Introduction
On account of the deteriorating living environment
and unfavorable living habits, cancer becomes the leading cause of
human mortality around the world (1).
Among all cancers, colorectal carcinoma (CRC) is the most frequent
malignant disease of the gastrointestinal tract and responsible for
600,000 deaths annually worldwide (2). It develops as the third most common
cancer in men (746,000 cases, 10% of all cancers) and the second
most common cancer in women (614,000 cases, 9.2% of all cancers)
(2). More than 50% of all CRC cases
occur in more developed regions, e.g., 345,000 new cases and
152,000 deaths were reported in the European Union (3). In some regions with previously low
incidence rates, e.g., Eastern Europe and East Asia, significantly
increasing numbers of CRC cases have been noted and attributed to
changes in risk factors and diet towards a lifestyle common to
Western countries (4). The individual
risk of CRC is essentially dependent on non-modifiable
dispositional factors such as age, sex, and family history as well
as in principle modifiable exposure to risk factors (3). Up to one-third of the CRC risk may be
attributable to hereditary factors, and another 30~50% of the CRC
risk is attributable to lifestyle factors such as smoking, high
consumption of red and processed meat, obesity, diabetes, and
excessive consumption of alcohol (5).
Sadly enough, the prognosis of CRC patients is
especially poor: Roughly two-third of CRC has been detected in an
inoperable, advanced stage, when the first clinical signs occur
(6). The current mainstay of
treatment for CRC is surgical resection with chemotherapy and
radiotherapy (7). Unfortunately, to
date, the therapies are far from optimal due to their limited
efficacy as well as toxic effects (8). For example, chemotherapeutic regimens
are always involved in delivering the drug to both tumor and normal
tissue, resulting in unexpected toxic effects such as neutropenia,
anemia, hand-foot syndrome, diarrhea, gastrointestinal toxicity,
mucositis, nausea, vomiting, fatigue, hematological disorders and
liver toxicity (9). The adverse
events not only worsen the patients' quality of life, but rather,
cause patients to refuse further chemotherapy (10). Therefore, the need to develop more
effective and safe agents for CRC treatment is urgent. In recent
years, a growing interest has arisen for the therapeutical
potential of natural resources to discover new anticancer agent,
which is promising to provide a favorable option for CRC patients
(11–14).
Traditional Chinese medicine (TCM) becomes a popular
complementary and alternative medicine for cancer treatment in
clinic. By using herbal medicines, TCM shows more therapeutic
benefits but lesser side effect and cost than the conventional
chemotherapeutics (15). As one of
the most famous TCM, Chinese ginseng (the dried root of Panax
ginseng C.A. Mey.) has been used for over 2,000 years as a
medicine in China and is popularly used in more than 35 countries
as food, health supplement, and natural remedy (16). It is claimed to be effective in
treating cancer, including CRC (17).
Many components are responsible for the anti-CRC effect of Chinese
ginseng, including protopanaxadiol, Rg1, Rb1, Rg3, Rh2, and
compound K. Protopanaxadiol is an active ginseng metabolite that
can enhance the anticancer effect of chemotherapy on CRC (18). Ginsenoside compounds, Rg1, Rb1, Rg3,
and Rh2, have been found to possess anti-CRC effect by inducing
cell apoptosis and cell cycle arrest and inhibiting metastasis
(19–22). Besides, ginsenoside compound K
possesses not only anti-proliferative and pro-apoptotic effect but
also synergistic activity with cancer cell-specific
apoptosis-inducing cytokine on CRC (23). Therefore, ginsenoside compounds are a
kind of ginseng component possessing anticancer effect on CRC. This
study focused on total ginsenosides (combination of ginsenoside
compounds) of Chinese ginseng and evaluated its effect on CRC cells
from the cellular and molecular levels.
Materials and methods
Chemicals and reagents
Powders of total ginsenosides of Chinese ginseng
(TGCG) (S25997; >80% of purity), ginsenoside Rb1 (Rb1) (B21050;
>98% of purity), ginsenoside Re (Re; B21055; >98% of purity),
ginsenoside Rd (Rd; B21054; >98% of purity), and ginsenoside Rg1
(Rg1) (B21057; >98% of purity) were obtained from Shanghai
Yuanye Biotechnology Co., Ltd (Shanghai, China; batch no.
2016Y08016). RPMI-1640 medium, fetal bovine serum (FBS), and 0.25%
trypsin were obtained from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany). Annexin V:FITC apoptosis detection
kit and cell cycle kit were purchased from BD Biosciences,
(Franklin Lakes, NJ, USA). ProLong® Diamond Antifade
Mountant with DAPI was purchased from Invitrogen; Thermo Fisher
Scientific, Inc. All antibodies were purchased from Cell Signaling
Technology Inc. (CST; Danvers, MA, USA). RNAiso Plus kit for real
time PCR was purchased from Takara (Dalian, China).
Chemoprofile analyses
The HPLC analysis was performed on an Agilent 1260
Infinity system (Agilent Technologies, Inc., Santa Clara, CA, USA).
Chromatographic separation was achieved on a Hypersil BDS-C18
column (250×4.6 mm, 5 µm; Shandon Scientific, Cheshire, UK) at
temperature of 30°C. The mobile phase consisted of (A) acetonitrile
and (B) 0.1% phosphoric acid solution with flow rate of 1.3 ml/min.
Samples were eluted with a gradient elution system at 0~30 min (19%
A), 30~35 min (19~24% A), and 35~60 min (24~60% A). The sample
injection volume was 10 µl and the detection wavelength was 205 nm.
The data was analyzed to determine the contents of Rb1, Re, Rd, and
Rg1 in TGCG.
Cell line and culture
Human CRC cell lines (HT-29, HCT-116, and SW620)
were obtained from Shanghai Cell Bank of Chinese Academy of
Sciences (Shanghai, China) and cultured in RPMI-1640 medium
containing 10% FBS at 37°C in a humidified 5% CO2
incubator. The medium was changed daily and the cells were treated
in their logarithmic growth phase.
MTT assay
MTT assay was performed to evaluate the cell
viability of HT-29, HCT-116, and SW620 cells. Cells were seeded on
96-well plates with density of 5×103 cells/well in 200
µl medium for 24 h and then treated with TGCG at different
concentrations (0, 25, 50, 100, 200, 400 µg/ml) for 24 h (all cell
lines), 48 h (HT-29), and 72 h (HT-29). Each 20 µl MTT solution
(5.0 mg/ml) was added to each well and incubated at 37°C for 4 h.
Then 150 µl DMSO was added in each well to dissolve the MTT
formazan crystals and the optical density (OD) value was measured
at 490 nm with a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Inhibitory rate (%)=[1-(TB-treated OD/untreated
OD)] ×100%. The 50% inhibitory concentrations (IC50) for
24, 48, and 72 h were calculated by regression analysis,
respectively.
Cell morphology and DAPI staining
HT-29 cells were seeded on 6-well plates with
density of 3×105 cells/well for 24 h, followed by TGCG
treatment at low, medium, and high doses for 24 h. The cells were
harvested and washed with PBS thrice and then fixed with 4%
paraformaldehyde in PBS for 30 min at room temperature. Then the
cells were permeabilized with 0.5% Triton X-100 in PBS for 10 min.
