Introduction
Prostate cancer (PCa) is the most commonly diagnosed
malignancy among African American men and the second-leading cause
of cancer-associated mortality during 2013 (1). For African Americans, the overall 5-year
survival rate of patients with prostate cancer is 100% when these
tumors are diagnosed at early stages, yet when the cancer has
spread to distant sites the 5-year survival rate decreases to 27%
(1). Although the present biomarker
for the diagnosis of PCa is prostate-specific antigen (PSA), it has
been demonstrated that PSA is not associated with rates of
mortality in PCa after 13 years (2).
The negative consequences of PSA screening lead to over-diagnosis,
overtreatment and treatment complications (3). Therefore, it is urgent to investigate
novel biomarkers for the diagnosis of early PCa and explore novel
therapeutic targets for the treatment of advanced PCa.
MicroRNAs (miRNAs/miRs), small noncoding single RNAs
measuring 21–25 nucleotides in length, are stably expressed in
clinical specimens, including serum (4), plasma (5)
and urine (6). miRNAs serve essential
regulatory roles through sequence-specific base pairing on the 3′
untranslated region of mRNAs, resulting in mRNA degradation or the
inhibition of translation (7).
Several studies have revealed that prostate cancer exhibits
specific expression profiles of miRNAs, including miR-106a,
miR-223, miR-20a, miR-21, miR-141 and miR-27a (8–10). Among
these miRNAs, the downregulation of miR-27a was identified in high
grade of prostate cancer (10), but
Fletcher et al (11)
demonstrated that androgen-regulated miR-27a acted as an oncogenic
miR (oncomiR) and increased prostate cancer cell growth via
targeting the tumor suppressor and androgen receptor corepressor,
prohibitin. In other types of cancer, including pancreatic cancer
(12), renal cell carcinoma (13) and osteosarcoma (14), miR-27a serves as an oncomiR and is
involved in cell proliferation, colony formation and metastasis.
However, in hepatocellular carcinoma, miR-27a was demonstrated to
be downregulated and to suppress tumor metastasis by inhibiting
epithelial-mesenchymal transition (15). Therefore, the present study focused on
miR-27a, and aimed to investigate its expression and role in
PCa.
In the present study, it was identified that miR-27a
was overexpressed in the tumor tissue and serum of patients with
PCa. The overexpression of miR-27a was associated with poor
survival of patients and an increase tumor cell proliferation.
Furthermore, it was identified that Sprouty2 (SPRY2) is a direct
target of miR-27a, and the induced expression of SPRY2 may rescue
the miR-27a-mediated increase in tumor cell proliferation of PCa
cells.
Materials and methods
Prostate carcinoma specimens and cell
lines
All specimens were collected from the individuals
who provided written informed consent according to the protocols
approved by the Ethics Review Board at Nanchang University
(Nanchang, China). A total of 60 patients (aged between 60 and 78,
median 69 years) with PCa and 60 healthy subjects from the Second
Affiliated Hospital of Nanchang University (Nanchang, China) were
included in this study between March 2013 and June 2015. Three
years of follow-up of the patients with PCa were performed. The
serum samples were collected from PCa patients with different Tumor
Node Metastasis (TNM) stages (16),
stage I (12 patients), stage II (13 patients), stage III (25
patients), stage IV (10 patients). No patients underwent any
treatment prior to the collection of serum samples. There was no
significant difference in the age distribution between the patients
with PCa and healthy subjects (data not shown). Cell-free serum was
isolated from 5 ml blood of patients and healthy subjects within 2
h via a two-step protocol (1,500 × g for 10 min, followed by 12,000
× g for 2 min, at 4°C) (17).
Finally, 450 µl serum was moved into nuclear-free tubes and stored
at −80°C.
Human PCa LNCaP and PC-3, and normal prostate
epithelial RWPE-1 cell lines were purchased from American Type
Culture Collection (Manassas, VA, USA). The cells were cultured in
RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) supplemented with 15% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences) at 37°C in 5% CO2.
RNA isolation
Circulating RNAs were extracted from 250 µl serum
using 750 µl TRIzol® LS reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol, and eluted with 35 µl pre-heated (65°C)
elution solution. A total of 10 µl of Caenorhabditis elegans
miR-39 (0.05 µM) (synthesized by Shanghai GenePharma Co., Ltd.,
Shanghai, China) was added to each tube subsequent to serum mixing
with TRIzol LS, and prior to the next step. Tissue RNA was isolated
using TRIzol reagent according to the manufacturer's protocol, and
eluted with 60 µl pre-heated (65°C) nuclease-free water. RNA
quantification was carried out using NanoDrop 1000 (Thermo Fisher
Scientific, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For miRNA, a Taqman MicroRNA Reverse Transcription
kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) was used
to perform the reverse transcription reaction according to the
manufacturer's protocol. qPCR reactions were performed in 20 µl
volume reaction containing 2 µl cDNA, 10 µl TaqMan 2X Perfect
Master Mix (Takara Bio, Inc., Otsu, Japan), 0.5 µl gene-specific
primers/probe (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and 7.5 µl nuclease-free water, and processed on a Bio-Rad IQ5
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) thermocycler with
the following parameters: 94°C for 1 min, followed by 40 cycles of
94°C for 15 sec and 60°C for 30 sec, and a melt curve with a range
of 60 to 94°C and 0.5°C was raised in each analysis. For mRNA,
PrimeScript RT reagent kits (Takara Bio, Inc.) and SYBR Green
Realtime PCR Master Mix (Takara Bio, Inc.) were used according to
the manufacturer's protocols. The 2−ΔΔCq method
(18) was used to calculate the
expression of miR-27a and SPRY2 relative to their references. The
primer sequences were as follow: SPRY2-F:
5′-ATCCAGAGACAAGACATGTAC-3′; SPRY2-R: 5′-TTCAGATGTGTTCTAAGCC-3′;
GAPDH-F: 5′-GCACCGTCAAGGCTGAGAAC-3′; GAPDH-R:
5′-GCCTTCTCCATGGTGGTGAA-3′. The primers of miR-27a and U6 were
purchased from Shanghai GenePharma Co., Ltd.
