Introduction
Ovarian cancer, one of the most common types of
cancer observed in females, has the highest mortality rate among
all gynecological malignancies and demonstrates rapid disease
progression (1). Approximately 70% of
patients with ovarian cancer are diagnosed in the advanced stages
of the disease and tumors are often accompanied by metastasis
(2). Cytoreductive surgery coupled
with adjuvant chemotherapy is widely applied as the standard
treatment for ovarian cancer (3,4). Although
the survival rate of patients with ovarian cancer has improved in
recent decades, almost all patients eventually experience tumor
recurrence due to resistance to chemotherapy agents, resulting in a
poor prognosis and a high mortality rate (5). Carboplatin is a chemotherapy drug used
in the treatment of a number of types of cancer, including ovarian,
lung, breast, cervical and esophageal cancer, and central nervous
system tumors, due to its easy administration, low toxicity and
high patient tolerance (6,7). Similarly to cisplatin, carboplatin
belongs to the group of platinum-based antineoplastic agents,
interacts with DNA in order to interfere with DNA repair, and
inhibits reproduction and general cell function, but demonstrates
fewer side effects compared with cisplatin (8). However, the development of resistance to
carboplatin in tumor cells causes patient insensitivity to
carboplatin chemotherapy and eventually reduces treatment outcome.
Therefore, the resistance of carboplatin is yet to be resolved and
the identification of suitable molecular targets responsible for
chemosensitivity to carboplatin is required in order for the
treatments and prognosis of ovarian cancer to be improved.
High mobility group protein box-1 (HMGB1) is a
highly conserved non-histone nuclear protein that exhibits dual
function (9). HMGB1 serves an
important structural function in chromatin organization, regulating
transcription by binding DNA and promoting protein assembly on
specific DNA targets within the nucleus (10–12). HMGB1
was also reported to be a critical cytokine in the cytoplasm that
is responsible for mediating a wide range of physiological and
pathological responses, including inflammation, infection and
injury response, and regulating cell differentiation and motility
(13–15). HMGB1 was reported to serve a crucial
role in numerous human diseases, including arthritis (16), sepsis (17), Alzheimer's disease (18), and cardiovascular disease (19). Additionally, HMGB1 was implicated in
the progression and development of several types of cancer,
including lymphoma (20), and breast
(21), lung (22), liver (23), stomach (24), colon (25), prostate (26) and ovarian cancer (27). HMGB1 overexpression was revealed to be
involved in the evasion of apoptosis, abnormal proliferation and
metastasis of tumor cells (28).
Higher HMGB1 expression levels were detected in ovarian cancer
tissues compared with expression in normal ovarian tissues
(29); and high HMGB1 expression was
reported to be associated with poor prognosis (30), suggesting that HMGB1 may be a
potential target for the diagnosis and treatment of ovarian cancer.
Furthermore, it was demonstrated that high HMGB1 expression in
cancer cells may be induced by several chemotherapeutic agents,
which may lead to the poor prognosis (31), suggesting that HMGB1 may be associated
with drug resistance. To the best of our knowledge, the association
between HMGB1 and resistance of ovarian cancer to carboplatin has
not previously been investigated. In the present study,
carboplatin-resistant ovarian cancer cells were developed and the
effects of HMGB1 on the sensitivity of the resistant ovarian cancer
cells to carboplatin were investigated.
Materials and methods
Cell culture
The human ovarian cancer SKOV3 cell line was
purchased from the Cell Resource Center, Institute of Basic Medical
Sciences, Chinese Academy of Medical Sciences (Beijing, China).
SKOV3 cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 µg/ml streptomycin and 100 U/ml penicillin
(Invitrogen; Thermo Fisher Scientific, Inc.) in a 37°C incubator
with humidified atmosphere containing 5% CO2.
Development of carboplatin-resistant
cells
To develop drug resistant cells, SKOV3 cells were
exposed to increasing concentrations of carboplatin ranging between
10 and 100 µg/ml in DMEM medium. To begin with, cells were cultured
on 60-mm culture plates for 24 h at 37°C. Carboplatin (10 µg/ml)
was then added for a further 48 h. Subsequently, the medium was
replaced with fresh drug-free DMEM medium. When cells reached 80%
confluency, cells were trypsinized, re-plated and re-treated with
20 µg/ml carboplatin. This process was repeated until clones
demonstrated resistance to 100 µg/ml carboplatin. Following
prolonged treatment (2 months) with increasing doses of
carboplatin, living clones were collected and named as
carboplatin-resistant SKOV3 cells (SKOV3-Carb), and were prepared
for follow-up experiments.
RNA interference of HMGB1
Small interfering (si)RNAs against HMGB1 (siHMGB1;
Sense: 5′-GGAAGUUUCUACUGUAUAGTT-3′; Antisense:
5′-CUAUACAGUAGAAACUUCCTT-3′) and the negative control (siCtrl;
Sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; Antisense:
5′-ACGUGACACGUUCGGAGAATT-3′) were designed and chemically
synthesized by Shanghai GenePharma Co., Ltd., (Shanghai, China). A
total of 75 pM siRNAs were transfected into SKOV3 and SKOV3-Carb
cells ~50–70% confluence in a six-well plate using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. Cells
were lysed 24 h after transfection, and the mRNA and protein
expression levels of HMGB1 were then analyzed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis, respectively.