An aliquot of the cells were mounted using ProLong®
Diamond Antifade Mountant (Thermo Fisher Scientific, Inc.) with
DAPI for 3 min in dark and then washed thrice. The unstained and
stained cells were observed under a fluorescence microscope (Carl
Zeiss, Göttingen, Germany). Five coverslips were used as replicates
of each group and the apoptotic nuclei of cells were
visualized.
Flow cytometry
TGCG-induced apoptosis of HT-29 cells was determined
by flow cytometry using an Annexin V/PI method, according to the
manufacturer's protocol. Briefly, HT-29 cells were seeded on 6-well
plates with density of 3×105 cells/well for 24 h and
then were treated with TGCG at low, medium, and high doses for
another 24 h. Thereafter, the cells were harvested and washed twice
with cold PBS, and then labeled with FITC Annexin V and PI in
binding buffer. Fluorescence intensity of the cells was detected by
BD FACSVerse™ flow cytometer (BD Biosciences). The apoptosis rate
(%) for each treatment was calculated.
Cell cycle analysis was also performed by flow
cytometry using BD cell cycle kit (BD Biosciences). HT-29 cells
were seeded on 6-well plates with density of 3×105
cells/well for 24 h and then were treated with TGCG at low, medium,
and high doses for another 24 h. The cells were harvested and
washed with PBS thrice and then were stained with PI/RNase staining
solution in accordance with the manufacturer's protocol of BD cell
cycle kit (BD Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Relative mRNA expressions of targeted genes in HT-29
cells were detected by qPCR assay on an ABI QuantStudio™ 7 Flex
Real-Time PCR System (Applied Biosystems; Thermo Fischer
Scientific, Inc.). The total RNA of the cells in each group was
extracted using TRIzol reagent and synthesized to cDNA via RT. PCR
reaction system had a 20.0 µl volume: 10.0 µl SYBR®
Premix Ex Taq II (Tli RnaseH Plus), 0.8 µl PCR Forward Primer, 0.8
µl PCR Reverse Primer, 2.0 µl template cDNA, 0.4 µl ROX Reference
Dye, and 6.0 µl ddH2O. The qPCR reaction condition was
set to 95°C for 30 sec initial denaturation, 40 cycles of 95°C for
5 sec denaturation, 60°C for 34 sec annealing, and 72°C for 40 sec
extension. At the end of each reaction, a melting curve analysis
was performed. β-actin was used as the reference gene and
2−Δ∆CT method was applied to analyze the relative mRNA
expressions (Table I).
 | Table I.Primer sequences used for
quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences used for
quantitative polymerase chain reaction analysis.
| Gene | Forward primer | Reverse primer |
|---|
| β-actin |
5′-CATGTACGTTGCTATCCAGGC-3′ |
5′-CTCCTTAATGTCACGCACGAT-3′ |
| CASP3 |
5′-AGAACTGGACTGTGGCATTGAG-3′ |
5′-GCTTGTCGGCATACTGTTTCAG-3′ |
| CASP8 |
5′-CTCCCCAAACTTGCTTTATG-3′ |
5′-AAGACCCCAGAGCATTGTTA-3′ |
| CCND1 |
5′-CAATGACCCCGCACGATTTC-3′ |
5′-CATGGAGGGCGGATTGGAA-3′ |
| CDKN2A |
5′-CATGGTGCGCAGGTTCTTG-3′ |
5′-CGGGATGTGAACCACGAAA-3′ |
| CDKN2B |
5′-AACACAGAGAAGCGGATTTC-3′ |
5′-AGGTCCAGTCAAGGATTTCA-3′ |
| CDK2 |
5′-GCTAGCAGACTTTGGACTAGCCAG-3′ |
5′-AGCTCGGTACCACAGGGTCA-3′ |
| CDK4 | 5′-
AAATCTTTGACCTGATTGGG-3′ | 5′-
CCTTATGTAGATAAGAGTGCTG-3′ |
| CDK6 |
5′-CTGAATGCTCTTGCTCCTTT-3′ |
5′-AAAGTTTTGGTGGTCCTTGA-3′ |
| MDM2 |
5′-ACCTCACAGATTCCAGCTTCG-3′ |
5′-TTTCATAGTATAAGTGTCTTTTT-3′ |
| MYC |
5′-GCCACGTCTCCACACATCAG-3′ |
5′-TGGTGCATTTTCGGTTGTTG-3′ |
| TOP1 |
5′-TCCGGAACCAGTATCGAGAAGA-3′ |
5′-CCTCCTTTTCATTGCCTGCTC-3′ |
| TP53 |
5′-TCAACAAGATGTTTTGCCAACTG-3′ |
5′-ATGTGCTGTGACTGCTTGTAGATG-3′ |
Western blot analysis
The total proteins of the TH-29 cells
(1.5×106) were extracted using a lysis buffer (50 mM
Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton, 0.1% SDS) with
proteinase inhibitor cocktail (Bimake, Houston, TX, USA) for 30 min
on ice. The proteins were separated by a denaturing sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 6–12%) and
then transferred onto a nitrocellulose membrane (Sartorius Stedim,
Goettingen, Germany). The membrane was blocked with 5%non-fat milk
for 2 h, followed by overnight incubation at 4°C with the primary
antibodies [Bax, cyclin-dependent kinase (Cdk2), Cdk4, c-Myc,
Cyclin D1, p15INK4b, p21WAF1,
p27Kip1, p53]. Following inculation with
peroxidase-conjugated goat anti-rabbit/mouse IgG at room
temperature for 2 h, proteins were visualized using Western
Lightning® Plus ECL (Perkin Elmer, Waltham, MA, USA) and
detected using X-film (Kodak, Tokyo, Japan) and scanned.
Statistical analysis
Data were expressed as mean ± SD and subjected to
one-way analysis of variance, followed by Fisher's least
significant difference comparison. All analyses were performed
using an updated version of DPS software (24). P<0.05 was considered to indicate a
statistically significant difference.
