Introduction
Small cell lung cancer (SCLC) is a type of invasive
malignant tumor, accounting for ~13% of lung cancer (1). It is characterized by marked
invasiveness, high grade malignancy, high risk of early metastasis
and poor prognosis. High metastases ability and relapse following
drug resistance are the primary causes for this poor prognosis in
patients with SCLC (2). The
initiation, progression, treatment resistance and relapse of tumors
are associated with metastasis. These are the primary factors
responsible for the intractable and stubborn properties of this
type of tumor (3). Identification of
biological characteristics and exploration of novel targets for
diagnosis and treatment methods are of important theoretical
significance and clinical value.
MicroRNA (miRNA) are single stranded noncoding
nuclear acid molecules with a length of 18–24 nucleotides and high
levels of evolutionary conservation. Certain miRNAs constitute the
RNA-induced silencing complex (RISC), together with other proteins
(4). Subsequent to the RlSC protein
complex combining with mRNA, the complex will begin to splice mRNA
or block mRNA translation, consequently causing mRNA degradation or
translation inhibition (5). It
participates in a variety of important biological processes
including cell differentiation, proliferation and apoptosis,
hormone secretion and tumor formation in plants and animals
(6). The results of bioinformatics
predictions have indicated that each miRNA may regulate 1,000s of
target genes (7). miRNAs have
potential effects on almost every genetic pathway and regulate
different biological processes.
An adhesion molecule is a bio-macromolecule that
mediates the binding between cells, and between cells and the
extracellular matrix, and serves an important role in maintaining
the normal structures of tissues and homeostasis of various
pathophysiological processes including the inflammatory and immune
responses, blood coagulation, formation of thrombosis and the
invasion and metastasis of malignant tumors (8). Based on their structures, adhesion
molecules may be grouped into various families: The integrin
family, selectin family, immunoglobulin superfamily, E-cadherin
family and other families, among which intercellular adhesion
molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)
are the most important types, and serve vital roles in regulating
the binding between cells, and between cells and the extracellular
matrix (9). In addition, ICAM-1 and
VCAM-1 are also involved in a number of physiological and
pathological processes including the immune response, inflammation
and the development and metastasis of tumors (10).
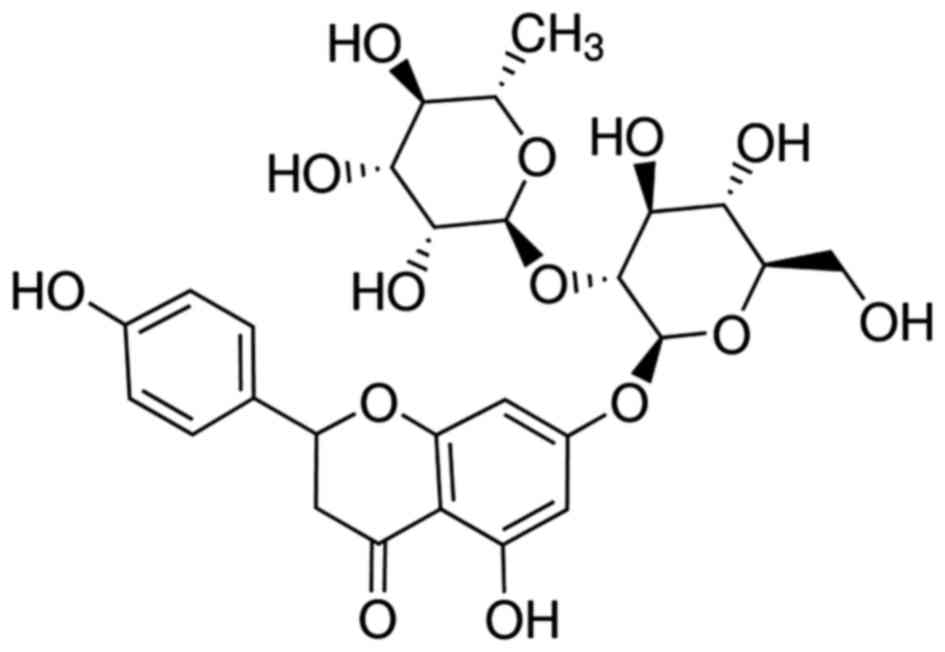
A previous study indicated that the antioxidant
activity of naringin (Fig. 1) is
markedly increased compared with that of compounds from
Pomelo (11). A further study
suggested that naringin exhibits anti-atherosclerosis and
anticancer effects, and also improves myocardial ischemia and
immune regulation (12). Naringin
exhibits notable anti-oxidant, anti-inflammatory and anti-tumor
effects (13). The present study
hypothesized that the anticancer effect of naringin involves the
suppression of cell growth and induction of apoptosis in human SCLC
cells, and analyzed the potential mechanisms of action.
Materials and methods
Cell culture
The human H69AR SCLC cell line was purchased from
the Affiliated Hospital of Hebei University (Baoding, China), and
grown in RPMI-1640 medium (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.)
and penicillin/streptomycin at 37°C in an environment containing 5%
CO2.
Cell proliferation assay
A total of 1.2×106 H69AR cells/well were
plated in 6-well plates and treated with 6, 12 and 25 µg/ml
naringin for 24 h at 37°C. The old medium was then removed, and 200
µl MTT (0.5 µmol/l) was added into the wells and incubated for 4 h
at 37°C. A total of 150 µl dimethyl sulfoxide was added into each
well and shaken for 20 min. The absorbance was detected using a
microplate reader (SpectraMax® M2; Molecular Devices,
LLC, Sunnyvale, CA, USA) and read at a wavelength of 570 nm.
Apoptosis and flow cytometry
A total of 1.2×106 H69AR cells/well were
plated in 6-well plates and treated with 6, 12 and 25 µg/ml
naringin for 24 h. H69 cells was washed and collected via
centrifugation 2,000 × g for 5 min at 4°C. H69 cells were fixed
with 4% paraformaldehyde for 15 min at room temperature and
re-suspended using buffer solution (eBioscience; Thermo Fisher
Scientific, Inc.), and then 5 µl Annexin V (eBioscience; Thermo
Fisher Scientific, Inc.) was added into every well and stained for
30 min in darkness, on ice at 37°C, and H69 cells were stained with
10 µl propidium iodide in darkness for 15 min at room temperature.