Western blot analysis
Total protein was extracted from the SKOV3 cells and
SKOV3-Carb cells using a Total Protein Extraction kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China), according to the
manufacturer's protocol. Following centrifugation at 12,000 × g for
10 min at 4°C, the supernatant was collected and quantified using a
bicinchoninic acid quantification kit (Beyotime Institute of
Biotechnology, Haimen, China). The proteins (50 µg) were separated
by 12% SDS-PAGE and were transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% skimmed dried milk in Tris-buffered saline with
Tween-20 for 1 h at room temperature, and were incubated with mouse
monoclonal HMGB1 (1:2,000; cat. no. ab77302; Abcam, Cambridge, UK)
and mouse monoclonal GAPDH (1:5,000; cat. no. sc365062; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) primary antibodies overnight
at 4°C. Membranes were subsequently incubated with a goat
anti-mouse horseradish peroxidase-conjugated immunoglobulin G
secondary antibody (1:2,000; cat. no. sc-2005; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature. Visualization was
performed using ECL-detecting reagent (GE Healthcare, Chicago, IL,
USA). The protein blots were quantified by densitometry using Image
J version 1.47 software (National Institutes of Health, Bethesda,
MD, USA), and the expression was normalized to the internal
reference GAPDH.
RT-qPCR
Total RNA was extracted from SKOV3 and SKOV3-Carb
cells using a Total RNA Mini Plus kit (A&A Biotechnology,
Gdynia, Poland), according to the manufacturer's protocol. cDNA was
obtained by RT-qPCR using a RevertAid™ First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc., Pittsburgh, PA,
USA) in 50 µl reactions with an initial denaturation at 94°C for 3
min, followed by 35 cycles of 94°C for 30 sec, 60°C for 30 sec, and
72°C for 30 sec and a final elongation step of 72°C for 5 min, and
then was amplified using a TaqMan® Gene Expression assay
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with
fluorogenic fluorescein amidite-labeled probes using specific
primers for target proteins. PCR involved 40 amplification cycles
of 94°C for 10 sec, 53°C for 30 sec, and 72°C for 40 sec, followed
by final extension at 72°C for 10 min. The primers were as follows:
HMGB1 F, 5′-TACCGCCCCAAAATCAAAGG-3′ and R,
5′-TCTCATAGGGCTGCTTGTCA-3′; GAPDH F, 5′-CATGGCCTTCCGTGTTCCTA-3′ and
R, 5′-CCTGCTTCACCACCTTCTTGAT-3′. The real-time fluorescence
detection was performed using the ABI PRISM 7700 Sequence Detector
(Perkin-Elmer Applied Biosystems; Thermo Fisher Scientific, Inc.).
HMGB1 mRNA expression was calculated using the formula
2−ΔΔCq (32) and was
normalized to the level of GAPDH. The relative levels of HMGB1 mRNA
are presented as a percentage of the control.
Proliferation assay
Cell proliferation was evaluated using an MTT assay
(Sigma Aldrich; Merck KGaA, Darmstadt, Germany). A total of 2,000
untransfected SKOV3 and SKOV3-Carb cells were seeded onto each well
of a 96-well plate in 100 µl DMEM and were incubated with or
without 10, 30, 60, 120, 240, 480 µg/ml carboplatin for 24, 48 and
72 h at 37°C in a 5% CO2 incubator. Subsequently, cells
were incubated with 20 µl of 5 mg/ml MTT (Sigma-Aldrich; Merck
KGaA) for 4 h at 37°C incubator, prior to being lysed for 10 min by
addition of 200 µl dimethyl sulfoxide (OriGene Technologies, Inc.,
Rockville, MD, USA) used to dissolve the purple formazan.
Absorbance was measured at 490 nm using a Rainbow microplate reader
(Tecan Group Ltd., Grödig, Austria). Cell proliferation was
expressed as a percentage of the untreated control.
Apoptosis assay
Cells were cultured to 80% confluence and were
treated with 50 µg/ml carboplatin for 48 h at 37°C. Apoptosis was
analyzed using an Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) assay, according to the manufacturer's
protocol. The amount of phosphatidylserine on the outer surface of
the plasma membrane (a biochemical alteration unique to the
membranes of apoptotic cells) and the amount of PI, a dye that
easily enters dead cells or cells in the late stages of apoptosis
and binds DNA but not the plasma membrane of viable cells, were
detected. Fluorescence was detected using a FACSCalibur flow
cytometer by fluorescence activated cell sorter analysis, and data
were analyzed using CellQuestPro version 5.2 software (BD
Biosciences, San Jose, CA, USA). Cells with phosphatidylserine on
their surface were considered to be apoptotic.
Statistical analysis
Data were obtained from at least three experiments.
Statistical analysis was performed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA) for Microsoft™ Windows (Microsoft Corporation,
Redmond, WA, USA). Data are presented as the mean ± standard error
of the mean. One-way analysis of variance was used to assess
differences between groups. The Duncan method was employed for
pairwise comparison, followed by Bonferroni's correction. P<0.05
was considered to indicate a statistically significant
difference.
