Introduction
Colorectal cancer (CRC) is one of the most common
types of cancer in Korea and other developed countries (1,2). The
carcinogenesis of CRC is driven by genetic and epigenetic changes
in tumor cells and is also influenced by tumor-host interactions
(3–5).
Previous studies have reported the potential association of
molecular markers such as BRAF, KRAS, and MMR with prognosis in
patients with CRC (6,7). Identification of the predictors of poor
prognosis and disease recurrence is important for the successful
treatment of these patients and for the discovery of new
therapeutic strategies.
Analysis of host immunity against human malignancy
is increasingly important in cancer research and treatment
(8,9).
Immune checkpoints represent a major defense system of the tumor
against antitumor immunity in the host and play an important role
in suppressing the T-cell-mediated immune response in the tumor
microenvironment (9,10). Programmed cell death ligand-1 (PD-L1)
expression in tumor cells suppresses the cytotoxic activity of
CD8-positive T-cells (11–13). Up-regulation of PD-L1 has been
reported in malignancies including lung cancer, esophageal cancer,
renal cell carcinoma, ovarian cancer, CRC, and breast cancer and
may play a central role in tumor-immune system interactions
(14–19).
PD-L1 expression in tumor cells may be linked to a
weakened host immune response, resulting in immune escape and an
adverse prognosis in several malignancies (20–22).
However, the prognostic role of PD-L1 expression in CRC is less
clear, with some studies reporting conflicting results as to
whether PD-L1 expression indicates a better or worse prognosis. The
prognostic evaluation in these studies has been limited because
they included patients at various disease stages and different
study populations (23–27). The aim of this study was to evaluate
the correlation between PD-L1 expression and long-term oncologic
outcomes in CRC.
Materials and methods
Patients
Formalin-fixed paraffin-embedded block specimens
from surgical resection of the primary tumor were obtained from 175
patients with CRC who underwent curative surgery and adjuvant
chemotherapy at our institution between September 1999 and August
2004. Baseline clinicopathological characteristics and clinical
outcome data were retrospectively collected from the Colorectal
Cancer Database of the Department of Colorectal Cancer Surgery and
the Pathological Diagnosis Database in the Department of Pathology.
The study physicians reviewed all medical records related to CRC,
extracted clinical information including the American Joint
Committee on Cancer tumor, node, metastases (TNM) classification,
the numbers of positive and negative lymph nodes harvested and
tumor location, and determined cause of death in deceased
individuals.
Production of tissue microarray
block
Formalin-fixed, paraffin-embedded tissue samples
were produced for tissue microarray (TMA). Representative areas of
each tumor were marked on each hematoxylin and eosin-stained slide
and the corresponding area of the tissue blocks was sampled. The
designated area of each donor block was collected using a tissue
cylinder punch (3 mm diameter), and the samples were transferred to
a recipient block.
Immunohistochemistry
Sections (4 µm thickness) from the TMAs were cut in
10% formalin buffer, embedded in paraffin, mounted onto Superfrost
Plus glass slides (VWR Scientific, West Chester, PA, USA), and
incubated at 60°C for 15 min. The slides were deparaffinized in
xylene, rehydrated in graded alcohol solutions, and washed in tap
water. Endogenous peroxidase activity was blocked by the addition
of 3% H2O2. The slides were placed in a steam
cooker filled with 10 mM sodium citrate buffer (pH 6.0) for antigen
retrieval after treatment with a blocking agent (DAKO, Carpinteria,
CA, USA) for 10 min to block nonspecific protein binding.
Immunohistochemistry for antigen (PD-L1) was performed using an
autostainer (LV360-2D; LabVision Co., Fremont, CA, USA). Reagents
and the secondary antibody from the commercial LP Kit (TL-125-HD,
LabVision Co.) were used as provided by the manufacturer. The
primary antibody was a rabbit polyclonal antibody against PD-L1
(1:400, Anti-PD-L1; AnaSpec, Fremont, CA, USA). Immunopositivity
was evaluated by determining the proportions of positive cells
(low, ≤50%; and high, >50%).
Evaluation parameters
The sixth edition of the American Joint Committee on
Cancer (AJCC) classification system was used to determine the
pathological tumor depth (pT), the number of metastasized lymph
nodes (pN), and cancer stage. A postoperative clinical examination,
measurement of serum carcinoembryonic antigen CEA levels, chest
radiography every 3 months, and chest/abdominal computed tomography
(CT) every 6 months were performed during each follow-up
examination over a period of 3 years. After 3 years, the follow-up
interval was changed to 6 months. Recurrence was defined as the
presence of radiologically confirmed or histologically proven tumor
and the location of recurrence was defined as the first site of
recurrence after complete resection. Local recurrence was defined
as any tumor recurrence in the surgical field; local recurrence
with synchronous systemic recurrence was included systemic
recurrence. The overall survival (OS) time was defined as the time
from the date of surgery to the date of the latest follow-up visit
or the date of death due to any cause, while the disease-free
survival (DFS) time was defined as the time from surgery to any
type of recurrence.
Statistical analyses
Data were expressed as medians with ranges.
