Introduction
Prostate cancer is a malignant tumor with a
relatively high incidence rate in the male reproductive system, and
its diagnosis has been regarded as one of the medical problems to
be overcome (1). Particularly in
European population, the incidence rate of solid tumor in prostate
cancer is very high, and the number of patients has overtaken those
with colorectal and lung cancer. Thus, prostate cancer has severe
effects on male health, and is ranked first in incidence rate among
solid tumors in North America and Europe (2–4). In China,
the incidence rate, the cases, and death toll of prostate cancer
are less than the average of the world, but there is an increasing
trend in the number of patients and death toll due to the
westernization of diet, prolonged average of life span and precise
diagnostic technique (5). It is
expected that in the near future, the incidence rate and mortality
rate of prostate cancer in China will increase (6). Thus, facing the severe situation in
diagnosis and treatment of prostate cancer, researchers must
develop new diagnostic and therapeutic procedures and treatment
methods for prostate cancer.
Sesquiterpenes are common secondary metabolites
existing in nature. The larger number of derivatives with
sesquiterpene as mother nucleus presents multiple bioactivities,
including antitumor, anti-inflammation, and antibacterial (7). Citronella oil contains numerous
sesquiterpenes, such as β-bourbonene, β-elemene, β-caryophyllene.
β-elemene have proven to be useful as chemopreventive agent
clinically in lung cancer, gastrointestinal carcinomas and breast
cancer (8). In this present study, we
observed that β-bourbonene inhibited proliferation of prostate
cancer cells. This motivated us to investigate the effects of
β-bourbonene on apoptosis of prostate cancer PC-3M cells to clarify
the relevant mechanisms in apoptosis, results of which are expected
to provide evidence for clinical treatment of prostate cancer.
Materials and methods
Materials and reagents
β-bourbonene (Sigma; Merck KGaA, Darmstadt,
Germany); prostate cancer cell line PC-3M (Cell Bank of CAS,
Shanghai, China); Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA); Cell Counting
Kit-8 (CCK-8) (Sigma; Merck KGaA); primary antibodies and
horseradish peroxidase (HRP)-labeled secondary antibodies of Bax,
Bcl-2 (Proteintech Group, Inc., Wuhan, China); primer synthesis
(Takara Biotechnology Co., Ltd., Dalian, China), TRIzol and
SYBR-GreenER™ qPCR SuperMix Universal kits (Invitrogen; Thermo
Fisher Scientific, Inc.).
Cell culture
Human androgen-independent and highly metastatic
prostate cancer, PC-3M cells, were cultured in DMEM supplemented
with 20% fetal bovine serum in an incubator (37°C and 5%
CO2), and cells in this experiment were divided into
four groups: the blank control group (Control), the low-dose
β-bourbonene group (25 µg/ml), the mid-dose β-bourbonene group (50
µg/ml) and the high-dose β-bourbonene group (100 µg/ml).
Measurement of inhibition on cell
proliferation through CCK-8 method
Prostate cancer PC-3M cells in logarithmic phase
were trypsinized, and density of single-cell suspension was
counted, and 100 µl suspension with 0.2×105 cells was
inoculated per well into 96-well plate. After overnight incubation,
medium was replaced with final concentration of 25, 50, and 100
µg/ml β-bourbonene in a total volume of 100 µl. After 72 h,
complete medium was replaced without β-bourbonene, and cells were
cultured for another 3 days. At every 24 h, 10 µl CCK-8 reagent was
added into the corresponding wells of the plate, and the optical
density (OD) at wavelength of 450 nm was detected with a microplate
reader (Thermo Fisher Scientific, MA, USA).
Measurement of apoptosis of prostate
cancer PC-3M cells via TUNEL method
Prostate cancer PC-3M cells were inoculated in 6-cm
dish for drug treatment, fixing in 4% paraformaldehyde for 15 min,
rinsing with phosphate-buffer saline (PBS) twice (5 min/time),
treating with 100 µl Proteinase K (20 µg/ml) for 15 min (10 to 30
min), and washing again with PBS for 3 min. TUNEL reaction mixture
(500 µl) was added into the dish for 1 h of reaction at 37°C in a
dark humidifying box. After washing and blocking, 500 µl
avidin-labeled FITC was added and incubated for 30 min. Then, the
cell nucleus was stained with DAPI for 10 min. The cells were
observed under a laser confocal microscope and visions (Nikon,
Tokyo, Japan) were selected randomly for photography.
Detection of apoptosis of PC-3M cells
via Annexin V/PI dual staining method
PC-3M cells were inoculated into 6-well plate, where
cells were divided into groups, i.e. the control and
β-bourbonene-treated groups (25, 50, and 100 µg/ml). After 48 h of
treatment, the cells were trypsinized followed by centrifugation at
340 × g for 5 min at 4°C, and washing with PBS. Then, the cells
were incubated with the mixture of 5 µl Annexin V and 5 µl
propidium iodide (PI) for 15 min in the dark at room temperature.
Cell apoptosis was detected with a flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
Cell cycle analysis by flow
cytometry
PC-3M cells were seeded into a 6-well plate and
incubated overnight, followed by 0, 25, 50, and 100 µg/ml of
β-bourbonene. After 48 h treatment, the cells were trypsinized,
harvested for single-cell suspension, and fixed with cold 70%
ethanol at 4°C for 30 min. The cell pellet was incubated in a
solution containing 50 µg/ml PI, 0.2 mg/ml RNase, and 0.1% Triton
X-100 at room temperature in the dark for 30 min. Then, the cells
were analyzed by flow cytometer (BD Biosciences).
