Introduction
Cancer is a multi-gene-associated disease; the genes
involved in each malignancy compose a ‘cancer panel’. This ‘cancer
panel’ results in a complex protein regulation network that is able
to determine the patterns of cancer cell behavior (1,2).
Therefore, treatments targeting single genes may result in failure,
as there are compensatory effects elicited by other genes that
occur when a single pathway is blocked (3–5).
Therefore, it is necessary to identify these ‘cancer panels’ in
each type of cancer to promote an improved understanding of cell
signaling transduction networks and enable the development of
higher-efficacy treatments to control cancer cells.
PRCC is the second most common type of kidney
cancer. It is also the most malignant type, without any effective
therapies (6). Optimal treatment
involves surgical removal of the tumor when the disease is in the
early stages. However, there remains a lack of treatment options
for patients with advanced-stage PRCC (7). Personalized medicine has aimed to
distinguish the genetic differences or gene expression pattern
alterations in each patient to enable physicians to provide the
best treatment for the individual. Cancer is a heterogenetic
disease in terms of somatic mutations or gene expression profile
alterations in cancer cells (8).
Differences in the patterns of gene expression determine the course
of treatment to be administered. Therefore, the present study
collected data from different cancer databases and integrated the
data using a bioinformatics approach to identify a gene panel that
affects the progress of PRCC.
DNA topoisomerase IIα (TOP2A) encodes DNA
topoisomerase, which is an important enzyme that releases the
torsional stress when DNA undergoes DNA replication and
transcription (9). TOP2A
actively participates in cellular proliferation (10). It is a critical gene in carcinogenesis
(11,12). Additionally, mutations in TOP2A
are a common cause of the failure of drugs that target the
corresponding protein (11). There
are numerous data demonstrating that TOP2A is involved in a
range of cancer types, including breast, endometrial, colon and
ovarian cancer (13–16).
The kidney epitheilal cell is a differentiated cell
type. TOP2A is absent or expressed at low levels in kidney
epithelial cells (17). A previous
study revealed that TOP2A was upregulated in clear cell
renal cell carcinoma (CCRCC), and that its expression was
predictive of a poor patient outcome (18). Therefore, the present study aimed to
identify whether TOP2A was also upregulated in PRCC and its
function as a cancer driver, and attempted to mine data online
using a bioinformatics approach to examine the cancer panel
associated with TOP2A in PRCC.
Materials and methods
Bioinformatics analysis
The Cancer Genome Atlas (TCGA) was joint project
launched by the National Cancer Institute and National Human Genome
Research Institute, has generated comprehensive, multi-dimensional
maps of the key genomic changes in 33 types of human cancer
(https://cancergenome.nih.gov/) (19). The present study used the kidney
cancer dataset in TCGA. The Oncomine cancer database is a
comprehensive cancer database including almost all types of cancer
(20). The present study assessed the
copy number and gene expression levels of TOP2A in Oncomine
(https://www.oncomine.org/resource) by
searching the gene symbol and cancer type within the ‘Cancer vs.
Normal Analysis’ analysis type filter on 21th March 2017.
Furthermore, the outlier analysis tool of Oncomine was used to
identify the ‘outlier genes’ that are only expressed in a number of
cancer samples on 21th March 2017. The outlier set in
the TOP2A positive sample accounted for 5–25% among all
samples in the three independent studies (21–23). The
survival rate curves were created using OncoLnc (http://www.OncoLnc.org/) on 27th March 2017 (24). The high and low expression groups were
set at the upper and lower quartiles, respectively. The high TOP2A
expression group was set at >394.46; whilst, the low TOP2A
expression group was set at <99.61. Using OncoLnc, the survival
curve, the Cox coefficient and the false discovery rate (FDR) were
calculated on 27th March 2017. Multiple gene survival
analysis was performed using survival tool in cBioPortal for Cancer
Genomics (http://www.cbioportal.org) by
searching the genes name simultaneously on 1st April 2017 (25,26). The
data for the generation of the heat map was downloaded from
cBioPortal and hierarchical clustering was performed with MeV
software version 4.9.0 developed by GitHub on 11th April
2017 (http://mev.tm4.org/#/welcome). The
protein-protein interaction network was completed using the Search
Tool for the Retrieval of Interacting Genes/Proteins (STRING,
version 10.5) by inputting all the genes on 16th April 2017
(http://string-db.org/).
Statistical analysis
One-way analysis of variance was conducted to
analyze variance among multiple groups and a Student-Newman-Keuls
test was used for post-hoc comparisons between the groups. Unpaired
Student's t-test was performed for the comparison of mean values of
two groups. Pearson's correlation analysis was conducted to test
the correlation between genes. All these data analysis were
performed using Graphpad Prism 5.0 (GraphPad Software, Inc., La
Jolla, CA, USA) and the data were presented as the mean ± the
standard deviation. Kaplan Meier analysis was used to compare the
survival time between two groups with different expression levels
of the genes of interest by OncoLnc. The log-rank test was
conducted to compare the survival time distribution of the two
groups. Hierarchical clustering was conducted for the generation of
the gene expression signature heat map using MeV (Version 4.9.0)
developed by GitHub, Inc. (San Francisco, CA, USA). Multivariate
Cox regression analysis was used for evaluation of the gene
expression of the genes assessed here on the patient's risk of
mortality. The Cox coefficient, P-value, FDR and gene rank were
calculated using the OncoLnc multivariate Cox regressions model
tool (24). P<0.05 was considered
to indicate a statistically significant difference.
