Introduction
Endometriosis is an estrogen-dependent chronic
inflammatory disorder due to the presence of endometrial tissue
outside the uterine cavity (1), and
it affects 6–10% of women of childbearing age; however, this
percentage increases to 17% among infertile women and 40–60% of
women suffering from chronic pelvic pain (2–7). There is
solid evidence that endometriosis-affected patients have a doubled
risk of ovarian cancer, particularly of the endometrioid and
clear-cell histology, and this association is also supported by
molecular and histological evidence (5–12).
Although the hypothesis that ovarian cancers develop
from ‘neometaplasia’ of the ovarian surface mesothelium cannot be
ruled out (13), there is currently
compelling morphological and molecular evidence that any ovarian
carcinoma subtype may originate from Müllerian epithelium present
in the pelvis, which involves the ovary only at a later stage
(12). According to this hypothesis,
endometrioid carcinoma and clear-cell carcinoma may originate from
ectopic endometrial tissue localized in the ovary (endometriotic
cyst or endometrioma). To date, the exact mechanism by which this
malignant transformation occurs has not been clearly determined,
and further studies are required to elucidate the mechanisms
underlying this transformation.
The unfolded protein response (UPR) is a system by
which the endoplasmic reticulum (ER) responds to endogenous or
exogenous stress (14), and is
activated by the accumulation of misfolded proteins in the ER lumen
(14). Recent studies have revealed
the role of UPR in neoplastic transformation, since it promotes
cell survival in a hypoxic environment and plays a protective role
against cell death caused by ER stress (15). Cell survival in a hypoxic environment
is promoted by attenuation of pro-apoptotic signals, cellular
metabolism changes and stimulation of neo-angiogenesis. The
activation of UPR may promote cell survival or stimulate apoptosis,
depending on the context. This response occurs via the activation
of ER transmembrane receptors, namely protein kinase R (PKR)-like
endoplasmic reticulum kinase (PERK), activating transcription
factor 6 (ATF6α) and inositol-requiring enzyme 1-spliced X-box
binding protein 1 (IRE1α-XBP1). Under acute stress conditions, the
UPR system enhances the ability to fold in order to cope with an
increase in protein synthesis, which helps cancerous cells survive
(15). Under conditions of chronic ER
stress, if the normal function of the cell is not restored within a
certain time span or the disruption is prolonged (15), normal cells undergo apoptosis-mediated
cell death, while cancer cells activate strategies to neutralize
the apoptotic stimulus; in normal cells, there is an attenuation of
the ATF6α and IRE1α-XBP1 pathways, with an activation of an
apoptotic stimulus mediated by CCAAT/enhancer-binding protein
homologous protein (CHOP) (15).
Conversely, cancer cells exhibit a constitutive activation of
IRE1α-XBP1, or glucose-regulated protein 78 (GRP78)/BIP
overexpression, with anti-apoptotic effects. Although the UPR
molecular pathway has been studied in several tumor models, its
role in the pathogenesis of endometrioid ovarian carcinoma has not
yet been fully elucidated.
Therefore, this pilot study was conducted with the
aim of analyzing the expression profile of UPR genes in
endometrioid ovarian carcinoma and to evaluate its possible
involvement in the neoplastic progression of endometriosis.
Materials and methods
The present study was conducted on women of
childbearing age with a histopathological diagnosis of
International Federation of Gynecology and Obstetrics (FIGO) stage
IA (16) endometrioid ovarian
carcinoma, who underwent complete surgical staging (peritoneal
washing, bilateral salpingo-oophorectomy, hysterectomy, multiple
peritoneal biopsies of all abdominal fields, infracolic
omentectomy, and pelvic and para-aortic lymph node dissection up to
the renal veins) (17) between
January 2010 and December 2015 at the Woman's Health Sciences
Department, Gynecologic Section-Università Politecnica delle Marche
(Ancona, Italy), retrospectively recruited from our database.
Patient studies
In order to evaluate the possible involvement of the
UPR pathway in the oncogenesis of ovarian endometrioid carcinoma,
we compared the expression of ATF6, GRP78, CHOP (also referred to
as DNA damage-inducible transcript 3, DDIT3) and XBP1 from a sample
of ovary affected by endometrioid ovarian carcinoma and from a
sample of the healthy contralateral ovary of the same patient.
Subsequently, we compared the UPR gene expression between
endometriotic cysts and eutopic healthy endometrial tissues, with
the healthy ovary as reference.