Differences in clinicopathological features between low and
high-intensity PD-L1-positive CRCs were analyzed by Chi-square or
Fisher's exact tests for categorical variables and Student's t-test
for continuous variables. Survivals rate were determined using the
Kaplan-Meier method and log-rank tests were used to compare
survival rates among subgroups. Log-rank tests were also used for
univariate analysis and independent prognostic factors were
identified by multivariate analysis using the Cox proportional
hazards model to calculate hazard ratios. All statistical tests
were performed using IBM SPSS Statistics for Windows, v.21.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Ethical statement
The study was approved by the Institutional Review
Board of Keimyung University and Donsan Medical Center (IRB no.
2017-11-037) and performed in accordance with the principles of the
Declaration of Helsinki. The informed consent was waived.
Results
Patient and tumor characteristics
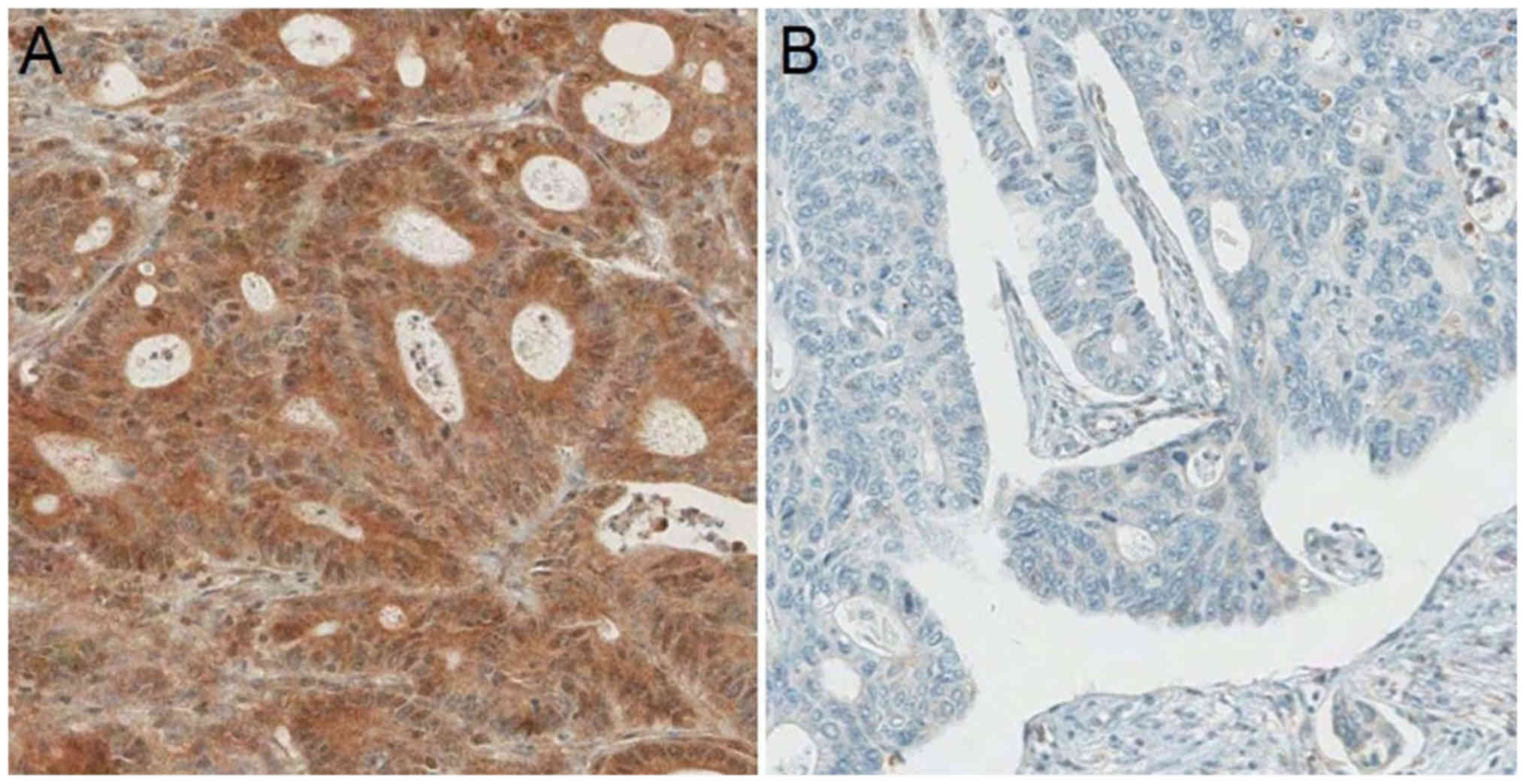
PD-L1 expression was categorized as low in 82
(46.9%) patients and high in 93 (53.1%) patients (Fig. 1). The patient and tumor
characteristics of the study cohort stratified by PD-L1 status are
shown in Table I. The demographic
characteristics were similar between the two cohorts for age, sex,
preoperative carcinoembryonic antigen level, tumor stage, nodal
stage, TNM stage, histology, lymphovascular invasion, and perinodal
extension. There was a trend in tumors to be located in the
right-sided colon, although without statistical significance, in
the high PD-L1 expression group compared to the low PD-L1
expression group (22.6 vs. 12.2%, P=0.073).
 | Table I.Characteristics of patients with CRC
according to programmed death-ligand 1 status. |
Table I.