Measurement of mRNA expression of
factor associated suicide (Fas) and its ligand (FasL) via
RT-PCR
Cultured cells were seeded on a 6-well plate at
density of 1×104/well, and after 24 h, the supernatant
was discarded. Following treatment with 25, 50 and 100 µg/ml of
β-bourbonene for 48 h, the cells in each group were collected for
extraction of the total RNA using RNApure Tissue Cell kit according
to manufacturer's instructions (CWBIO, Beijing, China). After
detection, the total RNA that was qualified using a
spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.) for this experiment, was used as a template for synthesis of
complementary DNA (cDNA) through reverse transcription in following
reaction conditions: incubation at 42°C for 15 min and 95°C for 3
min, and cooling on ice using the First-Strand cDNA Synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR was performed
on cDNA by using SYBR-GreenER™ qPCR SuperMix Universal kit
(Invitrogen; Thermo Fisher Scientific, Inc.) using the following
conditions: at 50°C for 2 min; at 95°C for 10 min; 40 cycles of
95°C for 15 sec and 60°C for 1 min; followed by 72°C for 10 min.
Data were analyzed through the 2−∆∆Cq method and
normalization to GAPDH as an endoge nous reference (9). Regular amplification was performed for
primer sequences in Table I.
 | Table I.Primer sequences of Fas and FasL mRNA
for RT-PCR. |
Table I.
Primer sequences of Fas and FasL mRNA
for RT-PCR.
| Genes | Primer sequences |
|---|
| Fas | F:
5′-GGCATCTGGACCCTCCTACCTCTG-3′ |
|
| R:
5′-CCTTGGAGTTGATGTCAGTCACTTGG-3′ |
| FasL | F:
5′-GGCCTGTGTCTCCTTGTGAT-3′ |
|
| R:
5′-TGCCAGCTCCTTCTGAAGTA-3′ |
| GAPDH | F:
5′-ACAACTTTGGTATCGTGGAAGG-3′ |
|
| R:
5′-GCCATCACGCCACAGTTTC-3′ |
Detection of protein expression levels
of Bax and Bcl-2 via western blot assay
The cultured cells were inoculated into a 6-well
plate at a density of 1×104/well, and after 24 h, the
supernatant was discarded. Following the treatment with 25 µg/ml,
50 µg/ml and 100 µg/ml of β-bourbonene for 48 h, the cells in each
group were collected and lyzed in a cell lysis buffer
(Sigma-Aldrich; Merck KGaA) containing 1 mM PMSF. Total protein
concentration was measured by Coomassie brilliant blue assay using
BSA as the quantitative standard (P0006; Beyotime Institute of
Biotechnology, Shanghai, China). From the protein samples, 50 µg
protein was electrophoresed on 10% SDS-PAGE (sodium dodecyl sulfate
polyacrylamide gel electrophoresis) with marker ladder (P0060S,
Beyotime Institute of Biotechnology). Proteins were then
transferred electrically on a polyvinylidene fluoride (PVDF)
membrane followed by blocking with blocking buffer (Candor
Bioscience GmbH, Wangen, Allgäu, Germany) for 1 h. Proteins were
then incubated with primary antibodies at 4°C overnight. The
dilution multiple of primary antibodies are 1:200, 1:500, 1:1,000,
1:500 and 1:1,500 for Fas, FasL, Bax, Bcl-2, and GAPDH,
respectively and then, the membrane was washed with Tween
20/Tris-buffer saline (TTBS). Then, the secondary antibody
(1:2,000) was added on the membrane for incubation at room
temperature for 1 h followed by washing with TTBS, color
development with ECL western blot substrate developing solution
(32109, Pierce; Thermo Fisher Scientific, Inc.) and photographing.
The protein bands were determined as relative quantity by an image
analysis system (Odyssey 3.0 software) and normalized to control
GAPDH. The following antibodies were used: Fas (1:200; cat. no.
BM4868) and GAPDH (1:500; cat. no. BM1623) were purchased from
Wuhan Boster Biological Technology (Wuhan, China), FasL (1:500;
cat. no. bs-0216R), Bax (1:1,000; cat. no. bsm-33283M), and Bcl-2
(1:500; cat. no. bsm-33047M) were purchased from BIOSS (Beijing,
China), goat anti-rabbit IgG (1:400; cat. no. 926-32211) and goat
anti-mouse IgG (1:400; cat. no. 926-32210) were purchased from
Licor (Lincoln, NE, USA).
Statistical processing
Data processed with Statistical Product and Service
Solutions 19.0 (SPSS 19.0). One-way ANOVA was used to identify
group difference and followed by Dunnett's post test for group
difference. Values are expressed as mean ± standard deviation (SD)
with replicate number of 3. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibitory effect of β-bourbonene on
cell proliferation
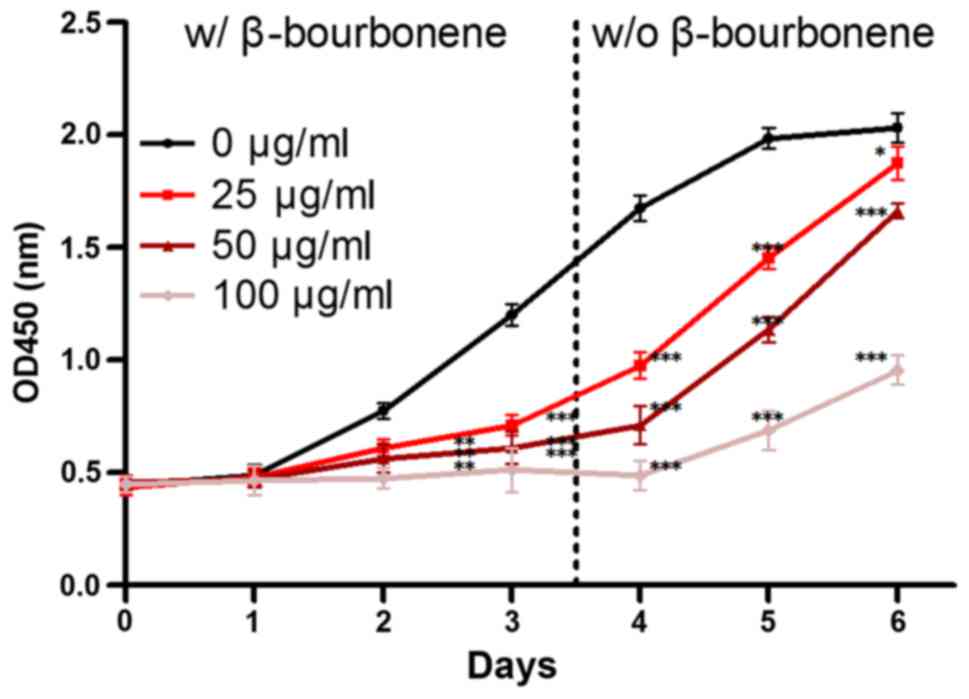
As shown in Fig. 1,
compared with the control group, PC-3M cell proliferation was
significantly inhibited in groups treated with 25, 50 and 100 µg/ml
β-bourbonene. The proliferation rate notably decreased in a
dose-dependent manner. After β-bourbonene was removed, the
proliferation profile of PC-3M cells was observed for the following
3 days. Compared to the proliferative activity (according to the
curve slope) since day 0 to day 3 with β-bourbonene treatment, the
proliferative activity at day 4 to day 6 dramatically increased
without treatment of β-bourbonene. The significantly different
proliferation profile with or without β-bourbonene treatment
indicated that the decreased proliferation before day 3 was induced
by β-bourbonene.
