determined using a bicinchoninic
acid assay (cat. no. P0010S; Beyotime Institute of Biotechnology).
A total of 15 µg protein per lane, was fractionated using SDS-PAGE
(10% gel) and was transferred onto polyvinylidene fluoride
membranes, blocking with 5% skim milk which dissolved in DMEM for 2
h. The membranes were then incubated with the appropriate
aforementioned primary antibodies (IL-1, CYP17, CYP19, pSTAT3-S727
and pSTAT3-Y705) for 24 h in 4°C, followed by incubation with
horseradish peroxidase-conjugated secondary antibody (cat. no.
sc-280786, dilution at 1:10,000 for 2 h in 4°C, Santa Cruz
Biotechnology, Inc.). Following three further washes in TBS, the
proteins were detected and visualized using an
electrochemiluminescence system (Pierce; Thermo Fisher Scientific,
Inc.) and GAPDH (cat. no. ab184531; dilution at 1:10,000; Abcam)
was used as an internal control.
IHC
The tissue sections were initially fixed in 10%
formalin solution at 4°C for 2 days and paraffin embedded. The
tissue sections were subsequently subjected to microtome sectioning
(5 µm). The sections were placed on glass slides and immersed with
100% ethanol (5 min) and boiling water (30 min) three times. The
endogenous peroxidase activity was blocked by immersing the
sections in freshly prepared 10% H2O2 and 10%
methanol in 1× PBS for 20 min. The sections underwent trypsin
treatment (0.1% trypsin in 0.1% CaCl2) for 10 min to
cleave the protein crosslinks to assess the antigen and epitope.
Nonspecific antigens were blocked using 4% bovine serum albumin
(BSA; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 2 h at
room temperature. The membranes were incubated with anti-PTGES2
(1:100; ProteinTech Group, Inc.) overnight at 4°C. Following
incubation, the sections were thoroughly washed with 1× PBS and
incubated with a goat anti-rabbit secondary antibody (dilution,
1:3,000; cat no. ab6721; Abcam) for 1 h at room temperature.
Following washing to prevent non-specific binding, the sections
were stained with diaminobenzidine (DAB; cat no. ab64238; Abcam).
The percentage of positively stained cells was rated as follows: 0
points, 0%; 1 point, 1-25%; 2 points, 26-50%; 3 points, 51-75%; and
4 points, ≥75%. The staining intensity was rated in the following
manner: 0 points, negative staining; 1 point, weak intensity; 2
points, moderate intensity; and 3 points, strong intensity.
Subsequently, immunoreactivity scores for each case were obtained
by multiplying the values of the two parameters described above.
The average score for all 5 random fields at ×200 magnification was
used as the histological score (HS). Tumors were categorized into 2
groups based upon the HS: The low-expression group (HS, 0-6) and
the high-expression group (HS, 7-12).
Transwell invasion assays
For Transwell invasion assays, the upper side of an
8-µm pore, 6.5-mm polycarbonate Transwell filter (Corning
Incorporated, Corning, NY, USA) chamber was uniformly coated with
Matrigel basement membrane matrix (BD Biosciences, Bedford, MA,
USA) for 2 h at 37°C prior to the cells being added. A total of
2×104 Ishikawa cells were seeded in serum-free DMEM/F-12
into the upper chamber of a Transwell filter (in triplicate) and
were incubated in 37°C for 48 h. Invasive cells, which had reached
the lower chamber, which contained DMEM/F-12 supplemented with 10%
FBS, were fixed in 4% paraformaldehyde, stained in 0.5% crystal
violet (Beyotime, 20°C, 10 min) and counted using a confocal
microscope (Olympus, Shibuya, Japan). A total of 5 fields was
counted for each Transwell filter. Each field was counted and
images were captured at ×200 magnification.
Transfection
Control groups and siCYP17 groups were created.
Ishikawa cells with DMEM medium only was used as the control group.
To inhibit the expression of the target gene, high-performance
liquid chromatography-purified small interfering CYP17 siRNAs (1 µl
in 50 µl DMEM medium; 5′-3′: GCUGGAGAAGAUCAUUUGU,) were prepared
according to the sequence of the target gene. A scrambled siRNA
with no homology to any known human mRNA was used as a negative
control (siCo). siRNA oligonucleotide duplexes were synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). The sequences of
the siRNA oligonucleotides are provided in Table I. Cells were seeded onto 6-well plates
at 70-80% confluence and grown overnight prior to transfection.
Transfection of the cells with the siRNA or siCo was accomplished
using the Lipofectamine 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocols in 48 h.
Plasmids
Flag-CYP17 was subcloned into a PiggyBac vector
(Shanghai GenePharma Co., Ltd.) and transfected with a help vector.
To obtain a stable and pure CYP17-overexpressing (CYP17 OE) cell
population, selection with 300 µg/ml hygromycin (Santa Cruz
Biotechnology, Inc.) was performed in 37°C until the control cells
died. For the immunofluorescence staining, CYP17-Flag was subcloned
to the pLenti-GIII-CMV-IRES-puro-SV40-GFP vector (Shanghai
GenePharma Co., Ltd.). To obtain a pure cell population, 2 µg/ml
puromycin (Santa Cruz Biotechnology, Inc.) selection was performed
after 24 h of transfection at 37°C. The DMEM/F12 medium was then
changed after 12 h when the blank control cells died. All the cells
were visually GFP-positive under a fluorescence microscope. CYP17
plasmids were obtained from Shanghai GenePharma Co., Ltd. The
plasmids were transfected with Lipofectamine 3000 into Ishikawa
cells according to the manufacturer's protocols.