Results
Development of carboplatin-resistant
ovarian cancer SKOV3 cells
To examine the mechanism underlying carboplatin
resistance in ovarian cancer, a carboplatin-resistant ovarian
cancer SKOV3 cell model (SKOV3-Carb) was generated. Cell
proliferation was assessed using an MTT assay, and the
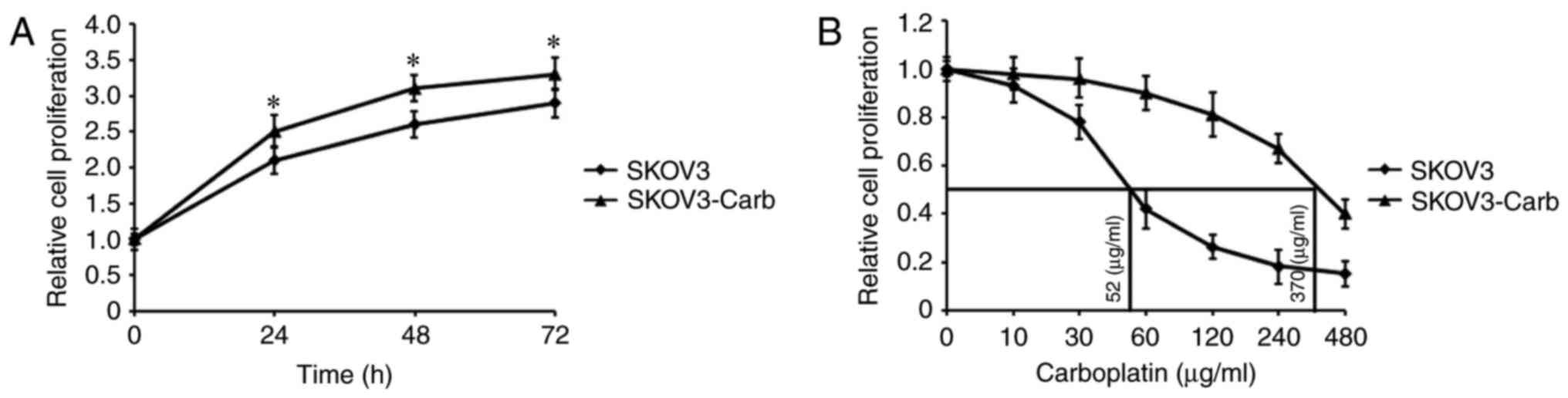
proliferation of SKOV3-Carb and SKOV3 cells are presented in
Fig. 1. The data demonstrated that
SKOV3-Carb cells significantly proliferated more rapidly than SKOV3
cells at 24, 48 and 72 h (P=0.037, P=0.018 and P=0.041,
respectively; Fig. 1A). Additionally,
the half-maximal inhibitory concentration (IC50) of
carboplatin was determined by exposing SKOV3-Carb and SKOV3 cells
to various concentrations of carboplatin for 72 h; the
IC50 values of the two types of cell were calculated to
be 370 and 52 µg/m, respectively (Fig.
1B).
Expression of HMGB1 in SKOV3-Carb and
SKOV3 cells
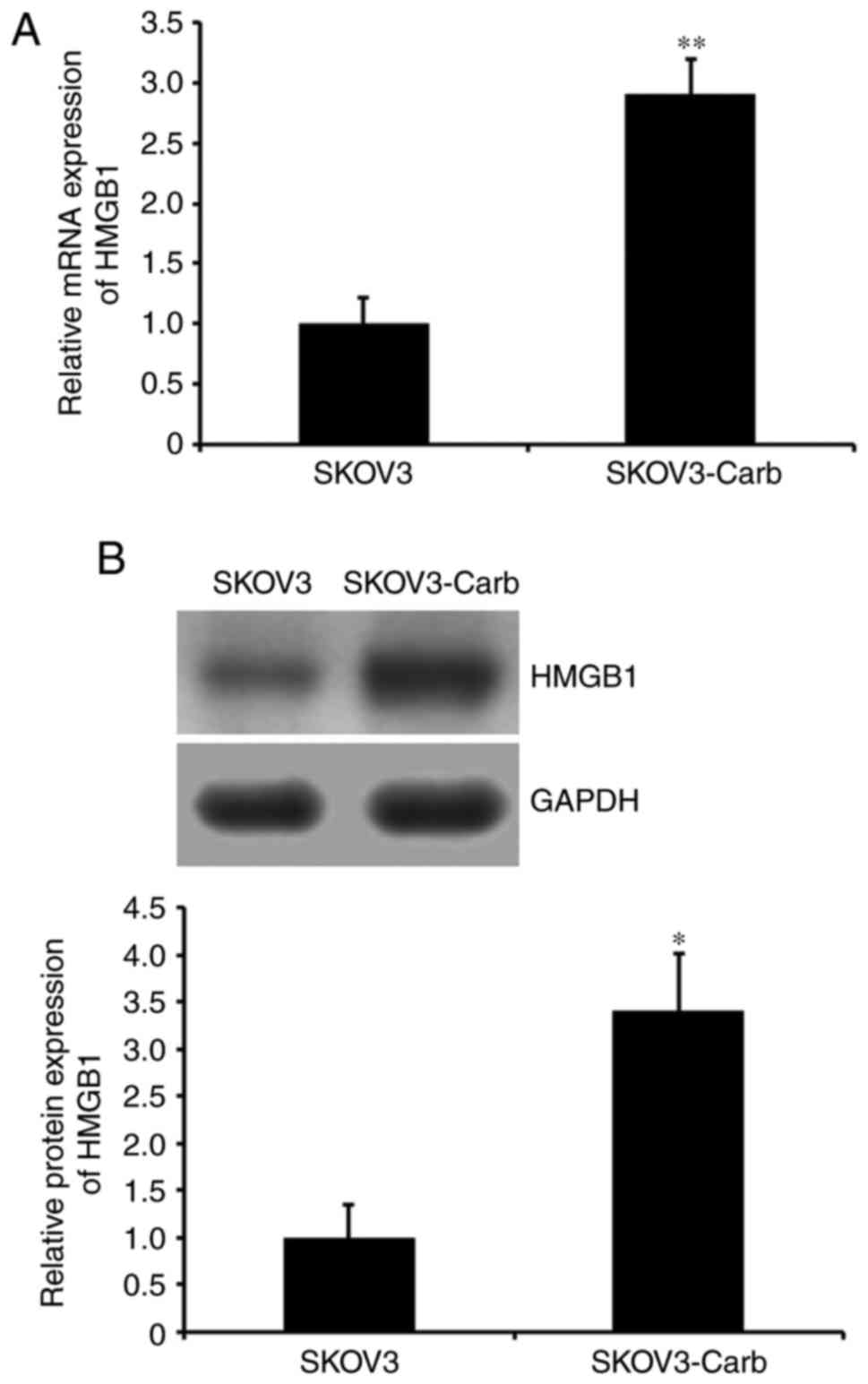
To determine whether HMGB1 is associated with the
development of carboplatin resistance in SKOV3-Carb cells, HMGB1
expression was analyzed. RT-qPCR and western blot analysis