Effect of β-bourbonene on cell cycle
arrest
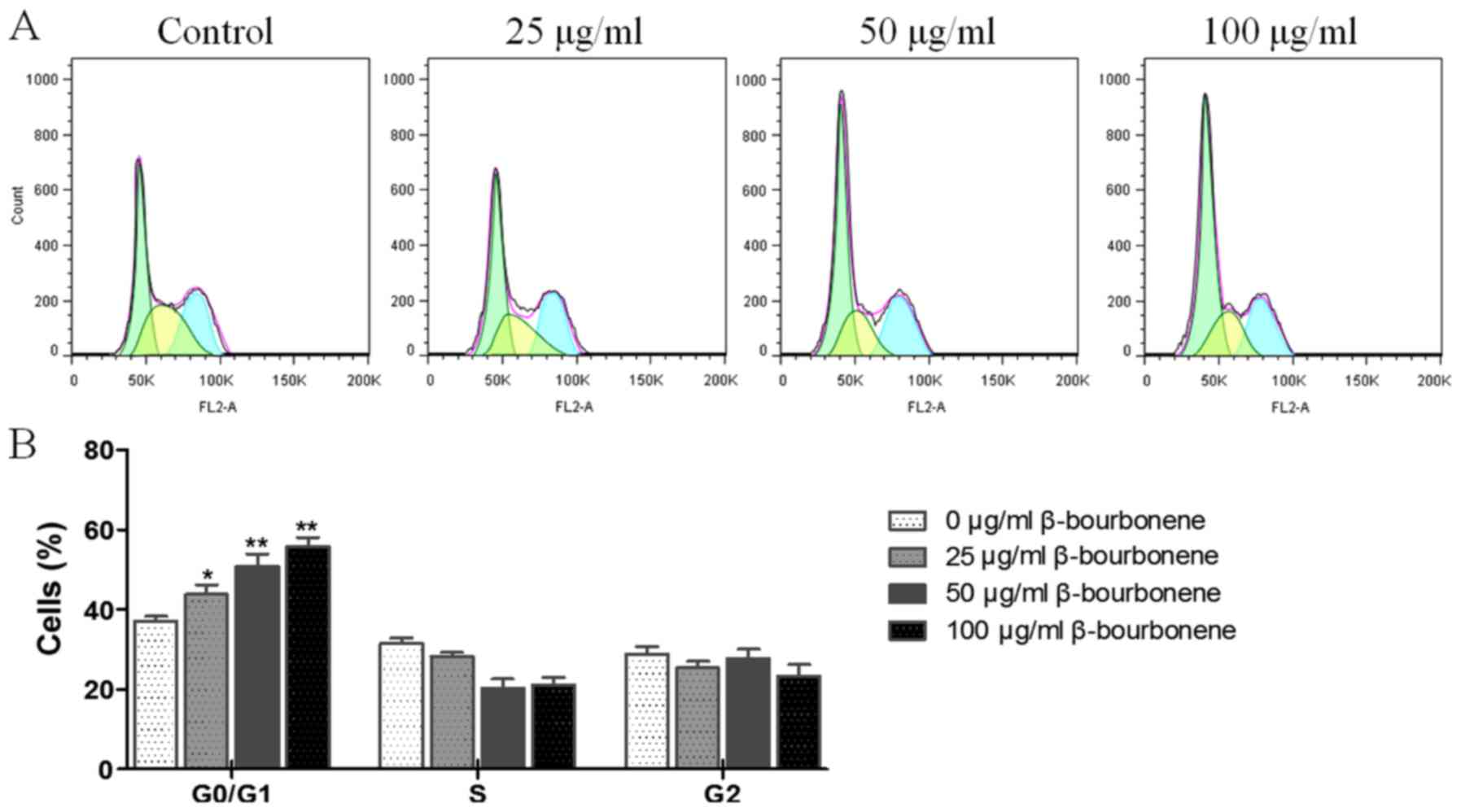
As shown in Fig. 2,
compared with the control group, the number of cells in G0/G1 phase
of PC-3M cells changed significantly in groups treated with 25, 50
and 100 µg/ml β-bourbonene (P<0.05). The increased trend was
dose-dependent. Cell amount in the S and G2 phase did not present
any trend. This result indicated that the progression of cell cycle
was arrested at G0/G1 phase.