ELISA
Concentrations of the estrogen, androgen and
progesterone hormones were detected in culture medium of PE cells
and Ishikawa cells using solid phase sandwich ELISA according to
the manufacturer's protocols (Cloud-Clone Corp.). The hormone assay
sensitivity was 0.1 pg/ml and the assay range was 1.03-20,000
pg/ml. For statistical analysis, culture medium was independently
collected three times. The ELISA kits used were as follows: ELISA
kit for Estradiol (estrogen, cat. no. CEA461Ge, 96T), ELISA kit for
Testosterone (cat. no. CEA458Ge, 96T) and ELISA kit for
Progesterone (cat. no. CEA459Ge, 96T), all purchased from
Cloud-Clone Corp.
Statistical analysis
All quantitative data are presented as the mean ±
standard deviation. Data were analyzed using one-way analysis of
variance (ANOVA) with a least significant difference test. All
statistical analyses were performed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed ≥3 times.
Results
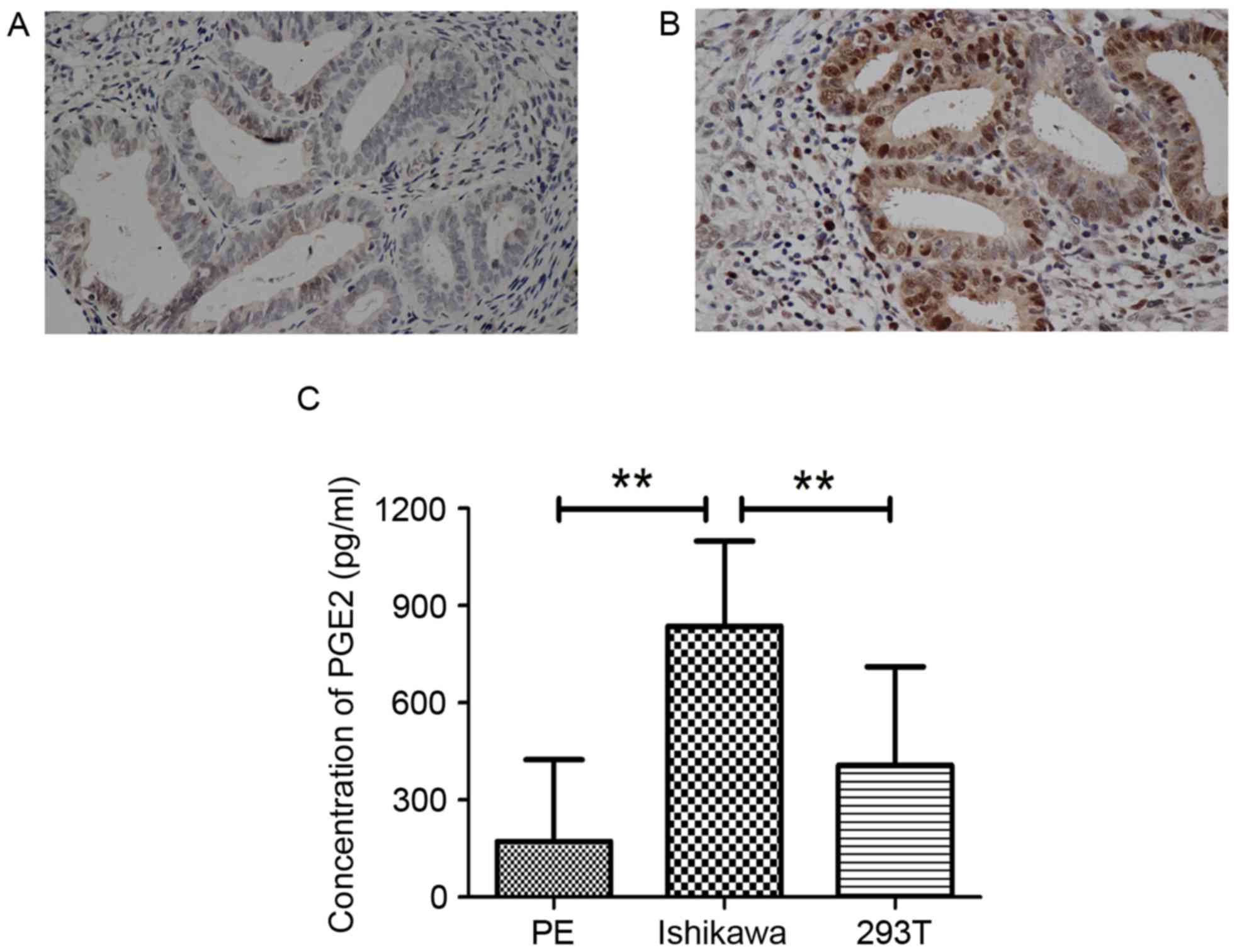
PTGES2 expression is increased in
endometrial cancer
PTGES2 is involved in the synthesis of PGE2. Recent
studies have indicated that PGE2 may be a mitogen with a role in a
number of cancer types (8–10). IHC was performed in normal endometria
and endometrial cancer tissues. Compared with that in the normal
endometria, the expression of PTGES2 was significantly upregulated
in the endometrial cancer tissues (Fig.