demonstrated that both mRNA and protein expression levels of HMGB1
in SKOV3-Carb cells were significantly higher compared with that in
SKOV3 cells (P=0.008 and P=0.035, respectively; Fig. 2A and B). The results of the present
study suggested that HMGB1 may be associated with the resistance
development of ovarian cancer SKOV3 cells to carboplatin.
HMGB1 silencing re-sensitizes
resistant cells to carboplatin
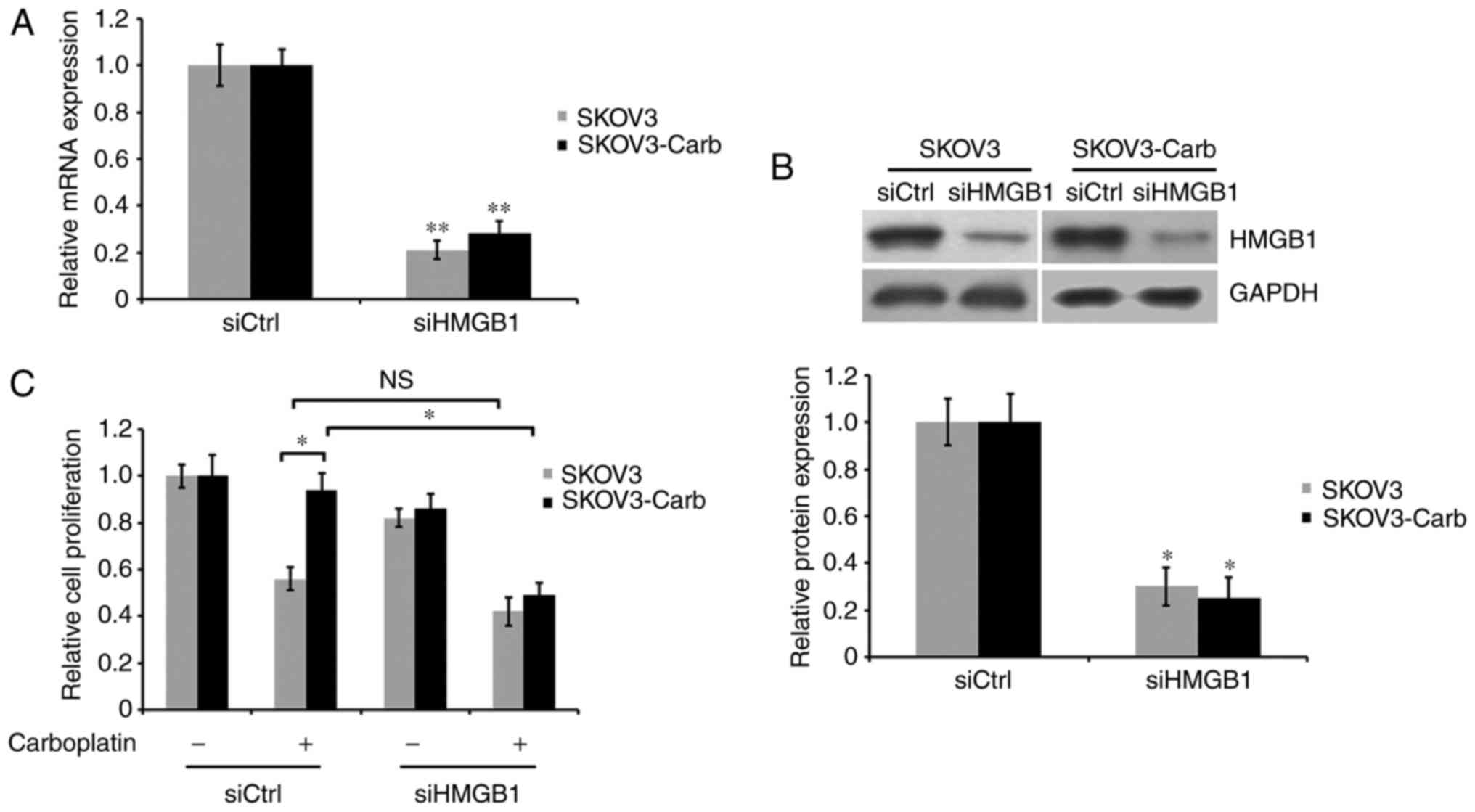
To investigate the effect of HMGB1 on the
development of carboplatin resistance in ovarian cancer SKOV3
cells, the corresponding siRNA of HMGB1 was used to decrease the
expression of HMGB1. RT-qPCR and western blot analysis indicated
that HMGB1 mRNA and protein expression in HMGB1-silenced cells was
significantly reduced by 80 and 71% for SKOV3 cells, 73 and 78% for
SKOV3-Carb cells, respectively, compared with expression in
non-silenced control cells (P=0.006 for SKOV3 and P=0.007 for
SKOV3-Carb, Fig. 3A; P=0.043 for
SKOV3 and P=0.031 for SKOV3-Carb, Fig.
3B). SKOV3-Carb and SKOV3 cells were transiently transfected