Results
The anti-proliferative effect of TGCG on CRC cells
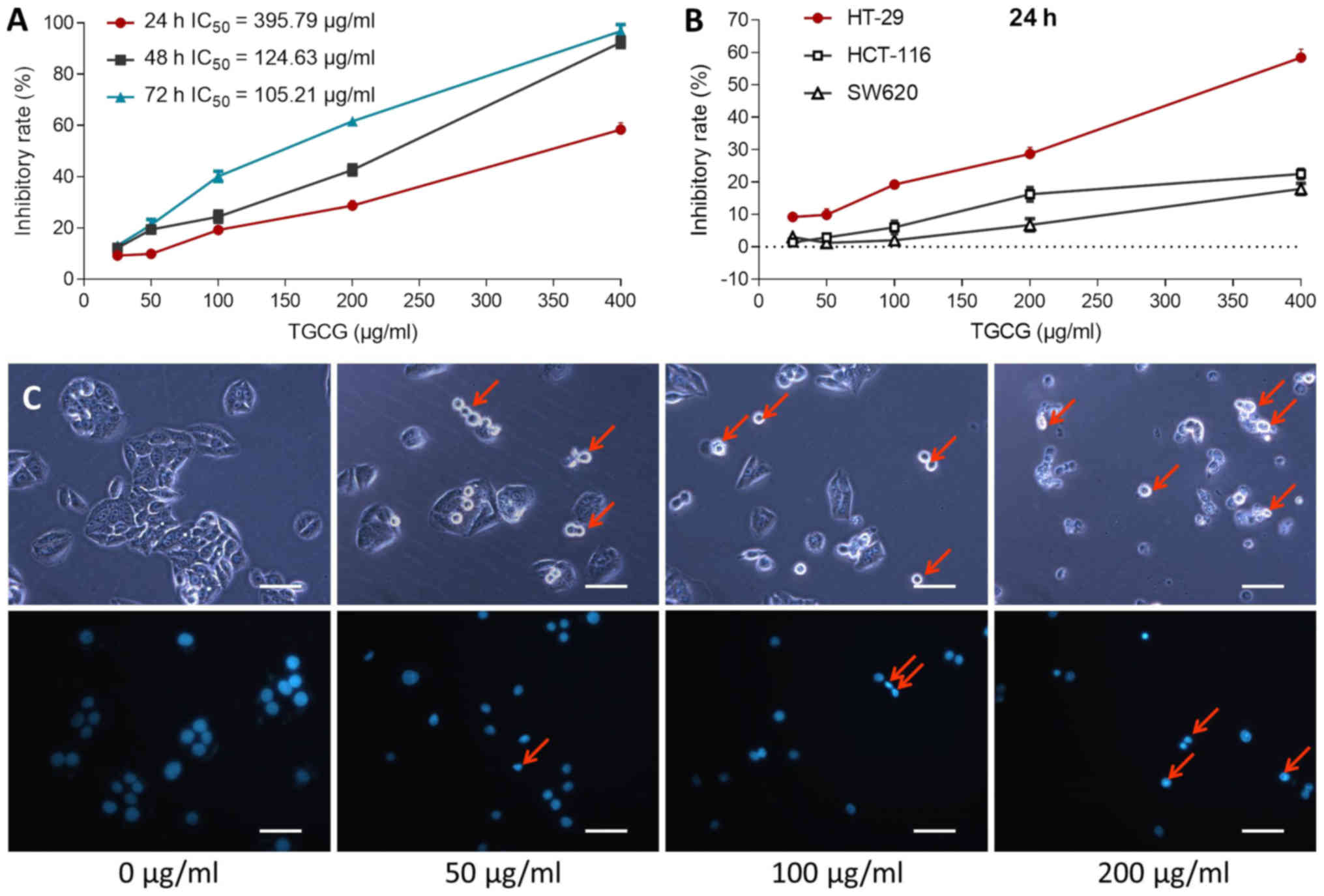
were determined by MTT assay. As shown in Fig. 1A, TGCG obviously inhibited the cell
viability of HT-29 at a dose range of 25 to 400 µg/ml and a time
range of 24 to 72 h. The inhibitory effect was increased with
increasing TGCG doses at each time point and also with increasing
time period at almost each dose point, indicating a time- and
dose-dependent manner of TGCG. The IC50 values were
395.79, 124.63, and 105.21 µg/ml for 24, 48 and 72 h treatment,
respectively. We employed 50, 100, and 200 µg/ml as low, medium,
and high doses of TGCG at 24 h for the following assays. As
compared with the inhibitory effects on HCT-116 and SW620, TGCG
showed a stronger effect on HT-29 (Fig.
1B).
The inhibitory effect of TGCG on TH-29 cells at
morphological level was observed with DAPI staining by fluorescence
microscopy. As shown in Fig. 1C,
detached cells in round and shrunken shape were seen with TGCG
treatment under light microscope (indicated by arrows). DAPI
staining showed typical apoptotic signs on TGCG-treated cells, such
as chromatin condensation, karyopyknosis and nuclear fragmentation
(indicated by arrows), indicating cell apoptosis induced by
TGCG.
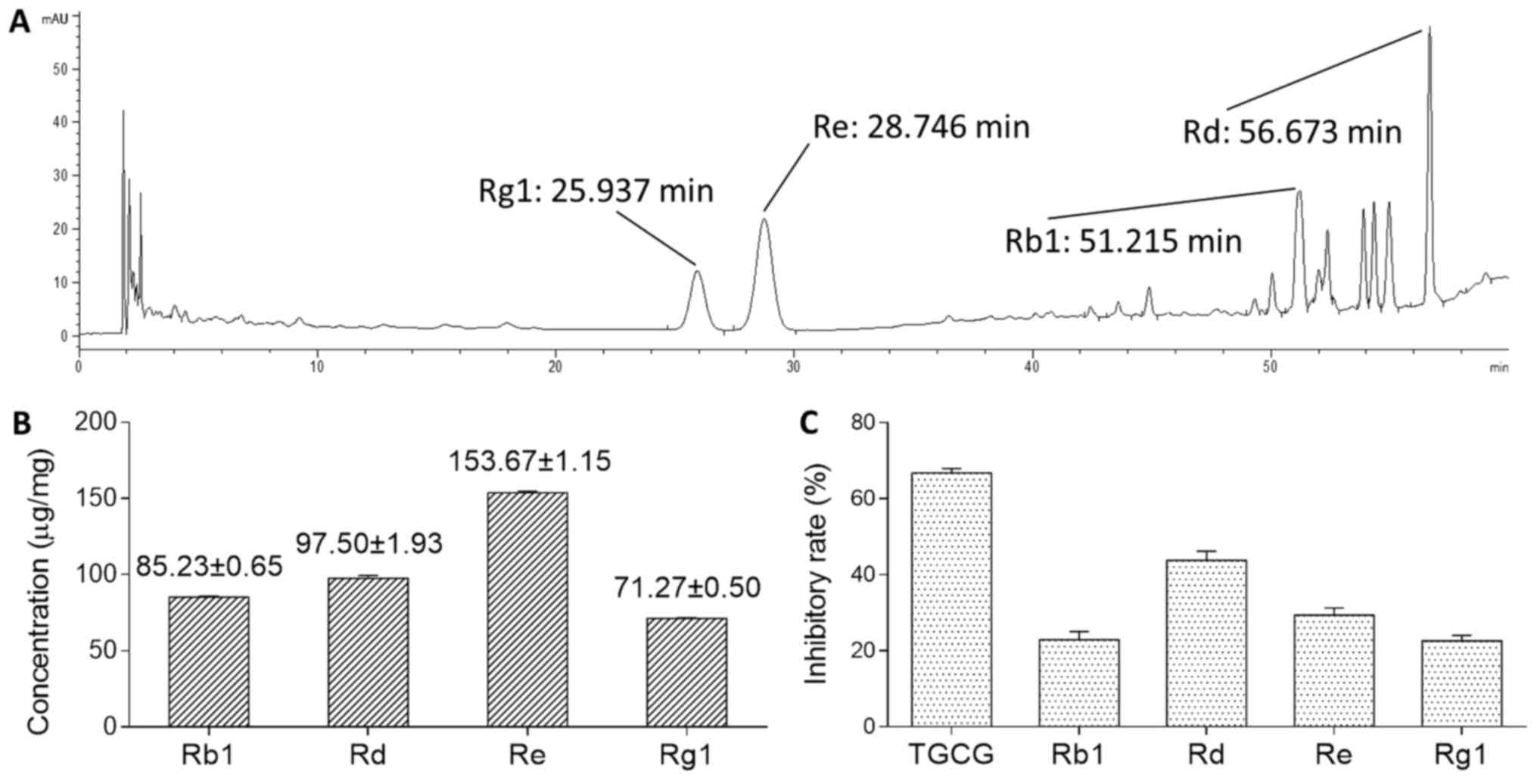
HPLC analysis showed the chemoprofile of TGCG
comprising Rb1, Rd, Re, and Rg1 (Fig.