Western blotting
LNCaP and PC-3 cells were lysed in
radioimmunoprecipitation assay lysis buffer (Pierce; Thermo Fisher
Scientific, Inc.) to extract total proteins which were measured
using ABC kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. A total of 60 µg of total proteins were
separated using SDS-PAGE (10% gel) and transferred to
polyvinylidene fluoride (PVDF) membranes. The PVDF membranes were
blocked with 3% bovine serum albumin (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature for 1 h and then incubated
with anti-SPRY2 (1:2,000; cat. no. ab50317, Upstate Biotechnology,
Inc., Lake Placid, NY, USA) or GAPDH (1:1,000; cat. no. sc-293335,
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies at 4°C
overnight. The PVDF membranes were then incubated with a
horseradish peroxidase-conjugated secondary antibody (goat anti
rabbit; cat no. ZB-2301; 1:10,000; Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing, China) and finally detected using
an enhanced chemiluminescence system (Amersham Pharmacia Biotech;
GE Healthcare, Chicago, IL, USA) and ChemiDoc MP System with Image
Lab Software version 6.0 (cat no. 170-8280; Bio-Rad Laboratories,
Inc.).
Oligonucleotide transfection
The oligonucleotides were purchased from GenePharma,
(Shanghai, China), including negative control, miR-27a inhibitor,
miR-27a mimics, small interfering (si)-SPRY2 and SPRY2 vectors
which contain the full-length SPRY2 cDNA sequence were constructed
by Sangon Biotech (Shanghai, China). The sequences of
oligonucleotides used were as follows: Negative control,
5′-UUCUCCGAACGUGUCACGUTT-3′; miR-27a inhibitors,
5′-GCGGAACUUAGCCACUGUGAA-3′; miR-27a mimics,
5′-UUCACAGUGGCUAAGUUCCGC-3′; si-SPRY2,
5′-CUCCAUUAGCUGAGUUCUAACAAG-3′. The oligonucleotides were
transfected into LNCaP and PC-3 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
24 h of transfection, the LNCaP and PC-3 cells were collected to
perform the experiments.
Cell proliferation assay
LNCaP and PC-3 cells were seeded at a density of
5,000/well in 96-well plates. The cells were transfected with the
NC, miR-27a inhibitor, miR-27a mimics, si-SPRY2, or co-transfected
with miR-27a mimics and a SPRY2 vector. Cell proliferation was
analyzed following transfection for 1, 2, 3, 4 or 5 days, using
Cell Counting Kit-8 (CCK8; Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocol.
Cell cycle analysis
The cell cycle of PCa cells were analyzed using cell
cycle and apoptosis analysis kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instruction.
Briefly, 48 h after transfection with NC, miR-27a mimics or