Results
TOP2A is upregulated, and high
expression of TOP2A contributes to poor outcome in PRCC
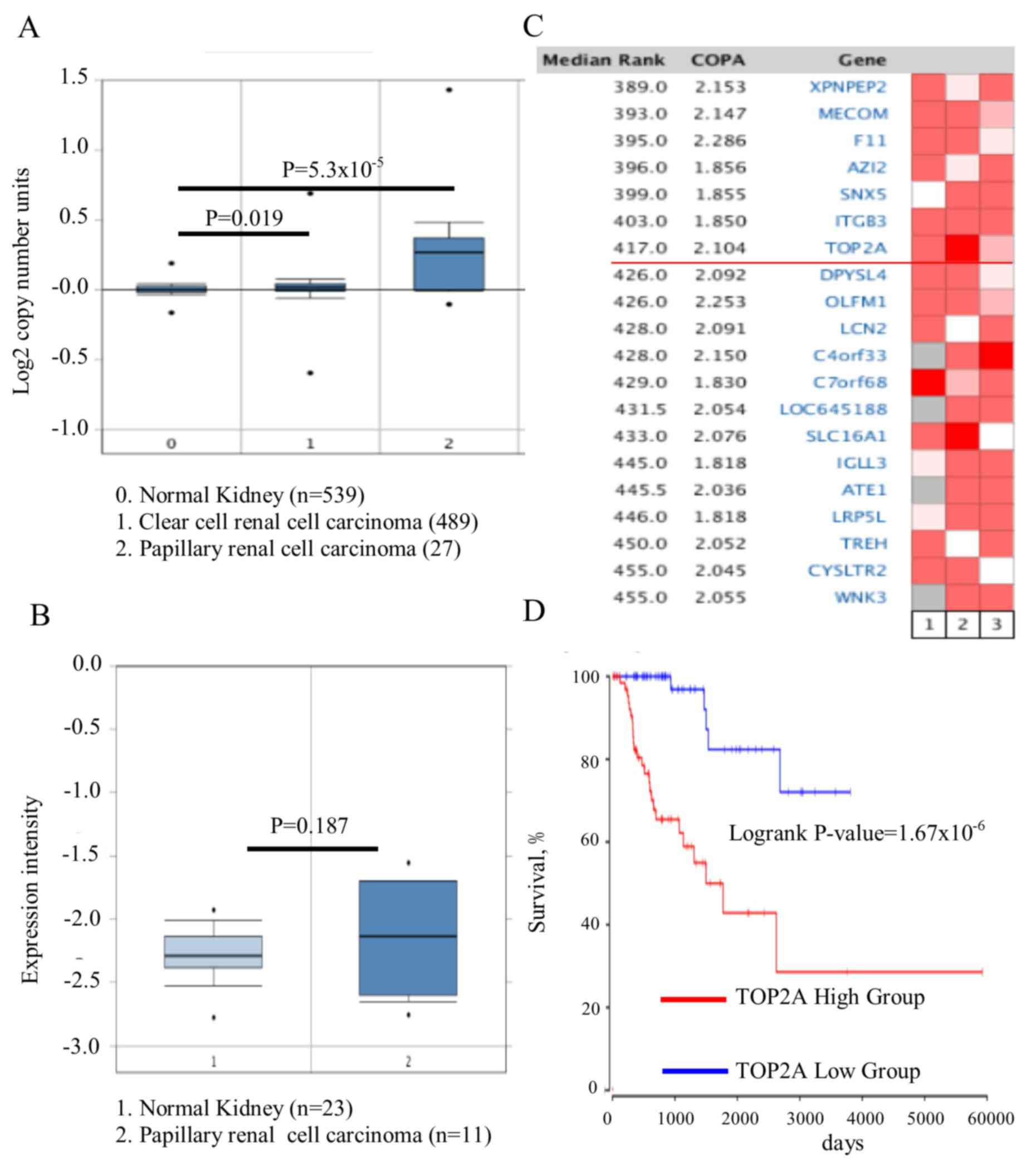
The Cancer Genome Atlas dataset of renal cell
carcinoma, which includes 1,017 cases, was analyzed by Oncomine and
it was identified that the TOP2A gene copy number in PRCC
was significantly increased, compared with kidney or CCRCC
(Fig. 1A; P=5.3×10−5).
Whether the increase in gene copy number contributed to the
upregulation of TOP2A was then assessed. The association
between the copy number and its expression level was then analyzed
in 23 normal kidney tissues and 11 PRCC tissues. No difference in
the expression of TOP2A was observed between the PRCC
tissues and normal kidney tissues (Fig.
1B; P=0.187). Owing to the heterogeneity of this cancer type,
whether the TOP2A gene was upregulated only in certain
patients with a specific genetic background. Therefore, the
Oncomine outlier analysis tool was utilized, and 193 patient tumor
tissues from 3 independent studies were analyzed (21–23). It
was identified that TOP2A was expressed in a subset of
patients with high expression of TOP2A (Fig. 1C); its association with the outcome of
patients was additionally investigated. The difference in survival
rates between the TOP2A high- and low-expression groups was
analyzed using the Cox regression model, and it was identified that
TOP2A expression was negatively associated with patient
outcome (Fig. 1D;
P=1.67×10−6). It was concluded that TOP2A was
upregulated in one subset of patients with PRCC, and was predictive
of poor prognosis.
