Introduction
Osteosarcoma, or osteogenic sarcoma, is a type of
aggressive malignant neoplasm that originates from bone (1). Osteosarcoma is a rare type of malignancy
that only accounts for about 1% of cancers overall, with an
incidence of around 5 per 1,000,000 (2). In the United States, osteosarcoma
affects about 1,000 new patients every year (3). Osteosarcoma mainly affects young people,
and primary conventional osteosarcoma is commonly observed in young
patients, while elderly patients are usually affected by secondary
osteosarcoma (4). Although various
treatment strategies such as chemotherapy, radiation therapy and
immunotherapy are efficient for the disease, treatment outcomes are
still unsatisfied (5) and the overall
5-years' survival rate is only about 30% (6). The development of osteosarcoma is a
complex process with various factors that involved. Therefore, the
in-depth analyses of the pathogenesis of osteosarcoma will
definitely improve the treatment of this disease.
MicroRNA, or miRNA, is a group of non-coding RNAs
that composed of approximately 22 nucleotides (7). miRNAs are widely distributed in animals,
plants and even some viruses to play different roles in almost all
critical physiological processes and even pathological changes by
its functions of post-transcriptional regulation of gene expression
and RNA silencing (8). Oncological
studies have also shown that miRNAs are involved in nearly all
aspects of the onset, development and progression of different
types of malignancies (9). miRNA-21
is usually highly expressed in various types of cancer to play its
oncogenic functions (10). However,
the involvement of miRNA-21 in osteosarcoma, the mechanism and its
prognostic as well as diagnostic values still have not been
systemically studied.
Materials and methods
Patients
A total of 46 patients with osteosarcoma were
selected in Guangzhou General Hospital of Guangzhou Military
Command from January 2010 to January 2012. Those patients were
diagnosed as osteosarcoma by pathological and imaging examinations.
All patients received surgical resections. Those patients included
26 males and 20 females, and age ranged from 10 to 65 years, with
an average age of 32±9.2 years. During surgical operations, cancer
tissues and adjacent normal tissues within 0.5 cm around the tumors
were collected. Extent of primary tumor was performed according to
the criteria established by American Cancer Society. There were 12
cases in T1, 19 cases in T2 and 15 cases in T3 stage. At the same
time, 20 healthy controls with similar age and gender distributions
were selected to serve as control group. The ethics committee of
Guangzhou General Hospital of Guangzhou Military Command approved
this study, and all patients signed informed consent. Follow-up
study was performed for 6 years to monitor the survival
conditions.
Preparation of serum
Fasting blood (5 ml) was extracted from each patient
and healthy control on the day of admission. Blood was kept at room
temperature for 1.5 h, followed by centrifugation at 2,000 × g for
20 min to collect serum. Serum samples were stored at −80°C before
RNA extraction.
Cell lines and cell culture
Human osteosarcoma cell lines MG-63 and U2OS were
purchased from American Type Culture Collection (ATCC; Manassas,
VA, USA). Cells were cultured with Eagle's Minimum Essential Medium
(cat. no. 30-2003; ATCC) containing 10% heat-inactivated fetal
bovine serum in an incubator (37°C, 5% CO2). Cells were
harvested during logarithmic growth phase for subsequent
experiments.
Establishment of miRNA-21 knockdown
cell lines
The pGCMV/EGFP-hsa-miR-21 interference plasmid and
control pGCMV/EGFP-hsa-miR-NC plasmid (empty plasmid without the
interference) were purchased from GenePharm (Shanghai, China).
Plasmid was transfected into cells with a dose of 50 ng/µl using
Lipofectamine® 2000 Reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Cell proliferation assay
Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was used to monitor cell
proliferation according to manufacturer's instructions. Briefly,
100 µl of cell suspension containing 3,000 cells was added into
each well of 96-well plate. Cells were re-incubated for 12 h, and
10 µl of CCK-8 was added 24, 48, 72 and 96 h later. Absorbance at
450 nm was measured using Epoch Microplate Spectrophotometer
(BioTek Instruments, Inc., Winooski, VT, USA) to calculate cell
proliferation rate.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to extract total RNA
from tissues and cells. RNA samples were tested by
NanoDrop™ 2000 Spectrophotometers (Thermo Fisher
Scientific, Inc.), and only RNA samples with a ratio of A260/A280
between 1.8 and 2.0 were used in reverse transcription to
synthesize cDNA using iScript™ cDNA Synthesis kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). PCR reactions were
performed using SYBR® Green Real-Time PCR Master Mixes
(Thermo Fisher Scientific, Inc.) on the ABI 7500 System. Primers
for miRNA-21 were purchased from Sigma-Aldrich (MIRAP00047;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Primers for
endogenous control β-actin were: 5′-GACCTCTATGCCAACACAGT-3′
(forward) and 5′-AGTACTTGCGCTCAGGAGGA-3′ (reverse). PCR reaction
conditions were: 95°C for 45 sec, followed by 40 cycles of 95°C for
18 sec and 60°C for 42 sec. Cq values were processed using
2−ΔΔCq method (11).
