Introduction
Endometrial cancer is a malignant tumor in the
female reproductive system with a high incidence rate, accounting
for approximately 25% of all the female reproductive system tumors,
and its incidence in developed countries (ranking fourth) is higher
than that in developing countries (ranking seventh) (1–2).
Endometrial cancer often occurs in perimenopausal and
postmenopausal women (3), and a
present investigation report has shown that the age of patients at
the onset of endometrial cancer becomes smaller and smaller
(4). Although the five-year survival
rate of patients has improved with the development of medical
technology, the prognosis of some endometrial cancer patients is
poor, causing death (5,6); thus, the early diagnosis of endometrial
cancer patients is crucial. The mortality rate of patients with
endometrial cancer in the advanced stage is much higher than that
of patients in the early stage (7).
Improving the specificity and sensitivity of the diagnosis of
endometrial cancer patients is an important means to improve the
survival rate of patients.
Ultrasound plays an extremely important role in the
diagnosis of tumors. Ultrasound has many advantages such as
noninvasiveness, high diagnostic sensitivity, no radioactivity and
simple methods. It has been widely used in the diagnosis of tumors
(8,9).
In addition, ultrasound can reduce the invasive examination for
patients and risks of invasive operation for patients through its
initial screening (10). Tumor
abnormal protein (TAP) is a kind of abnormal glycoprotein and
calmodulin complex expressed after the mutation of oncogenes and
tumor suppressor genes in cells (11). It has been reported that abnormal
glycoproteins are closely related to the occurrence, development,
invasion, metastasis and prognosis of cancers (12). Therefore, the detection of TAP in
serum can provide an important reference for the clinical diagnosis
of tumors.
In the present study, 248 patients with suspected
endometrial cancer who were admitted to the Gynecology Department
of The Second People's Hospital of Liaocheng (Linqing, China) from
September 2013 to September 2015 were selected, and the diagnostic
accordance rate of TAP combined with vaginal ultrasound was
analyzed.
Materials and methods
Clinical data
A total of 248 women patients (average age of
41.6±11.6 years) with suspected endometrial cancer who were
admitted to the Gynecology Department of The Second People's
Hospital of Liaocheng from September 2013 to September 2015 were
selected and randomly divided into the control (n=124) and the
observation group (n=124). The control group received conventional
ultrasound examination; the observation, underwent TAP combined
with conventional ultrasound examination, and all patients were
definitely diagnosed by hysteroscopy and diagnostic curettage
examination. Patients without tumor history, without liver, kidney
and other organ dysfunction, and without abnormal hemorrhage or
coagulation dysfunction before operation were included. Patients
with unqualified curettage specimens, receiving treatment before,
with excessive large tumor mass or with other pulmonary or chest
wall diseases were excluded. This study was approved by the Ethics
Committee of the Second People's Hospital of Liaocheng, and
patients or their family members signed informed consent.
Instruments and methods
Hitachi Hivision Avivs color Doppler ultrasound
diagnostic machine (Hitachi, Ltd., Tokyo, Japan) was applied for
conventional ultrasound examination so as to observe patients'
tumor diameter, shape, edge status, internal echo information and
whether there were space occupying lesions. At the same time, the
fasting whole blood was collected from the included patients in the
early morning, and TAP detection was conducted. Abnormal
carbohydrate chain glycoprotein detection kits were purchased from
Thermo Fisher Scientific, Inc., (Waltham, MA, USA), and the image
analyzer for TAP detection was purchased from Zhejiang Aicor
Medical Technology Co., Ltd. (Zhejiang, China).
Statistical analysis
The experimental results were analyzed by using SPSS
22.0 software (IBM Corp., Armonk, NY, USA), and the analysis of
variance followed by post hoc test (Least Significant Difference)
was used for compari-sons among multiple groups. Count data were
expressed as percentage and detected by χ2 test. The
sensitivity and specificity of conventional ultrasound and TAP
combined with conventional ultrasound in the diagnosis of
endometrial cancer were calculated, respectively. T0he diagnostic
values of conventional ultrasound and the combination of the two
methods in the diagnosis of endometrial cancer were studied by
using the receiver operating characteristic (ROC) curve. P<0.05
was considered to indicate a statistically sgnificant
difference.