1A and B; Table II). Statistical
analysis revealed that increased expression of PTGES2 was
significantly associated with the age of the patient (P=0.0092) and
the depth of myometrial invasion (P<0.0001), but not with any
other characteristics (FIGO stage, grade and nodal metastasis,
Table III). As PTGES2 is required
for the synthesis of PGE2, an ELISA was performed to determine the
PGE2 concentration in Ishikawa cells, a typical endometrial cancer
cell line. A significantly higher concentration of PGE2 was
observed in these cells compared with that observed in the PE cells
and the 293T cells (Fig. 1C). This
confirmed that endometrial cells grow in a high concentration of
PGE2. Therefore, the Ishikawa cells were selected for further
study.
 | Table II.Expression of PTGES2 in normal
endometria and endometrial cancer. |
Table II.
Expression of PTGES2 in normal
endometria and endometrial cancer.
|
|
| HS of PTGES2,
n |
|
|---|
|
|
|
|
|
|---|
| Groups | Patients, n | Low expression
group (HS<6) | High expression
group (HS≥6) | P-value |
|---|
| Normal
endometria | 66 | 49 | 17 |
|
| Endometrial
cancer | 152 | 56 | 96 |
<0.001a |
 | Table III.Associations between PTGES2
expression and clinicopathological characteristics in endometrial
cancer. |
Table III.
Associations between PTGES2
expression and clinicopathological characteristics in endometrial
cancer.
|
|
| HS of PTGES2,
n |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Patients, n | Low expression
group (HS<6) | High expression
group (HS≥6) | P-value |
|---|
| Total | 152 | 56 | 96 |
|
| Age, years |
|
|
|
|
|
≥55 | 78 | 21 | 57 | 0.0092a |
|
<55 | 74 | 35 | 39 |
|
| FIGO stage |
|
|
|
|
| Stage
I–II | 126 | 48 | 78 | 0.4804 |
| Stage
III–IV | 26 | 8 | 18 |
|
| Grade |
|
|
|
|
| G1 | 49 | 18 | 31 | 0.9856 |
|
G2-G3 | 103 | 38 | 65 |
|
| Myometrial invasion
(depth) |
|
|
|
|
| ≤1/2
myometrium | 48 | 35 | 13 |
<0.0001a |
| >1/2
myometrium | 104 | 21 | 83 |
|
| Nodal
metastasis |
|
|
|
|
|
Positive | 22 | 7 | 15 | 0.5990 |
|
Negative | 130 | 49 | 81 |
|
PGE2 promotes CYP17 expression in
endometrial cancer cells via STAT3
RT-qPCR was performed to test the expression of
certain potential mRNA targets identified in previous studies
(23–27). As the results indicated, CYP17
expression was significantly increased following PGE2 stimulation,
whereas the differences among other cytokines were not significant
(Fig. 2A). Subsequent western blot
analysis was performed to investigate CYP17 expression changes
following PGE2 stimulation (Fig. 2B).
As with the RT-qPCR results, CYP17 expression increased mainly in
these reported targets. In a previous study, prostaglandin E2
receptor 4 (EP4) was identified as the receptor of PGE2 (28). As EP4 is not a direct DNA promoter, it
is unable to directly stimulate CYP17 expression. Subsequently,
phosphorylation of the DNA binding protein, STAT3, was examined in