Relative expression level of miRNA-21 was normalized to endogenous
control β-actin.
Western blotting
Total protein extraction was performed using cell
lysis solutions (Thermo Fisher Scientific, Inc.), and protein
concentration was determined by BCA method. Electrophoresis (10%
SDS-PAGE gel) was performed with 30 µg of protein per lane. Gel
transfer to PVDF membrane was performed under 20 V for 1 h.
Membranes were blocked with 5% skimmed milk, followed by incubation
with primary antibodies including rabbit anti-p-PI3K antibody
(1:2,000, ab31392; Abcam, Cambridge, UK), anti-transforming growth
factor (TGF)-β1 (1:2,000, ab92486; Abcam) anti-GAPDH primary
antibody (1:1,000, ab37168; Abcam) overnight at 4°C. The next day,
membranes were washed for three times and further incubated with
anti-rabbit IgG-HRP secondary antibody (1:1,000, MBS435036;
MyBioSource, San Diego, CA, USA) at room temperature for 3 h. After
washing, ECL (Sigma-Aldrich; Merck KGaA) method was used to detect
signals. Images were processed using Image J software to calculate
the relative expression level of phosphatase and tensin homolog
(PTEN) and TGF-β1 according to endogenous control GAPDH.
Statistical analysis
The data was presented as the mean ± standard
deviation. SPSS v. 19.0 (SPSS, Inc., Chicago, IL, USA) was used for
all statistical analyses. Measurement data were expressed by mean ±
standard deviation, and comparisons between two groups were
performed using an unpaired t-test, and comparisons among multiple
groups were performed by one-way analysis of variance and the LSD
post hoc test. Gender was compared using a Chi-square test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of expression levels of
miRNA-21 in tumor and adjacent healthy tissues of osteosarcoma
patients
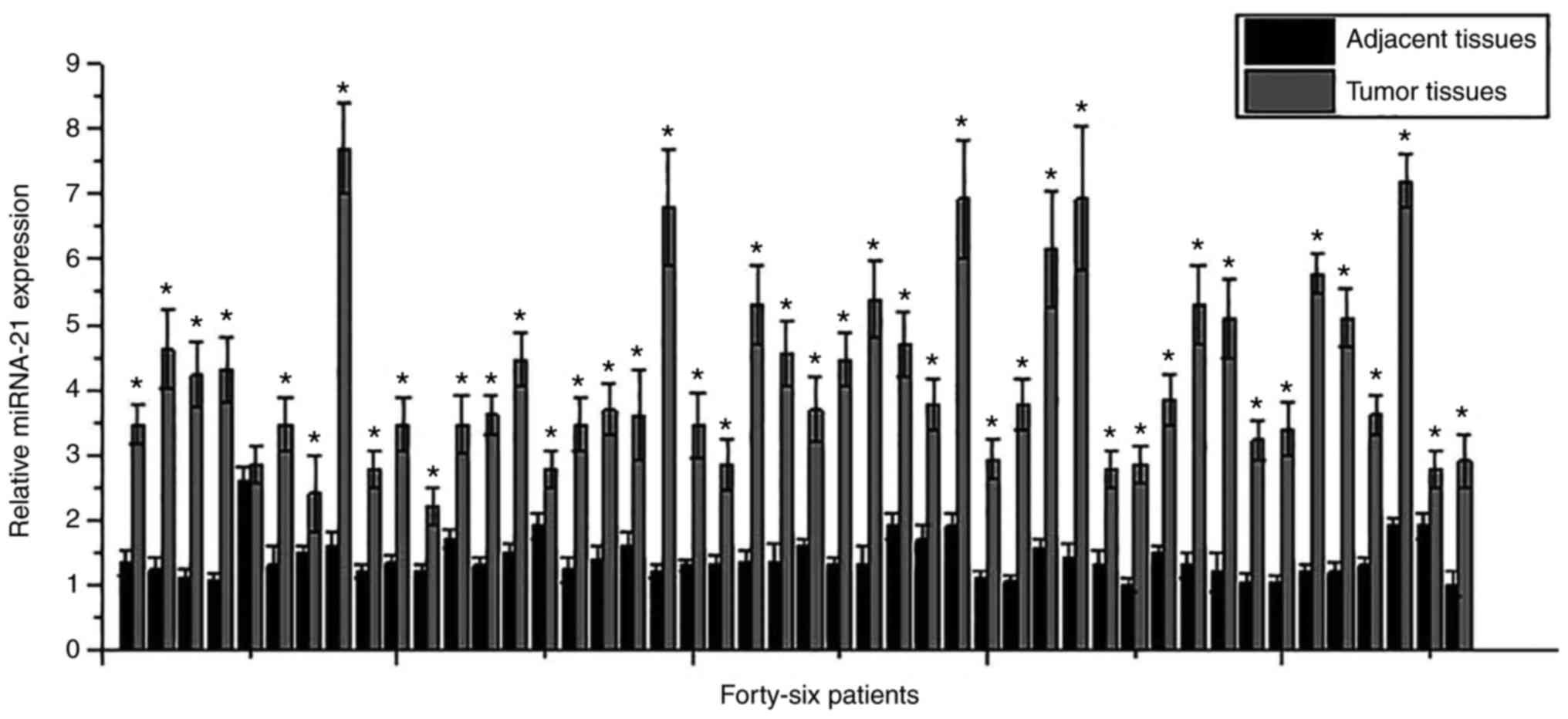
Expression of miRNA-21 in tumor tissues and adjacent
healthy tissues of osteosarcoma patients was detected by RT-qPCR.