Effect of β-bourbonene on apoptosis of
PC-3M cells
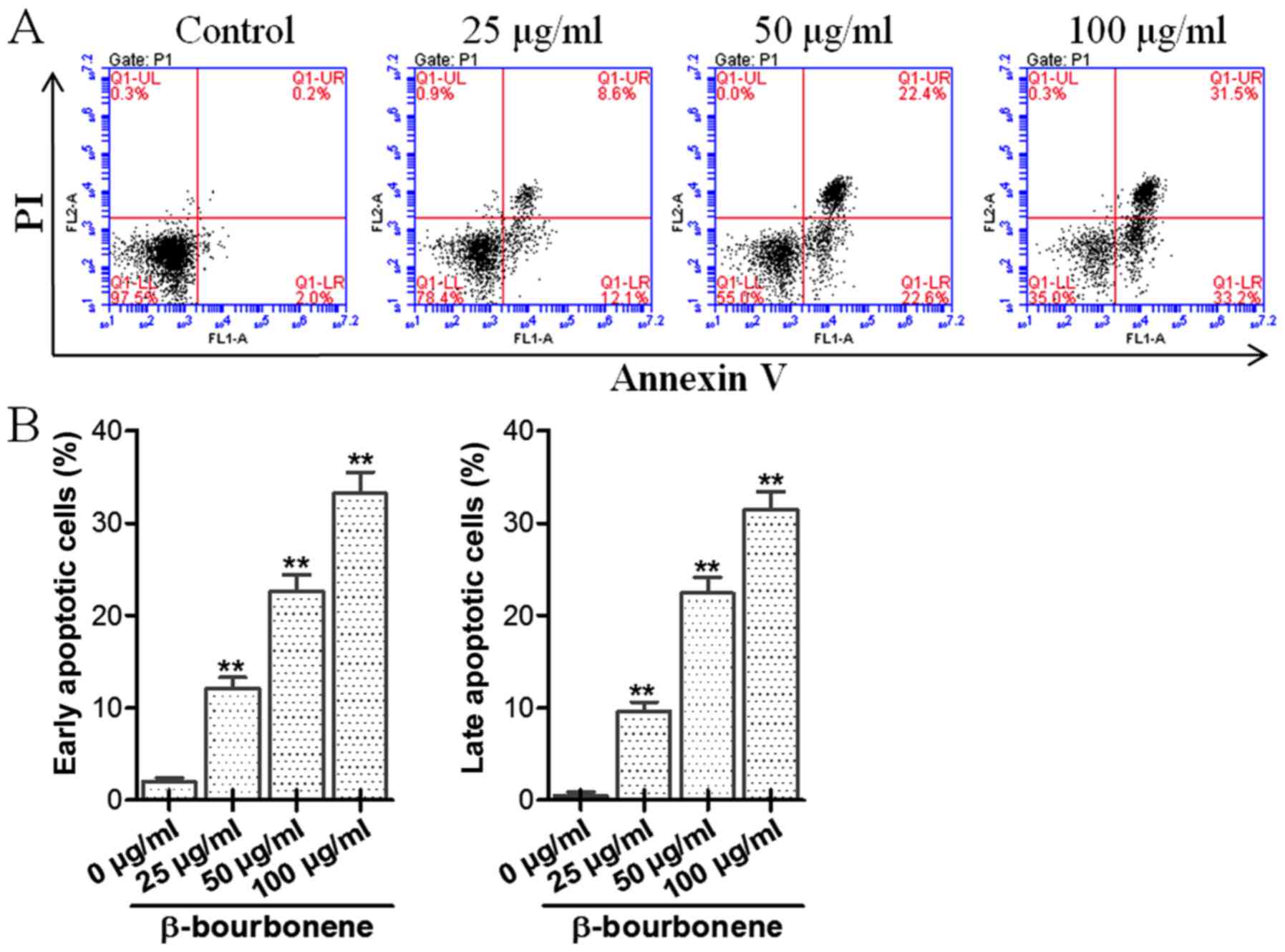
Annexin V/PI dual staining method was used to detect
the effect of β-bourbonene on apoptosis of PC-3M cells, and the
results are shown in Fig. 3. The four
quadrants of the dual parameter fluorescent dot plots represent
different states of the cells. The viable cell population was in
the lower left quadrant (Annexin V−/PI−). The
early apoptotic cells were in the lower right quadrant (Annexin
V+/PI−) and the ones in late apoptosis were
in the upper right quadrant (Annexin V+/PI+).
As shown in Fig. 3, compared with the
control group, significant increases in both early and late
apoptosis were seen in groups treated with β-bourbonene in
different concentrations, and the difference had statistical
significance (P<0.01), suggesting that β-bourbonene can
remarkably induce both early and late apoptosis in prostate cancer
PC-3M cells.