Ishikawa cells. The results demonstrated that two STAT3
phosphorylated residues at S727 and Y705 exhibited increased CYP17
expression in PGE2-stimulated Ishikawa cells. However, this
increase was more notable at S727 than at Y705 and the increase of
CYP17 was prevented by the presence of JSI-124, an inhibitor of
phosphorylation (Fig. 2C). These
results suggest that STAT3 may be a downstream protein of PGE2 and
that PGE2 increases CYP17 expression.
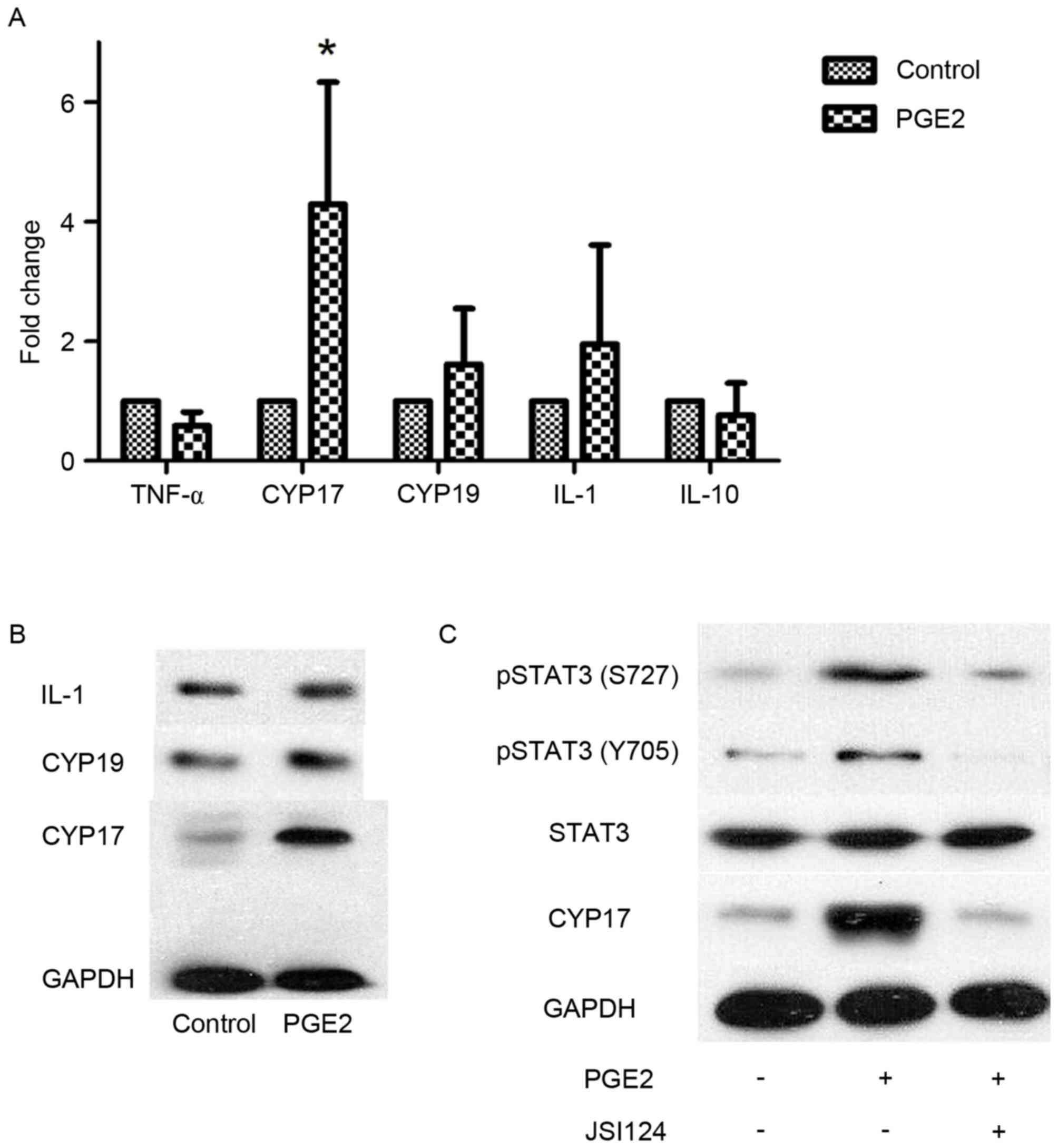 | Figure 2.PGE2 promotes CYP17 expression in
endometrial cancer cells via STAT3. (A) Reverse
transcription-quantitative polymerase chain reaction analysis for
TNF-α, CYP17, CYP19, IL-1 and IL-10 in Ishikawa cells following
stimulation with PGE2 (1×10−9 mol/l). *P<0.05,
analyzed by one-way analysis of variance. (B) Western blot analysis
for IL-1, CYP19 and CYP17 in Ishikawa cells following stimulation
with PGE2 (1×10−9 mol/). (C) Western blot analysis for
pSTAT3 S727, pSTAT3 Y705 and CYP17 in Ishikawa cells following
stimulation with PGE2 (1×10−9 mol/l) with or without
JSI-124 inhibition. PGE2, prostaglandin E2; CYP, cytochrome P450;
STAT3, signal transducer and activator of transcription 3; TNF-α,
tumor necrosis factor α; IL, interleukin; PGE2, prostaglandin E2;
pSTAT3, phosphorylated STAT3. |
High expression of CYP17 promotes
androgen secretion and invasion in endometrial cancer cells
CYP17 is known to be the key enzyme involved in the
synthesis of sex hormones. The concentrations of 3 hormones,
estrogen, androgen (testosterone) and progesterone, in Ishikawa
cells, were determined using ELISA. The results indicated that the
concentration of androgen in the Ishikawa cells was more elevated
than the concentrations of the other 2 hormones, compared with that
in the PE cells or the control group (Fig. 3A). For further study, Ishikawa cells
inhibited with CYP17 siRNA (siCYP17) and others overexpressing
CYP17 (CYP17 OE) were prepared (Fig.
3B-D). CYP17 OE Ishikawa cells had been transfected with
Flag-CYP17 plasmids, and exhibited an increased androgen
concentration (Fig. 3E).
Subsequently, invasion was evaluated using a Transwell assay, the
results of which indicated that siRNA Ishikawa cells had
significantly lost their invasion ability when compared with the
original Ishikawa cells following PGE2 stimulation (Fig. 4A and B). Ishikawa cells were
classified as follows: The control group, the low androgen group
(treated with 10−7 g/l androgen), the high androgen
group (treated with 10−5 g/l androgen) and the CYP17 OE
group. The Transwell results demonstrated that, compared with the
control groups, invasion in the high androgen group and the CYP17
overexpression group was significantly increased (Fig. 4C and D). This suggests that CYP17
overexpression and a high concentration of androgen promotes
invasion of endometrial cancer cells, initiated by PGE2
stimulation.