Samples of endometriotic ovarian cysts and eutopic
endometrium were obtained from patients undergoing laparoscopic
surgery for endometrial cyst removal following diagnosis of
moderate/severe endometriosis. None of the women were pregnant at
the time of surgery. In this subgroup of women, we collected two
types of tissue: a portion of the cyst following laparoscopic
removal, and a sample of endometrial tissue following hysteroscopic
biopsy. All the tissues were stored at −80°C.
The samples of healthy endometrial tissue were
collected from women of childbearing age, without previous
diagnosis of endometriosis, undergoing hysteroscopy for in
vitro fertilization due to male infertility. All the women
underwent diagnostic laparoscopy that ruled out endometriosis. A
sample of endometrial tissue was obtained by hysteroscopic
biopsy.
Healthy women and endometriosis patients were
consecutively enrolled from January 2016 onwards and, in order to
avoid the influence of confounding factors, patients with previous
diagnosis of hypertension, diabetes mellitus, renal disease,
proteinuria, cardiovascular, hepatic, endocrine (thyroid
abnormalities, prolactin imbalance, polycystic ovary syndrome) and
metabolic disorders, smokers, and individuals prone to alcohol
abuse were excluded from both groups. None of the included subjects
had taken any medications or hormone therapy during the 3 months
preceding surgery (progestin, oral contraceptives, danazol or
gonadotropin-releasing hormone agonists).
The present study was conducted in accordance with
The Code of Ethics of the World Medical Association (Declaration of
Helsinki). All participants signed an informed consent, granting
their permission to tissue sampling and data collection. The study
protocol was approved by the local Ethics Committee (Marche
Regional Ethics Committee, Riuniti di Ancona Hospital, Italy).
Preparation of paraffin-embedded
ovarian tissue samples
Total RNA was isolated from paraffin-embedded
ovarian tissues using the PureLink™ FFPE Total RNA Isolation kit
(cat. no. K156002; Life Technologies; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) that specifically purifies RNA from
paraffin-embedded tissues without the use of chemical solvents for
deparaffinization. Total RNA was quantified, and the purity of the
preparation was tested using NanoDrop (Thermo Fisher Scientific,
Inc.): Briefly, 1 µl of purified RNA was placed on the instrument,
260 and 260/230 nm ratio wavelength was measured and the instrument
software then calculated the amount of purified RNA. Reverse
transcription to cDNA was performed using Advanced iScript cDNA
synthesis kit for RT-qPCR (cat. no. 1725038; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The reverse transcription was performed
by preparing a reaction mixture containing ~1 µg RNA and random
primers provided by the kit. As the RNA obtained from
paraffin-embedded samples is typically very fragmented, the
efficacy of the amplification reaction by qPCR is generally low; to
avoid that, cDNA was pre-amplified using SsoAdvanced™ PreAmp
Supermix (cat. no. 1725160; Bio-Rad Laboratories, Inc.) in
conjunction with a pool of primers of target genes.
Preparation of frozen endometrial
tissue samples
The purification of total RNA collected from ~30 mg
of frozen (−80°C) endometrial tissues was performed using SV Total
RNA Isolation System kit (Z3101; Promega Corporation, Madison, WI,
USA), using diethyl pyrocarbonate-treated equipment. Total RNA was
quantified, and the purity of the preparation was tested using
NanoDrop. Reverse transcription to cDNA was performed with the
ImProm-II™ Reverse Transcription System kit (cat. no. A3800) using
2 µg RNA and random primers (cat. no. C1181) (both from Promega
Corporation.
Gene expression analysis by qPCR
qPCR was performed using SsoAdvanced™
SYBR®-Green SuperMix (cat. no. 1725271; Bio-Rad
Laboratories, Inc.), in a CFX96 thermocycler (Bio-Rad). The
reaction conditions are summarized as follows: 95°C for 30 sec, and
40 cycles at 95°C for 30 sec, and at 60°C for 30 sec. Melting
curves were analyzed after the reaction to assess the specificity
of the amplification products; the curves obtained showed no
evidence of dimers or non-specific signals. The primers used for
the study were designed using Primer 3 software. The primer
sequences are listed in Table I.
 | Table I.Primer sequences of the analyzed
genes. |
Table I.