2A). The contents of Rb1, Rd, Re, and Rg1 in TGCG are
85.23±0.65, 97.50±1.93, 153.67±1.15, and 71.27±0.50 µg/mg,
respectively (Fig. 2B). MTT assay
indicated that TGCG at 400 µg/ml exerted stronger inhibitory effect
than that of its ginsenoside component on HT-29 cells after 24 h
treatment (Fig. 2C).
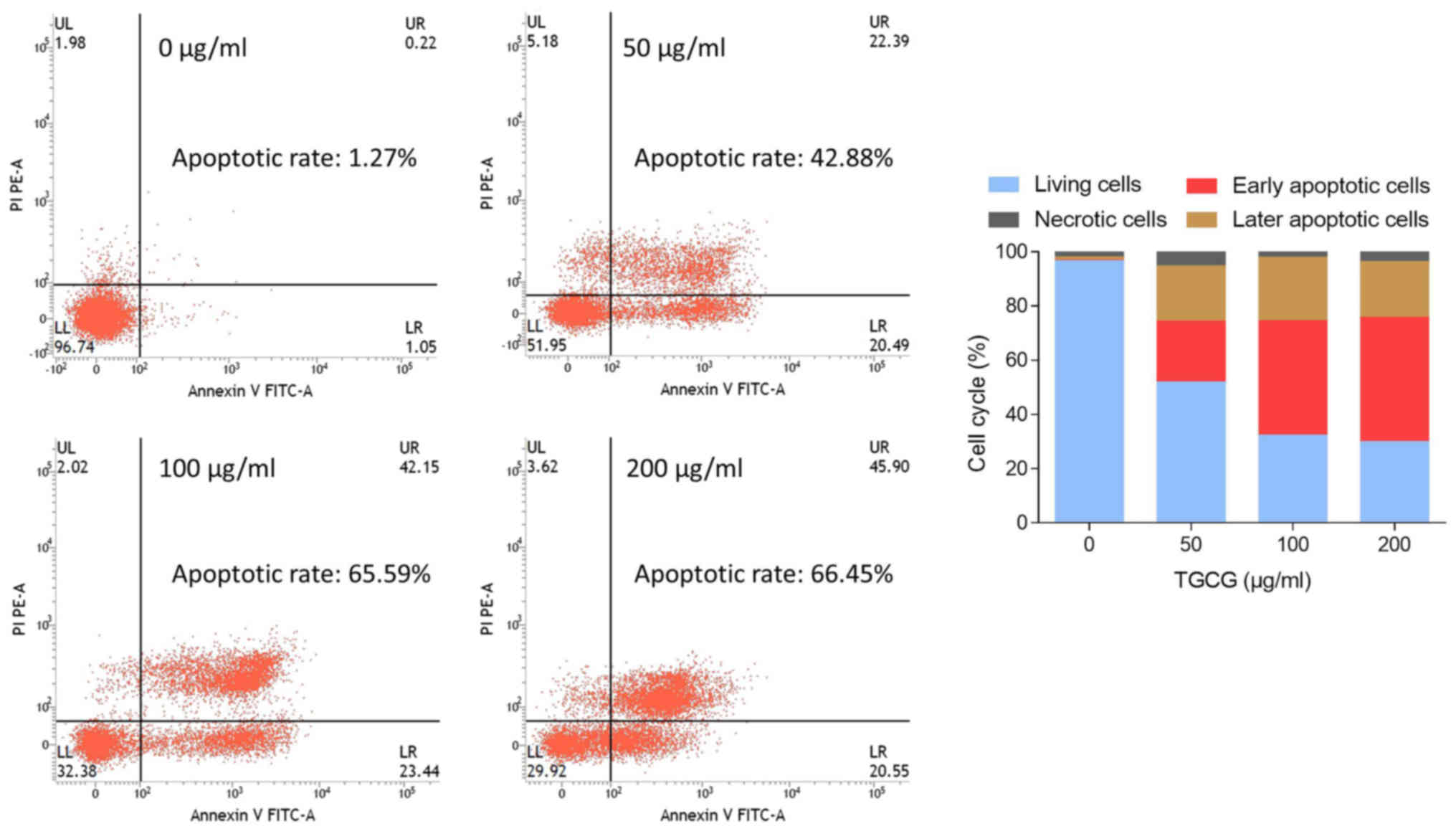
The apoptosis-inducing effect of TGCG on HT-29 cells
was further studied by Annexin V-FITC/PI double staining assay
using flow cytometry. As shown in Fig.
3, early and late apoptosis increased progressively from 1.27
to 66.45% with treatment of increasing doses of TGCG from 0 to 200
µg/ml, indicating a dose-dependent manner. Particularly, TGCG upon
100 µg/ml primarily induced early apoptosis (42.15%) in HT-29
cells.
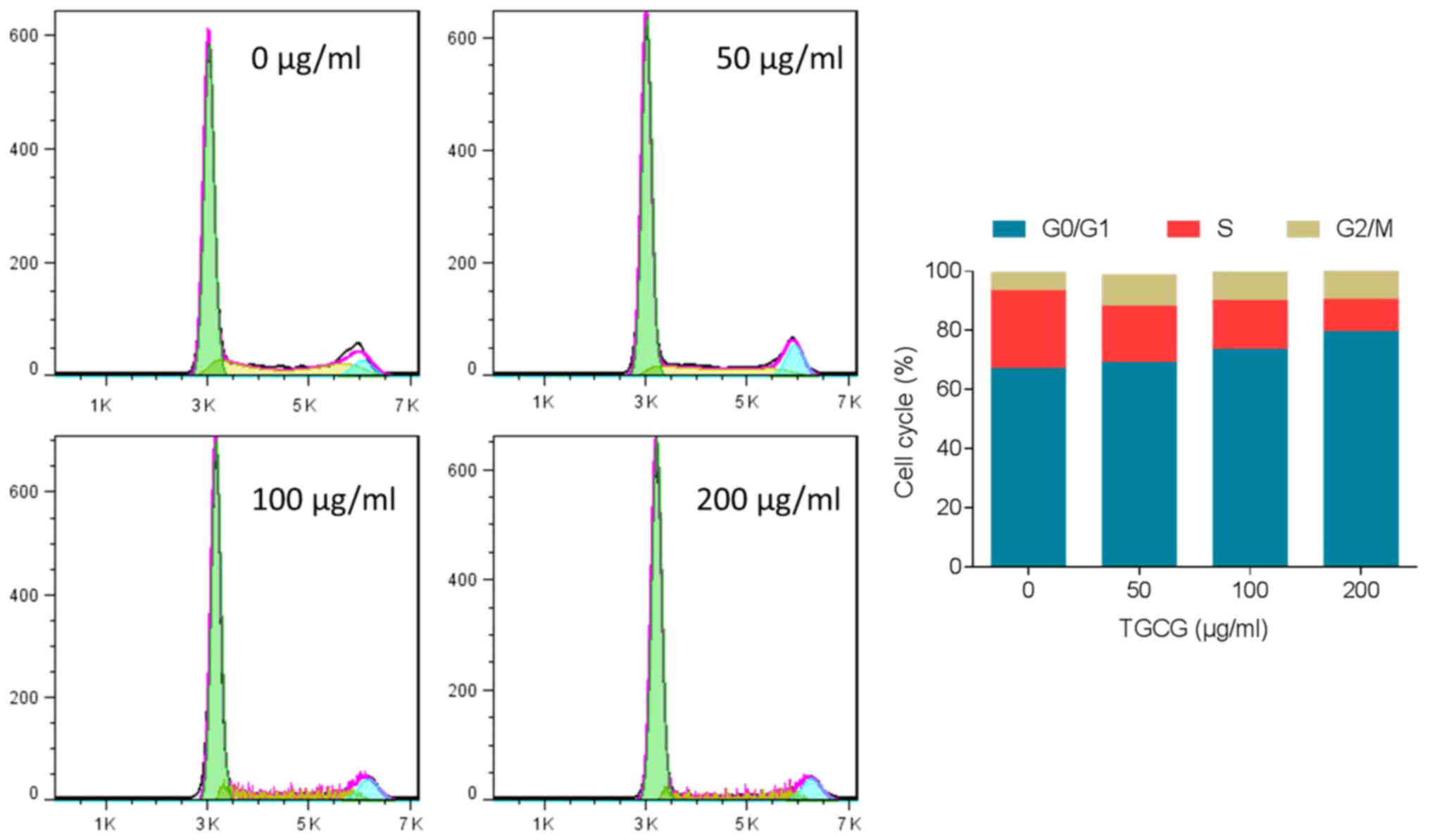
The effect of TGCG on HT-29 cell cycle progression
was performed by flow cytometry. As shown in Fig. 4, after 24 h treatment on HT-29 cells,
TGCG induced obvious G0/G1 phase accumulation
and S phase depletion in a dose-dependent manner. A visible
increase of cell number in G2/M phase was also found
with TGCG treatment. These trends indicated that TGCG could induce
cell cycle arrest in HT-29 cells.