Cells were measured using a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) and analyzed using Flowjo
7.6.1 (FlowJo, LLC, Ashland, OR, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
A total of 2×106 cells/well were plated
in 6-well plates and treated with 6, 12 and 25 µg/ml naringin for
24 h. Total RNA was isolated from treated H69 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and the expression level of miR-126 was determined by a
RT-qPCR assay. cDNA was transcribed using High-Capacity cDNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). qPCR was performed with FastStart SYBR Green
Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.) and ABI
7900 HT sequence detection system. The PCR thermocycler conditions
used were: 95°C for 10 min, followed by 35 cycles of 45 sec at
95°C, 45 sec at 60°C and 60 sec at 72°C, then samples were stored
at 4°C until use. The primer sequences used are as follows:
microRNA-126, forward, 5′-UCGUACCGUGAGUAAUAAUGCG-3′ and reverse,
5′-CAUUAUUACUUUUGGUACGCG-3′; U6, forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′. The 2−∆∆Cq method was used to
measure microRNA-126 expression (14).
Western blotting
Cells were collected and resuspended in RIPA assay
(Beyotime Institute of Biotechnology) for 20 min on ice. The cell
mixture was centrifuged at 12,000 × g at 4°C for 10 min, and the
supernatants were collected to determine the protein concentration
using Enhanced BCA Protein Assay Kit (catalog no., P0009, Beyotime
Institute of Biotechnology). A total of 50 mg of protein lysates
were separated in 10% SDS-PAGE and blotted onto polyvinylidene
difluoride membranes (Bio-Rad Laboratories, Inc.). The membranes
were blocked with 5% non-fat milk at room temperature for 1 h, and
incubated with the appropriate dilution of each primary antibody:
Phosphoinositide 3-kinase (PI3K; cat no. sc-7174; 1:500),
phosphorylation protein kinase B (p-AKT; cat no. sc-7985-R; 1:500),
phosphorylation mechanistic target of rapamycin (p-mTOR; cat no.
sc-101738; 1:1,000), nuclear factor (NF)-κB (cat no. sc-109;
1:500), VCAM-1 (cat no. sc-8304; 1:500) and GAPDH (cat no.
sc-25778; 1:1,000; all from Santa Cruz Biotechnology, Inc., CA,