Primer sequences of the analyzed
genes.
| Gene | Forward | Reverse |
|---|
| ATF6 |
TTCCTCCACCTCCTTGTCAG |
ACCCATCCTCGAAGTTCATGA |
| CHOP |
TGTTAAAGATGAGCGGGTGG |
TGCTTTCAGGTGTGGTGATG |
| GRP78 |
TGCCTACCAAGAAGTCTCAGA |
ACGAGGAGCAGGAGGAATTC |
| XBP-1 |
CTGAGTCCGCAGCAGGTG |
CCAAGTTGTCCAGAATGCCC |
| GAPDH |
TCCACTGGCGTCTTCACC |
GGCAGAGATGATGACCCTTTT |
Gene expression was reported as fold-change of
expression in the pathological ovarian tissues compared with
control (healthy contralateral ovary), setting 1 as the control
reference value. Relative gene expression was calculated using the
ΔΔCq method (18), converted for
statistical purposes to relative expression ratio using the
2−ΔΔCq formula. All gene expression data were normalized
to the expression of the endogenous reference gene,
glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
Statistical software SPSS v.20.0 (SPSS, Inc.,
Chicago, IL, USA) was used for data analysis. Fold-change
expression of UPR genes was presented as the mean ± standard error
of the mean. The one-sample signed-rank sum test was used for
comparison with reference tissues (healthy ovary, reference value =
1). The Mann-Whitney test for independent samples was used for
comparison of UPR genes fold-change expression between
endometriotic cysts and endometrioid carcinoma of the ovary. The
Kruskal-Wallis test with the Conover-Iman post hoc test was used
for the comparisons among endometriotic cysts, eutopic endometrium
and healthy endometrium. A P-value of <0.05 was considered to
indicate statistically significant differences.
Results
The UPR gene expression was examined in tissue
samples derived from a total of i) 6 patients diagnosed with FIGO
stage IA endometrioid ovarian carcinoma who met the inclusion
criteria; ii) the first 6 patients consecutively diagnosed with
ovarian endometriotic cysts; and iii) 6 healthy patients without
previous diagnosis of endometriosis.
Endometrioid carcinoma of the ovary
vs. contralateral healty ovary
We first compared the UPR gene expression between
endometrioid carcinoma of the ovary and the contralateral healthy
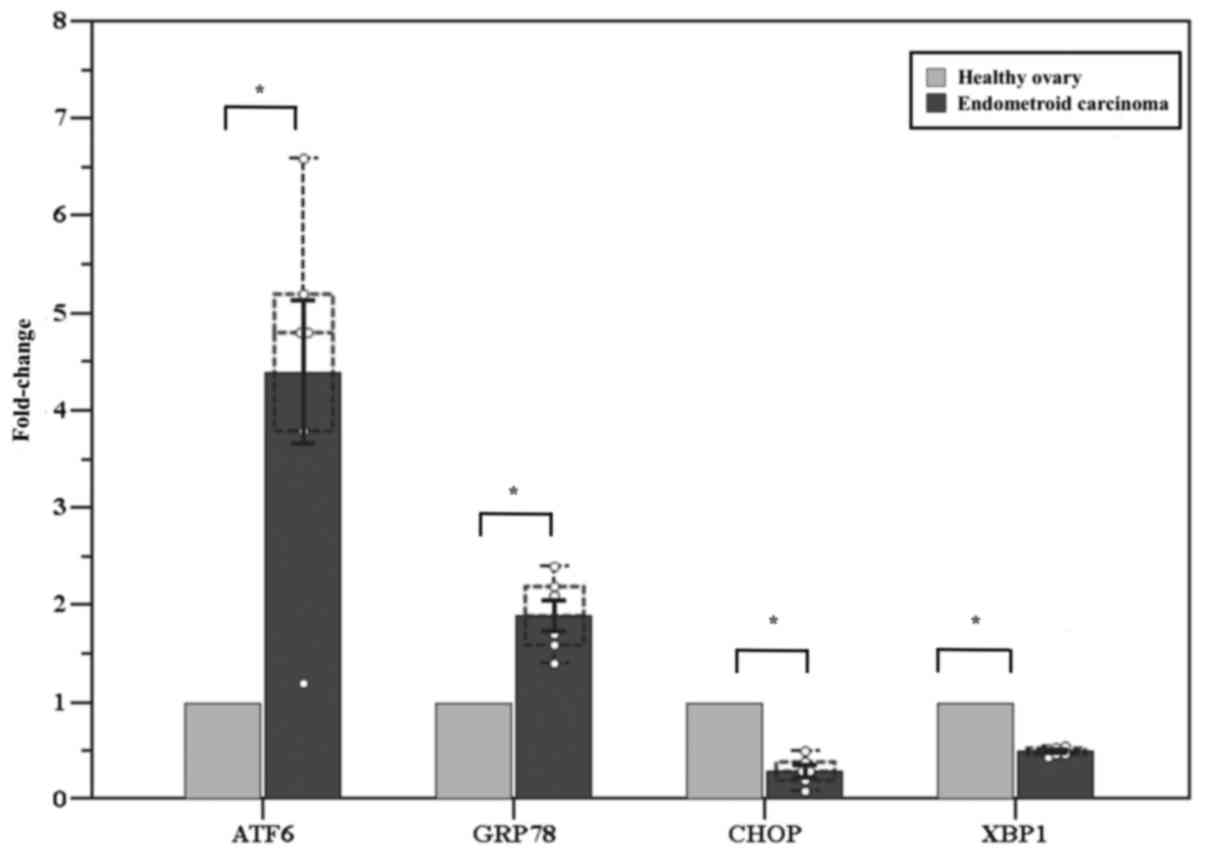
ovary of the same patient. A significantly higher expression of
ATF6 (fold-change: 4.4±0.7, P=0.01) and GRP78 (fold-change:
1.9±0.2, P=0.02) was observed in the affected ovary compared with
the healthy contralateral ovary, while CHOP and XBP1 exhibited
significantly lower expression (fold-change: 0.3±0.1, P<0.001
and 0.5±0.02, P<0.001, respectively) (Fig. 1).