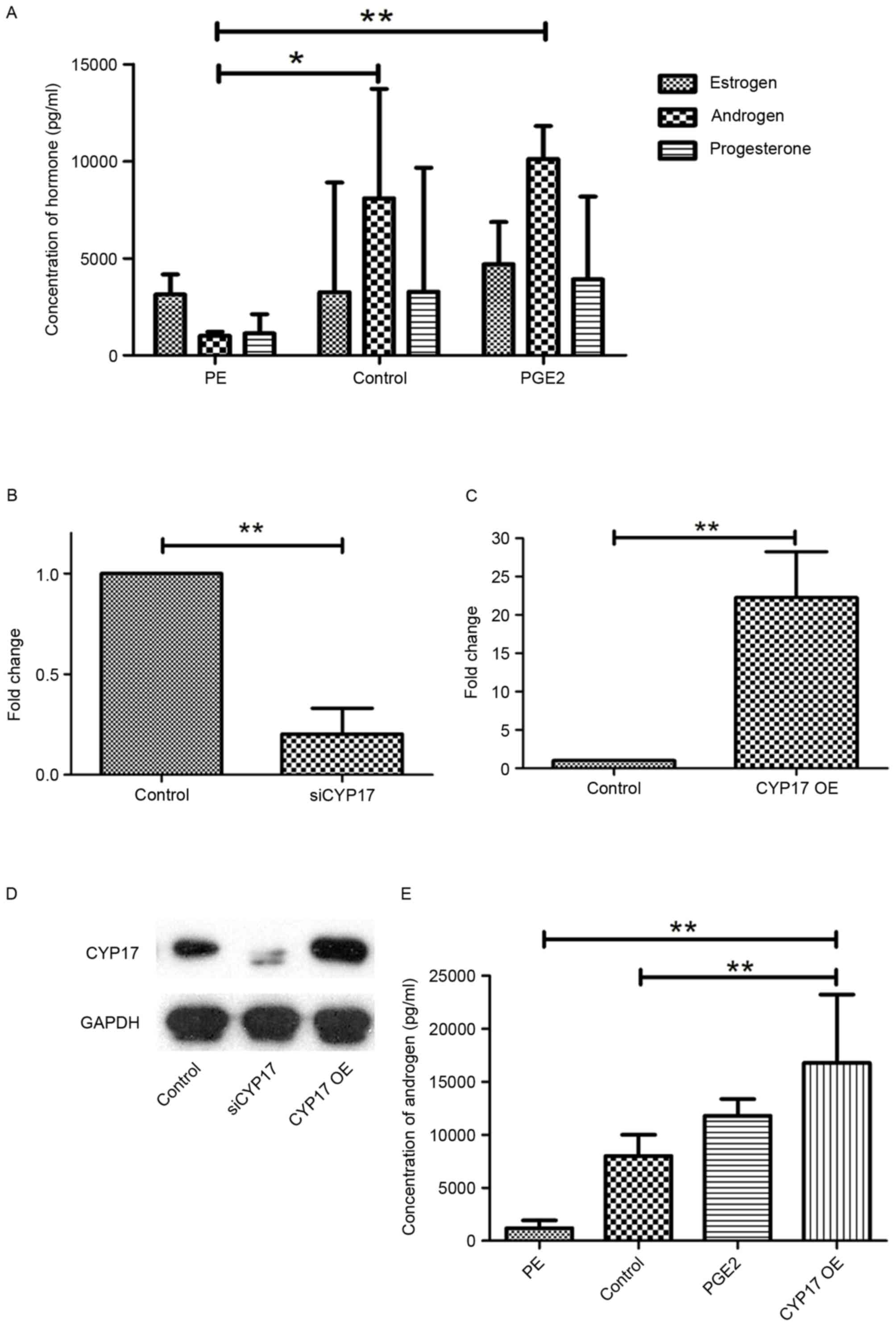 | Figure 3.High expression of CYP17 promotes the
secretion of androgens. (A) ELISA for estrogen, androgen and
progesterone concentrations in PE cells, Ishikawa cells (control)
and PGE2-stimulated Ishikawa cells. All cells were cultured for 48
h. Reverse transcription-quantitative polymerase chain reaction
analysis for Ishikawa cells (B) following transfection of siCYP17
and (C) in the CYP17 OE group. (D) Western blot analysis of
Ishikawa cells in control groups, siCYP17 groups and CYP17 OE
groups. (E) ELISA was performed to determine the concentration of
androgens in PE cells, Ishikawa cells (control), PGE2-stimulated
Ishikawa cells and CYP17 OE Ishikawa cells. All cells were cultured
for 48 h. *P<0.05, **P<0.01. CYP17, cytochrome P450 17α
hydroxylase; PE, primary endothelial; siCYP17, CYP17 small
interfering RNA; CYP17 OE, CYP17 overexpression. |
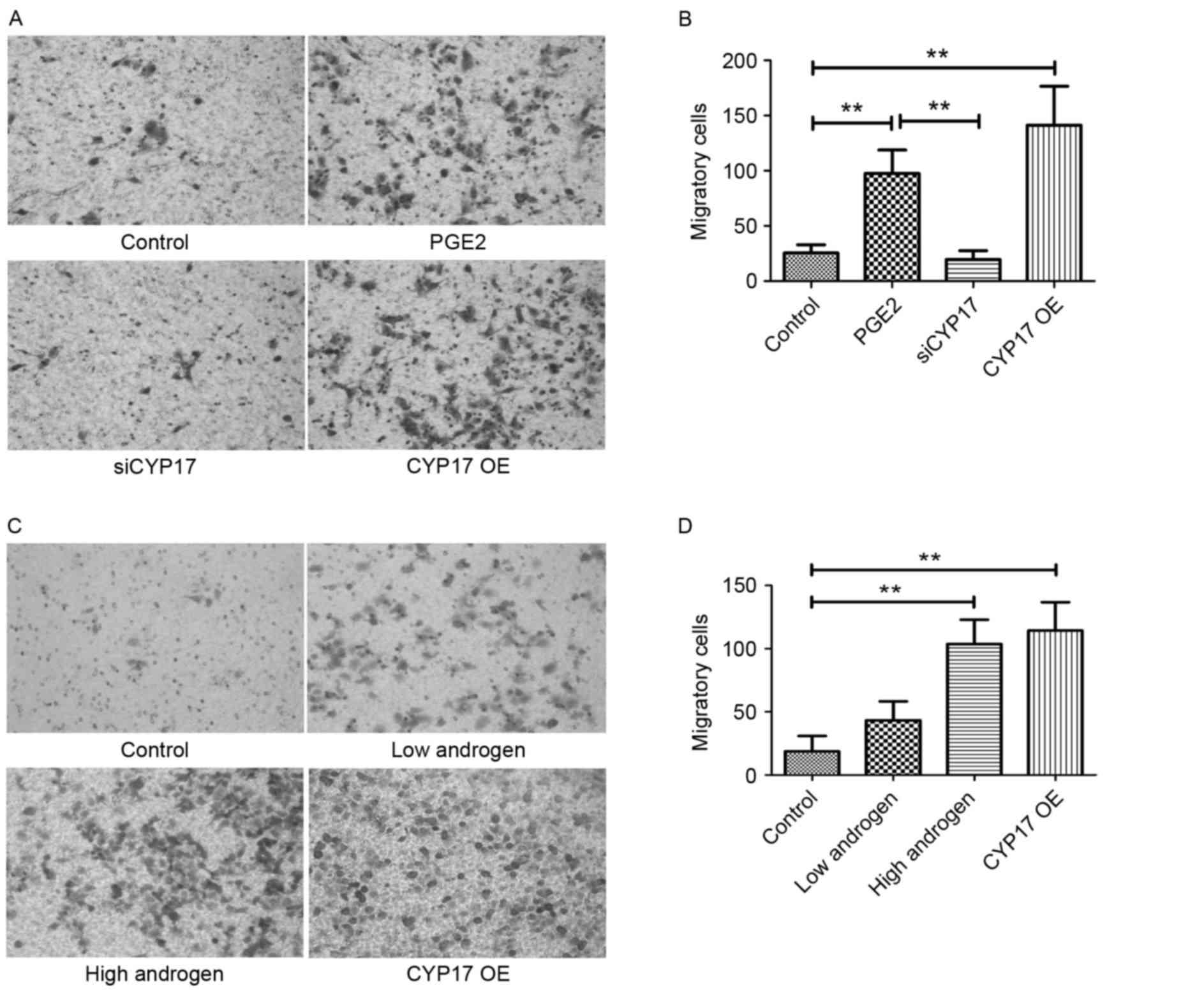 | Figure 4.High expression of CYP17 promotes
invasion in endometrial cancer cells. (A) Transwell migration assay
images of Ishikawa cells in control, PGE2-stimulated, siCYP17 and
CYP17 OE groups. Cells were stained with crystal violet. (B) The
number of Ishikawa cells in control, PGE2 stimulated, siCYP17 and
CYP17 OE groups (averaged across 5 random images). (C) Transwell
migration assay images of Ishikawa cells in control, low androgen
(treated with 10−7 g/l androgen for 48 h), high androgen
(treated with 10−5 g/l androgen for 48 h) and CYP17 OE
groups. Cells were stained with crystal violet. (D) The number of
Ishikawa cells in control, low androgen, high androgen and CYP17 OE
groups (averaged across five random images). **P<0.01, analyzed
by one-way analysis of variance. Micrographs were taken at ×200
magnification. CYP17, cytochrome P450 17α hydroxylase; PGE2,
prostaglandin E2; siCYP17, CYP17 small interfering RNA; CYP17 OE,
CYP17 overexpression. |
Discussion
The mediators and cellular effectors of inflammation
are vital components of the local tumor microenvironment (29). Inflammation that promotes tumor
development is an acknowledged enabling hallmark of cancer
(30). PGE2 is an important
inflammatory factor that acts as a tumor promoter in several cancer
types (31,32). In the present study, PTGES2, the
synthase of PGE2, was revealed to be highly expressed in
endometrial cancer cells, resulting in high expression of PGE2 in
the microenvironment of endometrial cancer cells. As demonstrated
in previous studies (28,32), EP4 is the key PGE2 receptor in
endometrial cancer, but the details of its function in cancer cells
are complex and remain unclear (28).
In the present study, PGE2 promoted CYP17 expression in endometrial