As shown in Fig. 1, although
differential expression levels of miRNA-21 in tumor tissues or
adjacent healthy tissues were found among patients, expression
levels of miRNA-21 were significantly higher in tumor tissues than
those in adjacent healthy tissues in 45 out of 46 patients. Those
data suggest that miRNA-21 overexpression is very likely involved
in the pathogenesis of osteosarcoma.
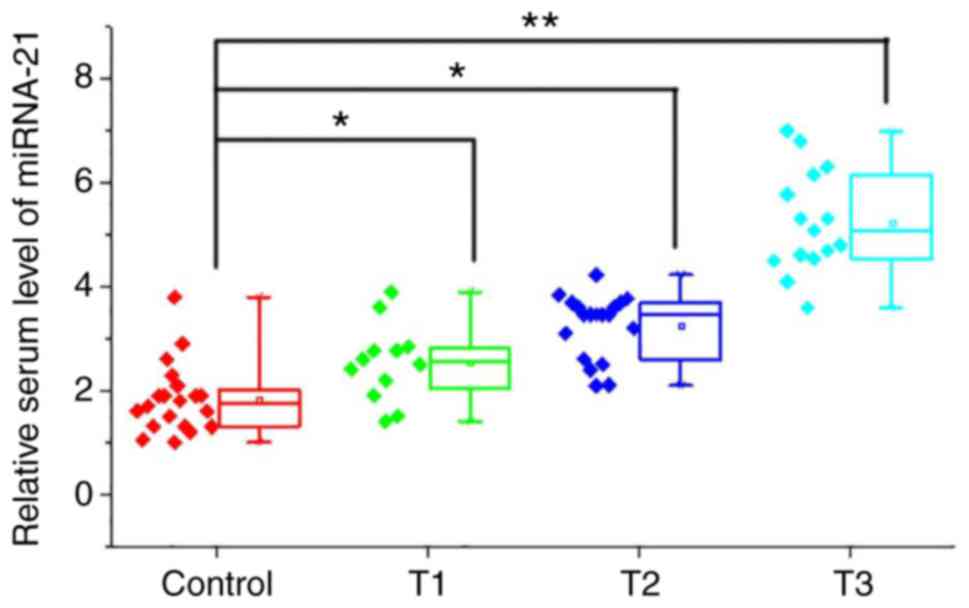
Serum levels of miRNA-21 increased
with the increased T stage of osteosarcoma
Patients with osteosarcoma (n=46) were divided into
T1, T2 and T3 groups according to T staging. Compared with healthy
controls, serum levels of miRNA-21 were significantly increased in
patients with different T stages (P<0.05 or P<0.01; Fig. 2). In addition, serum levels of
miRNA-21 were also increased with the increased T stage.
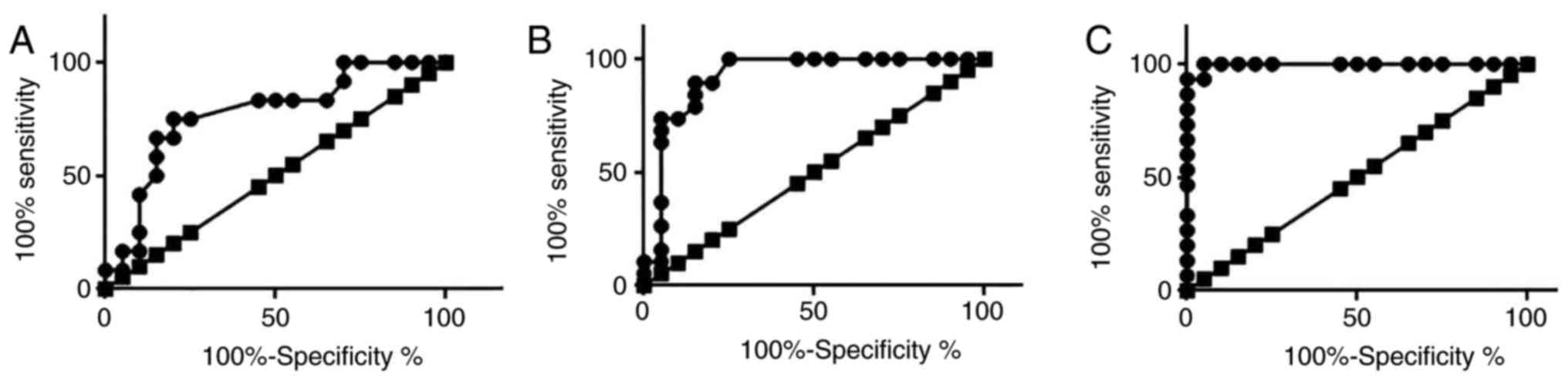
Diagnostic values of serum levels of
miRNA-21 for patients with different stages of osteosarcoma
ROC curve analysis was performed to evaluate the
diagnostic values of serum levels of miRNA-21 for patients with
different stages of osteosarcoma (Fig.