with control and HMGB1 siRNA, and were cultured in the presence or
absence of 50 µg/ml carboplatin for 72 h. Cell proliferation was
analyzed using an MTT assay. The results demonstrated that the
proliferative ability of HMGB1-silenced SKOV3-Carb cells was
significantly suppressed in response to carboplatin treatment in
comparison with carboplatin-treated SKOV3-Carb cells transfected
with the control siRNA. Additionally, the proliferative ability of
the SKOV3-Carb cells transfected with siCtrl was not markedly
affected by carboplatin treatment and revealed slightly increased
survival cells compared with the SKOV3 cells (P=0.038 and P=0.046,
respectively; Fig. 3C). However,
under identical experimental conditions no significant difference
was observed in the proliferation between HMGB1-silenced and
non-silenced SKOV3 cells.
HMGB1 silencing promotes the
SKOV3-Carb cell apoptosis induced by carboplatin treatment
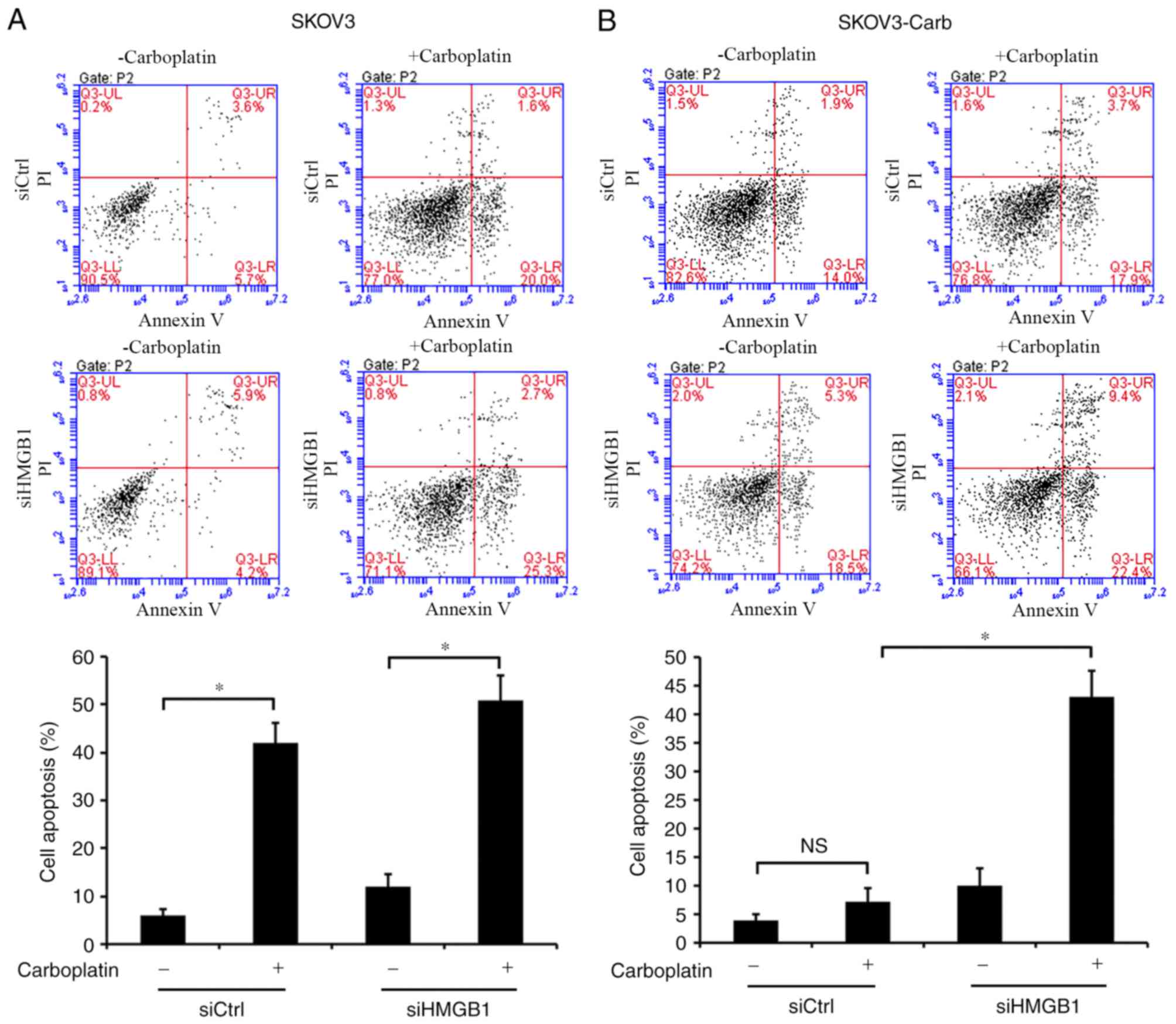
In order to elucidate the mechanism underlying HMGB1
silencing-induced sensitization of SKOV3-Carb cells to carboplatin,
apoptosis was evaluated using an Annexin V-FITC/PI assay.