Characteristics of patients with CRC
according to programmed death-ligand 1 status.
| Characteristics | Low expression
(N=82) | High expression
(N=93) | P-value |
|---|
| Age (years), median
(range) | 67 (32–86) | 70 (39–100) | 0.310 |
| Sex, n (%) |
|
| 0.711 |
| Male | 48 (58.5) | 57 (61.3) |
|
|
Female | 34 (41.5) | 36 (38.7) |
|
| Preoperative CEA
(ng/ml), median (range) | 2.82 (0.3–416.8) | 3.50 (0.1–332.8) | 0.762 |
| Tumor location, n
(%) |
|
| 0.073 |
| Right
side | 10 (12.2) | 21 (22.6) |
|
| Left
side | 72 (87.8) | 72 (77.4) |
|
| Tumor stage, n
(%) |
|
| 0.325 |
| T1 | 0 (0) | 2 (2.2) |
|
| T2 | 11 (13.4) | 18 (19.4) |
|
| T3 | 64 (78.0) | 68 (73.1) |
|
| T4 | 7 (8.5) | 5 (5.4) |
|
| Nodal stage, n
(%) |
|
| 0.109 |
| N0 | 36 (43.9) | 46 (49.5) |
|
| N1 | 20 (24.4) | 30 (32.3) |
|
| N2 | 26 (31.7) | 17 (18.3) |
|
| TNM stage, n (%) |
|
| 0.100 |
| Stage
I | 6 (7.3) | 15 (16.1) |
|
| Stage
II | 30 (36.6) | 30 (32.3) |
|
| Stage
III | 35 (42.7) | 43 (46.2) |
|
| Stage
IV | 11 (13.4) | 5 (5.4) |
|
| Histology, n (%) |
|
| 0.184 |
|
Well-differentiated | 4 (4.9) | 4 (4.3) |
|
|
Moderately differentiated | 67 (81.3) | 84 (90.3) |
|
| Poorly
differentiated | 8 (9.8) | 2 (2.2) |
|
|
Mucinous | 3 (3.7) | 3 (3.2) |
|
| Lymphovascular
invasion, n (%) | 61 (74.4) | 64 (68.8) | 0.415 |
| Perinodal
extension, n (%) | 15 (18.3) | 10 (10.8) | 0.155 |
| Recurrence pattern,
n (%) |
|
| 0.199 |
| Local
recurrence | 7 (18.4) | 7 (38.9) |
|
|
Systemic recurrence | 28 (73.7) | 9 (50.0) |
|
| Local
and systemic recurrence | 3 (7.9) | 2 (8.9) |
|
Oncologic outcomes and recurrence
pattern according to PD-L1 expression
During the median follow-up period of 88 months
(range 1–196 months), the total numbers of deaths and relapse
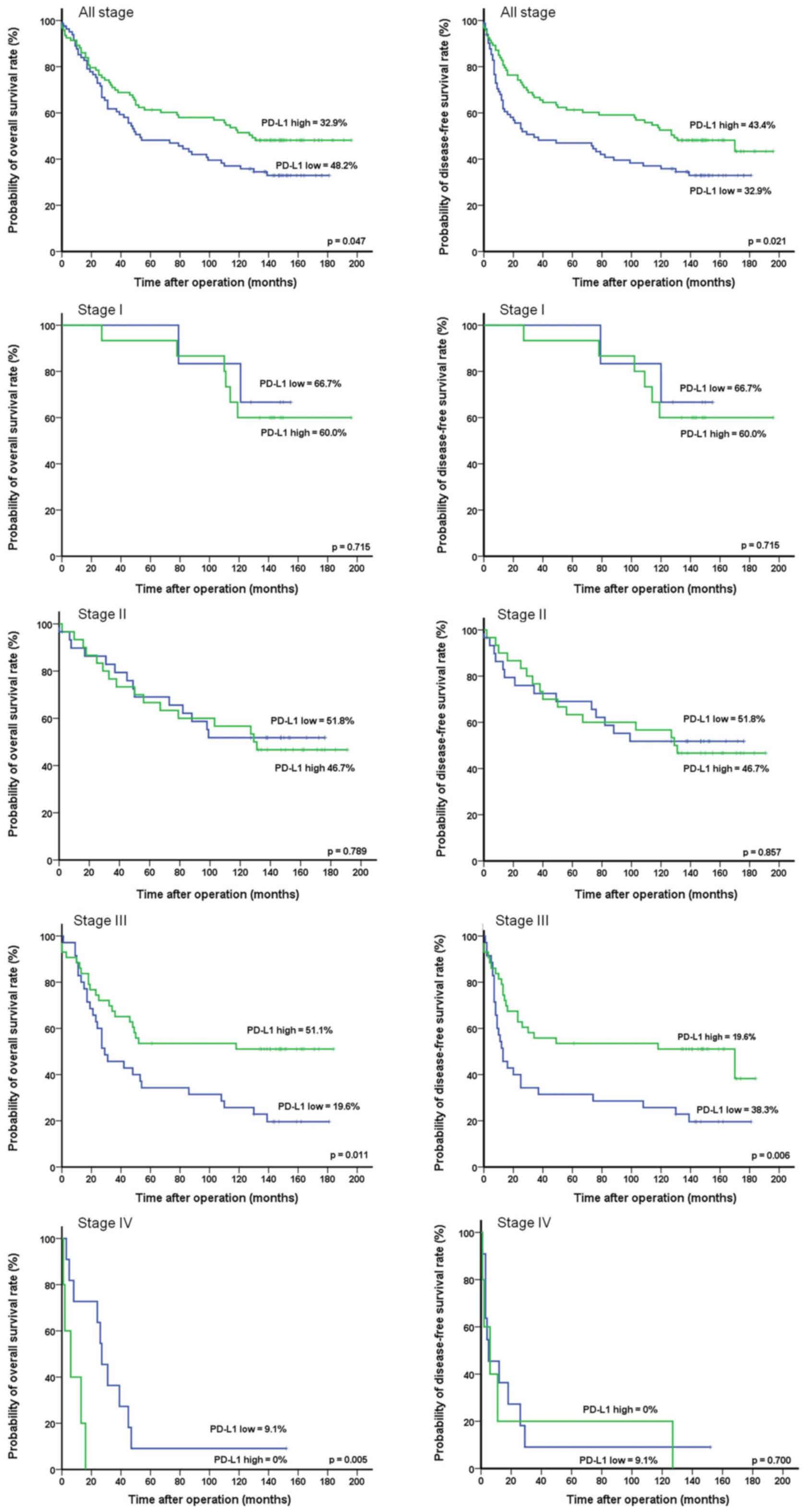
events were 102 and 57, respectively. The 10-year OS and DFS rates