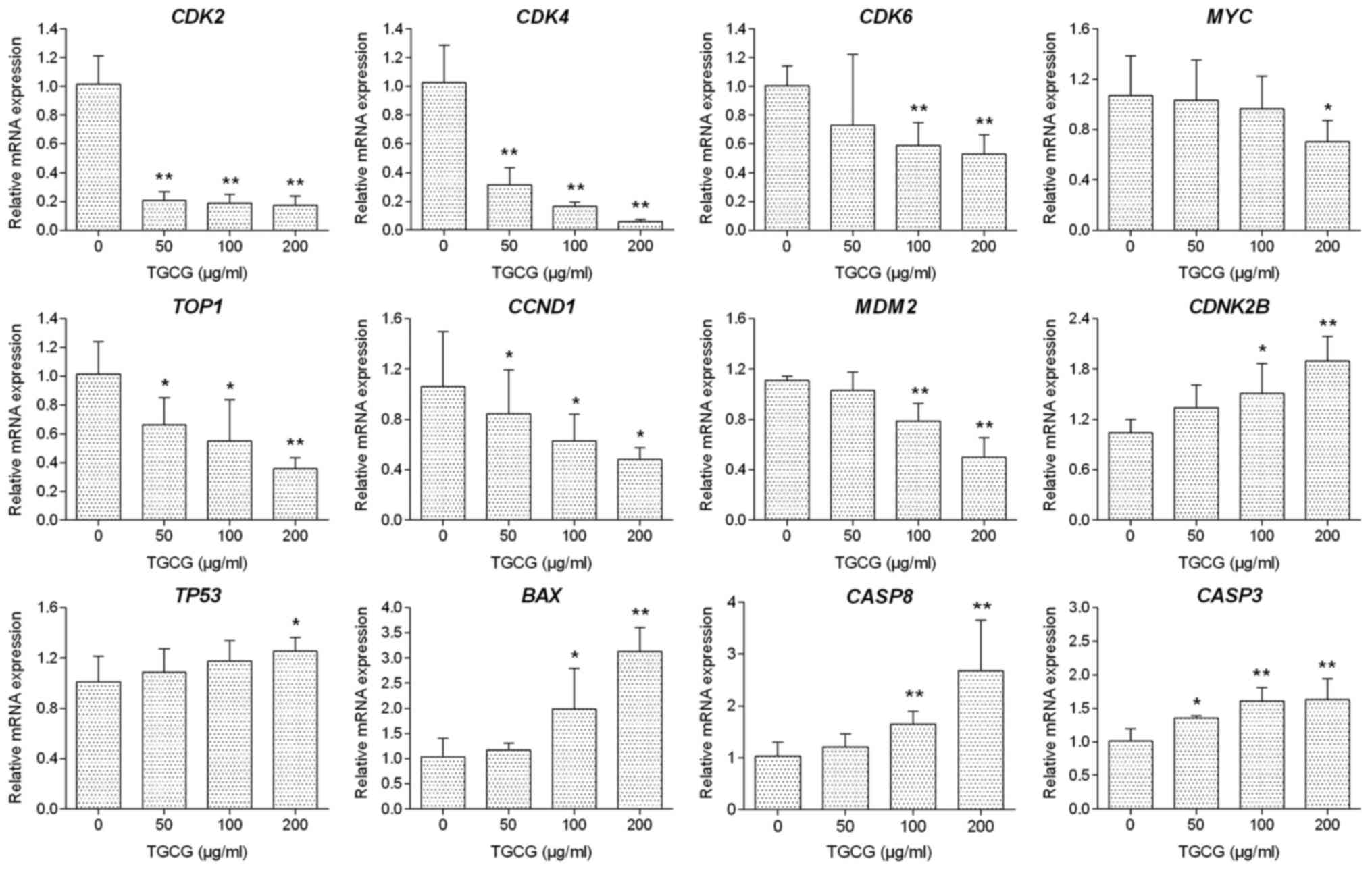
The regulatory effect of TGCG on the relative
expression of target genes in HT-29 cells was determined by qPCR
assay. As shown in Fig. 5, with 24 h
treatment, TGCG could significantly downregulate the
expression of CDK2, CDK4, CDK6, TOP1, MYC, MDM2, and
CCND1 mRNA transcripts and upregulate the expression of
BAX, CDNK2B, CASP8, CASP3, and TP53 mRNA transcripts
in HT-29 cells as compared to the untreated group. In most cases,
TGCG induced a dose-dependent manner in the mRNA modulation.