SKOV3-Carb and SKOV3 cells were treated with control and HMGB1
siRNA, and were exposed to 50 µg/ml carboplatin for 48 h. The data
indicated that carboplatin treatment induced the apoptosis of both
siHMGB1- and siCtrl-transfected SKOV3 cells compared with apoptosis
in the corresponding untreated control cells (P=0.036 and P=0.029,
respectively; Fig. 4A). Notably,
HMGB1 silencing significantly stimulated the apoptosis mediated by
carboplatin in SKOV3-Carb cells relative to that of
siCtrl-transfected SKOV3-Carb cells (P=0.012; Fig. 4B). However, carboplatin treatment did
not significantly induce apoptosis in siCtrl-transfected resistant
SKOV3-Carb cells compared with the untreated siCtrl-transfected
control cells. These results suggested that HMGB1 may be associated
with the resistance of ovarian cancer SKOV3 cells to carboplatin
and that HMGB1 silencing may increase the sensitivity of resistant
cells to carboplatin by increasing the apoptosis induced by
carboplatin treatment.
Discussion
Ovarian cancer is the fifth most common cause of
cancer-associated mortality in women worldwide, and has the highest
rates of mortality among all gynecological malignancies in 2014
(1). Non-metastatic ovarian cancer
may be curable with surgery, while surgery is insufficient for
cancer treatment in cases of invasion and metastasis (33). In these cases, chemotherapy is the
alternative treatment strategy; however, the development of drug
resistance during treatment widely limits chemotherapeutic
effectiveness, leading to a poor prognosis (33). Tumor cells treated with drugs may not
only obtain resistance to the agent originally used, but may also
exhibit cross resistance to other agents, which may be mediated by
numerous molecular mechanisms (34–36).
Therefore, investigating the underlying mechanism of
chemoresistance is important for improving cancer treatment.
In the present study, a drug-resistant ovarian cell
model was constructed using various concentrations of carboplatin,
which simulated the condition of drug resistance development in
vivo. The growth of carboplatin-resistant ovarian cancer SKOV3
cells, SKOV3-Carb and SKOV3 cells were compared to observe the
cellular resistance phenotype. It was demonstrated that the
proliferation rate of SKOV3-Carb cells was faster and was decreased
by carboplatin treatment to a lesser extent than that of SKOV3
cells. Additionally, it has been reported that elevated HMGB1
expression may be induced by numerous chemotherapeutic agents,
including cisplatin, Adriamycin and methotrexate in lung cancer
cells (31). Carboplatin, as well as
cisplatin, belongs to the group of platinum-based antineoplastic
agents. Therefore, it is possible that HMGB1 expression may also be
increased by carboplatin treatment. HMGB1 is a cancer-promoting
protein, closely associated with tumorigenesis and development, by
promoting cell proliferation and motility. Increased HMGB1
expression has been detected in several types of human cancer
(20–27). HMGB1 was reported to promote cell
proliferation and invasion, and to inhibit cell apoptosis in
osteosarcoma MG-63 cells (28).
Additionally, HMGB1 overexpression was reported to be associated
with the proliferation and metastasis of lung adenocarcinoma cells
(37). Furthermore, higher HMGB1
expression was observed in ovarian cancer compared with in normal
ovarian tissues (29), and high HMGB1
expression was reported to be correlated with poor prognosis
(30). The presented study
demonstrated that HMGB1 expression levels were increased in
resistant SKOV3-Carb cells compared with expression in SKOV3 cells,
suggesting that HMGB1 may be associated with the development of
resistance to carboplatin in ovarian cancer cells.