were significantly higher in the high PD-L1 expression group than
in the low PD-L1 expression group (OS: 48.2 vs. 32.9%, P=0.047;
DFS: 43.3 vs. 32.9%, P=0.021) (Fig.
2). According to the TNM stage subgroups, the OS rates in the
low and high PD-L1 expression groups, respectively, were 66.7 and
60.0% in stage I (P=0.715), 51.8 and 46.7% in stage II (P=0.789),
19.6 and 51.1% in stage III (P=0.011), and 9.1 and 0% in stage IV
(P=0.005). The DFS rates in the low and high PD-L1 expression
groups, respectively, were 66.7 and 60.0% in stage I (P=0.715),
51.8 and 46.7% in stage II (P=0.857), 19.6 and 38.3% stage III
(P=0.006), and 9.1 and 0% in stage IV (P=0.700). The numbers of
involved organs in recurrence did not differ between groups
(P=0.418; Table II). The local
recurrence rate was 6.1% in the low PD-L1 expression group and 7.5%
in the high PD-L1 expression group. The sites of local recurrence
in the low and high PD-L1 expression groups included the
anastomotic site (1.2 vs. 2.2%, P=0.636), perirectum (6.1 vs. 6.5%,
P=0.923), and pelvic cavity (3.7 vs. 1.1%, P=0.254). The systemic
recurrence rate was significantly higher in the low PD-L1
expression group than in the high PD-L1 expression group (42.7 vs.
12.9%, respectively, P=0.030). The sites of systemic recurrence
most commonly affected in the low and high PD-L1 expression groups
included the liver (25.6 vs. 7.5%, P=0.001), followed by the lungs
(6.1 vs. 3.2%, P=0.364), peritoneum (3.7 vs., 2.2%, P=0.550),
extraregional lymph nodes (3.7 vs. 2.2%, P=0.550), abdominal wall
(1.2 vs. 0%, P=0.286), adrenal glands (1.2 vs. 0%, P=0.286),
ureters (1.2 vs. 0%, P=0.286), ovaries (1.2 vs. 0%, P=0.286), and
supraclavicular lymph nodes (1.2 vs. 0%, P=0.286).
 | Table II.Recurrence patterns according to
programmed death-ligand 1 expression. |
Table II.
Recurrence patterns according to
programmed death-ligand 1 expression.