3). The area under the curve (AUC) of serum levels of miRNA-21
in the diagnosis of T1 osteosarcoma was 0.7750 with 95% confident
interval of 0.6037 to 0.9463 (P=0.01023), AUC for T2 osteosarcoma
was 0.9224 with 95% confident interval of 0.8276 to 1.017
(P<0.0001), and AUC for T3 osteosarcoma was 0.9967 with 95%
confident interval of 0.9856 to 1.008 (P<0.0001). Those data
suggest that serum levels of miRNA-21 can be used to accurately
diagnose osteosarcoma, especially cases in advanced stages.
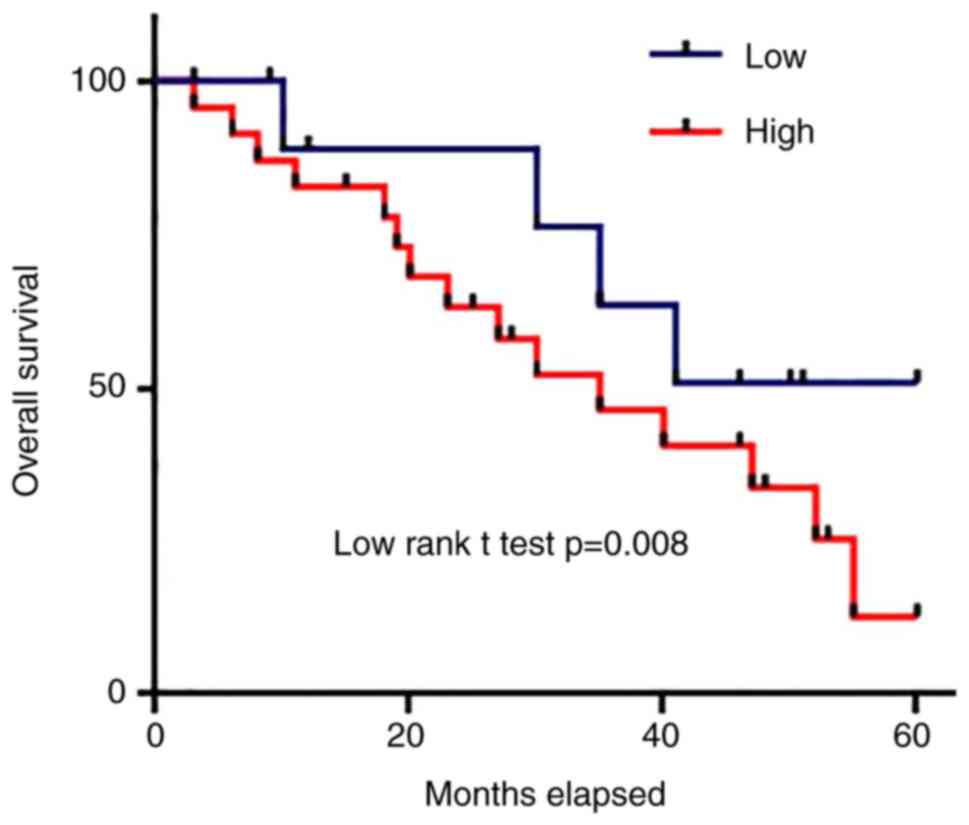
Prognostic values of serum levels of
miRNA-21 for patients with osteosarcoma
According to the median serum level of miRNA-21,
patients were divided into high level and low level groups.
Survival curves of those two groups were plotted using Kaplan-Meier
method and were compared by log rank t-test to evaluate the
prognostic value of serum miRNA-21 for osteosarcoma. As shown in
Fig. 4, overall survival rate of
osteosarcoma patient with high serum level of miRNA-21 was
significantly lower than that of patients with low serum level of
miRNA-21 (P<0.05).