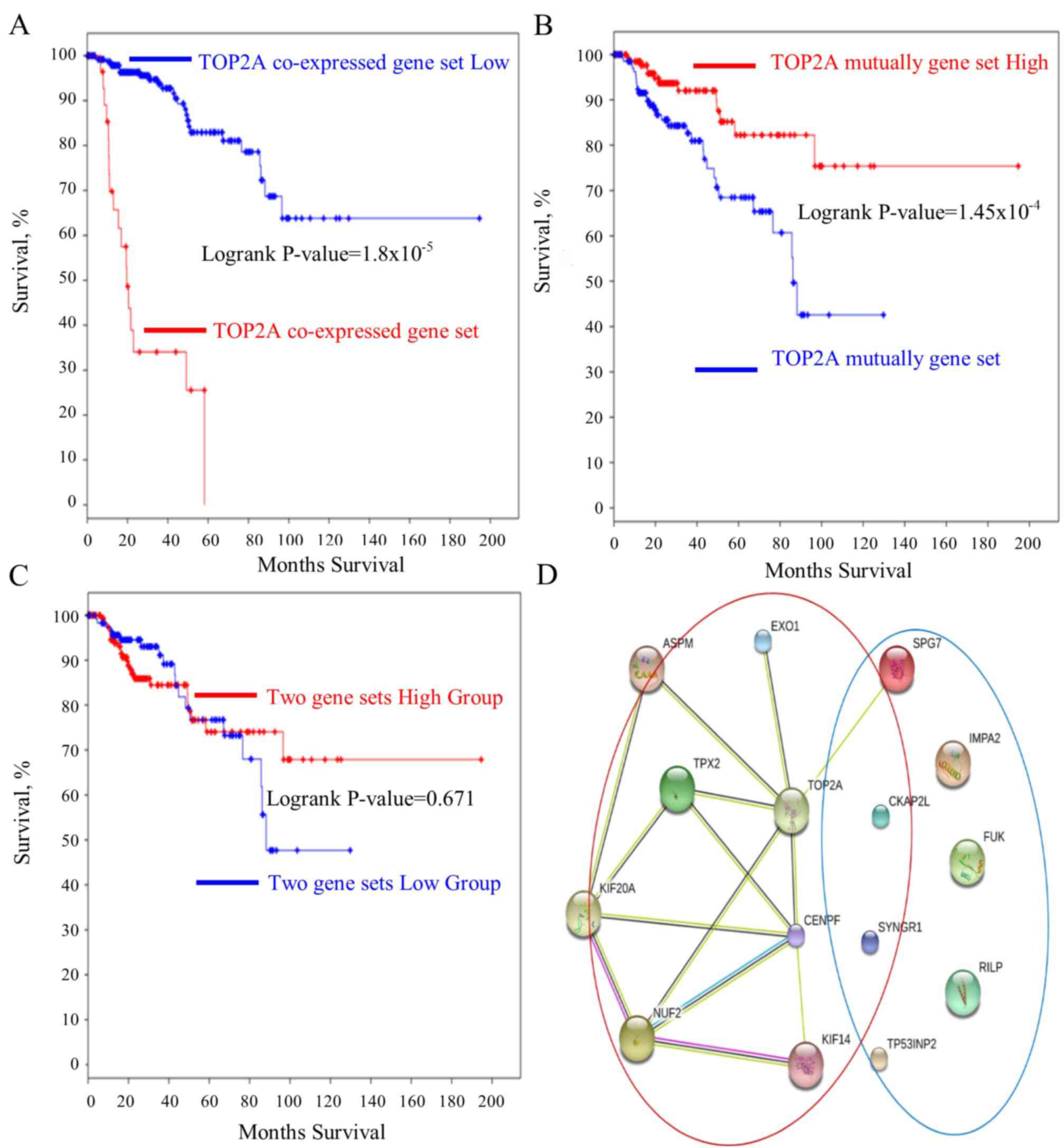
Co-expressed/mutually
exclusively-expressed genes with TOP2A and their role in PRCC
Pearson's correlation analysis was performed, and it
was identified that the expression levels of a large number of
genes were correlated with TOP2A. Genes with correlation
coefficients >0.9 were screened out for additional analysis. The
genes co-expressed with the TOP2A were: Abnormal spindle
microtubule assembly (ASPM), exonuclease 1 (EXO1),
TPX2, microtubule nucleation factor (TPX2), kinesin family
member 14 (KIF14), cytoskeleton associated protein 2 like
(CKAP2L), KIF20A, NUF, NDC80 kinetochore complex
component (NUF2) and centromere protein F (CENPF).
The genes that were inversely expressed with TOP2A were also
probed. Pearson's correlation analysis was performed, and genes
with correlation coefficients <-0.3 were selected. These genes
included tumor protein P53 inducible nuclear protein 2
(TP53INP2), Rab interacting lysosomal protein (RILP),
fucokinase (FUK), inositol monophosphatase 2 (IMPA2),
SPG7, paraplegin matrix AAA peptidase subunit (SPG7) and
synaptogyrin 1 (SYNGR1). A heat map for visualizing the
association between these genes was generated. The gene expression
profiles in the patient tumor tissues were characterized, and the
two gene sets of genes that were mutually exclusively-expressed in
tumor tissues were identified (Fig.
2). Furthermore, the two sets of genes were compared in CCRCC
and PRCC. Not only was the expression level rank but also the Cox
regression coefficient was significantly lower in the CCRCC
compared with PRCC (Table I).
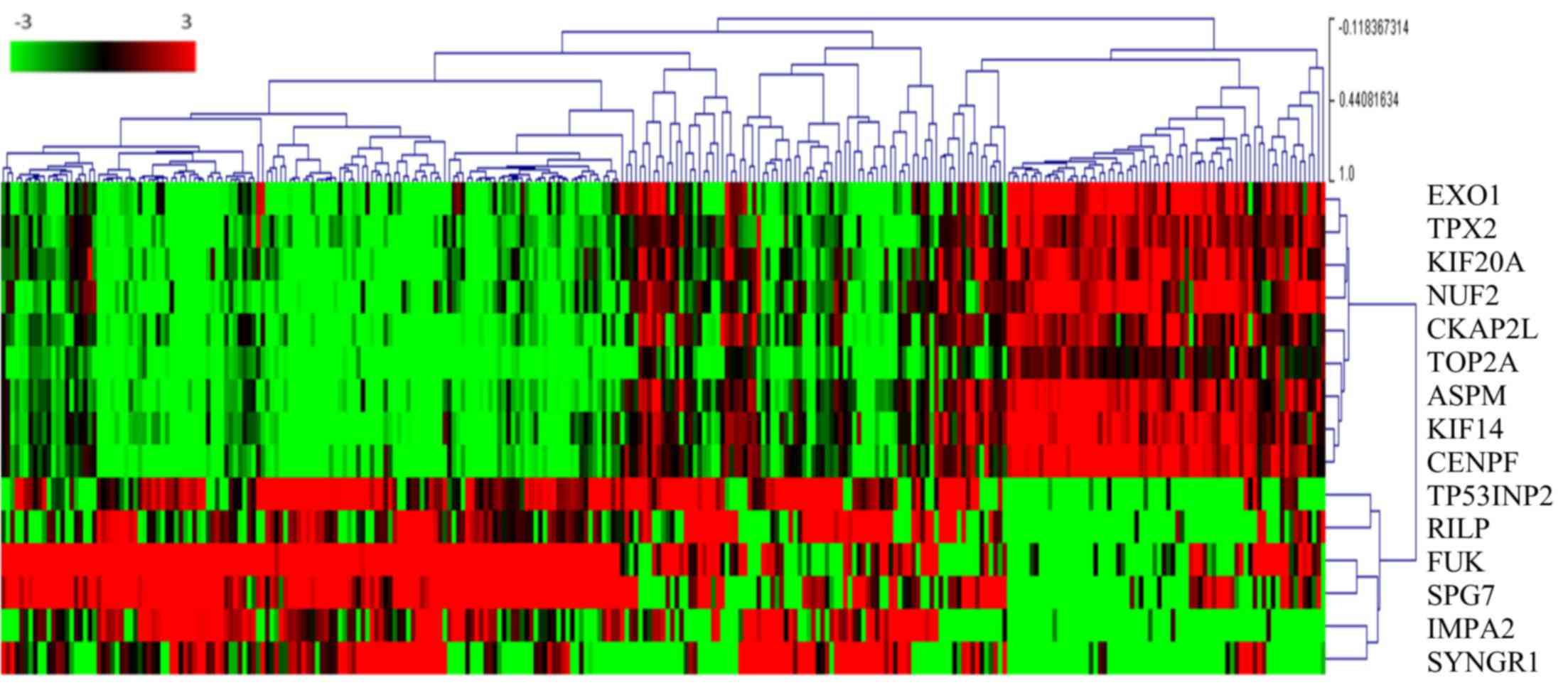 | Figure 2.TOP2A co-expressed and
mutually expressed genes. The heat map depicts the Log2(expression
level) of TOP2A-associated genes in patients with papillary
renal cell carcinoma (each column represents one case). Green to
red transition represents the range from −3 to 3 of gene expression
after Log2 transformation. The number next to the branches
represents the gene expression correlation with other cases. The