cancer cells via STAT3.
The results of the present study indicated that
CYP17 was more highly expressed following PGE2 stimulation. PGE2 is
known to serve a role in endometrial cancer cells through its
receptor, EP4 (28). With this in
mind, in the present study, it was subsequently observed that STAT3
phosphorylation was increased with increased CYP17 expression.
Therefore, STAT3 may promote CYP17 expression, via EP4, following
PGE2 stimulation in endometrial cancer cells. Subsequently, the
concentration of androgens in endometrial cancer cells was
determined and was revealed to be increased following CYP17
overexpression. As a result, invasion of endometrial cancer cells
increased, accompanying cell behavior changes.
The cytochrome P450 family 17, subfamily A, member 1
gene provides instructions for making a member of the CYP enzyme
family. Similar to other CYP enzymes, CYP17 is involved in the
synthesis of steroid hormones (33).
This group of hormones includes sex hormones, such as androgen and
estrogen, which are required for normal sexual development and
reproduction. The CYP17 enzyme performs two important reactions in
this process, and existing evidence indicates that unopposed
androgens contribute to the tumorigenesis and promotion of
endometrial cancer (34,35). Patients with polycystic ovary
syndrome, who usually have high androgen levels, have an increased
risk of developing endometrial cancer (35,36). The
results of the present study support this association and propose a
potential mechanism that may begin with inflammation in the
endometrium. Inflammation of the endometrium increased PGE2 levels
in the microenvironment and resulted in increased CYP17 expression
in endometrial cells and a higher concentration of total androgen
in the extracellular environment. This change further results in
tumorigenesis and invasion of endometrial cancer cells.
With regards to the mechanism by which PGE2 acts
upon cellular gene expression, the present study observed that
STAT3 serves an important role in promoting CYP17 expression in
endometrial cancer cells. Previous studies have demonstrated that
the STAT3 signaling pathway is persistently activated in tumors
(37,38). pSTAT3 forms homodimers or
heterodimers, translocates into the nucleus and transactivates the
expression levels of cyclin D1, vascular endothelial growth factor
and matrix metalloproteinases-2/−9 genes. The protein products of
these genes are involved in the regulation of tumor cell growth,
apoptosis, angiogenesis and metastasis (39–42). It
was clearly demonstrated that STAT3 is activated in endometrial
cancer cells (43), and that STAT3
can stimulate PGE2 production (44).