Results
Basic data
In this study, 248 patients at an average age of
41.6±11.6 years with suspected endometrial cancer were included.
After the pathological diagnosis by curettage, it was found that
there were 60 cases of endometrial polyps, 96 of endometrial
hyperplasia, 17 of uterine fibroids and 75 cases of endometrial
cancer. In addition, the menopause data of patients were collected,
in which there were 43 premenopausal patients (17.34%), confirming
that the age of patients at the onset of endometrial cancer becomes
smaller (Table I).
 | Table I.Clinical data of patients. |
Table I.
Clinical data of patients.
| Clinical data | Control group
(n=124) |
|---|
| Age (years) | 41.6±11.6 |
| Definite diagnostic
results |
|
|
Endometrial polyps | 60 |
|
Endometrial hyperplasia | 96 |
| Uterine
fibroids | 17 |
|
Endometrial cancer | 75 |
| Nationality n
(%) |
|
| Han | 219 (88.31) |
| National
minority | 29 (11.69) |
| Place of residence n
(%) |
|
| City | 165 (66.53) |
|
Countryside | 83 (33.47) |
| Menopausal status n
(%) |
|
|
Premenopause | 43 (17.34) |
|
Postmenopause | 205 (82.66) |
Diagnostic results of the two
programs
Of 248 patients receiving hysteroscopy and
diagnostic curettage examination, there were 75 patients with
early-stage endometrial cancer and 173 benign patients (including
60 patients with endometrial polyps, 96 with endometrial
hyperplasia and 17 with uterine fibroids). The total diagnostic
accordance rate of conventional ultrasound for endometrial lesions
was 87.90% (n=218), and for early-stage endometrial carcinoma was
90.67% (n=68). For patients with endometrial polyps, the rate was
88.33% (n=53); with endometrial hyperplasia was 87.50% (n=84); with
uterine fibroids was 76.47% (n=13). For TAP combined with vaginal
ultrasound for endometrial lesions, the rate was 94.35% (n=234),
and for early-stage endometrial cancer was 94.67% (n=71). For
diagnosis for patients with endometrial polyps the rate was 95.00%
(n=57), with endometrial hyperplasia it was 93.75% (n=90), and with
uterine fibroids it was 94.12% (n=16); The diagnostic accordance
rates of TAP combined with conventional ultrasound for endometrial
lesions were higher than those of simple conventional ultrasound
(P<0.05) (Table II). Imaging
manifestations of conventional ultrasound (Table III).
 | Table II.Diagnostic accordance rates of TAP
combined with conventional ultrasound and conventional
ultrasound. |
Table II.
Diagnostic accordance rates of TAP
combined with conventional ultrasound and conventional
ultrasound.
| Rate | Conventional
ultrasound | TAP combined with
conventional ultrasound | P-value |
|---|
| Total diagnostic
accordance rate | 218 (87.90) | 234 (94.35) | 0.031 |
| Accordance rate of
endometrial cancer | 68 (90.67) | 71 (94.67) | 0.047 |
| Accordance rate of
endometrial hyperplasia | 84 (87.50) | 90 (93.75) | 0.032 |
| Accordance rate of
endometrial polyps | 53 (88.33) | 57 (95.00) | 0.024 |
| Accordance rate of
uterine fibroids | 13 (76.47) | 16 (94.12) | 0.017 |
 | Table III.Imaging manifestations of conventional
ultrasound. |
Table III.