| Variables | Low expression
(N=82) | High expression
(N=93) | P-value |
|---|
| Nο. of involved
organs |
|
| 0.418 |
| 1 | 35 (42.7) | 15 (16.1) |
|
| 2 | 4 (4.9) | 4 (4.3) |
|
| 3 | 1 (1.2) | 0 (0) |
|
| Recurrence
pattern |
|
| 0.030 |
| Local
recurrence | 5 (6.1) | 7 (7.5) |
|
|
Anastomotic site | 1 (1.2) | 2 (2.2) | 0.636 |
|
Perirectum | 5 (6.1) | 6 (6.5) | 0.923 |
| Pelvic
cavity | 3 (3.7) | 1 (1.1) | 0.254 |
| Systemic
recurrence | 35 (42.7) | 12 (12.9) |
|
|
Liver | 21 (25.6) | 7 (7.5) | 0.001 |
|
Lung | 5 (6.1) | 3 (3.2) | 0.364 |
|
Peritoneum | 3 (3.7) | 2 (2.2) | 0.550 |
|
Extraregional lymph
nodea | 3 (3.7) | 2 (2.2) | 0.550 |
|
Abdominal wall | 1 (1.2) | 0 (0) | 0.286 |
| Adrenal
gland | 1 (1.2) | 0 (0) | 0.286 |
|
Ureter | 1 (1.2) | 0 (0) | 0.286 |
|
Ovary | 1 (1.2) | 0 (0) | 0.286 |
|
Supraclavicular lymph
node | 1 (1.2) | 0 (0) | 0.286 |
Univariate and multivariate survival
analyses of prognostic factors
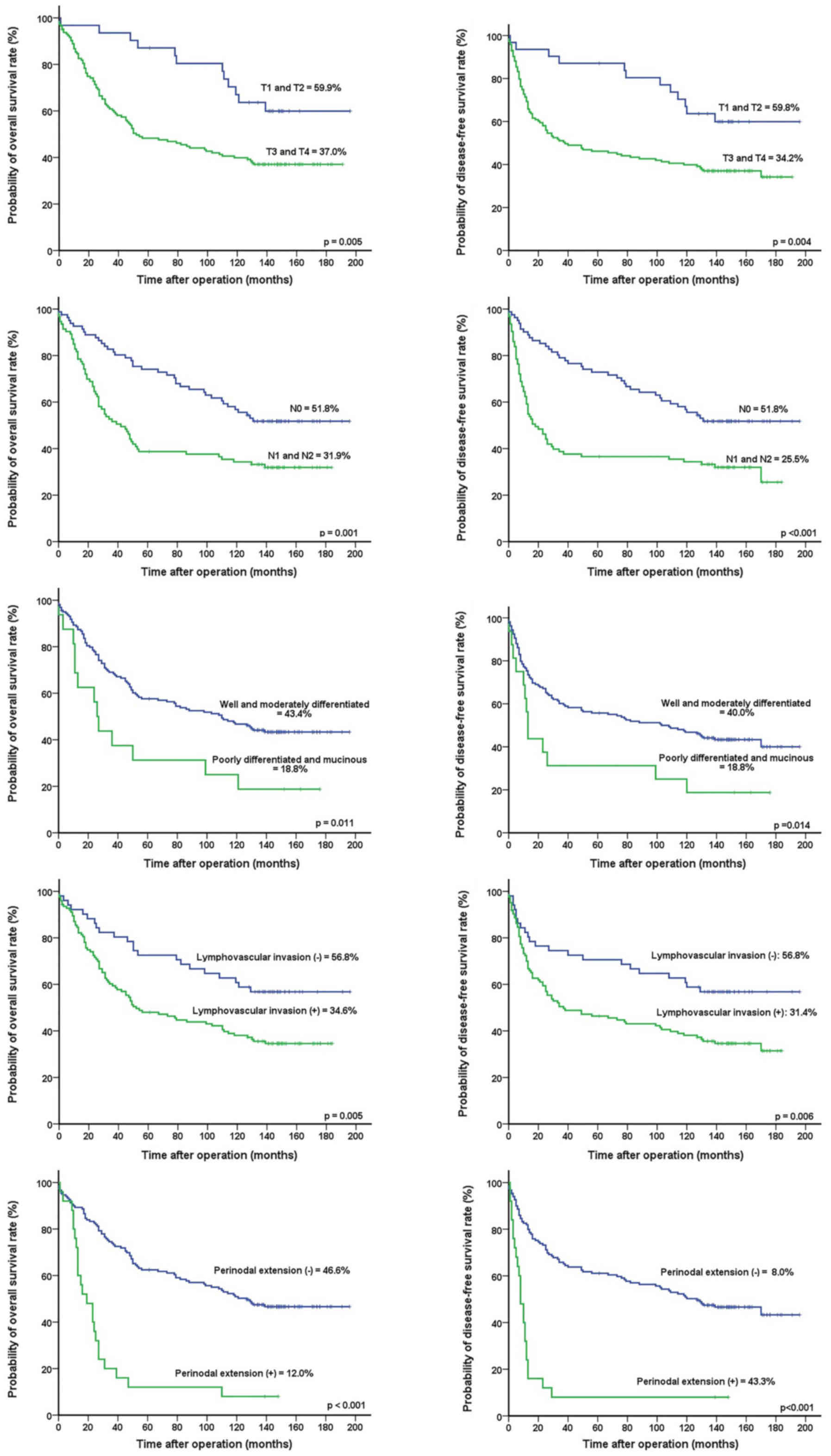
Univariate analyses revealed that T-stage (P=0.005
and P=0.004, respectively), N-stage (P=0.001 and P<0.001,
respectively), tumor differentiation (P=0.011 and P=0.014,
respectively), lymphovascular invasion (P=0.005 and P=0.006,
respectively), PD-L1 expression (P=0.047 and P=0.021,
respectively), and perinodal extension (P<0.001 and P<0.001,
respectively) were significantly associated with OS and DFS
(Table III) (Fig. 3). Multivariate analysis found that
T-stage [hazard ratio (HR), 1.888; 95% confidence interval [CI],
1.022–3.488, P=0.042] and perinodal extension (HR, 2.927; 95% CI,
1.693–5.060, P<0.001) were independent prognostic factors for OS
and PD-L1 expression (HR, 1.586; 95% CI, 1.069–2.353, P=0.022),
while T-stage (HR, 1.903; 95% CI, 1.033–3.508, P=0.039) and
perinodal extension (HR, 3.057; 95% CI, 1.776–5.259, P<0.001)
were independent prognostic factors for DFS (Table IV).