Endometriotic cysts vs. healthy ovary
vs. endometrioid carcinoma of the ovary
We subsequently evaluated the UPR gene expression in
endometriotic cysts compared with healthy ovary and endometrioid
carcinoma of the ovary. The expression of ATF6 and GRP78 was
significantly higher in the endometriotic cysts compared with that
in the healthy ovary (fold-change: 5.2±0.5, P<0.001 and
14.6±2.2, P=0.002, respectively). CHOP expression in endometriotic
cysts was similar to that in the healthy ovary (fold-change:
0.8±0.1, P=0.06), while XBP1 was overexpressed (fold-change:
4.2±0.2, P<0.001). The comparison between endometriotic cysts
and endometrioid carcinoma of the ovary is presented in Table II: ATF6 expression was similar
between the two tissues, while GRP78, CHOP and XBP1 were more
highly expressed in endometriotic cysts (Table II).
 | Table II.Fold-change gene expression analysis
of unfolded protein response genes in endometriosic cysts and
endometrioid carcinoma of the ovary. |
Table II.
Fold-change gene expression analysis
of unfolded protein response genes in endometriosic cysts and
endometrioid carcinoma of the ovary.
| Gene | Endometriosic
cysts | Endometrioid
carcinoma | P-value |
|---|
| ATF6 | 5.2±0.5 | 4.4±0.7 | 0.42 |
| GRP78 | 14.6±2.2 | 1.9±0.4 | <0.01 |
| CHOP | 0.8±0.1 | 0.3±0.1 | <0.01 |
| XBP1s | 4.2±0.2 | 0.5±0.3 | <0.01 |
Endometriotic cysts vs. eutopic
endometrium vs. healthy endometrium vs. healthy ovary
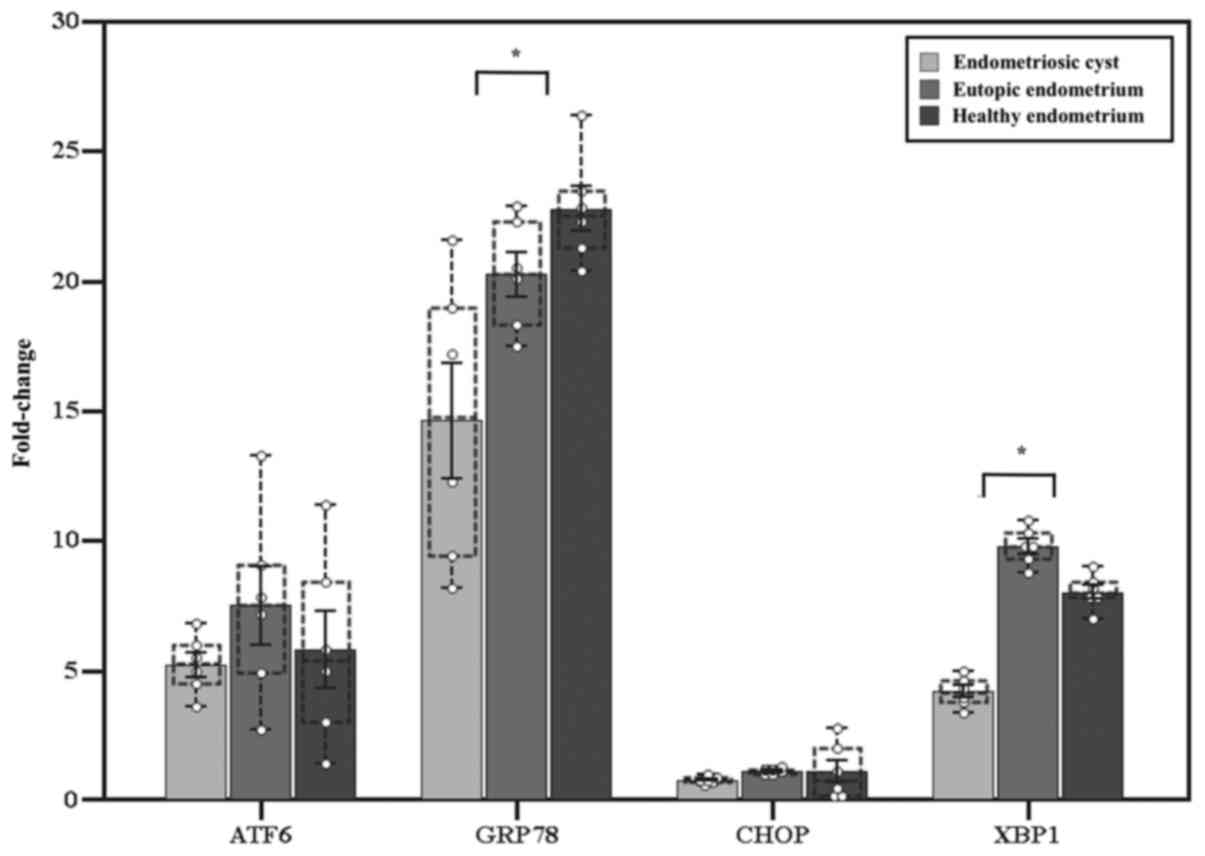
The fold-change in the expression of ATF6 was
significantly higher in endometriotic cysts, eutopic endometrium
and healthy endometrium compared with that in healthy ovarian
tissue (5.0±0.5, P=0.03; 7.5±1.5, P=0.03; and 5.8±1.5, P=0.03,
respectively), without significant differences between them
(P=0.51). GRP78 was more highly expressed in endometrial-derived
tissues (14.6±2.2, P=0.03; 20.3±0.9, P=0.03; and 22.8±0.9, P=0.03,
respectively), with a significantly higher expression in
endometrial tissue compared with endometriotic cysts (P=0.01). XBP1
s was also overexpressed (4.2±0.2, P=0.03; 9.8±0.3, P=0.03; and