USA) overnight at 4°C. The membranes were probed with anti-rabbit
horseradish peroxidase-conjugated secondary antibodies (cat no.
sc-2004; 1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h at 37°C.
The protein bands were visualized using BeyoECL Star (Beyotime
Institute of Biotechnology) and analyzed using Image_Lab_3.0
software (Bio-Rad Laboratories, Inc.).
Transfection with miR-126
A total of 2×106 cells/well were plated
in 6-well plates and transfected with miR-126
(5′-UCGUACCGUGAGUAAUAAUGCG-3′, Sangon Biotech Co., Ltd., Shaghai,
China) and control miRNA mimics (5′-CAGUACUUUUGUGUAGUACAA-3′,
Sangon Biotech Co., Ltd.) at a final concentration of 50 nM using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 6 h at 37°C. Then, the old RPMI-1640 medium
was removed and new RPMI-1640 medium (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 25 µg/ml naringin, was
introduced following transfection complexes for 24 h.
Statistical analysis
Data were presented as the mean ± standard
deviation. Data were analyzed via one-way analysis of variance with
Tukey's post-hoc test using SPSS software, version 16.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Anticancer effect of naringin on cell
proliferation of the H69 cell line
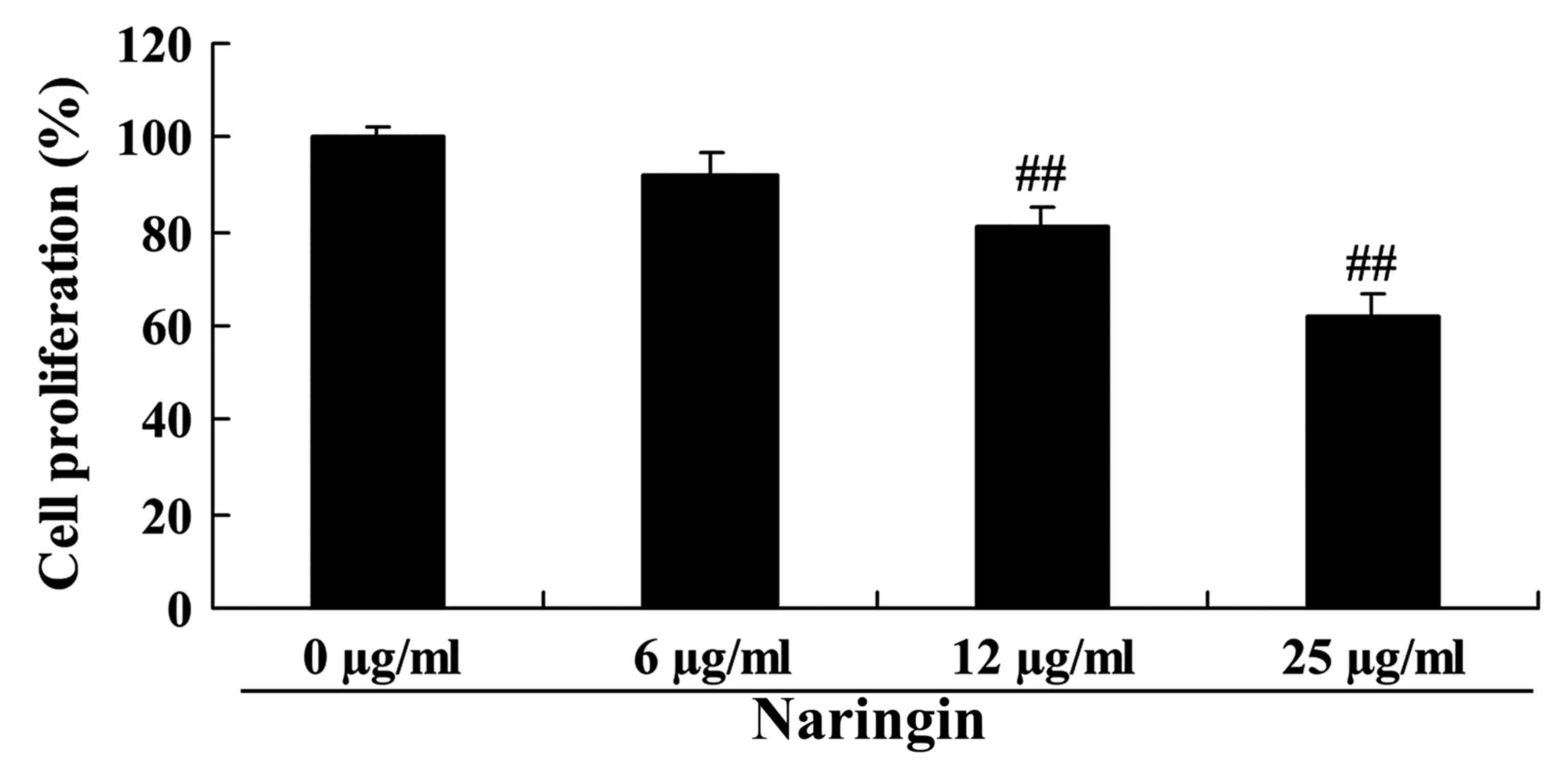
To understand the anticancer effect of naringin,
namely the suppression of cell growth in small cell lung cancer
cells, the SCLC H69 cell line was treated with 6, 12 and 25 µg/ml
naringin for 24 h. The results demonstrated that naringin
suppressed cell growth of the small cell SCLC lung cancer H69 cell
line in a dose-dependent manner (Fig.
2). In particular, the suppression of cell growth in the SCLC
H69 cell line was statistically significant following treatment
with 12 or 25 µg/ml naringin for 24 h, compared with control.
Anticancer effect of naringin on
apoptosis of the H69 cell line
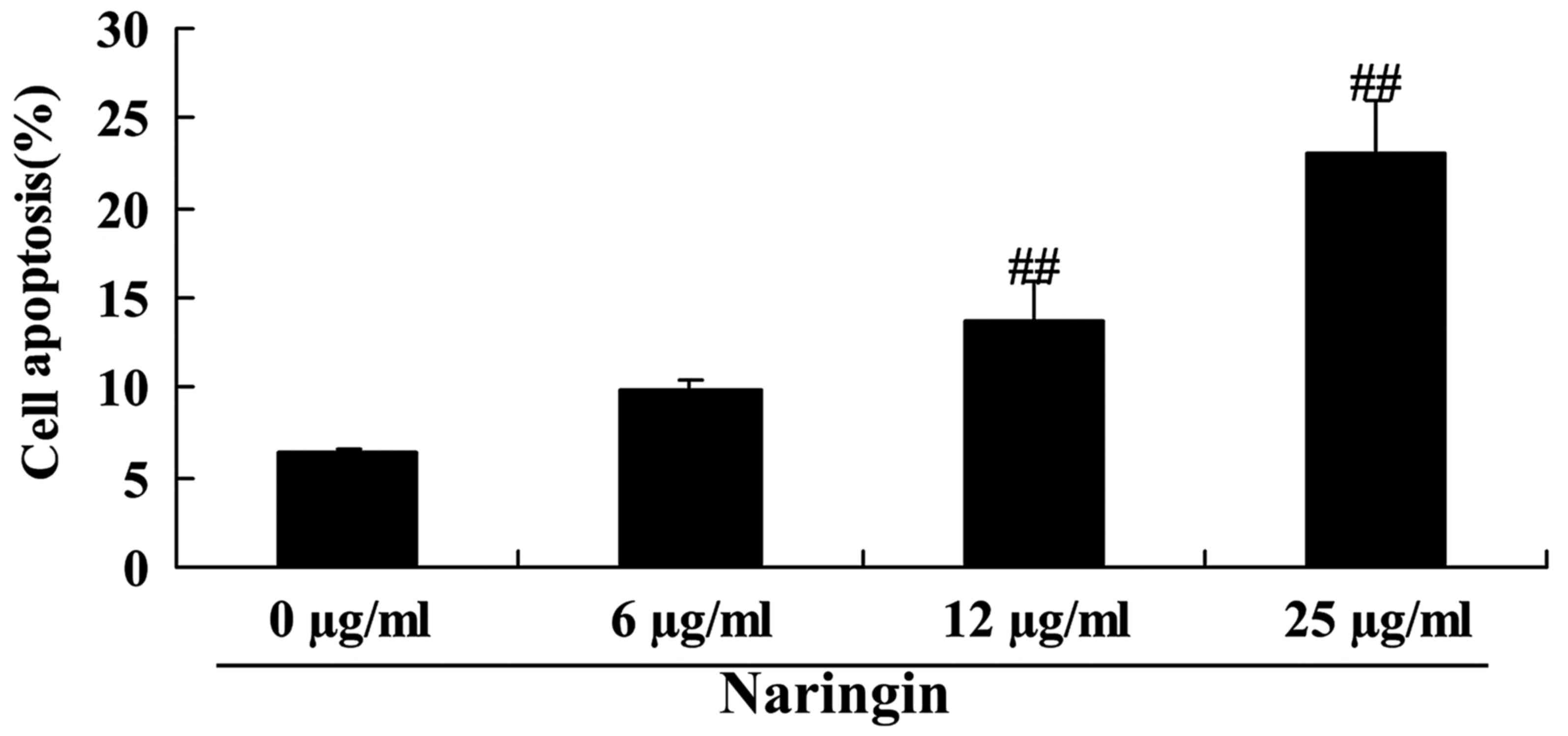
To investigate the inhibitory effects of naringin on
SCLC H69 cell apoptosis, levels of apoptosis were detected using
flow cytometry. Naringin-induced cell apoptosis of the SCLC H69
cell line was observed, which was statistically significant when
treated with 12 or 25 µg/ml naringin at 24 h, compared with control
(Fig. 3).