 | Table III.Prognostic factors of survival in
univariate analysis. |
Table III.
Prognostic factors of survival in
univariate analysis.
| Prognostic
factor | No. (n=175) | OS (%) | P-value | DFS (%) | P-value |
|---|
| Age (years) |
|
| 0.554 |
| 0.466 |
|
≤60 | 47 | 48.9 |
| 48.9 |
|
|
>60 | 128 | 38.0 |
| 33.8 |
|
| Sex |
|
| 0.173 |
| 0.130 |
|
Male | 105 | 37.1 |
| 33.1 |
|
|
Female | 70 | 46.8 |
| 46.8 |
|
| Location |
|
| 0.916 |
| 0.747 |
|
Right-sided | 31 | 41.9 |
| 41.9 |
|
|
Left-sided | 144 | 40.9 |
| 37.2 |
|
| Tumor stage |
|
| 0.005 |
| 0.004 |
| T1 and
T2 | 31 | 59.9 |
| 59.8 |
|
| T3 and
T4 | 144 | 37.0 |
| 34.2 |
|
| Nodal stage |
|
| 0.001 |
| <0.001 |
| N0 | 82 | 51.8 |
| 51.8 |
|
| N1 and
N2 | 93 | 31.9 |
| 25.5 |
|
| Histology |
|
| 0.011 |
| 0.014 |
| Well
and moderately differentiated | 159 | 43.4 |
| 40.0 |
|
| Poorly
differentiated and mucinous | 16 | 18.8 |
| 18.8 |
|
| Lymphovascular
invasion |
|
| 0.005 |
| 0.006 |
| No | 51 | 56.8 |
| 56.8 |
|
|
Yes | 124 | 34.6 |
| 31.4 |
|
| PD-L1
expression |
|
| 0.047 |
| 0.021 |
|
Low | 82 | 32.9 |
| 32.9 |
|
|
High | 93 | 48.2 |
| 43.3 |
|
| Perinodal
extension |
|
| <0.001 |
| <0.001 |
| No | 150 | 46.6 |
| 43.3 |
|
|
Yes | 25 | 12 |
| 8 |
|
 | Table IV.Prognostic factors of survival in
multivariate analysis. |
Table IV.
Prognostic factors of survival in
multivariate analysis.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Prognostic
factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Tumor stage |
|
|
|
|
| T3 and
T4 vs. T1 and T2 | 1.888
(1.022–3.488) | 0.042 | 1.903
(1.033–3.508) | 0.039 |
| Histology |
|
|
|
|
| Poorly
differentiated and mucinous vs. well and moderately
differentiated | 1.647
(0.906–2.995) | 0.102 | 1.409
(0.768–2.586) | 0.268 |
| Lymphovascular
invasion | 1.615
(0.992–2.629) | 0.054 | 1.593
(0.980–2.591) | 0.061 |
| Programmed
death-ligand 1 expression |
|
|
|
|
| Low vs.
high | 1.274
(0.852–1.904) | 0.238 | 1.586
(1.069–2.353) | 0.022 |
| Nodal stage |
|
|
|
|
|
Positive vs. ≤ negative | 1.306
(0.829–2.056) | 0.249 | 1.430
(0.912–2.243) | 0.119 |
| Perinodal
extension |
|
|
|
|
|
Positive vs. ≤ negative | 2.927
(1.693–5.060) | <0.001 | 3.057
(1.776–5.259) | <0.001 |
Discussion
PD-L1 expression has been reported in tumor cells or
tumor-infiltrating immune cells in several malignancies, including
CRC, and may play a central role in immune-oncologic interactions
(19,28,29). The
present study examined the effects of PD-L1 expression on long-term
oncologic outcomes and recurrence patterns among patients with CRC.
We found that PD-L1 expression was significantly associated with
tumor recurrence and poor prognosis and was an independent
prognostic factor for DFS, especially in stage III CRC. Moreover,
the systemic recurrence rate was significantly higher and hepatic
recurrence was more common in the low group than in the high
expression group.
Previous studies have reported conflicting results
as to whether PD-L1 expression indicates a better or worse
prognosis in CRC, possibly due to differences in study populations
and designs (21,23,30). In
the present study, the prognostic impact of PD-L1 expression on
long-term oncologic outcomes in CRC differed according to the TNM
stage. In early stages, including stages I and II, there was no
significant between-group difference; however, the long-term
oncologic outcomes of patients with stage III disease was
significantly better in the high PD-L1 expression group. In this
context, our results underscore the specificities of tumor immune
system interactions and microenvironments in CRC according to
disease stage.