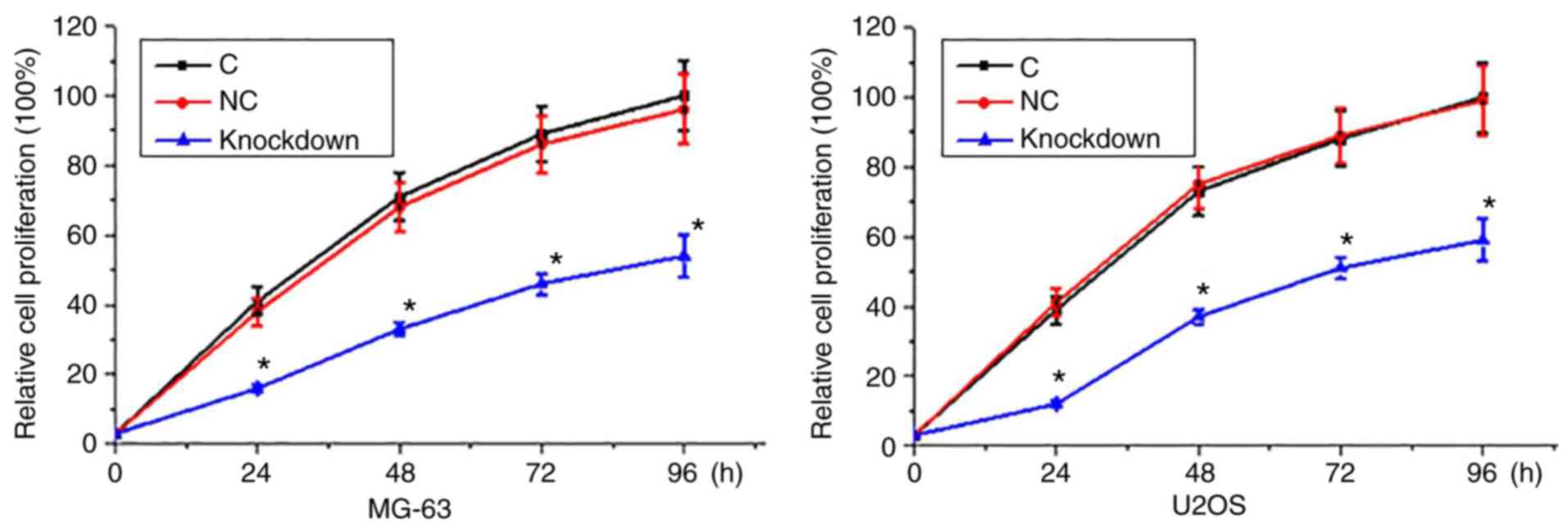
miRNA-21 knockdown inhibited
proliferation of osteosarcoma
CCK-8 assay was performed to measure cell
proliferation abilities of two osteosarcoma cell lines MG-63 and
U2OS. As shown in Fig. 5, compared
with control cells (cells without transfection) and negative
control cells (empty plasmid transfection), proliferation rate of
osteosarcoma cells was significantly decreased after the miRNA-21
knockdown.
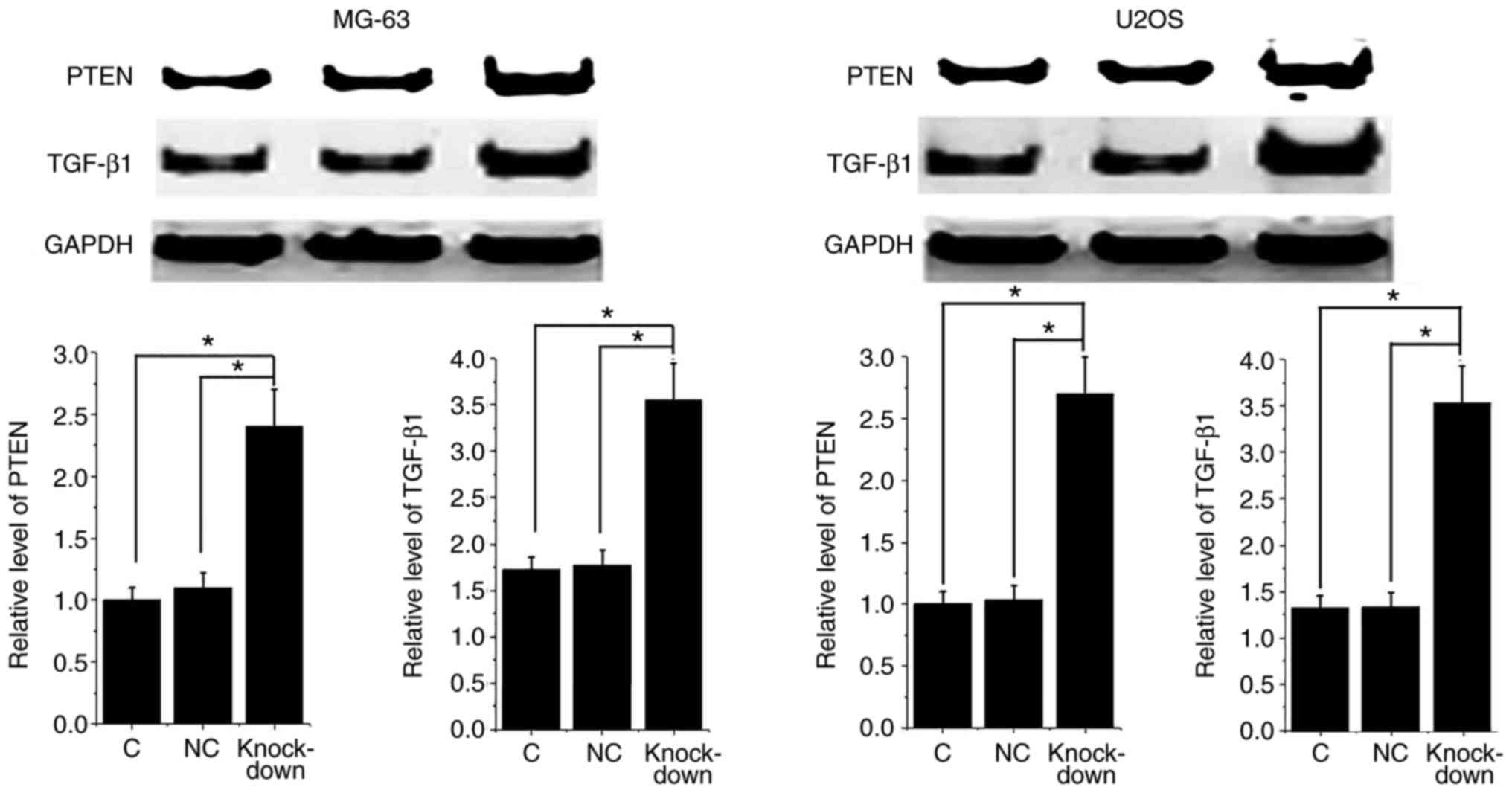
miRNA-21 knockdown promoted PTEN and
TGF-β1 expression in osteosarcoma cells
It is known that miRNA-21 can target PTEN to achieve
its biological function in glioblastoma (12). In addition, miRNA-21 can also regulate
TGF-β1 pathway to negatively regulate Treg cells (13). PTEN and TGF-β1 expression have been
proved to participate in proliferation of certain types of tumor