8.0±0.3, P=0.03, respectively), with a higher level of expression
in endometrial tissue compared with endometriotic cysts
(P<0.001). CHOP expression was similar among all examined
tissues (0.8±0.1, P=0.06; 1.4±0.1, P=0.13; and 1.1±0.4, P=0.84,
respectively) (Fig. 2).
Discussion
In the present pilot study, we observed a difference
in UPR gene expression between endometrioid ovarian carcinoma and
healthy ovarian tissue. More specifically, ATF6 and GRP78 were more
highly expressed in endometrioid ovarian carcinoma, while the
expression of CHOP and XBP1 was lower. The simultaneous increase of
ATF6 and GRP78 in cancer samples is in line with the current
literature (15,19–21).
Indeed, several studies indicated that, among the different
overexpressed proteins following UPR activation, GRP78 plays a
crucial role in tumor proliferation, survival, metastasis and
resistance to a wide variety of treatments (19,22–24). The
reduction of CHOP expression also reflects an important fact:
Although its function in oncogenesis has not yet been clearly
determined, a number of studies demonstrated that CHOP induction in
response to prolonged ER stress causes apoptosis of pre-malignant
cells, thus preventing tumor progression (25). Therefore, reduced CHOP expression,
observed in endometrioid ovarian carcinoma, would be expected, as
the reduction of apoptosis is a prerequisite for malignant
transformation and tumor progression.
The role of XBP1 in neoplastic transformation has
not yet been fully elucidated, although its oncosuppressive role
appears to be predominant. While some studies highlighted the
importance of the IRE1/XBP1 axis for cell survival in hypoxic
environment and tumor growth (26,27),
others reported that several tumor types harbored mutations of
IRE1α, leading to loss of kinase and/or endo-ribonuclease activity,
splicing inhibition and reduction of XBP1, and promoting
tumorigenesis (28–30). Thus, the decreased expression of XBP1
detected in endometrioid cancer is in line with a consistent part
of the literature.