Imaging manifestations of conventional
ultrasound.
|
| Endometrial
polyps | Endometrial
hyperplasia | Uterine fibroids | Endometrial
cancer |
|---|
| Uterine volume | No obvious
increase | No obvious change;
increase in some parts | Increased | Obviously
increased |
| Echo | Hypoecho Equal echo
Hyperecho | Slightly
enhanced | Hypoecho; hyperecho
in tiny minority | Unevenly
thickened |
| Intimal thickness
change | No significant | Thickened | Thickened | Thickened |
| Lesion border | Clear | Clear uterine cavity
line | Relatively clear | Rough surface; some
unclear or even vanished borders |
| Lesion area
liquidation/calcification | Calcification in few
lesion areas | No
liquidation/calcification |
Liquidation/calcification on in few lesion
areas | Liquidation in some
lesion areas |
TAP detection results
The image analyzer for TAP detection was used to
analyze the polarity of TAP detection results, which showed that
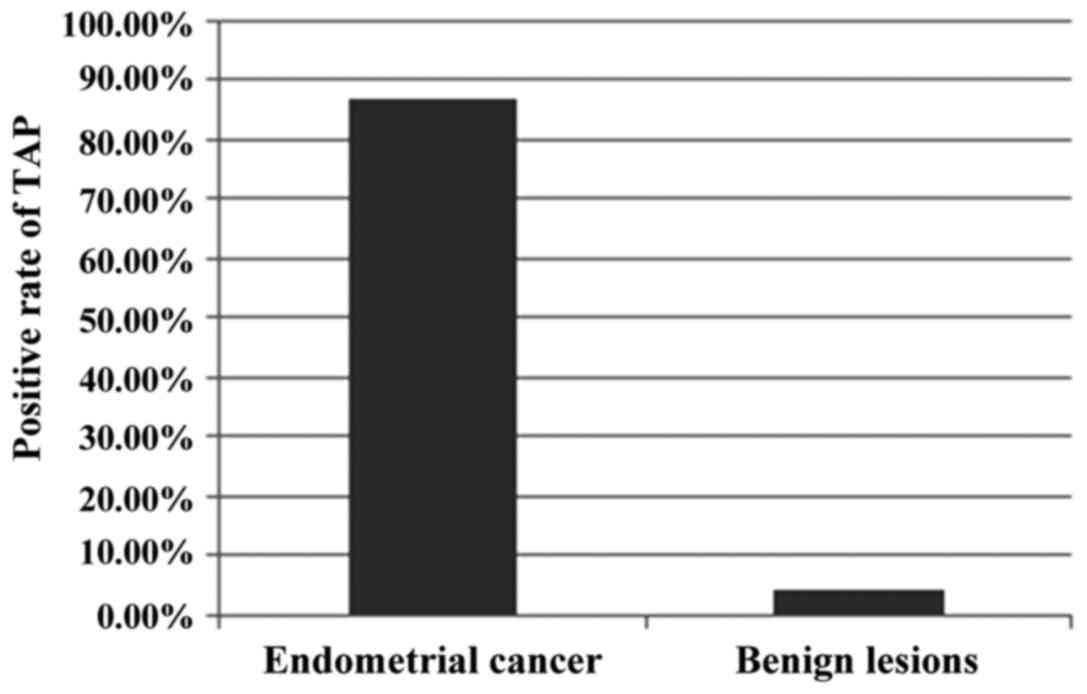
the positive rate of TAP detection was 86.67% (n=65) in patients
with endometrial cancer and 4.37% (n=8) with benign lesions; there
was a significant difference between the two groups of patients
(P<0.05). Among the patients with benign lesions and positive
TAP detection results, there were 5 cases of uterine fibroids, 2 of
endometrial polyps and 1 of endometrial hyperplasia (Fig. 1).