higher a correlation value is for a case, the more similar the
genes expression profile in the same cluster. EXO1,
exonuclease 1; TPX2, TPX2, microtubule nucleation factor;
KIF20A, kinesin family member 20A; NUF2, NUF, NDC80
kinetochore complex component; CKAP2L, cytoskeleton
associated protein 2 like; TOP2A, DNA topoisomerase IIα;
ASPM, abnormal spindle microtubule assembly; KIF14,
kinesin family member 14; CENPF, centromere protein F;
TP53INP2, tumor protein P53 inducible nuclear protein 2;
RILP, Rab interacting lysosomal protein; FUK,
fucokinase; SPG7, SPG7, paraplegin matrix AAA peptidase
subunit; IMPA2, inositol monophosphatase 2; SYNGR1,
synaptogyrin 1. |
 | Table I.Comparisons of Cox coefficient,
P-value, FDR and gene rank between the papillary renal cell
carcinoma and clear renal cell carcinoma samples using a Cox
regression model. |
Table I.
Comparisons of Cox coefficient,
P-value, FDR and gene rank between the papillary renal cell
carcinoma and clear renal cell carcinoma samples using a Cox
regression model.
| A, Co-expressed
genes |
|---|
|
|---|
| Gene/cancer
types | Cox
coefficient | P-value | Corrected FDR | Rank |
|---|
| TOP2A |
|
|
|
|
|
PRCC | 1.238 |
5.10×10−11 |
2.14×10−7 | 3 |
|
CCRCC | 0.259 |
3.00×10−3 |
1.22×10−2 | 4,086 |
| TPX2 |
|
|
|
|
|
PRCC | 1.23 |
4.20×10−10 |
3.21×10−7 | 21 |
|
CCRCC | 0.34 |
2.20×10−4 |
1.57×10−3 | 2,332 |
| EXO1 |
|
|
|
|
|
PRCC | 1.039 |
2.90×10−9 |
9.93×10−7 | 48 |
|
CCRCC | 0.16 |
6.00×10−2 |
1.27×10−1 | 7,875 |
| KIF14 |
|
|
|
|
|
PRCC | 1.069 |
1.80×10−9 |
7.05×10−7 | 42 |
|
CCRCC | 0.317 |
2.50×10−4 |
1.74×10−3 | 2,392 |
| KIF20A |
|
|
|
|
|
PRCC | 1.266 |
9.60×10−11 |
2.25×10−7 | 7 |
|
CCRCC | 0.377 |
3.00×10−5 |
3.39×10−4 | 1,471 |
| ASPM |
|
|
|
|
|
PRCC | 1.259 |
1.10×10−10 |
2.26×10−7 | 8 |
|
CCRCC | 0.286 |
7.60×10−4 |
4.16×10−3 | 3,044 |
| CKAP2L |
|
|
|
|
|
PRCC | 1.026 |
1.10×10−8 |
2.41×10−6 | 73 |
|
CCRCC | 0.173 |
4.00×10−2 |
9.22×10−2 | 7,212 |
| NUF2 |
|
|
|
|
|
PRCC | 1.09 |
5.70×10−10 |
3.46×10−7 | 27 |
|
CCRCC | 0.393 |
6.40×10−6 |
1.03×10−4 | 1,033 |
| CENPF |
|
|
|
|
|
PRCC | 1.137 |
3.00×10−10 |