It was reported that interference with HMGB1
significantly accelerated apoptosis and suppressed cellular
motility ability in breast cancer MCF-7 cells (38). Absence of HMGB1 inhibited the growth
and invasion of colon cancer LoVo cells (39). In the present study, to verify the
aforementioned hypothesis, the expression of HMGB1 was effectively
inhibited by employing its corresponding siRNA. The results of the
present study revealed that HMGB1 silencing enhanced the inhibitory
effect on proliferation and promoted the apoptosis induced by
carboplatin treatment in resistant SKOV3 cells and thus, restored
the carboplatin sensitivity to the cells. This suggested that HMGB1
may serve an important role in the modulation of carboplatin
resistance in ovarian cancer SKOV3 cells. Therefore, the results of
the present study indicated that the chemotherapeutic agent,
carboplatin, combined with the targeted therapy of
resistance-associated genes, including HMGB1, may serve as a more
effective therapy for ovarian cancer. However, whether cross
resistance of SKOV3-Carb cells to other therapeutic drugs is
generated or whether other factors are involved in the development
of resistance is yet to be investigated, which may be important in
the prognosis and treatment of cancer. The present study provided a
promising clinical therapeutic strategy for ovarian cancer. To
verify the universality of the therapeutic strategy in ovarian
cancer, additional ovarian cancer cell lines are required to
complete this study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All the works about this manuscript were carried out
by WS, including conception and design of the research and revision
of the manuscript, acquisition of data, analysis and interpretation
of data and statistical analysis and drafting the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: GLOBOCAN 2008 v1.2. Cancer incidence,
mortality and prevalence worldwideIARC CancerBase No. 10
(Internet). IARC Press; Lyon: 2010
|
|
2
|
Chen WQ, Zhang SW, Zou XN and Zhao P:
Cancer incidence and mortality in China, 2006. Chin J Cancer Res.
23:3–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
du Bois A, Quinn M, Thigpen T, Vermorken
J, Avall-Lundqvist E, Bookman M, Bowtell D, Brady M, Casado A,
Cervantes A, et al: 2004 consensus statements on the management of
ovarian cancer: final document of the 3rd international gynecologic
cancer intergroup ovarian cancer consensus conference (GCIG OCCC
2004). Ann Oncol. 16:viii7–viii12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li R and Linghu H: Treatment progress in
epithelial ovarian cancer. J Int Obstet Gynecol. 37:277–280.
2010.(in Chinese).
|
|
5
|
Brouwer-Visser J, Lee J, McCullagh K,
Cossio MJ, Wang Y and Huang GS: Insulinlike growth factor 2
silencing restores taxol sensitivity in drug resistant ovarian
cancer. PLoS One. 9:e1001652014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wheate NJ, Walker S, Craig GE and Oun R:
The status of platinum anticancer drugs in the clinic and in
clinical trials. Dalton Trans. 39:8113–8127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Apps MG, Choi EH and Wheate NJ: The
state-of-play and future of platinum drugs. Endocr Relat Cancer.
22:R219–R233. 2015.PubMed/NCBI
|
|
8
|
Wikipedia, the free encyclopedia:
Carboplatin. https://en.wikipedia.org/wiki/CarboplatinJune
2–2016
|
|
9
|
Taniguchi N, Yoshida K, Ito T, Tsuda M,
Mishima Y, Furumatsu T, Ronfani L, Abeyama K, Kawahara K, Komiya S,
et al: Stage-specific secretion of HMGB1 in cartilage regulates
endochondral ossification. Mol Cell Biol. 27:5650–5663. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Muller S, Scaffidi P, Degryse B, Bonaldi
T, Ronfani L, Agresti A, Beltrame M and Bianchi ME: New EMBO
members' review: the double life of HMGB1 chromatin protein:
architectural factor and extracellular signal. EMBO J.
20:4337–4340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang D, Kang R, Zeh HJ III and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta.
1799:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bell CW, Jiang W, Reich CF and Pisetsky
DS: The extracellular release of HMGB1 during apoptotic cell death.
Am J Physiol Cell Physiol. 291:C1318–C1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
LeBlanc PM, Doggett TA, Choi J, Hancock
MA, Durocher Y, Frank F, Nagar B, Ferguson TA and Saleh M: An
immunogenic peptide in the a-box of HMGB1 protein reverses
apoptosis-induced tolerance through RAGE receptor. J Biol Chem.
289:7777–7786. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lotze MT and DeMarco RA: Dealing with
death: HMGB1 as a novel target for cancer therapy. Curr Opin
Investig Drugs. 4:1405–1409. 2003.PubMed/NCBI
|
|
16
|
Park SY, Lee SW, Kim HY, Lee WS, Hong KW
and Kim CD: HMGB1 induces angiogenesis in rheumatoid arthritis via
HIF-1α activation. Eur J Immunol. 45:1216–1227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh A, Feng Y, Mahato N, Li J, Wu C and
Gong J: Role of high-mobility group box 1 in patients with acute
obstructive suppurative cholangitis-induced sepsis. J Inflamm Res.