Sensitivity and specificity of the two
programs
The sensitivity and specificity of conventional
ultrasound to endometrial cancer were 85.7 and 89.7%, respectively,
and those of TAP combined with vaginal ultrasound to endometrial
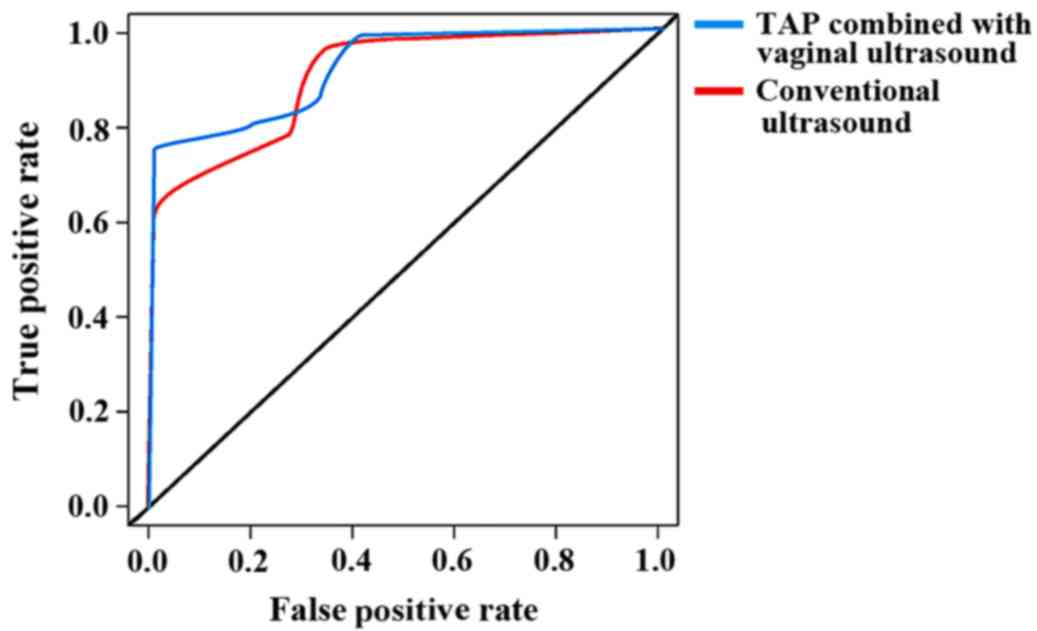
cancer were 90.0 and 92.8%, respectively. ROC analysis results
revealed that the AUC of convention ultrasound in the diagnosis of
endometrial cancer was 0.754 [95% confidence interval (CI):
0.211–2.534], and that of TAP combined with vaginal ultrasound in
the diagnosis of endometrial cancer was 0. 814 (95% CI:
0.517–0.932); the difference between the two programs was
significant (P=0.011) (Fig. 2).
Discussion
Prognoses of endometrial cancer in the advanced
stage and some special cases of endometrial cancer are very poor,
and their 5-year survival rates are relatively low, so the early
diagnosis of patients with endometrial cancer is very necessary
(6,7).
TAP is a type of complex that is expressed and released by tumor
cells and can be found in the blood of tumor patients, which
provides a very convenient specimen for the early screening of
tumors (13,14). TAP has become a hot research object
for researchers, especially on the screening and early diagnosis of
tumors, since it was discovered by the Union of Soviet Socialist
Republics (USSR) scholars, Kostyantin, A. and Galakhin (15,16).
However, there are few diagnostic studies in patients with
endometrial cancer in the early stage. This study was expected to
provide a basis for clinical diagnosis of endometrial cancer in the
early stage and improve the survival rate of patients.