3.21×10−7 | 15 |
|
CCRCC | 0.289 |
1.10×10−3 |
5.50×10−3 | 3,283 |
|
| B, Mutually
exclusive genes |
|
|
TP53INP2 |
|
|
|
|
|
PRCC | −0.77 |
2.00×10−6 |
1.30×10−4 | 253 |
|
CCRCC | −0.262 |
1.70×10−3 |
7.78×10−3 | 3,644 |
| RILP |
|
|
|
|
|
PRCC | −0.657 |
1.40×10−4 |
2.59×10−3 | 885 |
|
CCRCC | 0.099 |
2.20×10−1 |
3.40×10−1 | 10,750 |
| FUK |
|
|
|
|
|
PRCC | −0.688 |
1.40×10−5 |
4.86×10−4 | 467 |
|
CCRCC | 0.035 |
6.40×10−1 |
7.43×10−1 | 14,316 |
| IMPA2 |
|
|
|
|
|
PRCC | −0.678 |
8.40×10−6 |
3.41×10−4 | 403 |
|
CCRCC | −0.319 |
1.70×10−4 |
1.29×10−3 | 2,167 |
| SPG7 |
|
|
|
|
|
PRCC | −0.917 |
4.60×10−7 |
4.56×10−5 | 165 |
|
CCRCC | 0.364 |
1.80×10−6 |
3.98×10−5 | 751 |
| SYNGR1 |
|
|
|
|
|
PRCC | −0.506 |
1.60×10−3 |
1.39×10−2 | 1,891 |
|
CCRCC | 0.054 |
5.20×10−1 |
6.42×10−1 | 13,508 |
TOP2A cancer panel predicts prognosis
in PRCC
The roles of the genes in the TOP2A ‘panel’
in PRCC remain elusive. A survival curve analysis for each gene
between their respective high- and low-expression groups was
performed. It was identified that these genes were good prognostic
markers. Notably, these genes performed better in predicting
prognosis of the patient in PRCC compared with CCRCC (Table I). ASPM, TPX2, CENPF,
hyaluronan mediated motility receptor (HMMR), EXO1,
KIF14, KIF20A, NUF2, cytoskeleton associated protein 2 like
(CKAP2L) predicted the shortest survival time of patients
(Fig. 3A-I). However, the
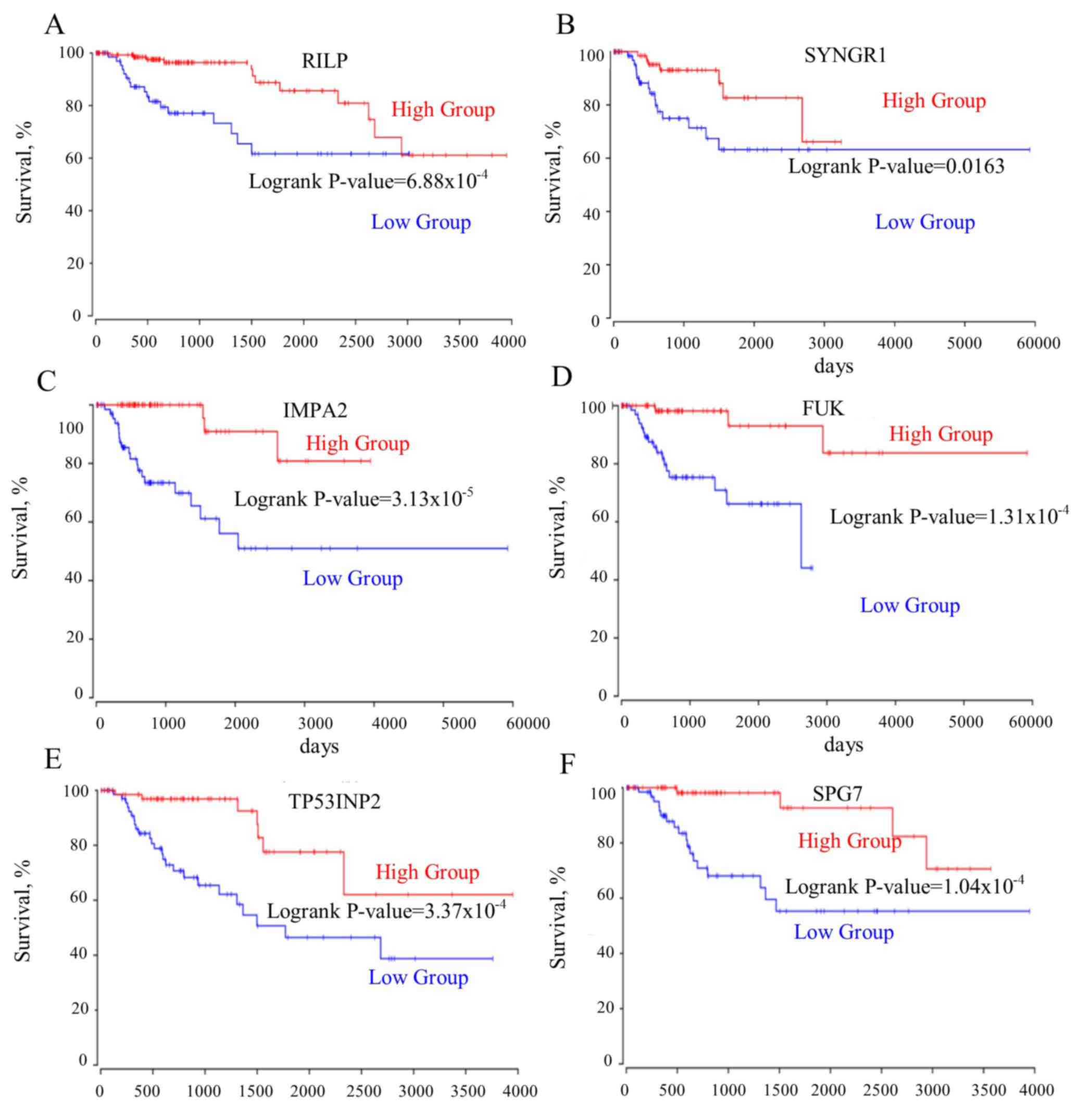
upregulation of the mutually exclusive genes (RILP, SYNGR1,
IMPA2, FUK, TP53INP2, SPG7) prolonged the patient survival time
(Fig. 4A-F). Furthermore, the
mutually exclusive gene expression in the patients with TOP2A high
expression may counteract the decreased survival time observed in
the TOP2A-cancer panel gene expression analysis (Fig. 5A-C). The gene interaction network of
the TOP2A-associated genes was analyzed with the STRING
protein interaction analysis tool, and it was observed that the
gene co-expressed with TOP2A form a ‘cancer panel’. The
downregulated genes may serve as ‘tumor repressor panel’ (Fig. 5D).