cells (14,15). In this study, miRNA-21 knockdown
significantly promoted the expression of PTEN and TGF-β1 in both of
osteosarcoma cell lines (Fig. 6).
PTEN is considered to be a tumor suppressor gene (12) and TGF-β1 has anti-proliferative
activity. Those data indicate that inhibition of miRNA-21 can
inhibit osteosarcoma cell proliferation by reducing the expression
level of PTEN and TGF-β1.
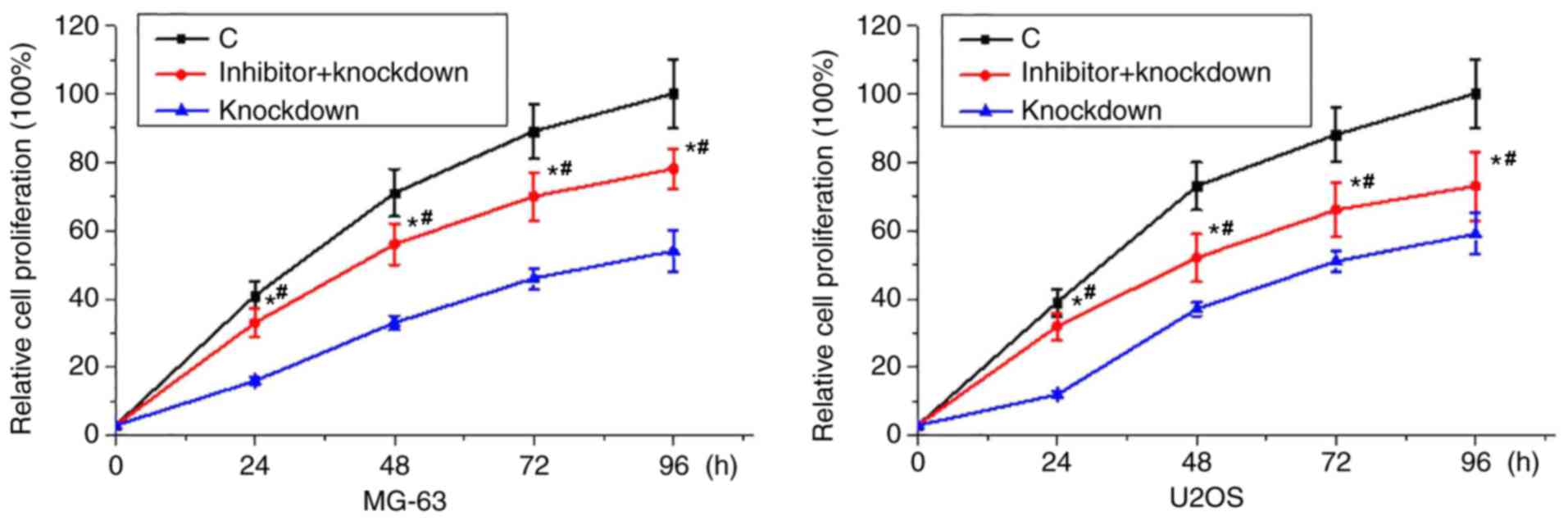
TGF-β1 inhibitor treatment reduced the
inhibitory effects of miRNA-21 knockdown on osteosarcoma cell
proliferation
Our data have shown that miRNA-21 knockdown-mediated
increased expression level of TGF-β1 is related with the reduced
proliferation rate of osteosarcoma cells. Therefore, TGF-β1
inhibitor SB431542 (10 nM) was used to treat cells of two
osteosarcoma cell lines. As shown in Fig.