In summary, the alterations in the expression of UPR
genes observed in endometrioid carcinoma (increased expression of
ATF6 and GRP78, and reduced expression of CHOP and XBP1) appear to
promote cell survival pathways and tumor progression.
Considering the association of endometrioid cancer
with endometriosis, supported by years of epidemiological research
(4,5)
and by accumulating evidence supporting that this histotype of
ovarian cancer arises from endometriosis cells localized in the
ovary rather than ovarian cells, it is possible to hypothesize that
even endometriotic cysts may harbor UPR gene alterations that may
be involved in the neoplastic progression to endometrioid ovarian
cancer. In order to test this hypothesis, we compared the UPR gene
expression pattern between endometriotic cysts and endometrioid
carcinoma, using healthy ovarian tissue as reference.
The endometriotic cysts exhibited a simultaneous
increase in ATF6 and GRP78, no difference in CHOP expression and
overexpression of XBP1 in comparison with the healthy ovary; this
UPR gene expression pattern was partly different from that of
endometrioid cancer. More specifically, while ATF6 expression was
similar between the two tissues, GRP78 was more highly expressed in
endometriosis compared with endometrioid carcinoma. Conversely,
CHOP expression tended to decrease in ovarian cancer compared with
that in both endometriotic cysts and healthy ovarian tissue, which
was in line with the role of CHOP demonstrated in different tumor
models and discussed above. Finally, the expression of XBP1 was
notably higher in endometriotic cysts compared with endometrioid
ovarian carcinoma. The latter two differences in the expression of
CHOP and XBP1 are compatible with neoplastic transformation of
endometriotic cysts and may be acquired following ovarian
localization of ectopic endometrial cells. However, the first two
differences appear to indicate increased anti-apoptotic activity in
endometriotic cysts, which may be partly maintained in endometrioid
carcinoma, but may also be present in eutopic endometrial cells and
be implicated in their migration to the ovary (5,31).
In order to investigate when the alterations of UPR
genes are acquired in the pathogenetic process from eutopic
endometrium to endometriotic cysts and endometrioid ovarian
carcinoma, and whether they result from migration outside the
uterus or are physiologically expressed in the analyzed tissues, we
compared their expression pattern within endometriotic cysts,
eutopic endometrium and healthy endometrium using healthy ovarian
tissue as reference.
We noted a greater basal UPR activation in
endometrial tissue (except for CHOP) compared with ovarian tissue,
which may be due to an innate diversity of the analyzed tissues. It
is well known that UPR is constitutively active in cells with
secretory functions and subjected to hormonal stimulation, such as
endometrial cells (32–35).
No difference in CHOP expression was observed
between endometriotic cysts, eutopic endometrium, healthy
endometrium and healthy ovary, while it was reduced only in
endometrioid ovarian cancer, confirming the oncosuppressive role of
CHOP, as previously described; therefore, alterations of this gene
appear to be acquired at a late stage of neoplastic progression,
after the ovarian localization of ectopic endometrial cells.
XBP1 exhibited a significantly higher expression in
eutopic and healthy endometrial tissue in comparison to
endometriotic cysts. Conclusively, XBP1 has a high baseline
expression in healthy endometrium, being a secretory tissue, it
then gradually decreases in endometriosis and, to a higher degree,
in ovarian carcinoma. Since the exact role of XBP1 is not clearly
defined in the literature, it is important to further investigate
its potential oncosuppressive role, considering that its reduction
has been previously demonstrated in other tumor models.
In conclusion, our results support the hypothesis
that alterations in the UPR genes CHOP and XBP1 are involved in the
neoplastic progression of endometrioid ovarian cancer and are
acquired following ovarian localization of ectopic endometrial
cells. The evidence indicating that ATF6 and GRP78 are not
increased in ovarian carcinoma compared with endometriosis does not
allow considering these two genes as being directly involved in
neoplastic transformation from endometriosis to endometrioid
ovarian carcinoma. However, the reduction of CHOP in ovarian
carcinoma, characterized by reduction of apoptosis, confirms its
pro-apoptotic role. Conversely, XBP1 is overexpressed in
endometrial tissues, which are secretory tissues, and is gradually
reduced in endometriosis and, even more considerably, in
endometrioid ovarian carcinoma, further supporting the concept of
XBP1 as a marker of neoplastic transformation. Elucidating these
mechanisms may represent an important step for a better
understanding of cancer pathogenesis and for the development of
customized therapies in the future.