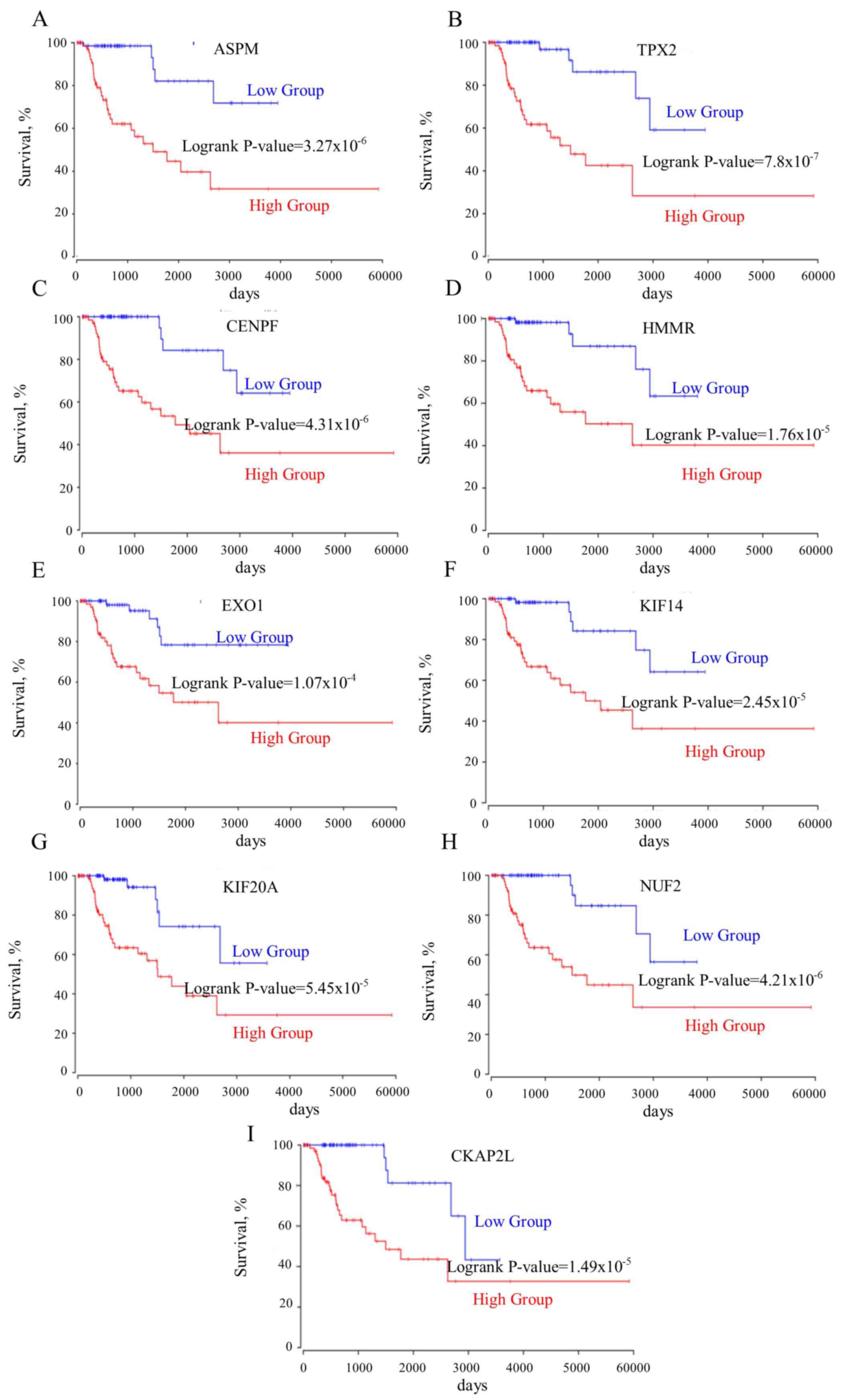 | Figure 3.(A-I) All genes co-expressed with
TOP2A are negatively associated with the survival rates of
patients. ASPM, abnormal spindle microtubule assembly;
TPX2, TPX2, microtubule nucleation factor; CENPF,
centromere protein F; HMMR, hyaluronan mediated motility
receptor; EXO1, exonuclease 1; KIF14, kinesin family
member 14; KIF20A, kinesin family member 20A; NUF2,
NUF, NDC80 kinetochore complex component; CKAP2L,
cytoskeleton associated protein 2 like; TOP2A, DNA
topoisomerase IIα. |
Discussion
Oncogene mutations usually drive carcinogenesis, but
gene alteration always results in the regulation of the expression
of other associated genes. The expression of these associated genes
may comprise a network, and their output may affect the behavior of
cancer cells. These genes serve a vital role in establishing the
properties of different types of cancer usually was defined as
‘cancer panels’ (27). The two most
common types of kidney cancer are CCRCC and PRCC (28). CCRCC is sensitive to chemotherapeutics
and patients generally exhibit improved outcomes following therapy
compared with without therapy (29).
However, PRCC often exhibits resistance to current
chemotherapeutics (30). It was
reported that TOP2A was upregulated in prostate cancer,
breast cancer and serves as an indicator of a poor outcome
(11,31). Previously, a number of studies have
revealed that TOP2A is upregulated in CCRCC and is a poor
predicative marker for pgrognosis (18,32).
However, to the best of our knowledge, there are no stdies on
whether TOP2A is dysregulated in PRCC. The present study
identified that TOP2A is upregulated in kidney cancer and is
significantly increased in PRCC compared with CCRCC. Additionally,
TOP2A functioned well in predicting the prognoses of
patients with PRCC compared with in CCRCC. This result indicated
that TOP2A may be involved in PRCC.
The ‘cancer panel’ established in the present study
included genes that were involved in similar biological functions
and contributed to cancer progression. These genes function
concomitantly to affect the behavior of cancer cells.
Identification of the associations between these genes and their
interaction network may enable an improved understanding of how
TOP2A causes the development of PRCC. To intervene in cancer
progression, the interruption of one driver gene is not sufficient
for the complete inhibition of cancer progression. Integral
disruption of all of the genes involved in cancer progression is
more important for the successful treatment of cancer, than
focusing on a single target gene. Therefore, the present study
analyzed the genes that were closely associated with TOP2A.