Detection of the effect of
β-bourbonene on cell apoptosis via TUNEL staining
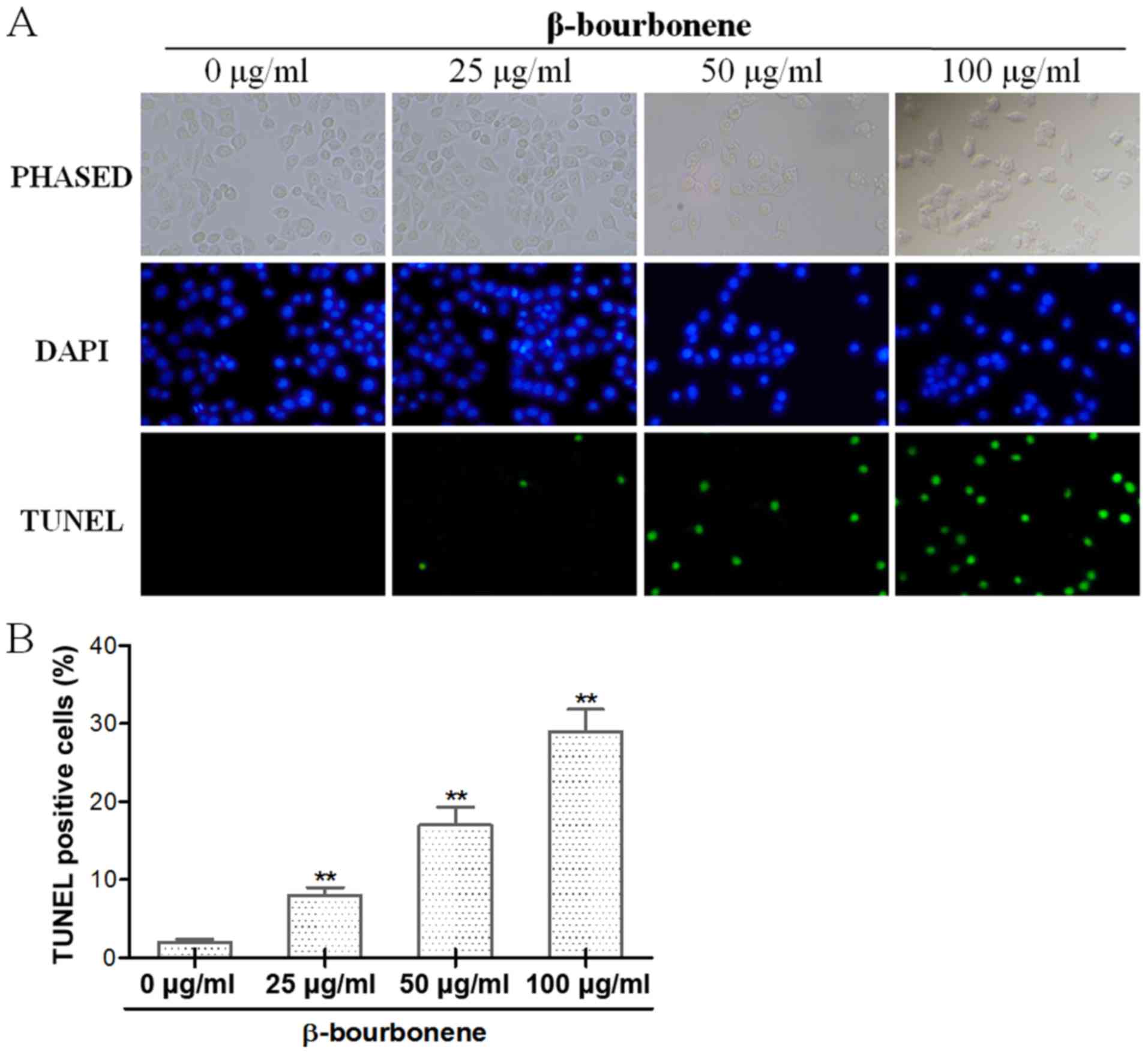
As shown in Fig. 4A,
the cells with green-stained nuclei were regarded as apoptotic
cells, and all cell nuclei were stained with DAPI to show the total
number of PC-3M cells. The results showed that in comparison with
the control group, the number of apoptotic cells was gradually
increased against an elevation in drug concentration in groups
treated with β-bourbonene in concentrations of 25, 50 and 100 µg/ml
for 48 h (Fig. 4A and B). Beyond
that, PC-3M cells showed shrinkage and detachment as drug
concentration increased, indicating the cytotoxic effect of
β-bourbonene.
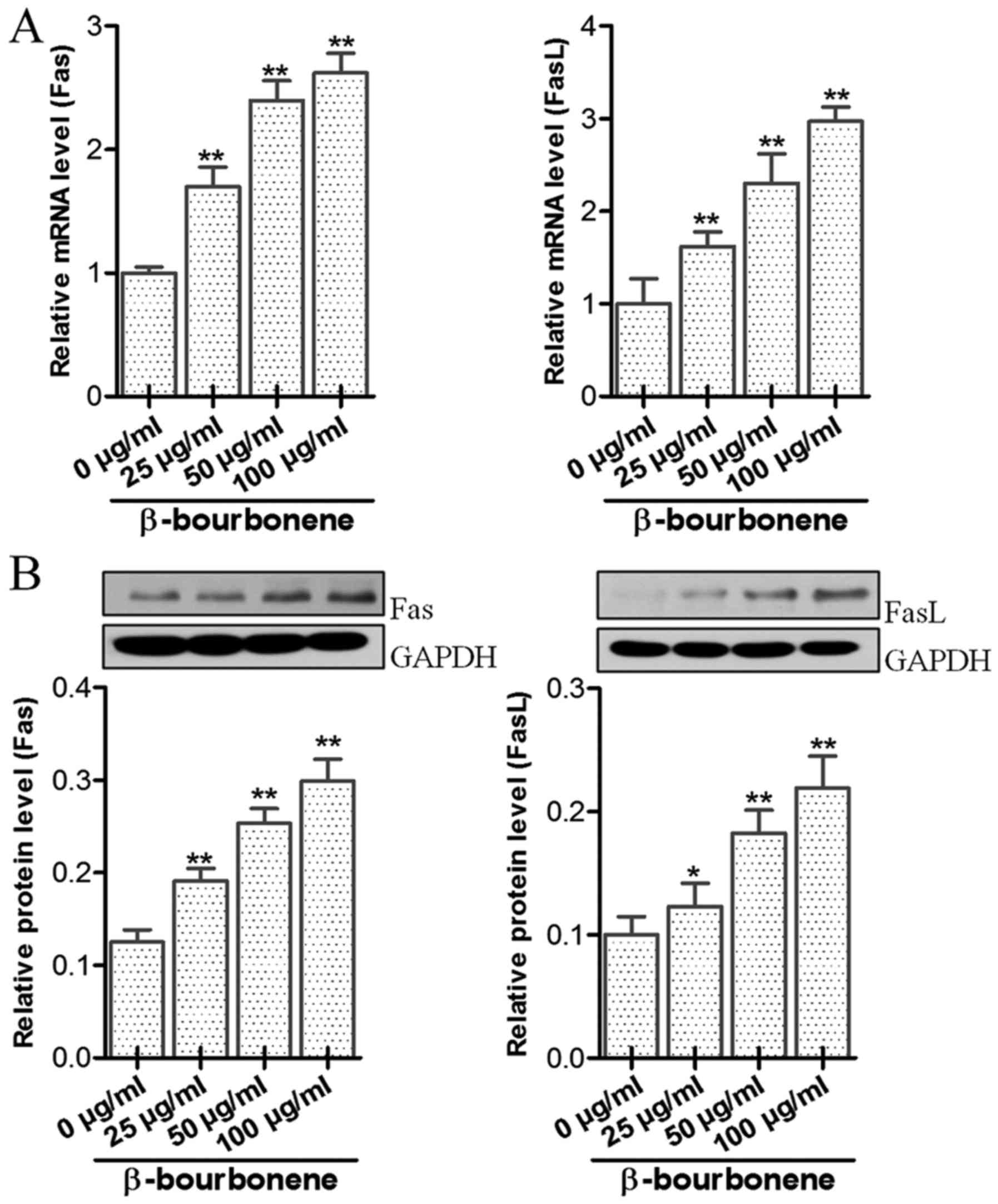
Effects of β-bourbonene on mRNA and
protein expression levels of Fas and FasL in cells
Results of mRNA and protein levels of Fas and FasL
are shown in Fig. 5. In groups that
were treated with β-bourbonene in concentrations of 25, 50 and 100
µg/ml for 48 h, mRNA expression levels of Fas and FasL were
signficantly higher than those in the control group (P<0.01).