Anticancer effect of naringin on
miR-126 in the H69 cell line
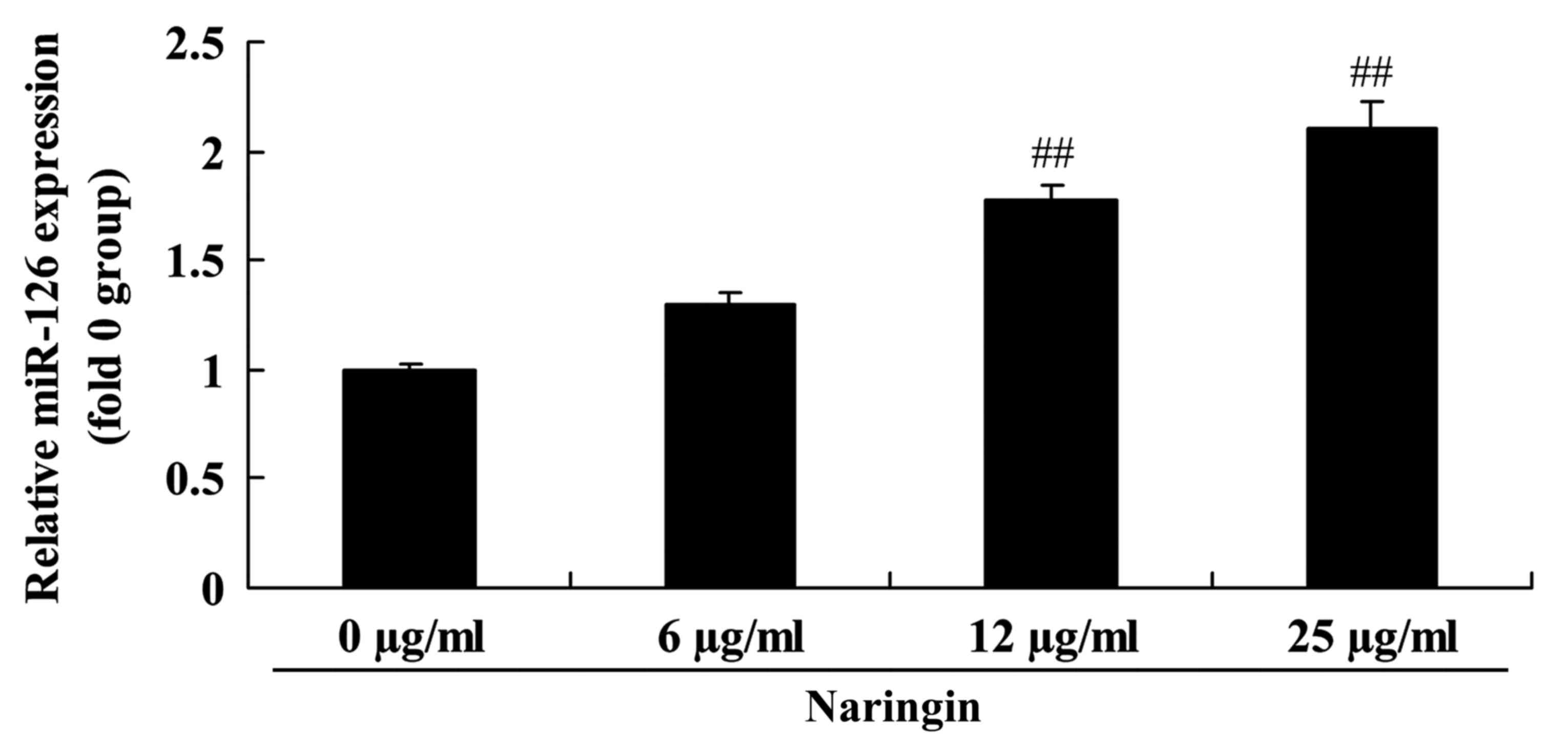
To additionally evaluate the mechanism of the
anti-tumor effects of naringin in the SCLC H69 cells, the
expression of miR-126 was measured using RT-qPCR. As demonstrated
in Fig. 4, the expression of miR-126
was markedly increased following treatment with 12 or 25 µg/ml
naringin.
Anticancer effect of naringin on
PI3K/AKT, mTOR, NF-κB and VCAM-1 in H69 cells
To verify the mechanism of action of naringin on the
PI3K/AKT pathway in H69 cells, PI3K/AKT, mTOR, NF-κB and VCAM-1
protein levels were measured using western blotting. Compared with
the 0 µg/ml naringin-treated group, PI3K, phosphorylated (p)-AKT,
p-mTOR, NF-κB and VCAM-1 protein levels were significantly
suppressed by treatment with 12 or 25 µg/ml naringin (Fig. 5).