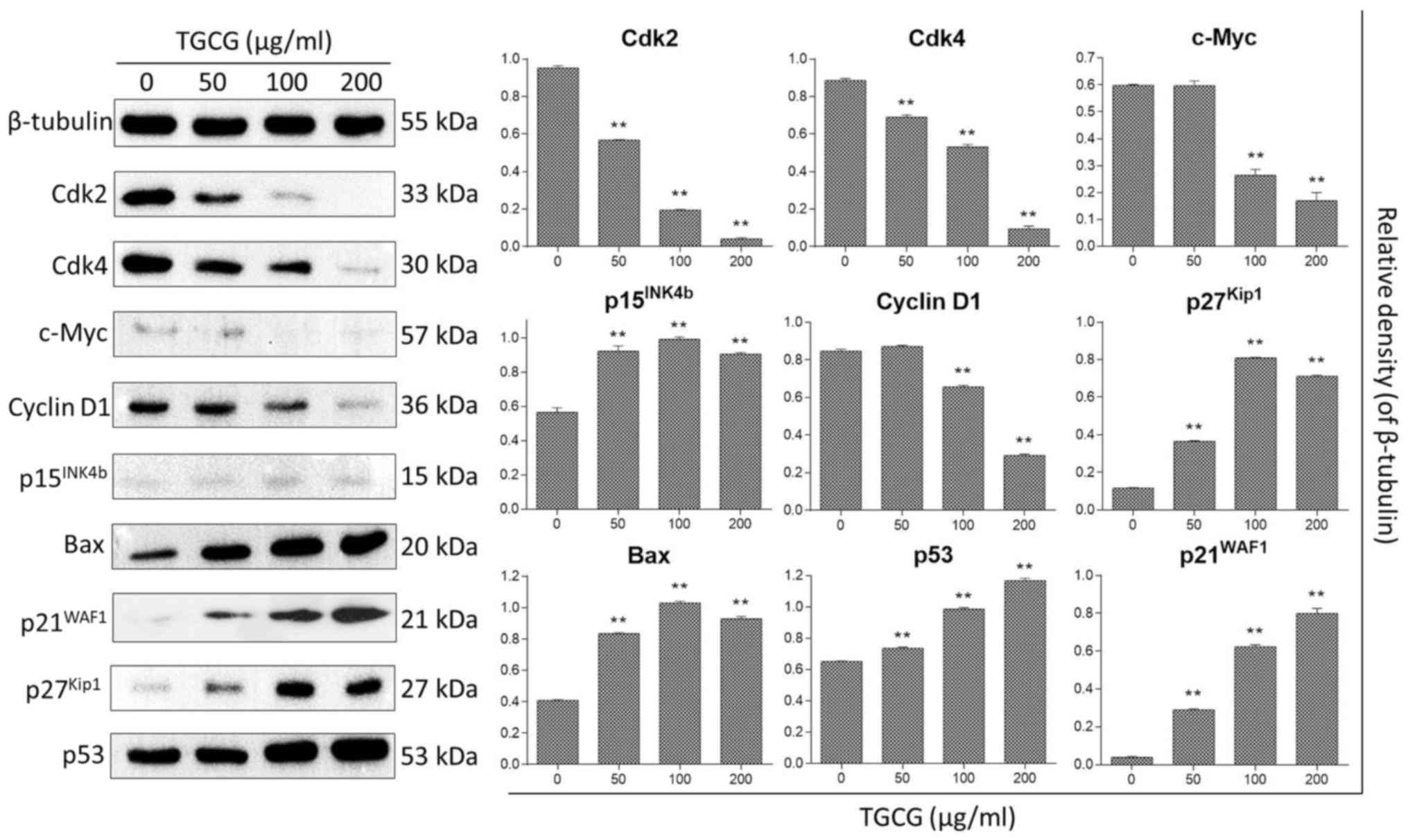
The regulatory effect of TGCG on protein expression
of HT-29 cells was determined by western blot assay. Proteins
related to cell apoptosis and cell cycle were analyzed by different
experiments under the same condition and the control (β-tubulin)
was found similarly expressed in each experiment. The expression
profiles of tested proteins were assembled together in Fig. 6 with one representative control. With
24 h treatment, TGCG could significantly downregulate the
expression of cell cycle checkpoint proteins (Cdk2, Cdk4, and
Cyclin D1) and upregulate the expression of cell apoptosis- and
cell cycle related proteins (Bax, p21WAF1,
p27Kip1, c-Myc, p15INK4b, and p53) as
compared to the untreated group.
Discussion
An epidemiological survey on 1,000 subjects revealed
reduced risks of various cancers, including CRC, in those who
received ginseng, when compared with those who did not use it
(25). Many studies have been
conducted on the anti-CRC effects of ginseng from its crude
extracts to single components. For instance, both fermented ginseng
extract and ginseng polysaccharide fraction showed
anti-proliferative and anti-invasive effects on HT-29 cells
(26). A steamed extract of ginseng
root induced mitochondrial damage and cell apoptosis by producing
reactive oxygen species (ROS) in CRC cells (27). As a main component of ginseng,
ginsenoside has been shown to exert strong anticancer activity by
blocking cell cycle progression at G1 phase or
G1/S boundary in breast and liver cancer cells through
activation of p21WAF1, p27Kip1, and p53
(28–30). It can also induce cancer cell
apoptosis by altering mitochondrial membrane integrity, releasing
cytochrome c, and activating caspase proteases (31,32).
Nevertheless, little report has determined whether ginsenoside has
anti-CRC effect.
In this study, TGCG arrested cell cycle of CRC cells
at G0/G1 and G2/M phases by
inhibiting cell cycle activators (c-Myc, Cdk2, Cdk4, Cdk6, and
Cyclin D1) and activating cell cycle inhibitors
(p15INK4b, p21WAF1, and p27Kip1).
Since Cdk2, Cdk4, Cdk6, Cyclin D1, p15INK4b,
p21WAF1, and p27Kip1 could be encoded by the
downstream genes of c-Myc, TGCG was concluded to induce cell cycle
arrest through a c-Myc-mediated mechanism (33–35).
Cyclin D1 and c-Myc are the downstream targets of Wnt/β-catenin
signaling pathway, the inhibition of which causes suppression of
tumor growth, epithelial mesenchymal transition (EMT), and cell
motility of CRC (36). Besides,
c-Myc, Cyclin D1, Cdk2, and Cdk4 are also the downstream cell cycle
factors of PI3K/Akt signaling pathway in CRC, while p21 and p27 are
its downstream cell cycle inhibitors (37,38).
Furthermore, c-Myc and Cyclin D1 are cell proliferative effectors
in the downstream of NF-κB signaling pathway in CRC (39). Therefore, Wnt/β-catenin, PI3K/Akt, and
NF-κB signaling pathways might possibly participate in the action
mechanism of TGCG underlying the observed effect on cell cycle and
proliferation of CRC. It has been reported that ginseng extract
could enhance the anti-proliferative effect of 5-fluorouracil
(5-FU) on CRC cells and attenuate nausea and vomiting induced by
chemotherapeutics, indicating an effect-enhancing and
toxicity-reducing activity (40–42). 5-FU
is a key drug that causes perturbation of ribosome biogenesis in
CRC cells by activating ribosomal proteins and p21, leading to
p21-mediated cell cycle arrest and apoptosis (43,44). It is
known that c-Myc is a key regulator of ribosome biogenesis and
emerging data indicate that 5-FU induces apoptosis through
ribosomal protein-mediated regulation of NF-κB. In this study, TGCG
has been shown to function on c-Myc and p21, thereby indicating a
possible mechanism that TGCG regulates c-Myc expression to activate
p21/ribosomal protein and leads to cell cycle arrest of CRC.