Protein levels of Fas and FasL were also notably enhanced by
various concentrations of β-bourbonene compared to the control
group (P<0.05).
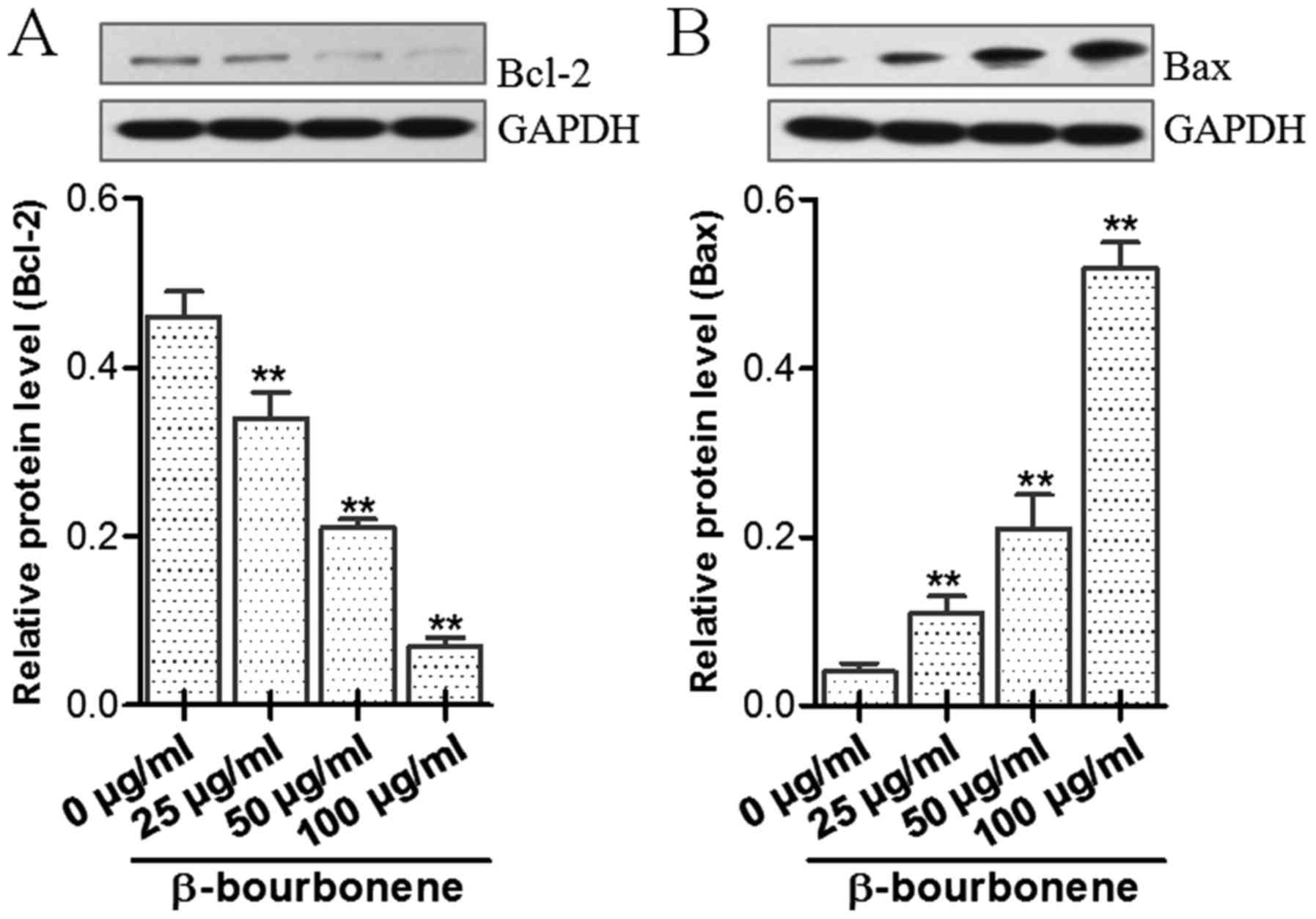
Effect of β-bourbonene on protein
expression levels of Bax and Bcl-2
As shown in Fig. 6,
after treatment with β-bourbonene in concentrations of 25, 50 and
100 µg/ml for 48 h, the protein expression levels of Bax and Bcl-2
in each group were significantly increased and decreased,
respectively, with an increase in concentration of β-bourbonene,
showing a significant dose-dependent manner.