7, proliferation rate of osteosarcoma cells was lower than that
of control cells but higher than that of cells with SB431542
treatment and miRNA-21 knockdown. Those data suggest that TGF-β1
inhibitor treatment can reduce the inhibitory effects of miRNA-21
knockdown on osteosarcoma cell proliferation.
Discussion
miRNAs have been proved to play critical roles in
the pathogenesis of osteosarcoma (16). Expression of microRNA-199a-3p was
downregulated in osteosarcoma tissues comparing with adjacent
healthy tissues, and the increased expression level of
microRNA-199a-3p is responsible for increased proliferation and
migration rates of cancer cells (17). miRNA-218 is also downregulated in
osteosarcoma to play a role as tumor suppressor by inhibiting tumor
cell migration and invasion (18).
miRNA-21 is usually highly expressed in various types of cancer to
play its oncogenic functions (19,20). In
our study, expression levels of miRNA-21 were significantly higher
in tumor tissues than those in adjacent healthy tissues in 45 out
of 46 patients. In addition, serum levels of miRNA-21 were also
significantly higher in osteosarcoma patients than those in normal
controls. Besides that, serum levels of miRNA-21 were increased
with the increased T stage of osteosarcoma. All those results
suggestion that upregulation of miRNA-21 expression is involved in
the development of osteosarcoma. miRNA-21 plays its oncogenic
functions at least partially by promoting the proliferation of
cancer cells, so as to accelerate the growth of tumors (21). In this study, inhibition of miRNA-21
expression significantly promoted the proliferation of two
osteosarcoma cell lines, indicating that inhibition of miRNA-21
expression may serve as a potential target for the clinical
treatment of osteosarcoma.
Development of pathological processes is usually
accompanied by changes of certain substances in blood, and the
detection of those substances may provide useful information for
diagnosis and prognosis of certain diseases (22). Abnormal expression of miRNA-21 has
been proved to serve as promising diagnostic biomarkers for certain
types of cancers such as cervical cancer (23). In our study, ROC curve analysis showed
that serum levels of miRNA-21 can be used to effectively diagnose
osteosarcoma, and the diagnostic value was increased with the
increased T stage of osteosarcoma. Increased expression level of
miRNA-21 has been proved to be closely correlated with poor
prognosis of some types of cancers such as glioma (24). In our study, survival curves of those
two groups were plotted using Kaplan-Meier method and compared by
log rank t test to evaluate the prognostic value of serum miRNA-21
for osteosarcoma. We found that the overall survival rate of
osteosarcoma patients with high serum level of miRNA-21 was
significantly lower than that of patients with low serum level of
miRNA-21. Those data suggest that serum miRNA-21 may serve as a
promising prognostic and diagnostic biomarker for osteosarcoma.
In the study of triple-negative breast cancer,
miRNA-21 was proved to promote the proliferation of cancer cells by
targeting PTEN to reduce its expression level (21). TGF-β1 plays different roles in
different aspects of the cancer pathogenesis. On one hand, TGF-β1
reduces cell proliferation rate by inhibiting cell cycle. On the
other hand, TGF-β1 promotes cell migration and invasion (15). In this study, miRNA-21 knockdown
significantly promoted the expression of PTEN and TGF-β1 in both of
the osteosarcoma cell lines. In addition, TGF-β1 inhibitor
treatment significantly reduced the inhibitory effects of miRNA-21
knockdown on osteosarcoma cell proliferation. Those data indicate
that inhibition of miRNA-21 can inhibit osteosarcoma cell
proliferation by reducing the expression level of PTEN and TGF-β1.
We tried to identify the target site of miRNA-21 on TGF-β1, but no
promising target sequence was found. Therefore miRNA-21 may target
the upstream regulator of TGF-β1 to indirectly regulate its
expression.
It is worth to note that the application of miRNA-21
as a prognostic marker for osteosarcoma has been evaluated be a
previous study (25). Several other
studies also reported the involvement of miRNA-21 in osteosarcoma
(20,26). However, in those studies the mechanism
of the action of miRNA-21 in osteosarcoma has not been
investigated. Our study reported the mechanism of the function of
miRNA-21 in this disease.
In conclusion, expression levels of miRNA-21 were
significantly higher in tumor tissues than those in adjacent
healthy tissues of most osteosarcoma patients. Serum levels of
miRNA-21 were increased with the increased T stage of osteosarcoma.
Serum miRNA-21 can be used to effectively diagnose osteosarcoma and
predict the prognosis of the disease. miRNA-21 knockdown inhibited
proliferation of osteosarcoma and promoted the expression of PTEN
and TGF-β1 proteins in those cells, while TGF-β1 inhibitor
treatment reduced the inhibitory effects of miRNA-21 knockdown on
osteosarcoma cell proliferation. Therefore, we conclude that
miRNA-21 expression inhibition can inhibit osteosarcoma cell
proliferation by targeting PTEN and regulating TGF-β1 pathway.