It was also found that TGCG induced cell apoptosis
of CRC by activating p53-mediated apoptotic pathway where
TOP1 and MDM2 were downregulated and TP53, BAX,
CASP3, and CASP8 were upregulated. TOP1 encodes
DNA topoisomerase I for DNA repair during DNA synthesis and meiotic
division. It is highly expressed in cancer cells making transient
single-strand DNA breaks to solve topological problems of DNA,
while DNA strand breaks (DSBs) formed with inhibition of its
expression (45). DSBs can trigger
DNA damage with induction of apoptosis in cancer cells (46), which might be an initial event in the
action of TGCG. TP53 encodes the tumor suppressor p53 that
can be activated in response to DNA damage. It is a crucial
transcription factor responsible for the prevention of cancer
formation due to its role in conserving stability of genome
(47). When DNA suffers irreparable
damage, p53 can bind DNA and initiate cell apoptosis, which in turn
activates transcription of many apoptotic genes, including the
members of Bcl-2 family (e.g., BAX) and caspase family
(e.g., CASP3 and CASP8) (48). However, the transcriptional activity
of p53 can be inhibited by an E3 ubiquitin ligase (Mdm2) encoded by
MDM2. Mdm2 is a negative regulator of p53 and is always
overexpressed in tumor cells, which would shuttle p53 out of the
nucleus and terminate p53-mediated apoptosis (49). In this study, TGCG activated p53 and
inhibited MDM2 expression to induce cell apoptosis following
activation of p53-downstreamed target genes (BAX, CASP3 and
CASP8). Bax functions as an apoptosis activator tending to
interact with mitochondria and release cytochrome c and
pro-apoptotic factors to activate caspases (50). Caspase-8 encoded by CASP8 plays
an initial role in the caspase cascade which activates
execution-phase of cell apoptosis. Overexpression of CASP8
induces apoptotic cell death in tumor cells, and it most likely
acts upon caspase-3. Caspase-3 encoded by CASP3 acts as the
predominant ‘executioner caspase’ in apoptotic cell death. It can
be activated in the apoptotic cells both by extrinsic (death
ligand) and intrinsic (mitochondrial) pathways (51).
Taken together, our results indicate that TGCG
induced cell cycle arrest at G0/G1 and
G2/M phases and apoptosis in HT-29 cells via c-Myc- and
p53-associated mechanism, respectively, possibly in response to DNA
damage. Recently, increasing studies have been focused on the
anti-CRC activity of the single compound of Chinese ginseng and
obtained positive outcomes. We found that TGCG exerted stronger
anti-CRC effect than that of each compound of Chinese ginseng (Rb1,
Re, Rd, and Rg1), indicating a synergistic action of those
compounds in TGCG. It makes TGCG a promising candidate for anti-CRC
application and drug development due to its better effect, easier
preparation procedure, and lower cost.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Zhejiang
Provincial Natural Science Foundation of China (grant nos.
LY17H270001 and LY17H270016), the Major Science and Technology
Special Project of Zhejiang Province (grant no. 2014C03035), the
National Natural Science Foundation of China (grant no. 81673997),
the Zhejiang Provincial Major Science and Technology Project of
Medical and Health of China (grant no. 201487674) and the Zhejiang
Provincial Science and Technology Project of Traditional Chinese
Medicine of China (grant nos. 2015ZA193, 2013ZB098, 2016ZZ011,
2016ZQ010, and 2013ZQ007).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TL and WS were the principal scientists conducted
the experiments. XD, WY and JC assisted with the experiments. LS
and QY conceived, designed and revised the study and drafted the
manuscript. TE made substantial contributions to the experimental
design and revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kolligs FT: Diagnostics and epidemiology
of colorectal cancer. Visc Med. 32:158–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Platz EA, Willett WC, Colditz GA, Rimm EB,
Spiegelman D and Giovannucci E: Proportion of colon cancer risk
that might be preventable in a cohort of middle-aged US men. Cancer
Causes Control. 11:579–588. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baak JP, Gyllenhaal C, Liu L, Guo H and
Block KI: Prognostic proof and possible therapeutic mechanisms of
herbal medicine in patients with metastatic lung and colon cancer.
Integr Cancer Ther. 10:NP1–NP11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mano MS and Duhoux F: Colon cancer: Update
on adjuvant therapy. Clin Colorectal Cancer. 7:178–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwamoto T: Clinical application of drug
delivery systems in cancer chemotherapy: Review of the efficacy and
side effects of approved drugs. Biol Pharm Bull. 36:715–718. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ades S: Adjuvant chemotherapy for colon
cancer in the elderly: Moving from evidence to practice. Oncology
(Williston Park). 23:162–167. 2009.PubMed/NCBI
|
|
10
|
Martin AR, Carides AD, Pearson JD, Horgan
K, Elmer M, Schmidt C, Cai B, Chawla SP and Grunberg SM: Functional
relevance of antiemetic control. Experience using the FLIE
questionnaire in a randomised study of the NK-1 antagonist
aprepitant. Eur J Cancer. 39:1395–1401. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chien TJ, Liu CY, Lu RH, Kuo CW, Lin YC
and Hsu CH: Therapeutic efficacy of Traditional Chinese medicine,
‘Kuan-Sin-Yin’, in patients undergoing chemotherapy for advanced
colon cancer-A controlled trial. Complement Ther Med. 29:204–212.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin YY, Lee IY, Huang WS, Lin YS, Kuan FC,
Shu LH, Cheng YC, Yang YH and Wu CY: Danshen improves survival of
patients with colon cancer and dihydroisotanshinone I inhibit the
proliferation of colon cancer cells via apoptosis and skp2
signaling pathway. J Ethnopharmacol. 209:305–316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su Z, Zhou C, Qin S, Jia E and Wu Z: The
significant pathways and genes underlying the colon cancer
treatment by the traditional Chinese medicine PHY906. Evid Based
Complement Alternat Med. 2017:87538152017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Li XM, Bai Z, Chi BX, Wei Y and
Chen X: Curcumol induces cell cycle arrest in colon cancer cells
via reactive oxygen species and Akt/GSK3β/cyclin D1 pathway. J
Ethnopharmacol. 210:1–9. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin H, Liu J and Zhang Y: Developments in
cancer prevention and treatment using traditional Chinese medicine.
Front Med. 5:127–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baeg IH and So SH: The world ginseng
market and the ginseng (Korea). J Ginseng Res. 37:1–7. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dai D, Zhang CF, Williams S, Yuan CS and
Wang CZ: Ginseng on cancer: Potential role in modulating
inflammation-mediated angiogenesis. Am J Chin Med. 45:13–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang CZ, Zhang Z, Wan JY, Zhang CF,
Anderson S, He X, Yu C, He TC, Qi LW and Yuan CS: Protopanaxadiol,
an active ginseng metabolite, significantly enhances the effects of
fluorouracil on colon cancer. Nutrients. 7:799–814. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JG, McKinney KQ, Pavlopoulos AJ, Park
JH and Hwang S: Data supporting the identification of
anti-metastatic drug and natural compound targets in isogenic
colorectal cancer cells. Data Brief. 1:73–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang CZ, Xie JT, Zhang B, Ni M, Fishbein
A, Aung HH, Mehendale SR, Du W, He TC and Yuan CS: Chemopreventive
effects of Panax notoginseng and its major constituents on
SW480 human colorectal cancer cells. Int J Oncol. 31:1149–1156.
2007.PubMed/NCBI
|
|
21
|
Zhu C, Liu F, Qian W, Zhang T and Li F:
Combined effect of sodium selenite and ginsenoside Rh2 on HCT116
human colorectal carcinoma cells. Arch Iran Med. 19:23–29.