The results of the present study support a role for STAT3 in
endometrial cancer, suggesting that a STAT3 inhibitor may be
beneficial in the treatment of advanced-stage endometrial cancer
when combined with PGE2/EP4 agonists.
In summary, the present study demonstrated a
probable mechanism of invasion in endometrial cancer cells. The
high expression of PGE2 in the microenvironment of endometrial
cancer cells, possibly caused by inflammation, results in CYP17
overexpression in endometrial cancer cells via STAT3.
Overexpression of CYP17 results in increased synthesis of
androgens, which increases invasion of endometrial cancer cells.
These findings also suggest that inflammation in the tumor
microenvironment and high androgen levels serve important roles in
tumorigenesis and invasion.
Acknowledgements
Not applicable.
Funding
The work was supported by Anhui Provincial Natural
Science Foundation (grant no. 1808085QH274).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in the published article.
Authors' contributions
JK and DW conceived and designed the study. ZS, ML,
CP, PX and YZ performed the experiments. MW provided the mutants.
JK wrote the paper. XZ reviewed and edited the manuscript, and
performed part of experiments such as IHC.JK reviewed and edited
the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Ethical approval was given by the medical ethics
committee of Anhui Provincial Hospital. All patients provided
consent to participate in this research.
Patient consent for publication
Patients in the manuscript provided written informed
consent for the publication of any associated data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von Euler US: On the specific
vaso-dilating and plain muscle stimulating substances from
accessory genital glands in man and certain animals (prostaglandin
and vesiglandin). J Physiol. 88:213–234. 1936. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Legler DF, Bruckner M, Uetz-von Allmen E
and Krause P: Prostaglandin E2 at new glance: Novel insights in
functional diversity offer therapeutic chances. Int J Biochem Cell
Biol. 42:198–201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Che Q, Liu BY, Liao Y, Zhang HJ, Yang TT,
He YY, Xia YH, Lu W, He XY, Chen Z, et al: Activation of a positive
feedback loop involving IL-6 and aromatase promotes intratumoral
17β-estradiol biosynthesis in endometrial carcinoma
microenvironment. Int J Cancer. 135:282–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu M, Che Q, Liao Y, Wang H, Wang J, Chen
Z, Wang F, Dai C and Wan X: Oncostatin M activates STAT3 to promote
endometrial cancer invasion and angiogenesis. Oncol Rep.
34:129–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doherty GA, Byrne SM, Molloy ES, Malhotra
V, Austin SC, Kay EW, Murray FE and Fitzgerald DJ: Proneoplastic
effects of PGE2 mediated by EP4 receptor in colorectal cancer. BMC
Cancer. 9:2072009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frasor J, Weaver AE, Pradhan M and Mehta
K: Synergistic up-regulation of prostaglandin E synthase expression
in breast cancer cells by 17beta-estradiol and proinflammatory
cytokines. Endocrinology. 149:6272–6279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rask K, Zhu Y, Wang W, Hedin L and
Sundfeldt K: Ovarian epithelial cancer: A role for PGE2-synthesis
and signalling in malignant transformation and progression. Mol
Cancer. 5:622006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frank DA: STAT3 as a central mediator of
neoplastic cellular transformation. Cancer Lett. 251:199–210. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaluvally-Raghavan P, Jeong KJ, Pradeep
S, Silva AM, Yu S, Liu W, Moss T, Rodriguez-Aguayo C, Zhang D, Ram
P, et al: Direct upregulation of STAT3 by MicroRNA-551b-3p
deregulates growth and metastasis of ovarian cancer. Cell Rep.
15:1493–1504. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mora LB, Buettner R, Seigne J, Diaz J,
Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, et
al: Constitutive activation of Stat3 in human prostate tumors and
cell lines: Direct inhibition of Stat3 signaling induces apoptosis
of prostate cancer cells. Cancer Res. 62:6659–6666. 2002.PubMed/NCBI
|
|
16
|
Luwor RB, Stylli SS and Kaye AH: The role
of Stat3 in glioblastoma multiforme. J Clin Neurosci. 20:907–911.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pilati C and Zucman-Rossi J: Mutations
leading to constitutive active gp130/JAK1/STAT3 pathway. Cytokine
Growth Factor Rev. 26:499–506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guengerich FP: Cytochrome p450 and
chemical toxicology. Chem Res Toxicol. 21:70–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vasaitis TS, Bruno RD and Njar VC: CYP17
inhibitors for prostate cancer therapy. J Steroid Biochem Mol Biol.
125:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alex AB, Pal SK and Agarwal N: CYP17
inhibitors in prostate cancer: Latest evidence and clinical
potential. Ther Adv Med Oncol. 8:267–275. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koh WJ, Greer BE, Abu-Rustum NR, Apte SM,
Campos SM, Chan J, Cho KR, Cohn D, Crispens MA, Dupont N, et al:
Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw.