si-SPRY2, the cells were harvested and fixed in ice-cold 70%
ethanol overnight, and then the cells were resuspended in propidium
staining solution (Beyotime Institute of Biotechnology) containing
40 µg/ml propidium iodide, 250 µg/ml RNase and 2 mM EDTA, and
incubated for 30 min at 37°C. Cell cycle was analyzed using flow
cytometer (FACSCalibur; BD Biosciences, Franklin Lakes, NJ,
USA).
Statistical analysis
miR-27a expression in the sera from patients and
healthy subjects was compared using the Mann-Whitney test. miR-27a
expression in pair tissues was analyzed using two-tailed Student's
t-test. The one-way analysis of variance and the
student-Newman-Keuls test was used to analyze more than two groups.
Pearson's correlation was used to analyze the association between
the expressions of miR-27a and SPRY2 mRNA. Receiver-operating
characteristic (ROC) curves were used to assess the
sensitivity/specificity of miR-27a for the diagnosis of PCa.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses and graphs were performed and
produced using GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
miR-27a is overexpressed in the serum
and tumor tissue of patients with PCa
To the best of our knowledge, the expression of
miR-27a in serum of patients with PCa remains unknown. To measure
the serum levels of miR-27a, its expression was detected in 60
serum samples of patients with PCa and 60 healthy subjects. The
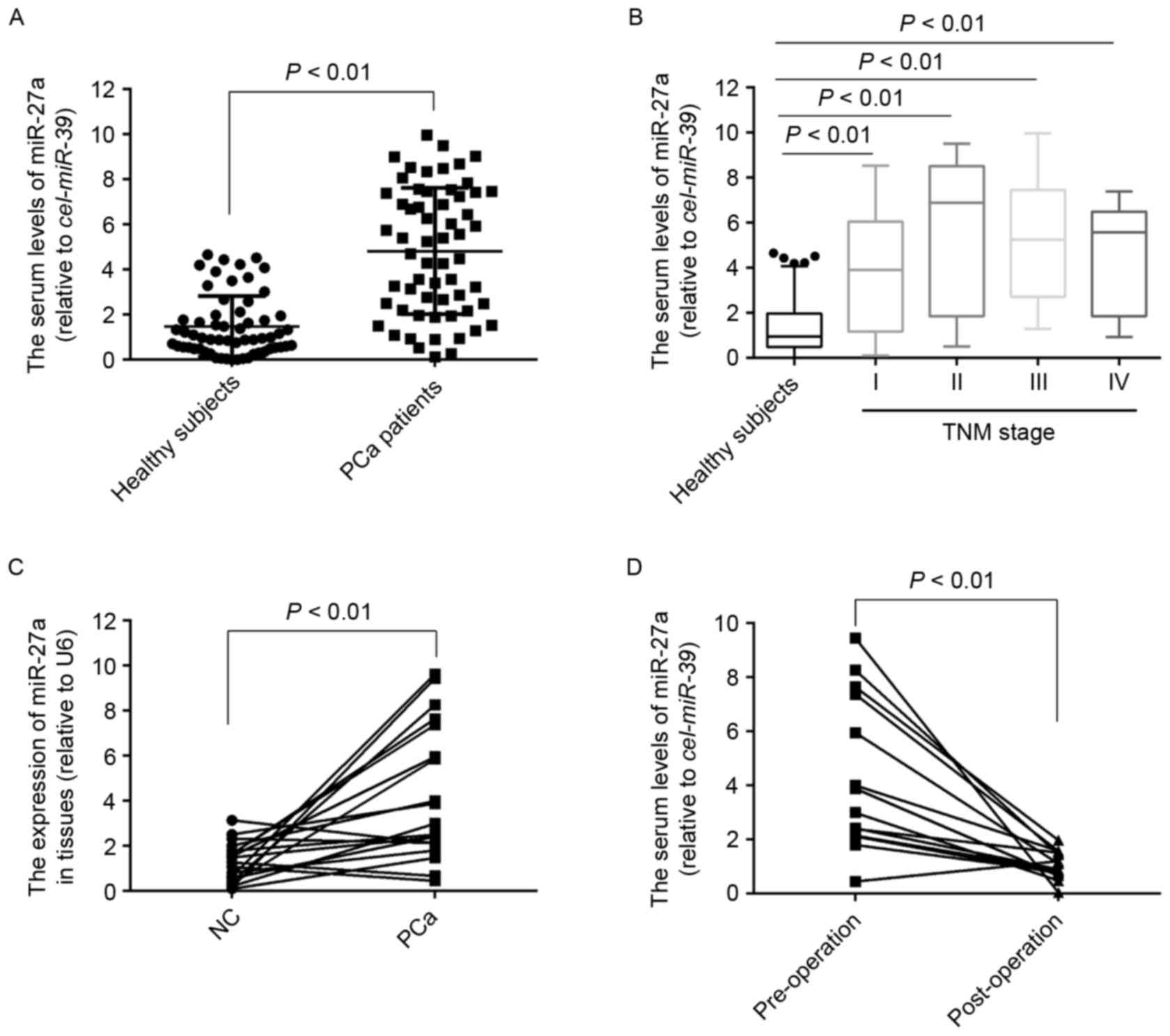
results demonstrated that the serum levels of miR-27a were
significantly higher in the patients with PCa compared with in the
healthy subjects (P<0.01; Fig.
1A), which was also observed in the PCa patients with stage I
(P<0.01; Fig. 1B), suggesting that
the expression profiles of miR-27a in serum may act as novel
non-invasive biomarkers for the diagnosis of early PCa.
Furthermore, the expression of miR-27a in 20 pairs of PCa tissues
and the matched normal tissues was also detected, and the results
indicated that the expression of miR-27a was also significantly
increased in the tumor tissues compared with the normal tissues
(Fig. 1C). To determine whether
miR-27a in serum was primarily derived from PCa tissues, the
expression of miR-27a in 14 serum samples of patients who underwent
surgery was measured for 3 months, and the results demonstrated
that the serum levels of miR-27a was significantly decreased
compared with the pre-operative levels (Fig. 1D), suggesting that the increased
levels of miR-27a in serum was caused by the PCa tissues.
Serum level of miR-27a is a potential