2016.PubMed/NCBI
|
|
22
|
Lee JG, McKinney KQ, Pavlopoulos AJ, Park
JH and Hwang S: Identification of anti-metastatic drug and natural
compound targets in isogenic colorectal cancer cells. J Proteomics.
113:326–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen L, Meng Y, Sun Q, Zhang Z, Guo X,
Sheng X, Tai G, Cheng H and Zhou Y: Ginsenoside compound K
sensitizes human colon cancer cells to TRAIL-induced apoptosis via
autophagy-dependent and -independent DR5 upregulation. Cell Death
Dis. 7:e23342016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang QY and Feng MG: DPS data processing
system: Experimental design, statistical analysis and data mining.
Beijing Science Press; Beijing, China: 2007
|
|
25
|
Yun TK, Choi SY and Yun HY:
Epidemiological study on cancer prevention by ginseng: Are all
kinds of cancers preventable by ginseng? J Korean Med Sci. 16
Suppl:S19–S27. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng H, Li S, Fan Y, Gao X, Hao M, Wang
J, Zhang X, Tai G and Zhou Y: Comparative studies of the
antiproliferative effects of ginseng polysaccharides on HT-29 human
colon cancer cells. Med Oncol. 28:175–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li B, Wang CZ, He TC, Yuan CS and Du W:
Antioxidants potentiate American ginseng-induced killing of
colorectal cancer cells. Cancer Lett. 289:62–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SE, Lee YH, Park JH and Lee SK:
Ginsenoside-Rs4, a new type of ginseng saponin concurrently induces
apoptosis and selectively elevates protein levels of p53 and
p21WAF1 in human hepatoma SK-HEP-1 cells. Eur J Cancer. 35:507–511.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi S, Kim TW and Singh SV: Ginsenoside
Rh2-mediated G1 phase cell cycle arrest in human breast cancer
cells is caused by p15 Ink4B and p27 Kip1-dependent inhibition of
cyclin-dependent kinases. Pharm Res. 26:2280–2288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao JL, Lv GY, He BC, Zhang BQ, Zhang H,
Wang N, Wang CZ, Du W, Yuan CS and He TC: Ginseng saponin
metabolite 20(S)-protopanaxadiol inhibits tumor growth by targeting
multiple cancer signaling pathways. Oncol Rep. 30:292–298. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park HM, Kim SJ, Kim JS and Kang HS:
Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced
apoptosis in hepatoma cells through mitochondrial signaling
pathways. Food Chem Toxicol. 50:2736–2741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Z, Li Z, Wu X, Zhang CF, Calway T,
He TC, Du W, Chen J, Wang CZ and Yuan CS: TRAIL pathway is
associated with inhibition of colon cancer by protopanaxadiol. J
Pharmacol Sci. 127:83–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dang CV: c-Myc target genes involved in
cell growth, apoptosis, and metabolism. Mol Cell Biol. 19:1–11.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grandori C, Cowley SM, James LP and
Eisenman RN: The Myc/Max/Mad network and the transcriptional
control of cell behavior. Annu Rev Cell Dev Biol. 16:653–699. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shao Q, Kannan A, Lin Z, Stack BC Jr, Suen
JY and Gao L: BET protein inhibitor JQ1 attenuates Myc-amplified
MCC tumor growth in vivo. Cancer Res. 74:7090–7102. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gu J, Cui CF, Yang L, Wang L and Jiang XH:
Emodin inhibits colon cancer cell invasion and migration by
suppressing epithelialmesenchymal transition via the Wnt/β-catenin
pathway. Oncol Res. Jan 4–2018.(Epub ahead of print). View Article : Google Scholar
|
|
37
|
Chen Y, Jiang J, Zhao M, Luo X, Liang Z,
Zhen Y, Fu Q, Deng X, Lin X, Li L, et al: microRNA-374a suppresses
colon cancer progression by directly reducing CCND1 to inactivate
the PI3K/AKT pathway. Oncotarget. 7:41306–41319. 2016.PubMed/NCBI
|
|
38
|
Izutani Y, Yogosawa S, Sowa Y and Sakai T:
Brassinin induces G1 phase arrest through increase of p21 and p27
by inhibition of the phosphatidylinositol 3-kinase signaling
pathway in human colon cancer cells. Int J Oncol. 40:816–824.
2012.PubMed/NCBI
|
|
39
|
Yang Z, Li C, Wang X, Zhai C, Yi Z, Wang
L, Liu B, Du B, Wu H, Guo X, et al: Dauricine induces apoptosis,
inhibits proliferation and invasion through inhibiting NF-kappaB
signaling pathway in colon cancer cells. J Cell Physiol.
225:266–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mehendale S, Aung H, Wang A, Yin JJ, Wang
CZ, Xie JT and Yuan CS: American ginseng berry extract and
ginsenoside Re attenuate cisplatin-induced kaolin intake in rats.
Cancer Chemother Pharmacol. 56:63–69. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fishbein AB, Wang CZ, Li XL, Mehendale SR,
Sun S, Aung HH and Yuan CS: Asian ginseng enhances the
anti-proliferative effect of 5-fluorouracil on human colorectal
cancer: Comparison between white and red ginseng. Arch Pharm Res.
32:505–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gu C, Qiao J, Zhu M, Du J, Shang W, Yin W,
Wang W, Han M and Lu W: Preliminary evaluation of the interactions
of Panax ginseng and Salvia miltiorrhiza Bunge with
5-fluorouracil on pharmacokinetics in rats and pharmacodynamics in
human cells. Am J Chin Med. 41:443–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pagliara V, Saide A, Mitidieri E, di Villa
Bianca d'Emmanuele R, Sorrentino R, Russo G and Russo A: 5-FU
targets rpL3 to induce mitochondrial apoptosis via
cystathionine-β-synthase in colon cancer cells lacking p53.
Oncotarget. 7:50333–50348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Russo A, Saide A, Cagliani R, Cantile M,
Botti G and Russo G: rpL3 promotes the apoptosis of p53 mutated
lung cancer cells by down-regulating CBS and NFκB upon 5-FU
treatment. Sci Rep. 6:383692016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Holden JA: DNA topoisomerases as
anticancer drug targets: From the laboratory to the clinic. Curr
Med Chem Anti-Canc Agents. 1:1–25. 2001. View Article : Google Scholar
|
|
46
|
Cheng MH, Yang YC, Wong YH, Chen TR, Lee
CY, Yang CC, Chen SH, Yang IN, Yang YS, Huang HS, et al: B1, a
novel topoisomerase II inhibitor, induces apoptosis and cell cycle
G1 arrest in lung adenocarcinoma A549 cells. Anticancer Drugs.
23:191–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Surget S, Khoury MP and Bourdon JC:
Uncovering the role of p53 splice variants in human malignancy: A
clinical perspective. Onco Targets Ther. 7:57–68. 2013.PubMed/NCBI
|
|
48
|
Levine AJ and Oren M: The first 30 years
of p53: Growing ever more complex. Nat Rev Cancer. 9:749–758. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang X, Arooz T, Siu WY, Chiu CH, Lau A,
Yamashita K and Poon RY: MDM2 and MDMX can interact differently
with ARF and members of the p53 family. FEBS Lett. 490:202–208.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Weng C, Li Y, Xu D, Shi Y and Tang H:
Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
in Jurkat leukemia T cells. J Biol Chem. 280:10491–10500. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Salvesen GS: Caspases: Opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|