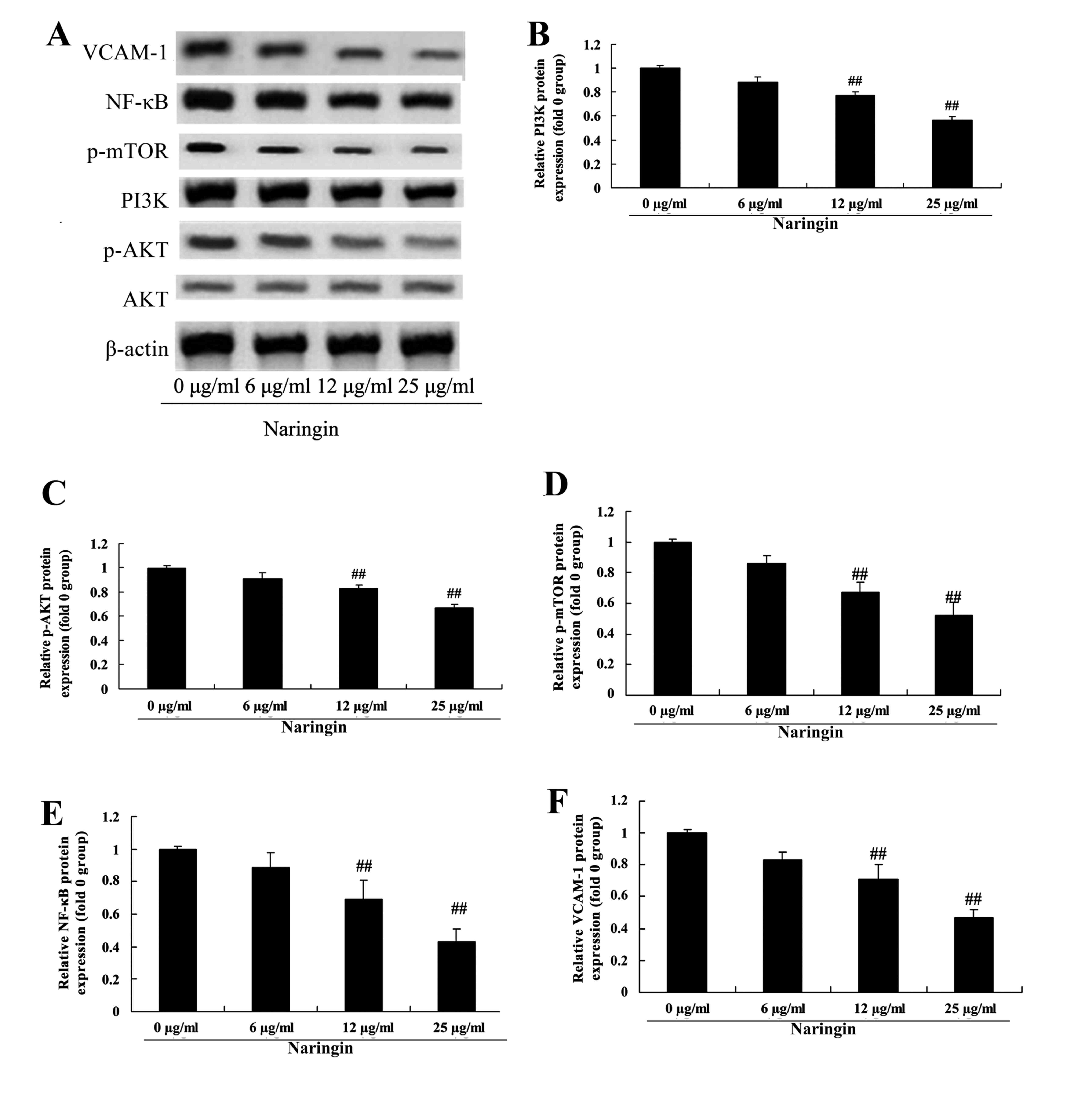 | Figure 5.Anticancer effect of naringin on
PI3K/AKT, mTOR, NF-κB and VCAM-1 levels in H69 cells. (A) Western
blot analysis of the protein levels of PI3K, p-AKT, p-mTOR, NF-κB
and VCAM-1 and statistical analysis of the protein levels of (B)
PI3K, (C) p-AKT, (D) p-mTOR, (E) NF-κB and (F) VCAM-1.
##P<0.01 vs. 0 µg/ml naringin-treated group. VCAM-1,
vascular cell adhesion molecule 1; NF-κB, nuclear factor κB; mTOR,
mechanistic target of rapamycin; PI3K, phosphoinositide 3-kinase;
AKT, protein kinase B; p, phosphorylated. |
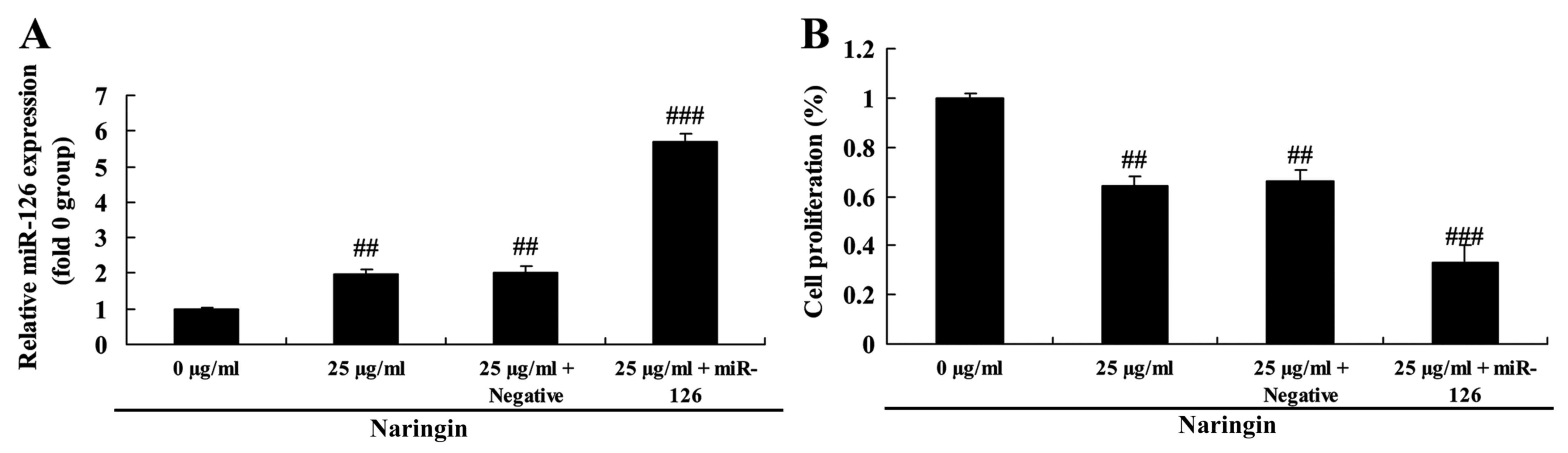
Overexpression of miR-126 on the
effect of naringin on cell growth
To identify the role of miR-126 in the effect of
naringin on cell growth of H69 cells, miR-126 and negative control
miRNA were transfected into H69 cells. When compared with an
additional small interfering control, the expression of miR-126 was
markedly increased in H69 cells (Fig.