biomarker for the diagnosis of early PCa and associated with poor
survival of patients
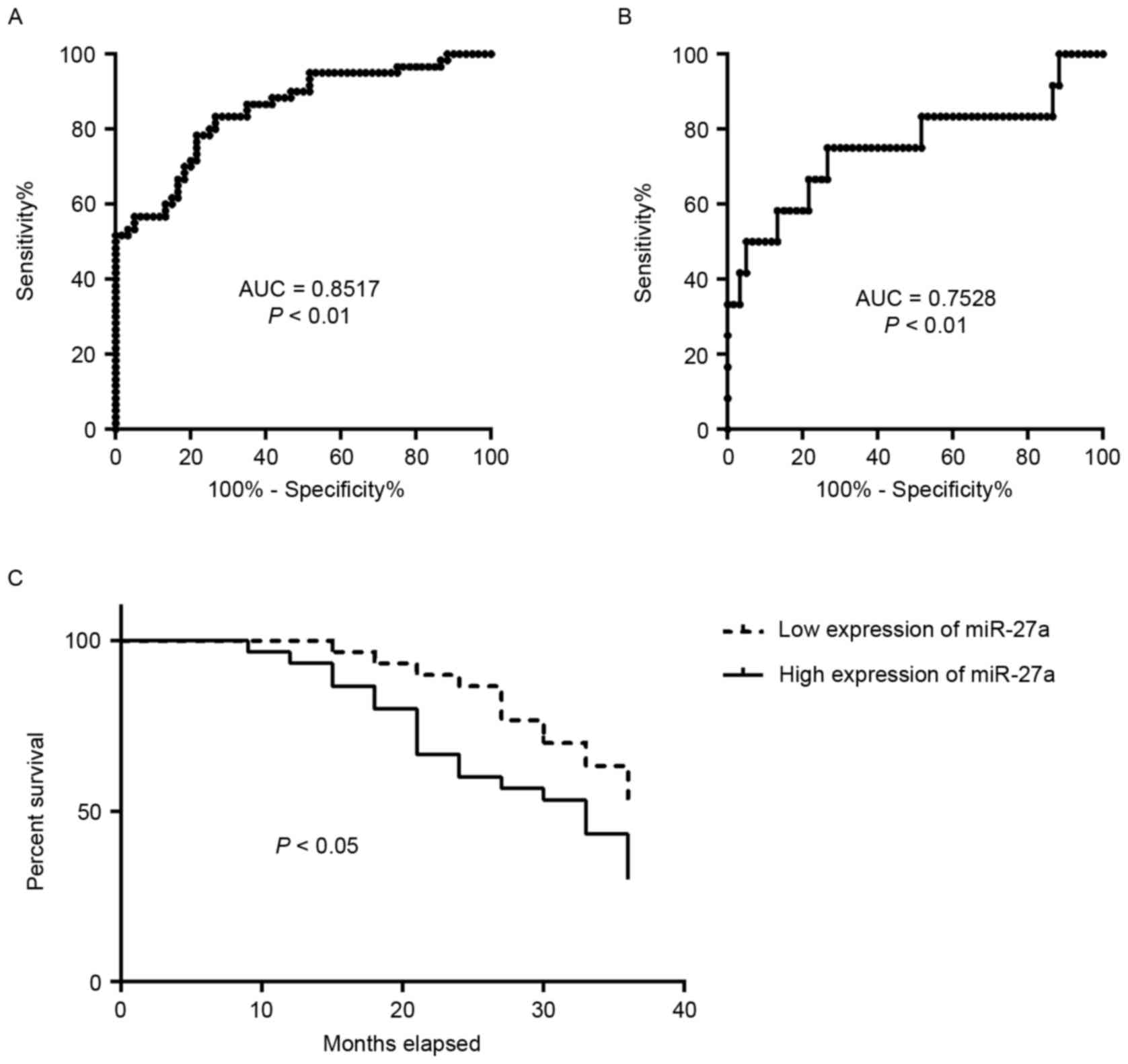
The diagnostic capabilities of the expression
profiles of miR-27a in the sera in distinguishing patients with PCa
from normal subjects were additionally analyzed. ROC curve analyses
revealed that the serum level of miR-27a was a valuable biomarker
for distinguishing patients with PCa from healthy subjects, with
AUC of 0.8517 [95% confidence interval (CI): 0.7842–0.9191]. The
cutoff value of 2.136 was considered optimal, and the sensitivity
and specificity were 78.33 and 78.33%, respectively (Fig. 2A). The serum level of miR-27a was also
a valuable biomarker for distinguishing PCa patients with TNM stage
I from the healthy subjects with AUC of 0.7528 (95% CI:
0.5681–0.9375). The cutoff value of 1.854 was considered optimal,
and the sensitivity and specificity were 75 and 73.33%,
respectively (Fig. 2B). It was then
determined whether the serum level of miR-27a was associated with
patient survival. The 60 patients with PCa were divided into two
groups, low expression of miR-27a (range, 0.1109–4.6972) and high
expression of miR-27a (range, 5.2489–9.9653), using the median
expression of miR-27a. The analysis of patient survival between the
two groups was performed. The results indicated that patients with
PCa with high expression of miR-27a exhibited significantly poorer
survival rates compared with those with low expression (P<0.05;
Fig. 2C). These data suggested that
the expression profile of miR-27a in serum may be a potential
biomarker for the diagnosis and prognosis of patients with PCa.
miR-27a directly inhibits SPRY2
expression, and their levels of expression are inversely correlated
in PCa tissues
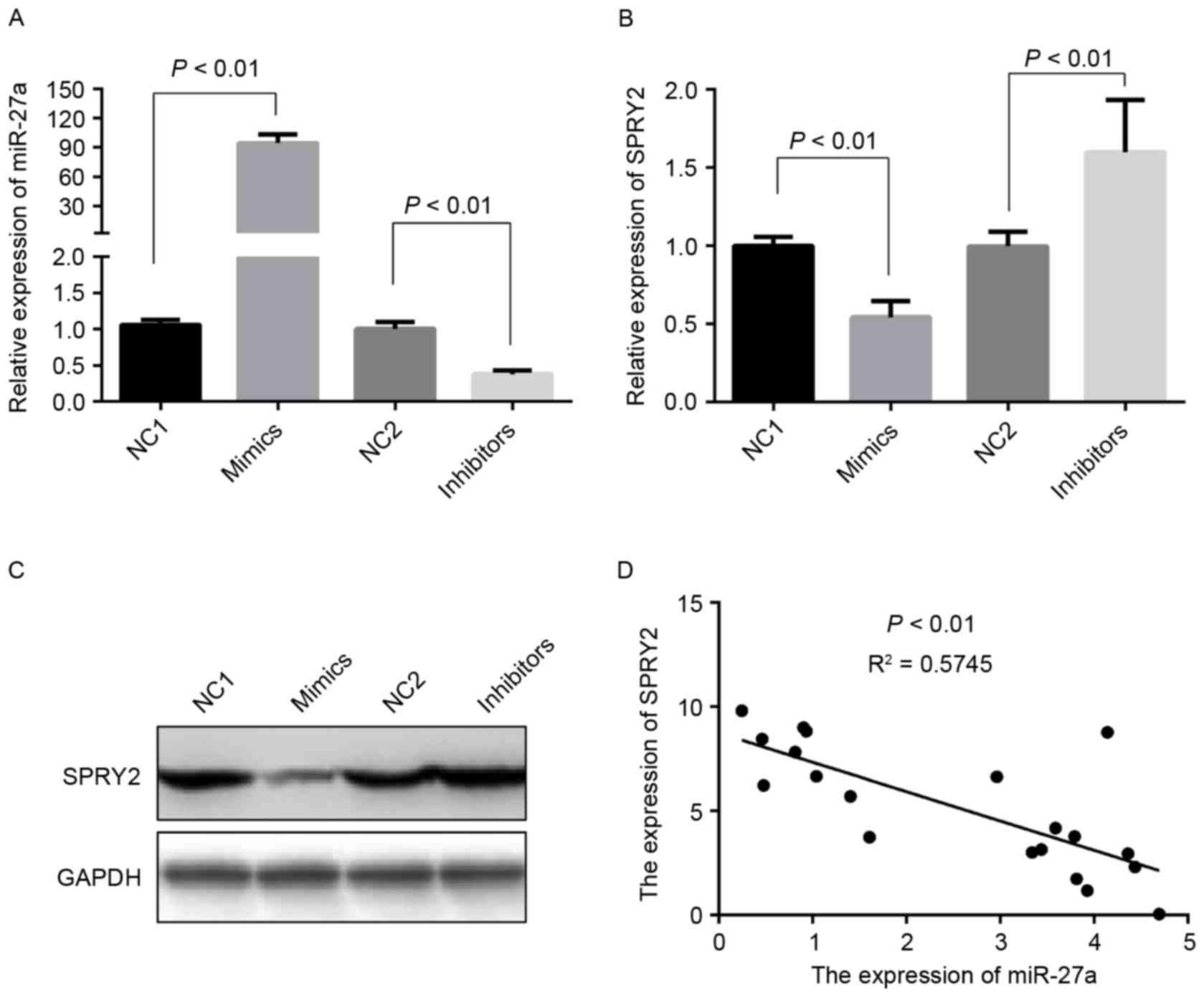
SPRY2, a tumor suppressor gene, is downregulated in
PCa tissues, and SPRY2 overexpression may suppress cell
proliferation in prostate cancer (19). Additionally, SPRY2 is hypothesized to
be a target of miR-27a: miR-27a altered cell growth, colony
formation and migration in pancreatic cancer through directly
targeting SPRY2 (12). However, it is
unclear whether the expression of SPRY2 is regulated by miR-27a in
PCa cells. Therefore, the expression of SPRY2 was initially
detected in the PCa cells transfected with miR-27a mimics or
inhibitors (Fig. 3A). Compared with
the negative control groups, the levels of mRNA and protein
expression of SPRY2 were markedly decreased, and increased,
respectively (Fig. 3B and C). The
correlation between the expression levels of miR-27a and SPRY2 were