Genes that were significantly co-expressed with TOP2A were
selected, and it was hypothesized that these genes may be
simultaneously involved in PRCC progression. To confirm the
function of these genes in PRCC, the survival curves for the high-
and low-expression groups of each gene were generated. Notably, it
was identified that the expression of these genes significantly
reduced the survival time of patients. Therefore, the expression
levels of these genes was not only associated with TOP2A,
but also upregulation of these genes would reduce the survival
rates of the patients. This result indicated that TOP2A may
prompt PRCC progression in conjunction with other genes; however,
whether TOP2A regulates the expression of these genes
requires additional investigation.
Considering the genes in the ‘TOP2A-cancer panel’,
the present study aimed to identify the processes they are involved
in, and to understand how these genes function together to
determine cancer cell properties. The ASPM gene is closely
associated with spindle function, which is involved in cell mitosis
(33). The TPX2 gene is a
spindle assembly factor that servers as a critical role in
G2/M transition of cell cycle (34). The HMMR gene encodes a protein
that forms a complex with BRCA1/2, which promotes cell
proliferation and increases the risk of cancer (35). The CENPF gene is required for
chromosome segregation in cell mitosis, which regulates DNA
replication and cell cycle progression (36). EXO1 encodes an exonuclease that
is responsible for DNA mismatch repair (37). KIF14 contributes to chromosome
segregation and spindle formation in the mitosis process (38). KIF20A functions as a spindle
assembly mediator, resulting in cell division (39). CKAP2L is involved in spindle
organization (40). NUF2
regulates chromosome segregation and centromere function in the
cell mitosis (41). Therefore, the
genes within the TOP2A-derived cancer panel function in the
regulation of cell mitosis. According to the protein-protein
network (Fig. 5D), the results of the
present study indicated that TOP2A may serve a vital role in
the regulation of cell proliferation through interaction with the
TOP2A cancer panel.
The present study compared the association between
the expression levels of these genes with the survival rates of
patients with CCRCC and PRCC. It was identified that these genes
that coexpressed with TOP2A significantly increase the
survival rate of patients with PRCC compared with patients with
CCRCC. However, the genes that inversely expressed with
TOP2A decrease the survival rates of patients with PRCC
compared with patients with CCRCC, which may provide a method for
distinguishing between renal cell carcinoma subtypes by the
expression of the TOP2A cancer panel genes. The present
study identified that TOP2A was a vital prognostic marker
for PRCC, and the genes involved in the network of TOP2A
were examined. This network of TOP2A genes may assist in
understanding how TOP2A affects cancer cells, and how
targeting these genes may provide an avenue for the treatment of
PRCC.
Acknowledgements
The results shown here are in whole or part based
upon data generated by the TCGA Research Network. The authors also
would like to thank Dr. Qingyu Zhang (Faculty of Health Sciences,
University of Macau, Macau, China) for providing advice on the data
interpretation and critical comments on the discussion.
Funding
This present study was by partially supported by the
Zhanjiang scientific research project (grant no. 2016C01005) and
Zhanjiang scientific special competition project (grant no.
2016A01011).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MY and ZH collected data and developed the
methodology. DW, ZI and XC interpreted the results. MY and JL
designed the work and wrote the manuscript.
Ethics approval and consent to publish
This study reinterpreted the data deposited in TCGA
and GEO without releasing the information of patients. According to
TCGA publication guidelines, there are no restrictions on the use
of TCGA data for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dalton E and Thompson J: AB054. Overview
of multi-gene panels for hereditary cancer. Ann Transl Med.
3:AB0542015.
|
|
2
|
Lincoln SE, Kobayashi Y, Anderson MJ, Yang
S, Desmond AJ, Mills MA, Nilsen GB, Jacobs KB, Monzon FA, Kurian
AW, et al: A systematic comparison of traditional and multigene
panel testing for hereditary breast and ovarian cancer genes in
more than 1000 patients. J Mol Diagn. 17:533–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Housman G, Byler S, Heerboth S, Lapinska
K, Longacre M, Snyder N and Sarkar S: Drug resistance in cancer: An
overview. Cancers (Basel). 6:1769–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo XE, Ngo B, Modrek AS and Lee WH:
Targeting tumor suppressor networks for cancer therapeutics. Curr
Drug Targets. 15:2–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Q, Madden NE, Wong AST, Chow BKC and
Lee LTO: The role of endocrine G protein-coupled receptors in
ovarian cancer progression. Front Endocrinol (Lausanne).
8:662017.PubMed/NCBI
|
|
6
|
Shuch B, Hahn AW and Agarwal N: Current
treatment landscape of advanced papillary renal cancer. J Clin
Oncol. 35:2981–2983. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross H, Martignoni G and Argani P: Renal
cell carcinoma with clear cell and papillary features. Arch Pathol
Lab Med. 136:391–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Friedman AA, Letai A, Fisher DE and
Flaherty KT: Precision medicine for cancer with next-generation
functional diagnostics. Nat Rev Cancer. 15:747–756. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lodish H, Berk A, Zipursky SL, Matsudaira
P, Baltimore D and Darnell J: The Role of topoisomerases in DNA
replication. Molecular Cell Biology. 4th edition. W.H. Freeman; New
York, NY: 2000
|
|
10
|
Chen T, Sun Y, Ji P, Kopetz S and Zhang W:
Topoisomerase IIα in chromosome instability and personalized cancer
therapy. Oncogene. 34:4019–4031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Resende MF, Vieira S, Chinen LT,
Chiappelli F, da Fonseca FP, Guimarães GC, Soares FA, Neves I,
Pagotty S, Pellionisz PA, et al: Prognostication of prostate cancer
based on TOP2A protein and gene assessment: TOP2A in prostate
cancer. J Transl Med. 11:362013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fountzilas G, Valavanis C, Kotoula V,
Eleftheraki AG, Kalogeras KT, Tzaida O, Batistatou A, Kronenwett R,
Wirtz RM, Bobos M, et al: HER2 and TOP2A in high-risk early breast
cancer patients treated with adjuvant epirubicin-based dose-dense
sequential chemotherapy. J Transl Med. 10:102012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito F, Furukawa N and Nakai T: Evaluation
of top2a as a predictive marker for endometrial cancer with
taxane-containing adjuvant chemotherapy. Int J Gynecol Cancer.