In the present study, 248 patients with suspected
endometrial cancer were included, and with other tumors were
excluded. TAP combined with conventional ultrasound was used for
diagnosis, whose results were compared with those of diagnostic
curettage detection, so as to analyze the value of TAP combined
with conventional ultrasound in the diagnosis of endometrial cancer
in the early stage. The study's results showed that the diagnostic
accordance rate of TAP for patients with endometrial cancer was
86.67%, and that of conventional ultrasound for patients with
endometrial cancer was 90.67%, and that of the combination of the
two was 94.35%; the diagnostic accordance rate of TAP and
conventional ultrasound for patients with endometrial cancer were
lower than that of the combination of the two (P<0.05),
indicating that TAP detection can improve the diagnostic effect of
conventional ultrasound on endometrial cancer. In addition, ROC
curve results also revealed that the combined diagnostic method had
a high diagnostic value (AUC=0.814). The sensitivity of the TAP
detection system is the expressed TAP when the number of tumor
cells reaches 100,000 and above, but it takes about one year for
the number of tumor cells to proliferate to 100,000 before the
tumor has not formed a mass, which is extremely important for the
treatment and survival of patients, especially of those with
malignant tumors with fast development and poor prognosis (17–20). There
are few researchers studying the effect of TAP detection in the
diagnosis of endometrial cancer in the early stage, so the
conclusions in this study still need to be further validated. This
study also had its advantages: All patients with past tumor history
were excluded to avoid the interference in TAP detection; a
self-controlled test was conducted so that the interference of test
samples was avoided. TAP is also used in many kinds of cancers,
such as gastric, thyroid and colorectal cancer, and it is of
positive significance in improving the accordance rate of tumor
diagnosis (21–22). These indirectly confirmed the
diagnostic value of TAP in the diagnosis of endometrial cancer.
Benign endometrial lesions were also analyzed, which showed that
approximately 20–40% of patients suffered from atypical endometrial
hyperplasia before the onset of endometrial cancer. TAP combined
with vaginal ultrasound also has a relatively diagnostic high
accordance rate (93.75%) for endometrial hyperplasia, and among
patients with TAP positive detection results, there was 1 case of
endometrial hyperplasia. Due to the limitation of experimental
time, the patients were not followed-up, and further investigation
is needed for confirmation of the results.
In summary, TAP combined with transvaginal
ultrasound in the diagnosis of early-stage endometrial cancer has a
higher accuracy rate, and it is worth promoting and applying in
clinical practice.
Acknowledgements
Not applicable.
Funding
This study was supported by the Liaocheng Health
Medical Letter [2016] No.3-146.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
AM and DF designed the study. DF and FY collected
and analyzed the patient data. AM prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Second People's Hospital of Liaocheng (Linqing, China) and
informed consent was signed by the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Burke WM, Orr J, Leitao M, Salom E, Gehrig
P, Olawaiye AB, Brewer M, Boruta D, Villella J, Herzog T, et al SGO
Clinical Practice Endometrial Cancer Working Group, ; Society of
Gynecologic Oncology Clinical Practice Committee, : Endometrial
cancer: A review and current management strategies: part I. Gynecol
Oncol. 134:385–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al ESMO-ESGO-ESTRO Endometrial Consensus Conference Working
Group, : ESMO-ESGO-ESTRO Consensus Conference on Endometrial
Cancer: Diagnosis, treatment and follow-up. Ann Oncol. 27:16–41.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCarroll ML, Armbruster S, Frasure HE,
Gothard MD, Gil KM, Kavanagh MB, Waggoner S and von Gruenigen VE:
Self-efficacy, quality of life, and weight loss in overweight/obese
endometrial cancer survivors (SUCCEED): A randomized controlled
trial. Gynecol Oncol. 132:397–402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chlebowski RT, Anderson GL, Sarto GE,
Haque R, Runowicz CD, Aragaki AK, Thomson CA, Howard BV,
Wactawski-Wende J, Chen C, et al: Continuous combined estrogen plus
progestin and endometrial cancer: The women's health initiative
randomized trial. J Natl Cancer Inst. 108:3502015. View Article : Google Scholar
|
|
5
|
Plataniotis G and Castiglione M; ESMO
Guidelines Working Group, : Endometrial cancer: ESMO Clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21 Suppl 5:41–45. 2010. View Article : Google Scholar
|
|
6
|
Carvajal-Carmona LG, O'Mara TA, Painter
JN, Lose FA, Dennis J, Michailidou K, Tyrer JP, Ahmed S, Ferguson
K, Healey CS, et al National Study of Endometrial Cancer Genetics
Group (NSECG), ; Australian National Endometrial Cancer Study Group
(ANECS), ; RENDOCAS; Australian Ovarian Cancer Study (AOCS), ;
GENICA Network, : Candidate locus analysis of the TERT-CLPTM1L
cancer risk region on chromosome 5p15 identifies multiple
independent variants associated with endometrial cancer risk. Hum
Genet. 134:231–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suri V and Arora A: Management of
endometrial cancer: A review. Rev Recent Clin Trials. 10:309–316.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Siddiqui MM, Rais-Bahrami S, Turkbey B,
George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL,
Linehan WM, et al: Comparison of MR/ultrasound fusion-guided biopsy
with ultrasound-guided biopsy for the diagnosis of prostate cancer.