8:71–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jang A, Liew H, Kim YM, Choi H, Kim S, Lee
SH, Ohshima T, Mikoshiba K and Suh YH: p35 deficiency accelerates
HMGB-1-mediated neuronal death in the early stages of an
Alzheimer's disease mouse model. Curr Alzheimer Res. 10:829–843.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park S, Yoon SJ, Tae HJ and Shim CY: RAGE
and cardiovascular disease. Front Biosci (Landmark Ed). 16:486–497.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meyer A, Staratschek-Jox A, Springwald A,
Wenk H, Wolf J, Wickenhauser C and Bullerdiek J: Non-Hodgkin
lymphoma expressing high levels of the danger-signalling protein
HMGB1. Leuk Lymphoma. 49:1184–1189. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brezniceanu ML, Volp K, Bosser S, Solbach
C, Lichter P, Joos S and Zornig M: HMGB1 inhibits cell death in
yeast and mammalian cells and is abundantly expressed in human
breast carcinoma. FASEB J. 17:1295–1297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng A, Tu Z and Yin B: The effect of
HMGB1 on the clinicopathological and prognostic features of
non-small cell lung cancer. Oncotarget. 7:20507–20519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cheng P, Dai W, Wang F, Lu J, Shen M, Chen
K, Li J, Zhang Y, Wang C, Yang J, et al: Ethyl pyruvate inhibits
proliferation and induces apoptosis of hepatocellular carcinoma via
regulation of the HMGB1-RAGE, and AKT pathways. Biochem Biophys Res
Commun. 443:1162–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akaike H, Kono K, Sugai H, Takahashi A,
Mimura K, Kawaguchi Y and Fujii H: Expression of high mobility
group box chromosomal protein-1 (HMGB-1) in gastric cancer.
Anticancer Res. 27:449–457. 2007.PubMed/NCBI
|
|
25
|
Kang HJ, Lee H, Choi HJ, Youn JH, Shin JS,
Ahn YH, Yoo JS, Paik YK and Kim H: Non-histone nuclear factor HMGB1
is phosphorylated and secreted in colon cancers. Lab Invest.
89:948–959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gnanasekar M, Kalyanasundaram R, Zheng G,
Chen A, Bosland MC and Kajdacsy-Balla A: HMGB1: A promising
therapeutic target for prostate cancer. Prostate Cancer.
2013:1571032013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Liu X, Zhang J and Zhao Y:
Targeting HMGB1 inhibits ovarian cancer growth and metastasis by
lentivirus-mediated RNA interference. J Cell Physiol.
227:3629–3638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo ZS, Liu Z, Bartlett DL, Tang D and
Lotze MT: Life after death: targeting high mobility group box 1 in
emergent cancer therapies. Am J Cancer Res. 3:1–20. 2013.PubMed/NCBI
|
|
29
|
Machado L, Moseley P, Moss R, Nolan C,
Rampage J, Chan S and Durrant LG: High-mobility group protein 1
(HMGB1) is an independent predictor of poor survival in ovarian
cancerProceedings of the National Cancer Research Institute (NCRI)
Cancer Conference. Liverpool, UK: 2014
|
|
30
|
Zhi H, Ma HY and Chen XL: The changes and
clinical significance of serum levels of HMGB1, VEGF before and
after operation in patients with ovarian carcinoma. Hebei Med J.
36:1297–1299. 2014.(In Chinese).
|
|
31
|
Zhang R, Li Y, Wang Z, Chen L, Dong X and
Nie X: Interference with HMGB1 increases the sensitivity to
chemotherapy drugs by inhibiting HMGB1-mediated cell autophagy and
inducing cell apoptosis. Tumour Biol. 36:8585–8592. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Slack JL, Bi W, Livak KJ, Beaubier N, Yu
M, Clark M, Kim SH, Gallagher RE and Willman CL: Pre-clinical
validation of a novel, highly sensitive assay to detect
PML-RARalpha mRNA using real-time reverse-transcription polymerase
chain reaction. J Mol Diagn. 3:141–149. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harding M: Ovarian cancer. https://patient.info/doctor/ovarian-cancer-proDecember
2–2016
|
|
34
|
Szakacs G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Germann UA and Chambers TC: Molecular
analysis of the multidrug transporter, P-glycoprotein.
Cytotechnology. 27:31–60. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu Y, Zhi F, Xu G, Tang X, Lu S, Wu J and
Hu Y: Overcoming multidrug resistance in vitro and in vivo by a
novel P-glycoprotein inhibitor 1416. Biosci Rep. 32:559–566. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun KK, Ji C, Li X, Zhang L, Deng J, Zhong
N and Wu XY: Overexpression of high mobility group protein B1
correlates with the proliferation and metastasis of lung
adenocarcinoma cells. Mol Med Rep. 7:1678–1782. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ni P, Zhang Y, Liu Y, Lin X, Su X, Lu H,
Shen H, Xu W, Xu H and Su Z: HMGB1 silence could promote MCF-7 cell
apoptosis and inhibit invasion and metastasis. Int J Clin Exp
Pathol. 8:15940–15946. 2015.PubMed/NCBI
|
|
39
|
Li Z, Wang H, Song B, Sun Y, Xu Z and Han
J: Silencing HMGB1 expression by lentivirus-mediated small
interfering RNA (siRNA) inhibits the proliferation and invasion of
colorectal cancer LoVo cells in vitro and in vivo. Zhonghua Zhong
Liu Za Zhi. 37:664–670. 2015.(in Chinese). PubMed/NCBI
|