The results of this pilot study are encouraging and
may lay the foundation for the design of future studies with larger
samples, a wider spectrum of UPR genes, and using additional
methodologies such as western blotting and immunohistochemistry, in
order to confirm the obtained results and achieve a better
understanding of the pathogenesis of endometriosis cysts and
endometrioid ovarian carcinoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AC and FS conceived the study and revised the final
version of the manuscript. GDC, MS and AT collected the data and
drafted the manuscript. FL and EDL performed the experiments. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All participants provided signed informed consent
granting permission for tissue sampling and data collection. The
study protocol was approved by the Marche Regional Ethics Committee
of Riuniti di Ancona Hospital, Italy.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATF6
|
activating transcription factor 6
|
|
CHOP
|
CCAAT/-enhancer-binding protein
homologous protein
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose-regulated protein 78
|
|
IRE1
|
inositol-requiring enzyme 1
|
|
PERK
|
protein kinase R (PKR)-like
endoplasmic reticulum kinase
|
|
UPR
|
unfolded protein response
|
|
XBP1
|
spliced X-box binding protein 1
|
References
|
1
|
Buggio L, Somigliana E, Barbara G,
Frattaruolo MP and Vercellini P: Oral and depot progestin therapy
for endometriosis: Towards a personalized medicine. Expert Opin
Pharmacother. 18:1569–1581. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giudice LC: Clinical practice.
Endometriosis. N Engl J Med. 362:2389–2398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kennedy S, Bergqvist A, Chapron C,
D'Hooghe T, Dunselman G, Greb R, Hummelshoj L, Prentice A and
Saridogan E; ESHRE Special Interest Group for Endometriosis and
Endometrium Guideline Development Group, : ESHRE guideline for the
diagnosis and treatment of endometriosis. Hum Reprod. 20:2698–2704.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giudice LC and Kao LC: Endometriosis.
Lancet. 364:1789–1799. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pavone ME and Lyttle BM: Endometriosis and
ovarian cancer: Links, risks and challenges faced. Int J Womens
Health. 7:663–672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donnez J, Nisolle M, Gillet N, Smets M,
Bassil S and Casanas-Roux F: Large ovarian endometriomas. Hum
Reprod. 11:641–646. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waller KG, Lindsay P, Curtis P and Shaw
RW: The prevalence of endometriosis in women with infertile
partners. Eur J Obstet Gynecol Reprod Biol. 48:135–139. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sampson J: Endometrial carcinoma of the
ovary arising in endometrial tissue of that organ. Arch Surg.
10:1–72. 1925. View Article : Google Scholar
|
|
9
|
Kokcu A: Relationship between
endometriosis and cancer from current perspective. Arch Gynecol
Obstet. 284:1473–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dorner AJ, Wasley LC and Kaufman RJ:
Increased synthesis of secreted proteins induces expression of
glucose-regulated proteins in butyrate-treated Chinese hamster
ovary cells. J Biol Chem. 264:20602–20607. 1989.PubMed/NCBI
|
|
11
|
Poole EM, Lin WT, Kvaskoff M, De Vivo I,
Terry KL and Missmer SA: Endometriosis and risk of ovarian and
endometrial cancers in a large prospective cohort of U.S. nurses.
Cancer Causes Control. 28:437–445. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dubeau L and Drapkin R: Coming into focus:
The nonovarian origins of ovarian cancer. Ann Oncol. 24 Suppl
8:viii28–viii35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prat J: New insights into ovarian cancer
pathology. Annals of Oncology. 23 Suppl 10:x111–x117. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schröder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rutkowski DT, Arnold SM, Miller CN, Wu J,
Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D and Kaufman RJ:
Adaptation to ER stress is mediated by differential stabilities of
pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol.