6). miR-126 overexpression augmented the inhibitory effect of
naringin on cell growth in H69 cells, compared with negative
control (Fig. 6).
Overexpression of miR-126 on the
effect of naringin on PI3K/AKT, mTOR, NF-κB and VCAM-1 signaling
pathway
The role of miR-126 in p-AKT, p-mTOR, NF-κB and
VCAM-1 protein in H69 cells was investigated. As demonstrated in
Fig. 7, the decrease in the
phosphorylated protein levels suggested that the activity of the
PI3K/AKT/mTOR signaling pathway in the 25 µg/ml naringin-treated
group or negative control was decreased compared with of 0 µg/ml
naringin-treated group. Notably, the expression of p-AKT, p-mTOR,
NF-κB and VCAM-1 protein levels observed in the miR-126
overexpression group was markedly inhibited in H69 cells treated
with naringin, compared with that of the negative control (Fig. 7).
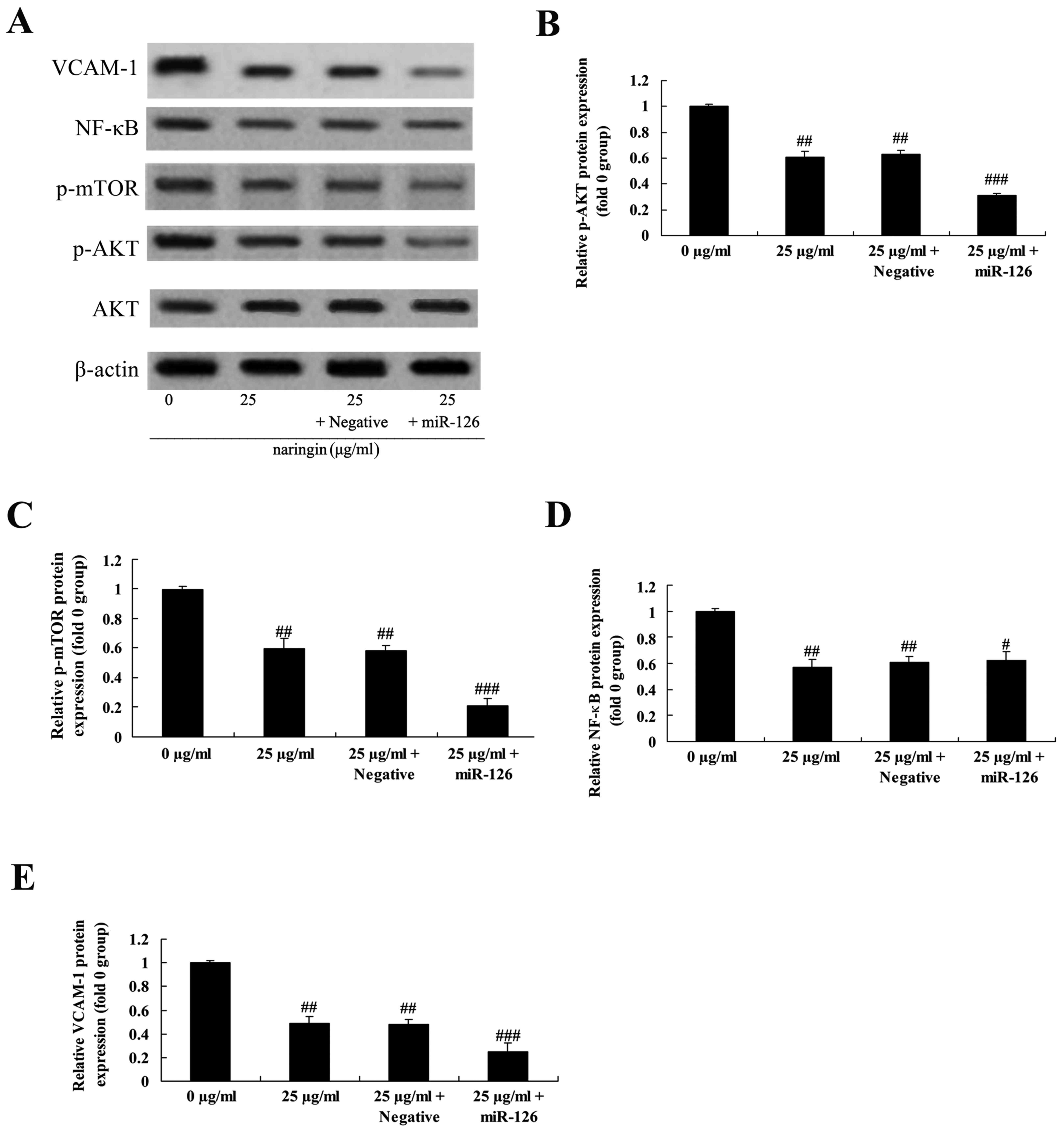 | Figure 7.Overexpression of miR-126 on the
effect of naringin on PI3K/AKT, mTOR, NF-κB and VCAM-1 protein
levels. The protein levels of p-AKT, NF-κB and VCAM-1 using (A)
western blot analysis and statistical analysis for (B) p-AKT, (C)
mTOR, (D) NF-κB and (E) VCAM-1. #P>0.05 vs. 25 µg/ml
naringin-treated group; ##P<0.01 vs. 0 µg/ml
naringin-treated group; ###P<0.01 vs. 25 µg/ml
naringin-treated group. VCAM-1, vascular cell adhesion molecule 1;
NF-κB, nuclear factor κB; mTOR, mechanistic target of rapamycin;
PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; p,
phosphorylated; miR, microRNA; Negative, negative mimics group. |
Discussion
Lung cancer is a highly malignant neoplastic
disease. In addition, its morbidity rate has demonstrated an
increasing trend year-on-year (5). In
previous years, with the increase in anti-smoking efforts, its
morbidity has demonstrated a decreasing trend. According to
statistics from the United States of America, there were ~172,570
patients with lung cancer, including 93,010 males and 79,560
females, ranking second among all incident tumor diseases (15). Of all cancer mortalities in the USA in
that year, males with lung cancer accounted for 31% and females for
27% (16). According to a study from
the World Health Organization in 2000, the global mortalities from
lung cancer accounted for 19% of all mortalities from malignant
tumors, and it was the most common cause of malignant tumor
mortality (17). To the best of the
author's knowledge, the present study demonstrated for the first
time that the anticancer effect of naringin involved the
suppression of cell proliferation and the induction of apoptosis in
H69 cells. Previous studies indicate that naringin suppressed cell
growth of HeLa cervical cancer cells (18), human triple-negative breast cancer
cells (19) and P388 cells (20). Therefore, it is hypothesized that
naringin may be used as an effective anticancer agent for therapy
in lung cancer.