additionally analyzed in PCa tissues, and the results demonstrated
that the expression of miR-27a was negatively associated with SPRY2
(Fig. 3D). These data suggested that
miR-27a/SPRY2 axis may serve an essential role in the proliferation
of PCa cells.
miR-27a promotes the proliferation
activity of PCa cells and this function is rescued by the
overexpression of SPRY2
miR-27a was suggested to act as an oncomiR and
affect PCa cell growth (11);
therefore the present study analyzed the function of miR-27a in PCa
cells, particularly the miR-27a/SPRY2 axis. The miR-27a mimics or
inhibitors were transfected into PCa cells, and the results
revealed that miR-27a mimics significantly promoted the
proliferation of PCa cells, whereas miR-27a inhibitors
significantly suppressed the proliferation of PCa cells compared
with the negative control groups (Fig.
4A), suggesting that miR-27a did act as an oncomiR in PCa. The
expression of SPRY2 was also inhibited or overexpressed by using
specific siRNA or SPRY2-overexpressed vectors in PCa cells,
respectively (Fig. 4B), and the
results indicated that the downregulation of SPRY2 significantly
increased the proliferation of PCa cells, whereas the upregulation
of SPRY2 significantly decreased the proliferation of PCa cells
(Fig. 4C). To additionally
investigate whether miR-27a exerting its function depended on the
expression of SPRY2, miR-27a mimics and SPRY2-overexpressed vectors
were co-transfected into PCa cells (Fig.
4D), and the results demonstrated that the proliferation of PCa
cells was rescued compared with the PCa cells transfected with
miR-27a mimics only (Fig. 4E). These
data suggested that the miR-27a/SPRY2 axis served an important role
in the proliferation of PCa cells.
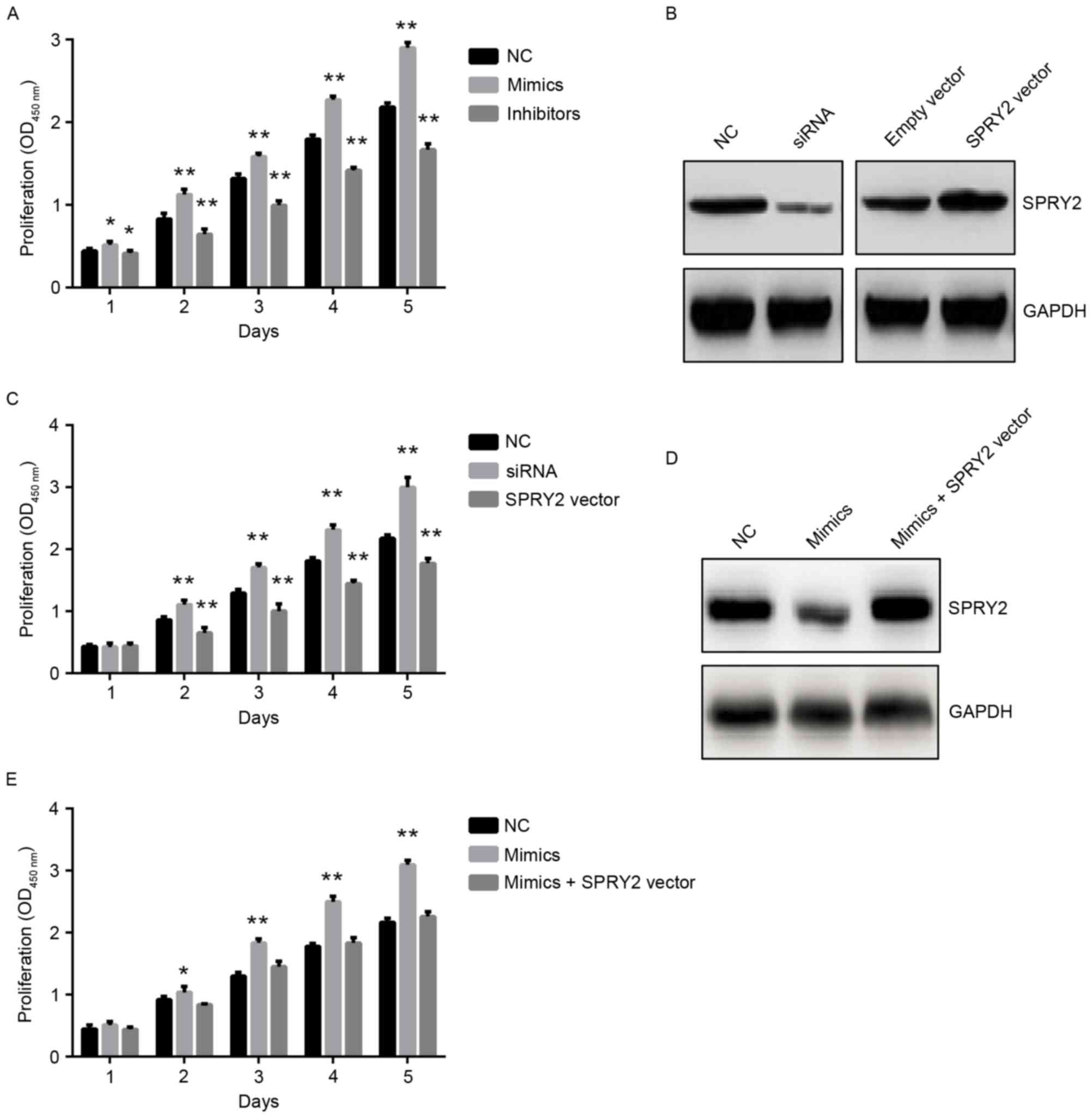 | Figure 4.miR-27a promotes the proliferation
activity of PCa cells and this function is rescued by the
overexpression of SPRY2. (A) The proliferation of PCa cells
transfected with NC, miR-27a mimics or miR-27a inhibitors by 1 to 5
days, respectively. *P<0.05 vs. NC; **P<0.01 vs. NC. (B) The
protein expression of SPRY2 in PCa cells transfected with NC, SPRY2
siRNA, Empty vector or SPRY2 vector for 48 h, respectively. (C) The
proliferation of PCa cells transfected with NC, SPRY2 siRNA or
SPRY2 vector for 1 to 5 days, respectively. (D) The protein
expression of SPRY2 in PCa cells transfected with NC, miR-27a
mimics or miR-27a mimics + SPRY2 vector by 48 h, respectively.
**P<0.01 vs. NC. (E) The proliferation of PCa cells transfected
with NC, miR-27a or miR-27a mimics + SPRY2 for 1 to 5 days,