JAMA. 313:390–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Claudon M, Dietrich CF, Choi BI, Cosgrove
DO, Kudo M, Nolsøe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC,
et al: Guidelines and good clinical practice recommendations for
contrast enhanced ultrasound (CEUS) - update 2008. Ultrasound Med
Biol. 39:187–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solomon SD and Saldana F: Point-of-care
ultrasound in medical education-stop listening and look. N Engl J
Med. 370:1083–1085. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El Hage F, Durgeau A and Mami-Chouaib F:
TAP expression level in tumor cells defines the nature and
processing of MHC class I peptides for recognition by
tumor-specific cytotoxic T lymphocytes. Ann N Y Acad Sci.
1283:75–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Guo X, Min Y and Xu J: § (TAP)
examination contributes to primary diagnosis of bladder cancer. Int
J Clin Exp Med. 8:18528–18532. 2015.PubMed/NCBI
|
|
13
|
Motoi T, Yoshida A, Motoi N, Kato I, Okuma
T, Tonooka A, Horiguchi S, Goto T and Hishima T: Abstract 3542:
Abnormal intracytoplasmic accumulation of autophagy-related protein
p62/SQSTM1 characterizes giant cells of giant cell tumor of bone.
Cancer Res. 76 Suppl 14:3542. 2016. View Article : Google Scholar
|
|
14
|
Doorduijn EM, Sluijter M, Querido BJ,
Oliveira CC, Achour A, Ossendorp F, van der Burg SH and van Hall T:
TAP-independent self-peptides enhance T cell recognition of
immune-escaped tumors. J Clin Invest. 126:784–794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moniaux N, Andrianifahanana M, Brand RE
and Batra SK: Multiple roles of mucins in pancreatic cancer, a
lethal and challenging malignancy. Br J Cancer. 91:1s633–1638.
2004. View Article : Google Scholar
|
|
16
|
Hakomori S: Glycosylation defining cancer
malignancy: New wine in an old bottle. Proc Natl Acad Sci USA.
99:10231–10233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dube DH and Bertozzi CR: Glycans in cancer
and inflammation-potential for therapeutics and diagnostics. Nat
Rev Drug Discov. 4:477–488. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rutkowski MR, Stephen TL, Svoronos N,
Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E,
Escovar-Fadul X, Nguyen JM, Cadungog MG, et al: Microbially driven
TLR5-dependent signaling governs distal malignant progression
through tumor-promoting inflammation. Cancer Cell. 27:27–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J and Huang XE: Clinical application
of serum tumor abnormal protein from patients with gastric cancer.
Asian Pac J Cancer Prev. 16:4041–4044. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Z, Cai J, Yu Y, Fang H, Si Y, Jankee
JJ and Shen M: Tumor abnormal protein as a novel biomarker in
papillary thyroid carcinoma. Clin Lab. 63:479–485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu XY and Huang XE: Clinical application
of serum tumor abnormal protein (TAP) in colorectal cancer
patients. Asian Pac J Cancer Prev. 16:3425–3428. 2015. View Article : Google Scholar : PubMed/NCBI
|