miRNA serve an important regulating role in cell
growth, differentiation, apoptosis, fat metabolism and other
cellular processes in plants and animals (5). Abnormal miRNA expression is closely
associated with the development of human tumors. It may function as
an oncogene or tumor suppressor gene during tumorigenesis (4). High expression levels of miR-16 in SCLC
have been verified by applying qPCR methods, and its potential
specific target genes have been indicated (21). The present study identified that
pretreatment with naringin activated miR-126 expression in the H69
cell line, and Tan et al (22)
suggested that naringin suppresses cell growth of chondrosarcoma
migration via activation of miR-126. These results indicate that
naringin may be a novel and useful anticancer agent for lung cancer
therapy that functions through miR-126 expression.
In previous years, the effects of the PI3K/Akt/mTOR
signaling pathway on human tumors have been of primary research
concern (23). Activation of the
PI3K/Akt/mTOR signaling pathway commonly occurs in human tumors
(24) through a variety of
mechanisms, including gene mutation, reduction of the expression of
the cancer suppressor gene Phosphatase and tensin homolog, mutation
or amplification of PI3K, mutation or amplification of Akt and gene
receptor activation (25). In
addition, the activation of PI3K/Akt/mTOR signaling pathway may
lead to poor prognostic effects in a variety of tumors. It may
cause drug resistance, reverse drug resistance inhibition of the
pathway and improve in vitro and in vivo chemotherapy
and radiation therapy effects (26).
Consequently, in-depth studies on the detailed mechanisms of this
pathway are required. In the present study, it was observed that
naringin suppressed the PI3K/Akt/mTOR and NF-κB signaling pathways
and activated miR-126 expression in H69 cells. Concurrently, it was
also identified that overexpression of miR-126 enhanced the
naringin-mediated suppression of cell proliferation and
PI3K/Akt/mTOR signaling pathway, however did not affect the NF-κB
signaling pathway. Lee et al (27) suggested that naringin inhibits the
PI3K/AKT/mTOR pathway in tumor necrosis factor-α-treated vascular
smooth muscle cells.
The invasion and metastases of malignant tumors are
complicated biological phenomena, in which cell adhesion serves a
vital role (8). VCAM-1 was activated
by inflammation (8). In addition, it
is also expressed in dendritic cells, including macrophages and
placental trophoblastic cells, B-lymphocytes, smooth muscle cells,
fibroblast cells and certain epithelial cells of the kidney
(28). VCAM-1 is involved in a
variety of biological processes, with a wide range of biological
functions, including the adhesion of white blood cells, regulation
of inflammatory response, activation and transduction of signals,
proliferation and differentiation of cells and tissues, immune
response, mobilization of hematopoietic stem cells and other
important physiological and pathological processes (29). In particular, numerous studies have
verified that VCAM-1 is highly expressed in tumors, and it has been
demonstrated that a high level of VCAM-1 expression is present in
the tissues and blood samples of breast carcinoma, nasopharyngeal
carcinoma and liver carcinoma (30).
In conclusion, the present study identified the
anticancer effect of naringin; that it suppresses cell growth and
induces apoptosis in SCLC cells through the miR-126/PI3K/AKT/mTOR
pathway, potentially through the overexpression of miR-126.
Additional studies are required to understand the anticancer effect
of naringin in the growth and apoptosis of tumors in
vivo.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JD designed the study; MC, WP, SH performed the
experiments; JD and MC, analyzed the data; JD wrote the
manuscript.
Ethics and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xue H, Wang H, Liu J, Liu H, Li C, Han L,
Lin C, Zhan Q, Zhao Z and Qian H: MTA1 downregulation inhibits
malignant potential in a small cell lung cancer cell line. Oncol
Rep. 33:885–892. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bremnes RM, Sundstrom S, Aasebø U, Kaasa
S, Hatlevoll R and Aamdal S: Norweigian Lung Cancer Study Group:
The value of prognostic factors in small cell lung cancer: Results
from a randomised multicenter study with minimum 5 year follow-up.
Lung Cancer. 39:303–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wood L, Palmer M, Hewitt J, Urtasun R,
Bruera E, Rapp E and Thaell JF: Results of a phase III,
double-blind, placebo-controlled trial of megestrol acetate
modulation of P-glycoprotein-mediated drug resistance in the
first-line management of small-cell lung carcinoma. Br J Cancer.
77:627–631. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barger JF and Nana-Sinkam SP: MicroRNA as
tools and therapeutics in lung cancer. Respir Med. 109:803–812.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Che C, Zhang L, Huo J and Zhang Y: RNA
interference targeting enhancer of polycomb1 exerts anti-tumor
effects in lung cancer. Int J Clin Exp Pathol. 8:361–367.
2015.PubMed/NCBI
|
|
6
|
Joshi P, Middleton J, Jeon YJ and Garofalo
M: MicroRNAs in lung cancer. World J Methodol. 4:59–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Y, Hu Q, Deng Z, Hang Y, Wang J and
Wang K: MicroRNAs in body fluids as biomarkers for non-small cell
lung cancer: A systematic review. Technol Cancer Res Treat.
13:277–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferjančič Š, Gil-Bernabé AM, Hill SA,
Allen PD, Richardson P, Sparey T, Savory E, McGuffog J and Muschel
RJ: VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary
metastasis in mice. Blood. 121:3289–3297. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang X, He D, Ming J, He Y, Zhou C, Ren
H, He X, Wang C, Jin J, Ji L, et al: High-density lipoprotein of
patients with breast cancer complicated with type 2 diabetes
mellitus promotes cancer cells adhesion to vascular endothelium via
ICAM-1 and VCAM-1 upregulation. Breast Cancer Res Treat.