respectively. *P<0.05 vs. NC; **P<0.01 vs. NC. miR, microRNA;
SPRY2, Sprouty2; PCA, prostate cancer; si, small interfering; NC,
negative control. |
miR-27a/SPRY2 axis regulates the cell
cycle of PCa cells
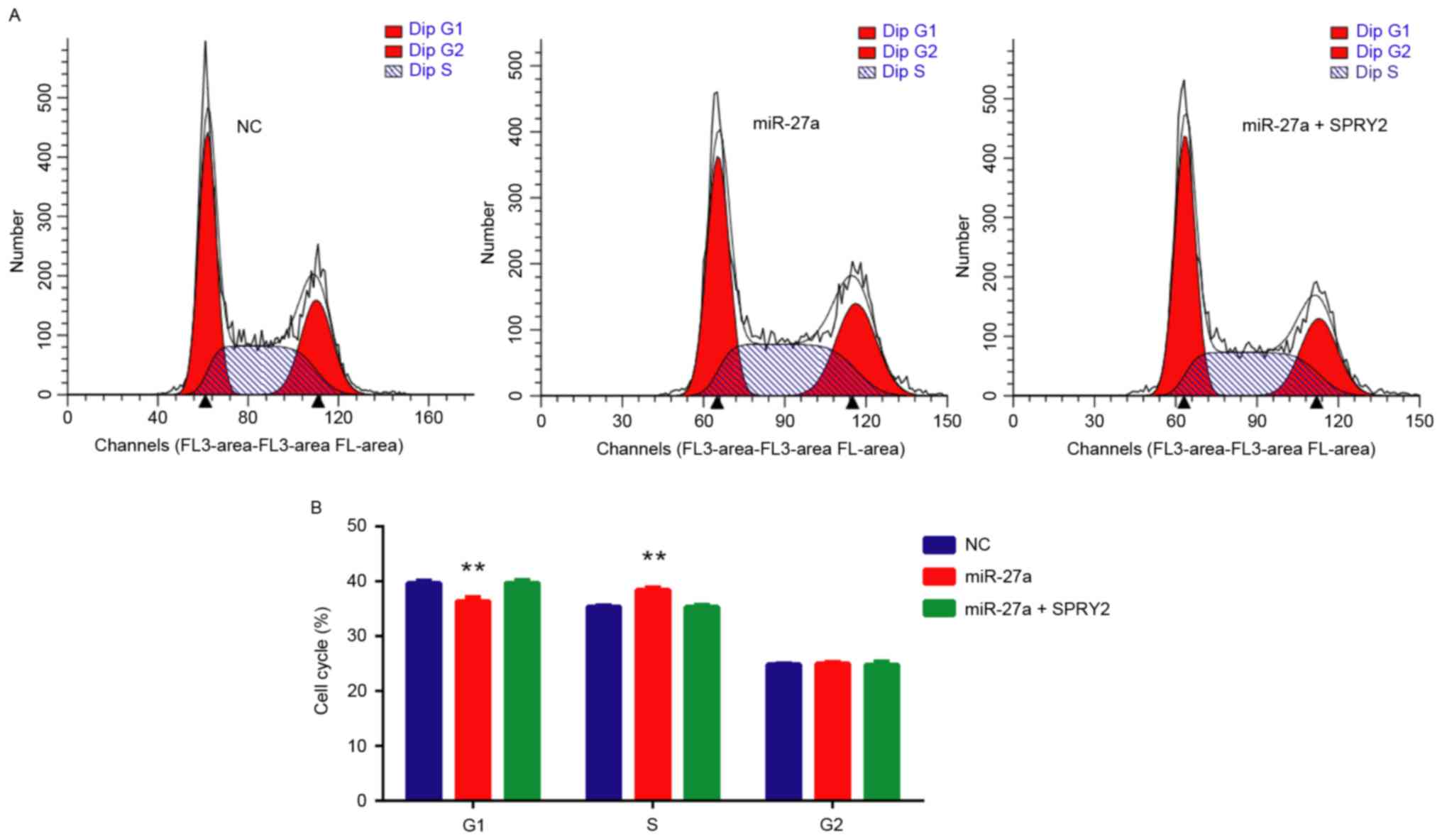
Whether miR-27a/SPRY2 did affect the cell cycle of
PCa cells was additionally investigated, and the results revealed
that miR-27a mimics significantly increased the number of S-stage
cells (P<0.01), whereas when the cells were co-transfected with
miR-27a mimics and SPRY2 vectors, the number of S-stage cells was
rescued compared with the negative control (Fig. 5). These data suggested that the
miR-27a/SPRY2 axis serves an important role in the cell cycle of
PCa cells.
Discussion
The present study identified that the expression of
miR-27a was increased in the tumor tissue and serum of patients
with PCa, and was correlated with poor survival of patients with
PCa. Furthermore, it was determined that miR-27a acted as an
oncomiR and promoted the proliferation activity of PCa cells. In
addition, miR-27a directly inhibited the mRNA and protein
expression of SPRY2, which is considered as a tumor suppressor in
PCa. In the majority of different types of cancer, miR-27a serves
as an oncomiR and promoted the progression of cancers. Fletcher
et al (11) first identified
that androgen regulated the overexpression of miR-27a in PCa and
that its upregulation was involved in cell growth. However, Walter
et al (10) demonstrated that
the downregulation of miR-27 was present in high-grade tumors. This
contradictory phenomenon formed the basis of the present study, to
investigate the in vivo expression of miR-27a in tumor
tissue of patients with PCa. The data indicated that miR-27a was
overexpressed in PCa tissues. In addition, the data demonstrated
that the overexpression of miR-27a was also present in the sera of
patients with PCa compared to the healthy subjects. Furthermore,
the data revealed that the overexpression of miR-27a in serum was
associated with poor survival of patients with PCa. These suggested
that the expression profile of miR-27a in sera may be a potential
biomarker for the diagnosis and prognosis of patients with PCa.
SPRY was identified in Drosophila as an
inhibitor of fibroblast growth factor signaling (20). SPRY2 was first identified to be
epigenetically suppressive in prostate cancer and to act as a tumor
suppressor (21). Although Ma et
al (12) suggested that SPRY2 is
a direct target of miR-27a in pancreatic cancer, the association
between SPRY2 and miR-27a in PCa remains unclear. The data of the