Our evaluation of the recurrence patterns according
to the PD-L1 expression status revealed a significantly higher
systemic recurrence rate in the low PD-L1 expression group than
that in the high PD-L1 expression group. Moreover, low PD-L1
expression was significantly associated with hepatic recurrence.
The high systemic recurrence rate and significant association with
hepatic recurrence in the low PD-L1 expression group may be
reflected in the poor prognosis.
CRC with MMR defects shows high microsatellite
instability (MSI) and accounts for 12 to 15% of colorectal
carcinomas. These tumors are infiltrated by higher numbers of
lymphocytes and are characterized by a more favorable prognosis
compared to those in MMR-proficient tumors. Some studies have
reported different impacts of PD-L1 expression on survival
according to microsatellite status. Dunne et al (26) demonstrated that PD-L1 expression was
associated with a significantly worse recurrence-free survival in
mismatch-repair-deficient tumors, whereas PD-L1 expression did not
show a statistically significant association with recurrence-free
survival in mismatch-repair-proficient tumors. In contrast, Droeser
et al (23) reported that high
PD-L1 expression in mismatch-repair-proficient CRC was associated
with early tumor stage, absence of lymph node metastases, lower
tumor grade, absence of vascular invasion, high numbers of
tumor-infiltrating CD8+ T cells, and an improved 5-year survival
rate. Unfortunately, we did not evaluate the status of germline
mutations in MLH1, MSH2, MSH6, and PMS2; thus,
further studies are needed to assess the possible association
between MSI status and PD-L1 expression in tumor cells.
Adjuvant chemotherapy has an established role in
patients with stage III and high-risk stage II CRC; however, its
use remains controversial in stage II disease due to its
restriction to a small subgroup of patients with high-risk
prognostic factors. Dunne et al (26) used relapse follow-up data to find that
patients with high PD-L1 expression had a significantly better
outcome than that in patients with low expression in the untreated
stage III population; however, the correlation between survival and
high PD-L1 expression was lost in the stage III patients treated
with adjuvant chemotherapy. They suggested that patients with high
PD-L1 expression should not be administered 5-FU-based adjuvant
chemotherapy following surgery. However, neither high nor low PD-L1
expression showed a statistically significant association with
recurrence-free survival in our study, regardless of adjuvant
chemotherapy (data not shown).
Our study has several limitations, including the
small number of patients, its retrospective nature, and the lack of
investigation of other immune-oncologic biomarkers such as MSI
status, K-ras and BRAF mutations, and the expression of other
immune checkpoints in tumor and immune cells. Further studies are
needed to determine the possible association of MSI status with
immune checkpoint molecules in tumor and immune cells in different
stages or statuses.
Analysis of our long-term data indicates that love
PD-L1 expression is significantly associated with tumor relapse and
poor prognosis in CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Keimyung
University Research Grant of 2017 (grant no. 20170567).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
SUB and ISH conceived and designed the study and
collected and assembled the data. SUB, ISH and SKB provided
administrative support. SKB and ISH provided the study materials
and enrolled the patients. SUB, ISH, SKB and NKK performed the data
analysis and interpretation. All authors wrote the manuscript and
final approved the version to be published.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Keimyung University and Donsan Medical Center (IRB
No. 2017-11-037) and performed in accordance with the principles of
the Declaration of Helsinki. All patients provided written informed
consent to allow the use of their tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shin A, Kim KZ, Jung KW, Park S, Won YJ,
Kim J, Kim DY and Oh JH: Increasing trend of colorectal cancer
incidence in Korea, 1999–2009. Cancer Res Treat. 44:219–226. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colussi D, Brandi G, Bazzoli F and
Ricciardiello L: Molecular pathways involved in colorectal cancer:
Implications for disease behavior and prevention. Int J Mol Sci.
14:16365–16385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Caro G, Marchesi F, Laghi L and Grizzi
F: Immune cells: Plastic players along colorectal cancer
progression. J Cell Mol Med. 17:1088–1095. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ogino S, Galon J, Fuchs CS and Dranoff G:
Cancer immunology-analysis of host and tumor factors for
personalized medicine. Nat Rev Clin Oncol. 8:711–719. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaanan A, Fléjou JF, Emile JF, Des GG,
Cuilliere-Dartigues P, Malka D, Lecaille C, Validire P, Louvet C,
Rougier P, et al: Defective mismatch repair status as a prognostic
biomarker of disease-free survival in stage III colon cancer
patients treated with adjuvant FOLFOX chemotherapy. Clin Cancer
Res. 17:7470–7478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sinicrope FA, Shi Q, Smyrk TC, Thibodeau
SN, Dienstmann R, Guinney J, Bot BM, Tejpar S, Delorenzi M,
Goldberg RM, et al: Molecular markers identify subtypes of stage
III colon cancer associated with patient outcomes.