Discussion
Currently, endocrine therapy remains the major
method for clinical treatment of prostate cancer, including
surgical resection and anti-androgen therapy, and its objective is
to reduce the level of androgen or the completion to the receptor
of androgen (10–12), thereby activating the biological
effect of androgen receptor. Despite that it is conducive to the
treatment of androgen-dependent prostate cancer, endocrine therapy
shows poor efficacy on the hormone-independent patients in advanced
prostate cancer (13,14). Moreover, our preliminary data (not
shown) indicated that β-bourbonene was not an antagonist of
androgen receptor. Thus, it is prioritized to develop a new
effective compound to treat hormone-independent prostate cancer.
For compound of β-bourbonene, it may be more meaningful to first
explore its effect and mechanism on androgen-independent cellular
model. In the present study, PC-3M, a highly metastatic cell line
was used as the objective. Both PC-3M and PC-3 cell lines are
androgen-independent (15), whereas
PC-3M shows higher metastasis than PC-3. Therefore, PC-3M is more
suitable for the study of advanced prostate cancer.
Bcl-2, as a gene with biological functions,
such as inhibitory effect on cell apoptosis (16), is pivotal in mechanism of cell
apoptosis. Bcl-2 can protect cells from death in diverse forms,
thereby improving the survival of cells and increasing the quantity
of cells. Thus, in some tumor cells, the upregulation of
Bcl-2 can help cells escape from death or prolong the life
span (17), indicating that
Bcl-2 is closely related to tumors. On the contrary,
Bax can promote cell apoptosis (18). Even though Bax and Bcl-2
belong to the same family, Bax can not only antagonize the
inhibitory effect of Bcl-2 on cell apoptosis, but also
directly act on cells to facilitate apoptosis. The homodimer of
Bcl-2 can inhibit cell apoptosis, while the dimers
constituted by highly expressed Bax proteins and Bcl-2 protein, or
Bax protein itself, will facilitate cell apoptosis (19). Kim et al (20) also found that the downregulation of
Bcl-2, as the target gene, can induce apoptosis of cells in
multi-onset myeloma. Fas, also known as factor associated suicide,
is a kind of cell death factor that can induce cell apoptosis, and
when it combines with FasL, the intracellular apoptosis-associated
signal pathway can be activated (21,22).
CCK-8 experiment revealed that compared with the
control group, β-bourbonene in different concentrations could
inhibit the proliferation of PC-3M cells, and with an increase in
concentration, the inhibition rate on proliferation was
significantly elevated. Result of G0/G1 arrest further verified the
anti-proliferation effect of β-bourbonene to PC-3M cells. Since
various proteins influence G0/G1 arrest, it is speculated according
to this result, that β-bourbonene can be regulated by multiple
molecules, such as the p21 (23). The
results of TUNEL staining showed that apoptosis emerged gradually
in cells in drug-treatment groups, and the increase in
concentration of drug led to an elevation in quantity of apoptotic
cells. According to the results of Annexin V/PI dual staining
experiment, we found that β-bourbonene could induce apoptosis in
PC-3M cells in a concentration-dependent manner. Therefore,
proliferation inhibition by β-bourbonene might be the combined
effect of cell cycle arrest and pro-apoptosis. In addition,
compared with the control group, the mRNA and protein expression
levels of Fas and FasL in the drug-treatment group were
significantly elevated, which further confirmed that β-bourbonene
can initiate the apoptosis pathway to induce apoptosis in PC-3M
cells through upregulation of Fas and FasL.
With the results of western blot assay, we found
that in comparison with the control group, the protein expression
level of Bax in the drug-treatment group was significantly elevated
with an increase in concentration of β-bourbonene, but the Bcl-2
expression was significantly decreased with the increase in
concentration of β-bourbonene. Zhang et al (24) reported that some antitumor drugs can
downregulate the expression of Bcl-2 to induce apoptosis in tumor
cells. Messaris et al (25)
also found that the abnormal downregulation of Bcl-2 and
upregulation of Bax in gastrointestinal epithelial tumors may be
the early variations in gastrointestinal tumors. The above results,
consistent with the results in this study, further proved that
β-bourbonene-induced apoptosis in PC-3M cells may be achieved
through upregulation of Bax protein and downregulation of Bcl-2
protein.
Moreover, adaptive upregulation of some
anti-apoptosis genes has been verified by substantial literature.
For example, the adaptive upregulation of Bcl-2 may exert a cell
protective effect against apoptosis due to androgen removal. In
this study, the inhibition of Bcl-2 by β-bourbonene indicated that
β-bourbonene might produce inhibitive effect to
androgen-independent prostate cancer.
In conclusion, the results of this study confirmed
that β-bourbonene can inhibit the proliferation of PC-3M cells in
prostate cancer, and induce cell apoptosis, which may be realized
through upregulating Fas, FasL, Bax, and downregulating Bcl-2.
However, the development of prostate cancer, especially
castration-resistant prostate cancer, is closely related to
androgen receptor. It is a limitation of this study to explore the
efficacy of β-bourbonene using a single cell line, and it is
necessary to detect the profile of β-bourbonene against androgen
receptor on androgen-receptor-overexpressing cells or
androgen-dependent cells. Therefore, attention should be paid more
on a comprehensive understanding for β-bourbonene efficacy in
vitro or in vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The original data used to support the findings of
this study are available from the corresponding author upon
request.
Authors' contributions
The concept and study design was performed by JZJ.