12:248–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Venturin GL, Chiku VM, Silva KL, de
Almeida BF and de Lima VM: M1 polarization and the effect of PGE2
on TNF-α production by lymph node cells from dogs with visceral
leishmaniasis. Parasite Immunol. 38:698–704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prosperi JR and Robertson FM:
Cyclooxygenase-2 directly regulates gene expression of P450 Cyp19
aromatase promoter regions pII, pI.3 and pI.7 and estradiol
production in human breast tumor cells. Prostaglandins Other Lipid
Mediat. 81:55–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang X, Jin J, Shen S, Xia Y, Xu P, Zou
X, Wang H, Yi L, Wang Y and Gao Q: Modulation of expression of
17-Hydroxylase/17,20 lyase (CYP17) and P450 aromatase (CYP19) by
inhibition of MEK1 in a human ovarian granulosa-like tumor cell
line. Gynecol Endocrinol. 32:201–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramalho TR, Filgueiras LR, Pacheco de
Oliveira MT, Lima AL, Bezerra-Santos CR, Jancar S and Piuvezam MR:
Gamma-terpinene modulation of LPS-stimulated macrophages is
dependent on the PGE2/IL-10 axis. Planta Med. 82:1341–1345. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Machado-Carvalho L, Martín M, Torres R,
Gabasa M, Alobid I, Mullol J, Pujols L, Roca-Ferrer J and Picado C:
Low E-prostanoid 2 receptor levels and deficient induction of the
IL-1β/IL-1 type I receptor/COX-2 pathway: Vicious circle in
patients with aspirin-exacerbated respiratory disease. J Allergy
Clin Immunol. 137:99–107.e7. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ke J, Yang Y, Che Q, Jiang F, Wang H, Chen
Z, Zhu M, Tong H, Zhang H, Yan X, et al: Prostaglandin E2 (PGE2)
promotes proliferation and invasion by enhancing SUMO-1 activity
via EP4 receptor in endometrial cancer. Tumour Biol.
37:12203–12211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JI, Lakshmikanthan V, Frilot N and
Daaka Y: Prostaglandin E2 promotes lung cancer cell migration via
EP4-betaArrestin1-c-Src signalsome. Mol Cancer Res. 8:569–577.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li S, Xu X, Jiang M, Bi Y, Xu J and Han M:
Lipopolysaccharide induces inflammation and facilitates lung
metastasis in a breast cancer model via the prostaglandin E2-EP2
pathway. Mol Med Rep. 11:4454–4462. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miller WR, Anderson TJ and Jack WJ:
Relationship between tumour aromatase activity, tumour
characteristics and response to therapy. J Steroid Biochem Mol
Biol. 37:1055–1059. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ito K, Miki Y, Suzuki T, McNamara KM and
Sasano H: In situ androgen and estrogen biosynthesis in endometrial
cancer: Focus on androgen actions and intratumoral production.
Endocr Relat Cancer. 23:R323–R335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goodman NF, Cobin RH, Futterweit W, Glueck
JS, Legro RS and Carmina E; American Association of Clinical
Endocrinologists (AACE), ; American College of Endocrinology (ACE),
; Androgen Excess and PCOS Society (AES), : American association of
clinical endocrinologists, american college of endocrinology and
androgen excess and pcos society disease state clinical review:
Guide to the best practices in the evaluation and treatment of
polycystic ovary syndrome-part 1. Endocr Pract. 21:1291–1300. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shao R, Li X and Billig H: Promising
clinical practices of metformin in women with PCOS and early-stage
endometrial cancer. BBA Clin. 2:7–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao SP, Mark KG, Leslie K, Pao W, Motoi N,
Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B and Bromberg
JF: Mutations in the EGFR kinase domain mediate STAT3 activation
via IL-6 production in human lung adenocarcinomas. J Clin Invest.
117:3846–3856. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berishaj M, Gao SP, Ahmed S, Leslie K,
Al-Ahmadie H, Gerald WL, Bornmann W and Bromberg JF: Stat3 is
tyrosine-phosphorylated through the interleukin-6/glycoprotein
130/Janus kinase pathway in breast cancer. Breast Cancer Res.
9:R322007. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sai K, Wang S, Balasubramaniyan V, Conrad
C, Lang FF, Aldape K, Szymanski S, Fokt I, Dasgupta A, Madden T, et
al: Induction of cell-cycle arrest and apoptosis in glioblastoma
stem-like cells by WP1193, a novel small molecule inhibitor of the
JAK2/STAT3 pathway. J Neurooncol. 107:487–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Okazaki H, Tokumaru S, Hanakawa Y,
Shiraishi K, Shirakata Y, Dai X, Yang L, Tohyama M, Hashimoto K and
Sayama K: Nuclear translocation of phosphorylated STAT3 regulates
VEGF-A-induced lymphatic endothelial cell migration and tube
formation. Biochem Biophys Res Commun. 412:441–445. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F,
Sawaya R and Huang S: Stat3 activation regulates the expression of
matrix metalloproteinase-2 and tumor invasion and metastasis.
Oncogene. 23:3550–3560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Song Y, Qian L, Song S, Chen L, Zhang Y,
Yuan G, Zhang H, Xia Q, Hu M, Yu M, et al: Fra-1 and Stat3
synergistically regulate activation of human MMP-9 gene. Mol
Immunol. 45:137–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Subramaniam KS, Omar IS, Kwong SC, Mohamed
Z, Woo YL, Mat Adenan NA and Chung I: Cancer-associated fibroblasts
promote endometrial cancer growth via activation of
interleukin-6/STAT-3/c-Myc pathway. Am J Cancer Res. 6:200–213.
2016.PubMed/NCBI
|
|
44
|
Gao J, Tian J, Lv Y, Shi F, Kong F, Shi H
and Zhao L: Leptin induces functional activation of
cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT
pathways in human endometrial cancer cells. Cancer Sci.
100:389–395. 2009. View Article : Google Scholar : PubMed/NCBI
|