Gastroenterology. 148:88–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Galon J, Mlecnik B, Bindea G, Angell HK,
Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et
al: Towards the introduction of the ‘Immunoscore’ in the
classification of malignant tumours. J Pathol. 232:199–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mlecnik B, Bindea G, Angell HK, Maby P,
Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M,
Fredriksen T, et al: Integrative analyses of colorectal cancer show
immunoscore is a stronger predictor of patient survival than
microsatellite instability. Immunity. 44:698–711. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Topalian SL, Drake CG and Pardoll DM:
Immune checkpoint blockade: A common denominator approach to cancer
therapy. Cancer Cell. 27:450–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ostrand-Rosenberg S, Horn LA and Haile ST:
The programmed death-1 immune-suppressive pathway: Barrier to
antitumor immunity. J Immunol. 193:3835–3841. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rooney MS, Shukla SA, Wu CJ, Getz G and
Hacohen N: Molecular and genetic properties of tumors associated
with local immune cytolytic activity. Cell. 160:48–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirano F, Kaneko K, Tamura H, Dong H, Wang
S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, et al: Blockade of
B7-H1 and PD-1 by monoclonal antibodies potentiates cancer
therapeutic immunity. Cancer Res. 65:1089–1096. 2005.PubMed/NCBI
|
|
14
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohigashi Y, Sho M, Yamada Y, Tsurui Y,
Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thompson RH, Gillett MD, Cheville JC,
Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen
L, et al: Costimulatory B7-H1 in renal cell carcinoma patients:
Indicator of tumor aggressiveness and potential therapeutic target.
Proc Natl Acad Sci USA. 101:17174–17179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+
T lymphocytes are prognostic factors of human ovarian cancer. Proc
Natl Acad Sci USA. 104:3360–3365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sabatier R, Finetti P, Mamessier E,
Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D and
Bertucci F: Prognostic and predictive value of PDL1 expression in
breast cancer. Oncotarget. 6:5449–5464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hino R, Kabashima K, Kato Y, Yagi H,
Nakamura M, Honjo T, Okazaki T and Tokura Y: Tumor cell expression
of programmed cell death-1 ligand 1 is a prognostic factor for
malignant melanoma. Cancer. 116:1757–1766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu P, Wu D, Li L, Chai Y and Huang J:
PD-L1 and survival in solid tumors: A meta-analysis. PLoS One.
10:e01314032015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu Z, Jin Z, Zhang M, Tang Y, Yang G,
Yuan X, Yao J and Sun D: Prognostic value of programmed
death-ligand 1 in sarcoma: A meta-analysis. Oncotarget.
8:59570–59580. 2017.PubMed/NCBI
|
|
23
|
Droeser RA, Hirt C, Viehl CT, Frey DM,
Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A,
Rosso R, et al: Clinical impact of programmed cell death ligand 1
expression in colorectal cancer. Eur J Cancer. 49:2233–2242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee LH, Cavalcanti MS, Segal NH, Hechtman
JF, Weiser MR, Smith JJ, Garcia-Aguilar J, Sadot E, Ntiamoah P,
Markowitz AJ, et al: Patterns and prognostic relevance of PD-1 and
PD-L1 expression in colorectal carcinoma. Mod Pathol. 29:1433–1442.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saigusa S, Toiyama Y, Tanaka K, Inoue Y,
Mori K, Ide S, Imaoka H, Kawamura M, Mohri Y and Kusunoki M:
Implication of programmed cell death ligand 1 expression in tumor
recurrence and prognosis in rectal cancer with neoadjuvant
chemoradiotherapy. Int J Clin Oncol. 21:946–952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dunne PD, McArt DG, O'Reilly PG, Coleman
HG, Allen WL, Loughrey M, Van Schaeybroeck S, McDade S,
Salto-Tellez M, Longley DB, et al: Immune-derived PD-L1 gene
expression defines a subgroup of stage II/III colorectal cancer
patients with favorable prognosis who may be harmed by adjuvant
chemotherapy. Cancer Immunol Res. 4:582–591. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koganemaru S, Inoshita N, Miura Y, Miyama
Y, Fukui Y, Ozaki Y, Tomizawa K, Hanaoka Y, Toda S, Suyama K, et
al: Prognostic value of programmed death-ligand 1 expression in
patients with stage III colorectal cancer. Cancer Sci. 108:853–858.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen JK, Cote GM, Choy E, Yang P, Harmon
D, Schwab J, Nielsen GP, Chebib I, Ferrone S, Wang X, et al:
Programmed cell death ligand 1 expression in osteosarcoma. Cancer
Immunol Res. 2:690–698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Curiel TJ, Wei S, Dong H, Alvarez X, Cheng
P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, et
al: Blockade of B7-H1 improves myeloid dendritic cell-mediated
antitumor immunity. Nature Med. 9:562–567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song M, Chen D, Lu B, Wang C, Zhang J,
Huang L, Wang X, Timmons CL, Hu J, Liu B, et al: PTEN loss
increases PD-L1 protein expression and affects the correlation
between PD-L1 expression and clinical parameters in colorectal
cancer. PLoS One. 8:e658212013. View Article : Google Scholar : PubMed/NCBI
|