26:325–330. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erriquez J, Becco P, Olivero M, Ponzone R,
Maggiorotto F, Ferrero A, Scalzo MS, Canuto EM, Sapino A, Verdun di
Cantogno L, et al: TOP2A gene copy gain predicts response of
epithelial ovarian cancers to pegylated liposomal doxorubicin:
TOP2A as marker of response to PLD in ovarian cancer. Gynecol
Oncol. 138:627–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tarpgaard LS, Qvortrup C, Nygård SB,
Nielsen SL, Andersen DR, Jensen NF, Stenvang J, Detlefsen S,
Brünner N and Pfeiffer P: A phase II study of epirubicin in
oxaliplatin-resistant patients with metastatic colorectal cancer
and TOP2A gene amplification. BMC Cancer. 16:912016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Xu B, Yuan P, Zhang P, Li Q, Ma F
and Fan Y: TOP2A amplification in breast cancer is a predictive
marker of anthracycline-based neoadjuvant chemotherapy efficacy.
Breast Cancer Res Treat. 135:531–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheburkin IuV, Kniazeva TG, Peter S,
Kniazev IuP, Karelin MI, Shkol'nik MI, Evtushenko VI, Hanson KP,
Ullrich A and Kniazev PG: Molecular portrait of human kidney
carcinomas: The gene expression profiling of protein-tyrosine
kinases and tyrosine phosphatases which controlled regulatory
signals in the cells. Mol Biol (Mosk). 36:480–490. 2002.(In
Russian). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen D, Maruschke M, Hakenberg O,
Zimmermann W, Stief CG and Buchner A: TOP2A, HELLS, ATAD2 and TET3
are novel prognostic markers in renal cell carcinoma. Urology.
102:265 e1–265 e7. 2017. View Article : Google Scholar
|
|
19
|
Grossman RL, Heath AP, Ferretti V, Varmus
HE, Lowy DR, Kibbe WA and Staudt LM: Toward a shared vision for
cancer genomic data. N Engl J Med. 375:1109–1112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones J, Otu H, Spentzos D, Kolia S, Inan
M, Beecken WD, Fellbaum C, Gu X, Joseph M, Pantuck AJ, et al: Gene
signatures of progression and metastasis in renal cell cancer. Clin
Cancer Res. 11:5730–5739. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lenburg ME, Liou LS, Gerry NP, Frampton
GM, Cohen HT and Christman MF: Previously unidentified changes in
renal cell carcinoma gene expression identified by parametric
analysis of microarray data. BMC Cancer. 3:312003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dannull J, Su Z, Rizzieri D, Yang BK,
Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E and Vieweg
J: Enhancement of vaccine-mediated antitumor immunity in cancer
patients after depletion of regulatory T cells. J Clin Invest.
115:3623–3633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anaya J: OncoLnc: Linking TCGA survival
data to mRNAs, miRNAs, and lncRNAs. PeerJ Computer Science.
2:e672016. View Article : Google Scholar
|
|
25
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:p112013. View Article : Google Scholar
|
|
26
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cbio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
LaDuca H, Stuenkel AJ, Dolinsky JS, Keiles
S, Tandy S, Pesaran T, Chen E, Gau CL, Palmaer E, Shoaepour K, et
al: Utilization of multigene panels in hereditary cancer
predisposition testing: Analysis of more than 2,000 patients. Genet
Med. 16:830–837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Capitanio U and Montorsi F: Renal cancer.
Lancet. 387:894–906. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ronnen EA, Kondagunta GV, Ishill N, Spodek
L, Russo P, Reuter V, Bacik J and Motzer RJ: Treatment outcome for
metastatic papillary renal cell carcinoma patients. Cancer.
107:2617–2621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang J, Xu B, Yuan P, Zhang P, Li Q, Ma F
and Fan Y: TOP2A amplification in breast cancer is a predictive
marker of anthracycline-based neoadjuvant chemotherapy efficacy.
Breast Cancer Res Treat. 135:531–537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parker AS, Eckel-Passow JE, Serie D,
Hilton T, Parasramka M, Joseph RW, Wu KJ, Cheville JC and Leibovich
BC: Higher expression of topoisomerase II alpha is an independent
marker of increased risk of cancer-specific death in patients with
clear cell renal cell carcinoma. Eur Urol. 66:929–935. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barr FA, Silljé HH and Nigg EA: Polo-like
kinases and the orchestration of cell division. Nat Rev Mol Cell
Biol. 5:429–440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kufer TA, Silljé HH, Körner R, Gruss OJ,
Meraldi P and Nigg EA: Human TPX2 is required for targeting
Aurora-A kinase to the spindle. J Cell Biol. 158:617–623. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Parvin JD, Kais Z, Arora M, Kotian S, Zha
A, Ransburgh D, Bozdag D, Catalyurek U and Huang K: Identification
of a breast cancer associated regulatory network. Proceedings of
the 2009 Ohio Collaborative Conference on Bioinformatics.
Bioinformatics Cleveland, OH: pp. 71–75. 2009, View Article : Google Scholar
|
|
36
|
Taylor SS, Scott MI and Holland AJ: The
spindle checkpoint: A quality control mechanism which ensures
accurate chromosome segregation. Chromosome Res. 12:599–616. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kolodner RD and Marsischky GT: Eukaryotic
DNA mismatch repair. Curr Opin Genet Dev. 9:89–96. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rath O and Kozielski F: Kinesins and
cancer. Nat Rev Cancer. 12:527–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu Y and Feng YM: The role of kinesin
family proteins in tumorigenesis and progression: Potential
biomarkers and molecular targets for cancer therapy. Cancer.
116:5150–5160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hong KU, Kim E, Bae CD and Park J:
TMAP/CKAP2 is essential for proper chromosome segregation. Cell
Cycle. 8:314–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
DeLuca JG, Dong Y, Hergert P, Strauss J,
Hickey JM, Salmon ED and McEwen BF: Hec1 and nuf2 are core
components of the kinetochore outer plate essential for organizing
microtubule attachment sites. Mol Biol Cell. 16:519–531. 2005.
View Article : Google Scholar : PubMed/NCBI
|