155:441–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tas F, Karabulut S, Bilgin E and
Duranyildiz D: Serum levels of vascular cell adhesion molecule-1
(VCAM-1) may have diagnostic, predictive, and prognostic roles in
patients with lung cancer treated with platinum-based chemotherapy.
Tumour Biol. 35:7871–7875. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bharti S, Rani N, Krishnamurthy B and Arya
DS: Preclinical evidence for the pharmacological actions of
naringin: A review. Planta Med. 80:437–451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanno S, Shouji A, Asou K and Ishikawa M:
Effects of naringin on hydrogen peroxide-induced cytotoxicity and
apoptosis in P388 cells. J Pharmacol Sci. 92:166–170. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Camargo CA, Gomes-Marcondes MC, Wutzki NC
and Aoyama H: Naringin inhibits tumor growth and reduces
interleukin-6 and tumor necrosis factor α levels in rats with
Walker 256 carcinosarcoma. Anticancer Res. 32:129–133.
2012.PubMed/NCBI
|
|
14
|
Yang J, Chen L, Ding J, Zhang J, Fan Z,
Yang C, Yu Q and Yang J: Cardioprotective effect of miRNA-22 on
hypoxia/reoxygenation induced cardiomyocyte injury in neonatal
rats. Gene. 579:17–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawaguchi T, Ando M, Asami K, Okano Y,
Fukuda M, Nakagawa H, Ibata H, Kozuki T, Endo T, Tamura A, et al:
Randomized phase III trial of erlotinib versus docetaxel as second-
or third-line therapy in patients with advanced non-small-cell lung
cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin
Oncol. 32:1902–1908. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gitlitz BJ, Tsao-Wei DD, Groshen S, Davies
A, Koczywas M, Belani CP, Argiris A, Ramalingam S, Vokes EE,
Edelman M, et al: A phase II study of halichondrin B analog
eribulin mesylate (E7389) in patients with advanced non-small cell
lung cancer previously treated with a taxane: A California cancer
consortium trial. J Thorac Oncol. 7:574–578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gohagan J, Marcus P, Fagerstrom R, Pinsky
P, Kramer B and Prorok P: Writing Committee, Lung Screening Study
Research Group: Baseline findings of a randomized feasibility trial
of lung cancer screening with spiral CT scan vs chest radiograph:
The Lung Screening Study of the National Cancer Institute. Chest.
126:114–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng L, Zhen Y, Chen Y, Zou L, Zhang Y, Hu
F, Feng J, Shen J and Wei B: Naringin inhibits growth and induces
apoptosis by a mechanism dependent on reduced activation of
NF-κB/COX-2-caspase-1 pathway in HeLa cervical cancer cells. Int J
Oncol. 45:1929–1936. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Yang B, Huang J, Xiang T, Yin X, Wan
J, Luo F, Zhang L, Li H and Ren G: Naringin inhibits growth
potential of human triple-negative breast cancer cells by targeting
β-catenin signaling pathway. Toxicol Lett. 220:219–228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanno S, Shouji A, Hirata R, Asou K and
Ishikawa M: Effects of naringin on cytosine arabinoside
(Ara-C)-induced cytotoxicity and apoptosis in P388 cells. Life Sci.
75:353–365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang W, Zhang Q, Zhang M, Zhang Y, Li F
and Lei P: Analysis for the mechanism between the small cell lung
cancer and non-small cell lung cancer combing the miRNA and mRNA
expression profiles. Thorac Cancer. 6:70–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tan TW, Chou YE, Yang WH, Hsu CJ, Fong YC
and Tang CH: Naringin suppress chondrosarcoma migration through
inhibition vascular adhesion molecule-1 expression by modulating
miR-126. Int Immunopharmacol. 22:107–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fumarola C, Bonelli MA, Petronini PG and
Alfieri RR: Targeting PI3K/AKT/mTOR pathway in non small cell lung
cancer. Biochem Pharmacol. 90:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Poornima P, Weng CF and Padma VV: Neferine
from Nelumbo nucifera induces autophagy through the inhibition of
PI3K/Akt/mTOR pathway and ROS hyper generation in A549 cells. Food
Chem. 141:3598–3605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Papadimitrakopoulou V: Development of
PI3K/AKT/mTOR pathway inhibitors and their application in
personalized therapy for non-small-cell lung cancer. J Thorac
Oncol. 7:1315–1326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Choi UJ, Jee BK, Lim Y and Lee KH:
KAI1/CD82 decreases Rac1 expression and cell proliferation through
PI3K/Akt/mTOR pathway in H1299 lung carcinoma cells. Cell Biochem
Funct. 27:40–47. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee EJ, Kim DI, Kim WJ and Moon SK:
Naringin inhibits matrix metalloproteinase-9 expression and AKT
phosphorylation in tumor necrosis factor-alpha-induced vascular
smooth muscle cells. Mol Nutr Food Res. 53:1582–1591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Visweswaran GR, Gholizadeh S, Ruiters MH,
Molema G, Kok RJ and Kamps JA: Targeting rapamycin to podocytes
using a Vascular Cell Adhesion Molecule-1 (VCAM-1)-harnessed
SAINT-based lipid carrier system. PLoS One. 10:e01388702015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yurdagul A Jr, Sulzmaier FJ, Chen XL,
Pattillo CB, Schlaepfer DD and Orr AW: Oxidized LDL induces
FAK-dependent RSK signaling to drive NF-κB activation and VCAM-1
expression. J Cell Sci. 129:1580–1591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong L, Mi HJ, Zhu H, Zhou X and Yang H:
P-selectin-mediated platelet activation promotes adhesion of
non-small cell lung carcinoma cells on vascular endothelial cells
under flow. Mol Med Rep. 5:935–942. 2012. View Article : Google Scholar : PubMed/NCBI
|