4:e3742006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prat J; FIGO Committee on Gynecologic
Oncology, : FIGO's staging classification for cancer of the ovary,
fallopian tube and peritoneum: Abridged republication. J Gynecol
Oncol. 26:87–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ledermann JA, Raja FA, Fotopoulou C,
Gonzalez-Martin A, Colombo N and Sessa C; ESMO, Guidelines Working
Group, : Newly diagnosed and relapsed epithelial ovarian carcinoma:
ESMO Clinical Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 Suppl 6:vi24–vi32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bifulco G, Miele C, Di Jeso B, Beguinot F,
Nappi C, Di Carlo C, Capuozzo S, Terrazzano G, Insabato L and
Ulianich L: Endoplasmic reticulum stress is activated in
endometrial adenocarcinoma. Gynecol Oncol. 125:220–225. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shuda M, Kondoh N, Imazeki N, Tanaka K,
Okada T, Mori K, Hada A, Arai M, Wakatsuki T, Matsubara O, et al:
Activation of the ATF6, XBP1 and grp78 genes in human
hepatocellular carcinoma: A possible involvement of the ER stress
pathway in hepatocarcinogenesis. J Hepatol. 38:605–614. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feldman DE, Chauhan V and Koong AC: The
unfolded protein response: A novel component of the hypoxic stress
response in tumors. Mol Cancer Res. 3:597–605. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jamora C, Dennert G and Lee AS: Inhibition
of tumor progression by suppression of stress protein GRP78/BiP
induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci USA.
93:7690–7694. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bernstein H, Payne CM, Bernstein C,
Schneider J, Beard SE and Crowley CL: Activation of the promoters
of genes associated with DNA damage, oxidative stress, ER stress
and protein malfolding by the bile salt, deoxycholate. Toxicol
Lett. 108:37–46. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reddy RK, Mao C, Baumeister P, Austin RC,
Kaufman RJ and Lee AS: Endoplasmic reticulum chaperone protein
GRP78 protects cells from apoptosis induced by topoisomerase
inhibitors: Role of ATP binding site in suppression of caspase-7
activation. J Biol Chem. 278:20915–20924. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huber AL, Lebeau J, Guillaumot P, Pétrilli
V, Malek M, Chilloux J, Fauvet F, Payen L, Kfoury A, Renno T, et
al: p58IPK-mediated attenuation of the proapoptotic
PERK-CHOP pathway allows malignant progression upon low glucose.
Mol Cell. 49:1049–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Romero-Ramirez L, Cao H, Nelson D, Hammond
E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, et
al: XBP1 is essential for survival under hypoxic conditions and is
required for tumor growth. Cancer Res. 64:5943–5947. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujimoto T, Yoshimatsu K, Watanabe K,
Yokomizo H, Otani T, Matsumoto A, Osawa G, Onda M and Ogawa K:
Overexpression of human X-box binding protein 1 (XBP-1) in
colorectal adenomas and adenocarcinomas. Anticancer Res.
27:127–131. 2007.PubMed/NCBI
|
|
28
|
Ghosh R, Wang L, Wang ES, Perera BG,
Igbaria A, Morita S, Prado K, Thamsen M, Caswell D, Macias H, et
al: Allosteric inhibition of the IRE1α RNase preserves cell
viability and function during endoplasmic reticulum stress. Cell.
158:534–548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niederreiter L, Fritz TM, Adolph TE,
Krismer AM, Offner FA, Tschurtschenthaler M, Flak MB, Hosomi S,
Tomczak MF, Kaneider NC, et al: ER stress transcription factor Xbp1
suppresses intestinal tumorigenesis and directs intestinal stem
cells. J Exp Med. 210:2041–2056. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu H, Abulimiti M, Liu H, Su XJ, Liu CH
and Pei HP: RITA enhances irradiation-induced apoptosis in
p53-defective cervical cancer cells via upregulation of IRE1α/XBP1
signaling. Oncol Rep. 34:1279–1288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bulun SE: Endometriosis. N Engl J Med.
360:268–279. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shaffer AL, Shapiro-Shelef M, Iwakoshi NN,
Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, et al:
XBP1, downstream of Blimp-1, expands the secretory apparatus and
other organelles and increases protein synthesis in plasma cell
differentiation. Immunity. 21:81–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sengupta S, Sharma CG and Jordan VC:
Estrogen regulation of X-box binding protein-1 and its role in
estrogen induced growth of breast and endometrial cancer cells.
Horm Mol Biol Clin Investig. 2:235–243. 2010.PubMed/NCBI
|
|
34
|
Bruner-Tran KL, Herington JL, Duleba AJ,
Taylor HS and Osteen KG: Medical management of endometriosis:
Emerging evidence linking inflammation to disease pathophysiology.
Minerva Ginecol. 65:199–213. 2013.PubMed/NCBI
|
|
35
|
Burney RO, Talbi S, Hamilton AE, Vo KC,
Nyegaard M, Nezhat CR, Lessey BA and Giudice LC: Gene expression
analysis of endometrium reveals progesterone resistance and
candidate susceptibility genes in women with endometriosis.
Endocrinology. 148:3814–3826. 2007. View Article : Google Scholar : PubMed/NCBI
|