present study indicated that the expression of miR-27a was
inversely correlated with the expression of SPRY2 in PCa tissues,
and miR-27a promoted the proliferation of PCa cells also through
inhibiting the expression of SPRY2, suggesting that the
miR-27a/SPRY2 axis serves an important role in the proliferation of
PCa cells. It was also hypothesized that SPRY2 is a direct target
of miR-27a. In addition, Liu et al (22) demonstrated that SPRY2 is also a direct
target of miR-27b, a relative of miR-27a. Due to this, SPRY2 was
not identified a direct target of miR-27a in the present study.
miRNAs serum profiles are valuable biomarkers for
the diagnosis of diseases, due to its non-invasion, stability and
specificity. The challenge is that there is no suitable internal
reference to normalize the expression of miRNAs in sera, but using
exogenous genes as reference may be feasible, including
Cel-miR-39, a miRNA in nematodes (17,23,24). The
present study measured the relative expression of miR-27a in the
sera of patients with PCa and healthy subjects using this approach.
The data indicated that the serum levels of miR-27a were
significantly correlated with the survival rate of patients with
PCa. In the present study, a limitation was that the number of
samples was small. Although 140 subjects were enrolled, including
80 patients with PCa and 60 healthy subjects, the conclusion that
the expression profile of miR-27a in serum acted as novel
non-invasive biomarker for the diagnosis of PCa needs to be
confirmed in large scale in the future.
Taken together, the overexpression of miR-27a was
identified in the tumor tissue and serum of patients with PCa and
was correlated with poor survival of patients. Furthermore, miR-27a
as an oncomiR promoted the proliferation and cell cycle of PCa
cells by targeting SPRY2. These suggested that the serum signature
of miR-27a may be a novel non-invasive biomarker for the diagnosis
of PCa and the miR-27a/SPRY2 axis may be a therapeutic target.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Project of Jiangxi Provincial Education Department (grant nos.
YC2012-S026 and GJJ14077).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
WG and ZH performed the experiments. HH, AZ, and SL
collected the patient samples. CC and XZ analyzed the data. GZ and
ZS designed the study and wrote this article.
Ethics and consent to participate
All specimens were collected from the individuals
who provided written informed consent according to the protocols
approved by the Ethics Review Board at Nanchang University
(Nanchang, China).