However, osteosarcoma is a rare type of cancer, leading to the
small sample size in this study. Future studies with bigger sample
size are still needed to further confirm the conclusions in this
study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH and YZ designed the experiments. XH, LL and YL
performed the experiments. XY, HC and QY analyzed the data. YZ
wrote the manuscript. All authors have read and approved the final
submitted manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Guangzhou General Hospital
of Guangzhou Military Command approved the study and all patients
provided written informed consent prior to their inclusion.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Botter SM, Neri D and Fuchs B: Recent
advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin PP and Patel S: OsteosarcomaBone
sarcoma. Springer; New York, NY: pp. 75–97. 2013, View Article : Google Scholar
|
|
5
|
Tsukahara T and Wada T: Immunotherapy for
osteosarcomaOsteosarcoma. Springer; Tokyo: pp. 31–41. 2016,
View Article : Google Scholar
|
|
6
|
Farfalli GL, Albergo JI, Lobos PA, Smith
DE, Streitenberger PD, Pallotta Rodríguez MG and Aponte-Tinao LA:
Osteosarcoma lung metastases. Survival after chemotherapy and
surgery. Medicina (B Aires). 75:87–90. 2015.(In Spanish).
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zimmerman AL and Wu S: MicroRNAs, cancer
and cancer stem cells. Cancer Lett. 300:10–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor. Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu
P, Wang B, Wang G, Jia Z, Pu P, et al: Downregulation of miR-21
inhibits EGFR pathway and suppresses the growth of human
glioblastoma cells independent of PTEN status. Lab Invest.
90:144–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li S, Fan Q, He S, Tang T, Liao Y and Xie
J: MicroRNA-21 negatively regulates Treg cells through a
TGF-β1/Smad-independent pathway in patients with coronary heart
disease. Cell Physiol Biochem. 37:866–878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Xie W, Xie C, Huang C, Zhu J, Liang
Z, Deng F, Zhu M, Zhu W, Wu R, et al: Curcumin modulates
miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7
breast cancer cell proliferation. Phytother Res. 28:1553–1560.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Ballim D, Rodriguez M, Cui R, Goding
CR, Teng H and Prince S: The anti-proliferative function of the
TGF-β1 signaling pathway involves the repression of the oncogenic
TBX2 by its homologue TBX3. J Biol Chem. 289:35633–35643. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duan Z, Choy E, Harmon D, Liu X, Susa M,
Mankin H and Hornicek F: MicroRNA-199a-3p is downregulated in human
osteosarcoma and regulates cell proliferation and migration. Mol
Cancer Ther. 10:1337–1345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin J, Cai L, Liu ZM and Zhou XS:
miRNA-218 inhibits osteosarcoma cell migration and invasion by
down-regulating of TIAM1, MMP2 and MMP9. Asian Pac J Cancer Prev.
14:3681–3684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Huang Z, Wu S, Zang X, Liu M and
Shi J: miR-33a is up-regulated in chemoresistant osteosarcoma and
promotes osteosarcoma cell resistance to cisplatin by
down-regulating TWIST. J Exp Clin Cancer Res. 33:122014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vanas V, Haigl B, Stockhammer V and
Sutterlüty-Fall H: MicroRNA-21 increases proliferation and
cisplatin sensitivity of osteosarcoma-derived cells. PLoS One.
11:e01610232016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang H, Xie J, Zhang M, Zhao Z, Wan Y and
Yao Y: miRNA-21 promotes proliferation and invasion of
triple-negative breast cancer cells through targeting PTEN. Am J
Transl Res. 9:953–961. 2017.PubMed/NCBI
|
|
22
|
Jacobson S, Kechris K, Sun W, Yang J, Chen
TH, Barr RG, Basta P, Bleecker ER, Couper D, Curtis JF, et al:
Blood biomarker quantitative trail loci in chronic obstructive
pulmonary disease. B93. Genetic signatures of Asthma: Key to
endotypes? Am Thorac Soc. 191:A36272015.
|
|
23
|
Han Y, Xu GX, Lu H, Yu DH, Ren Y, Wang L,
Huang XH, Hou WJ, Wei ZH, Chen YP, et al: Dysregulation of miRNA-21
and their potential as biomarkers for the diagnosis of cervical
cancer. Int J Clin Exp Pathol. 8:7131–7139. 2015.PubMed/NCBI
|
|
24
|
Shi R, Wang PY, Li XY, Chen JX, Li Y,
Zhang XZ, Zhang CG, Jiang T, Li WB, Ding W and Cheng SJ: Exosomal
levels of miRNA-21 from cerebrospinal fluids associated with poor
prognosis and tumor recurrence of glioma patients. Oncotarget.
6:26971–26981. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan J, Chen L, Chen X, Sun W and Zhou X:
Identification of serum microRNA-21 as a biomarker for
chemosensitivity and prognosis in human osteosarcoma. J Int Med
Res. 40:2090–2097. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ziyan W, Shuhua Y, Xiufang W and Xiaoyun
L: MicroRNA-21 is involved in osteosarcoma cell invasion and
migration. Med Oncol. 28:1469–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|