The conduct of experimental parts, supervision, resources,
materials, data collection and processing were performed by ZW, JJY
and FL. The analysis and interpretation of the data were performed
by JZJ and ZW. The literature search and writing the manuscript
were performed by ZW and JZJ. The critical review was performed by
JZJ. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
International Agency for Research on
Cancer, : GLOBOCAN 2008: Cancer Incidence, Mortality and Prevalence
Worldwide. https://www.iarc.fr/en/media-centre/iarcnews/2010/globocan2008.php
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gu F: Epidemiological survey of benign
prostatic hyperplasia and prostatic cancer in China. Chin Med J
(Engl). 113:299–302. 2000.PubMed/NCBI
|
|
4
|
Lichrenstein P, Holm NV, Verkasalo PK,
Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A and Hemminki
K: Environmental and heritable factors in the causation of cancer -
analyses of cohorts of twins from Sweden, Denmark, and Finland. N
Engl J Med. 343:78–85. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu F: Changing constituents of
genitourinary cancer in recent 50 years in Beijing. Chin Med J
(Engl). 116:1391–1393. 2003.PubMed/NCBI
|
|
6
|
Qi JL, Wang LJ, Zhou MG, Liu YN, Liu JM,
Liu SW, Zeng XY and Yin P: Disease burden of prostate cancer among
men in China, from 1990 to 2013. Zhonghua Liu Xing Bing Xue Za Zhi.
37:778–782. 2016.(In Chinese). PubMed/NCBI
|
|
7
|
Bosco A and Golsteyn RM: Emerging
anti-mitotic activities and other bioactivities of sesquiterpene
compounds upon human cells. Molecules. 22:E4592017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Z, Song Y, Che J, Liu X, Ning Y, Shan
C, Hou Y, Liu Y, Miao X and Cheng Y: Validation of a sensitive gas
chromatographic-mass spectrometric method for the simultaneous
determination of beta-elemene and beta-elemenal in human plasma. J
Chromatogr B Analyt Technol Biomed Life Sci. 877:408–414. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmittgen TD, Zakrajsek BA, Mills AG,
Gorn V, Singer MJ and Reed MW: Quantitative reverse
transcription-polymerase chain reaction to study mRNA decay:
Comparison of endpoint and real-time methods. Anal Biochem.
285:194–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Montgomery BT, Young CY, Bilhartz DL,
Andrews PE, Prescott JL, Thompson NF and Tindall DJ: Hormonal
regulation of prostate-specific antigen (PSA) glycoprotein in the
human prostatic adenocarcinoma cell line, LNCaP. Prostate.
21:63–73. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Young CYF, Montgomery BT, Andrews PE, Qui
SD, Bilhartz DL and Tindall DJ: Hormonal regulation of
prostate-specific antigen messenger RNA in human prostatic
adenocarcinoma cell line LNCaP. Cancer Res. 51:3748–3752.
1991.PubMed/NCBI
|
|
12
|
Chen CD, Welsbie DS, Tran C, Baek SH, Chen
R, Vessella R, Rosenfeld MG and Sawyers CL: Molecular determinants
of resistance to antiandrogen therapy. Nat Med. 10:33–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Taplin ME and Balk SP: Androgen receptor:
A key molecule in the progression of prostate cancer to hormone
independence. J Cell Biochem. 91:483–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weiss-Messer E, Merom O, Adi A, Karry R,
Bidosee M, Ber R, Kaploun A, Stein A and Barkey RJ: Growth hormone
(GH) receptors in prostate cancer: Gene expression in human tissues
and cell lines and characterization, GH signaling and androgen
receptor regulation in LNCaP cells. Mol Cell Endocrinol.
220:109–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lv T, Tian BQ, Zheng XM and Li SW: Changes
of androgen receptor expression in hormonal dependent prostatic
cancer and hormonal independent prostatic cancer. Wuhan Daxue
Xuebao Yixue Ban. 28:181–184. 2007.(In Chinese).
|
|
16
|
Cory S, Huang DCS and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Packham G and Cleveland JL: c-Myc and
apoptosis. Biochim Biophys Acta. 1242:11–28. 1995.PubMed/NCBI
|
|
18
|
Brady HJ and Gil-Gómez G: Bax. The
pro-apoptotic Bcl-2 family member, Bax. Int J Biochem Cell Biol.
30:647–650. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu QL, Abel P, Foster CS and Lalani EN:
bcl-2: Role in epithelial differentiation and oncogenesis. Hum
Pathol. 27:102–110. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SH, Ahn KS, Jeong SJ, Kwon TR, Jung
JH, Yun SM, Han I, Lee SG, Kim DK, Kang M, et al: Janus activated
kinase 2/signal transducer and activator of transcription 3 pathway
mediates icariside II-induced apoptosis in U266 multiple myeloma
cells. Eur J Pharmacol. 654:10–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Myong NH: Tissue microarray analysis of
Fas and FasL expressions in human non-small cell lung carcinomas;
with reference to the p53 and bcl-2 overexpressions. J Korean Med
Sci. 20:770–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang JS, Hsu YL, Kuo PL, Chiang LC and
Lin CC: Upregulation of Fas/Fas ligand-mediated apoptosis by
gossypol in an immortalized human alveolar lung cancer cell line.
Clin Exp Pharmacol Physiol. 31:716–722. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xi S, Dyer KF, Kimak M, Zhang Q, Gooding
WE, Chaillet JR, Chai RL, Ferrell RE, Zamboni B, Hunt J, et al:
Decreased STAT1 expression by promoter methylation in squamous cell
carcinogenesis. J Natl Cancer Inst. 98:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang B, Lai Y, Li Y, Shu N, Wang Z, Wang
Y, Li Y and Chen Z: Antineoplastic activity of isoliquiritigenin, a
chalcone compound, in androgen-independent human prostate cancer
cells linked to G2/M cell cycle arrest and cell apoptosis. Eur J
Pharmacol. 821:57–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Messaris E, Kekis P, Memos N, Chatzigianni
E, Menenakos E, Leandros E and Konstadoulakis MM: Sepsis:
Prognostic role of apoptosis regulators in gastrointestinal cells.
World J Surg. 31:787–794. 2007. View Article : Google Scholar : PubMed/NCBI
|