Consent for publication
The study participants provided consent for the data
to be published.
Competing interests
The authors declare that they have no competing
interests.
Author information
No additional information provided.
References
|
1
|
DeSantis C, Naishadham D and Jemal A:
Cancer statistics for African Americans, 2013. CA Cancer J Clin.
63:151–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayes JH and Barry MJ: Screening for
prostate cancer with the prostate-specific antigen test: A review
of current evidence. JAMA. 311:1143–1149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin XJ, Chong Y, Guo ZW, Xie C, Yang XJ,
Zhang Q, Li SP, Xiong Y, Yuan Y, Min J, et al: A serum microRNA
classifier for early detection of hepatocellular carcinoma: A
multicentre, retrospective, longitudinal biomarker identification
study with a nested case-control study. Lancet Oncol. 16:804–815.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li BS, Zuo QF, Zhao YL, Xiao B, Zhuang Y,
Mao XH, Wu C, Yang SM, Zeng H, Zou QM and Guo G: MicroRNA-25
promotes gastric cancer migration, invasion and proliferation by
directly targeting transducer of ERBB2, 1 and correlates with poor
survival. Oncogene. 34:2556–2565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sapre N, Macintyre G, Clarkson M, Naeem H,
Cmero M, Kowalczyk A, Anderson PD, Costello AJ, Corcoran NM and
Hovens CM: A urinary microRNA signature can predict the presence of
bladder urothelial carcinoma in patients undergoing surveillance.
Br J Cancer. 114:454–462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sharova E, Grassi A, Marcer A, Ruggero K,
Pinto F, Bassi P, Zanovello P, Zattoni F, D'Agostino DM, Iafrate M
and Ciminale V: A circulating miRNA assay as a first-line test for
prostate cancer screening. Br J Cancer. 114:1362–1366. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin C, Fang C, Weng H, Yuan C and Wang F:
Circulating microRNAs as novel biomarkers in the diagnosis of
prostate cancer: A systematic review and meta-analysis. Int Urol
Nephrol. 48:1087–1095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walter BA, Valera VA, Pinto PA and Merino
MJ: Comprehensive microRNA profiling of prostate cancer. J Cancer.
4:350–357. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fletcher CE, Dart DA, Sita-Lumsden A,
Cheng H, Rennie PS and Bevan CL: Androgen-regulated processing of
the oncomir miR-27a, which targets prohibitin in prostate cancer.
Hum Mol Genet. 21:3112–3127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma Y, Yu S, Zhao W, Lu Z and Chen J:
miR-27a regulates the growth, colony formation and migration of
pancreatic cancer cells by targeting Sprouty2. Cancer Lett.
298:150–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng H, Wang X, Zhang P, Sun T, Ren X and
Xia Z: miR-27a promotes cell proliferation and metastasis in renal
cell carcinoma. Int J Clin Exp Pathol. 8:2259–2266. 2015.PubMed/NCBI
|
|
14
|
Salah Z, Arafeh R, Maximov V, Galasso M,
Khawaled S, Abou-Sharieha S, Volinia S, Jones KB, Croce CM and
Aqeilan RI: miR-27a and miR-27a* contribute to metastatic
properties of osteosarcoma cells. Oncotarget. 6:4920–4935. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao N, Sun H, Sun B, Zhu D, Zhao X, Wang
Y, Gu Q, Dong X, Liu F, Zhang Y and Li X: miR-27a-3p suppresses
tumor metastasis and VM by down-regulating VE-cadherin expression
and inhibiting EMT: An essential role for Twist-1 in HCC. Sci Rep.
6:230912016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bostwick DG: Staging prostate cancer-1997:
Current methods and limitations. Eur Urol. 32 Suppl 3:S2–S14.
1997.
|
|
17
|
Li BS, Zhao YL, Guo G, Li W, Zhu ED, Luo
X, Mao XH, Zou QM, Yu PW, Zuo QF, et al: Plasma microRNAs, miR-223,
miR-21 and miR-218, as novel potential biomarkers for gastric
cancer detection. PLoS One. 7:e416292012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Barros Pita W, Leite FC, de Souza
Liberal AT, Pereira LF, Carazzolle MF, Pereira GA and de Morais MA
Jr: A new set of reference genes for RT-qPCR assays in the yeast
Dekkera bruxellensis. Can J Microbiol. 58:1362–1367. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel R, Gao M, Ahmad I, Fleming J, Singh
LB, Rai TS, McKie AB, Seywright M, Barnetson RJ, Edwards J, et al:
Sprouty2, PTEN, and PP2A interact to regulate prostate cancer
progression. J Clin Invest. 123:1157–1175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hacohen N, Kramer S, Sutherland D, Hiromi
Y and Krasnow MA: sprouty encodes a novel antagonist of FGF
signaling that patterns apical branching of the Drosophila airways.
Cell. 92:253–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McKie AB, Douglas DA, Olijslagers S,
Graham J, Omar MM, Heer R, Gnanapragasam VJ, Robson CN and Leung
HY: Epigenetic inactivation of the human sprouty2 (hSPRY2)
homologue in prostate cancer. Oncogene. 24:2166–2174. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu C, Liang S, Xiao S, Lin Q, Chen X, Wu
Y and Fu J: MicroRNA-27b inhibits Spry2 expression and promotes
cell invasion in glioma U251 cells. Oncol Lett. 9:1393–1397. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao B, Wang Y, Li W, Baker M, Guo J,
Corbet K, Tsalik EL, Li QJ, Palmer SM, Woods CW, et al: Plasma
microRNA signature as a non-invasive biomarker for acute
graft-versus-host disease. Blood. 122:3365–3375. 2013. View Article : Google Scholar : PubMed/NCBI
|