Introduction
Ampullary carcinoma is a rare histological type of
gastrointestinal (GI) malignancies accounting for approximately
0.2–0.5% worldwide (1,2). The radical and beneficial surgery is
pancreatoduodenectomy for resectable ampullary malignancies
(3,4).
Carbohydrate antigen 19-9 (CA19-9) and bilirubin are
well-established preoperative markers for indicting the prognosis
of ampullary carcinoma (5–7). However, they exhibit unreliable and
inconsonant characteristics in different studies with different
patient cohorts (8,9). The critical disadvantages of CA19-9 and
bilirubin are the deterioration in low sensitivity and specificity
in jaundiced patients (9–11) and the ball-valve effects, as elevated
CA19-9 and bilirubin associated with ampullary lesions can be
intermittent) (1).
Therefore, simple, easily available and credible
biomarkers for prognosis for patients with ampullary carcinoma are
needed (12). Systematic inflammatory
response (SIR) has been proven to be closely associated with cancer
initiation, promotion, malignant conversion, invasion and
metastasis (13–16). Several inflammatory biomarkers are
routinely available from the pre-treatment routine blood test,
including platelet count, platelet to lymphocyte ratio (PLR) and
neutrophil to lymphocyte ratio (NLR). PLR and NLR have been the
most frequently inflammatory biomarkers used to assess the
prognosis in a number of different types of cancer (7,17,18), including ovarian cancer. Nevertheless,
only one previous article has explored PLR as prognostic factor in
ampullary carcinoma (7), and, to the
best of our knowledge, there are no studies regarding NLR.
Therefore, it is necessary to provide more evidence regarding the
prognostic role of PLR and NLR in ampullary carcinoma.
Therefore, an aim of the present study was to
determine whether preoperative PLR and NLR are preoperative
predictive indicators for patients with ampullary carcinoma
following curative surgery, then providing additional evidence to
for physicians to make an improved clinical decision prior to
surgery and theoretically helping to identify tumor
progression.
Materials and methods
A total of 94 complete ampullary carcinoma (AC)
cases who received surgery were included in the present study and
diagnosed with AC by pathology. Retrospective data were extracted
from the consecutive 94 patients (50 males and 44 females; median
age 62 years; age range, 39–82 years) undergoing pylorus-preserving
pancreaticoduodenectomy (PPPD) or classical Kausch-Whipple
(Whipple) resection between January 2010 and September 2016 at the
Peking Union Medical University Hospital (Beijing, China). The
cases were excluded if: i) The patient succumbed within 30 days
after surgery; ii) there was incomplete information; iii) the
cancer was mixed type; iv) the patient received non-radical
surgery; v) the patient succumbed during follow-up. Patient
demographics, operative details, and the histological
characteristics of the resected specimen were all extracted and
numbered as Patient ID, due to no personal information being
collected. A positive resection margin (R1) was defined as tumor
involvement of margin under microscopic examination without any
involvement in physical examination. While, R2 was defined as tumor
involvement of the margin under microscopic examination, as well as
involvement in physical examination No R2 resections were
identified. Details of preoperative biliary drainage and adjuvant
therapy were also collected, and survival data were obtained. The
preoperative full blood count, CA19-9, and concurrent bilirubin
levels were recorded where available. The normal diagnostic
reference interval for serum CA19-9 was based on the previous study
and our laboratory specification. For pre-operative CA19-9 a
cut-off value of >150 kU/l was used to define the high-risk
group for CA19-9 when recorded in the absence of concurrent
cholestasis, such as bilirubin ≤35 µmol/l, and a cut-off value of
>300 kU/l was used in the presence of cholestasis, such as
bilirubin >35 µmol/l. For pre-operative CA19-9 a cut-off value
of >255.5 kU/l was used (19,20).
Additionally, the PLR optimum cut-off point 226.8
was determined represent the optimum stratification point at which
the survival differences between two groups was maximized. The
pathological stage of the ampullary carcinoma was based on the
update 8th edition of American Joint Committee On Cancer (AJCC)
(21) by two pathologists
independently who were blind to the study. The pathological report
was offered and authorized by the Affiliated Pathology Department
of Peking Union Medical College Hospital, Chinese Academy of
Medical Sciences and Peking Union Medical College (Beijing,
China).
Statistical analysis
Continuous data were described and present as the
median ± interquartile range (IQR) or 95% confidence intervals
(CI). Relationships between the two continuous variables were
analyzed using Spearman's rank correlation. Comparative analysis of
categorical data was based on χ2 or Fisher's exact
tests. Survival data were analyzed using log rank testing for
univariate analysis and Cox proportional hazards with
forward-stepwise regression for multivariate analysis. Survival
analysis by the continuous variable was categorized by the optimum
cut-off of baseline platelet/netrophil to lymphocyte ratio value,
which were defined by maximally selected Log-rank statistics
(22,23). Patients who died within 30 days after
surgery were excluded from survival analysis. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed using R software version 3.4.0
for Windows (GUI front-end), Graph Pad Prism 7.00 (GraphPad
Software, Inc., La Jolla, CA, USA) and IBM SPSS Statistics 20 for
Windows (IBM Corp., Armonk, NY, USA).
Results
Patient clinicopathological features
according to preoperative PLR
A total of 345 consecutive patients underwent
pancreatoduodenectomy (PPPD and Whipple) for pancreatic or
perampullary tumors during the study period. Among them, 97 cases
had histologically confirmed carcinoma arising from the ampulla of
Vater. A single patient (1.03% of the patient cohort) who died
within 30 days following surgery was excluded from the subsequent
survival analyses. Two patients were also excluded for incomplete
information. Finally, 94 complete AC cases were included in this
study. There were a total of 71 censored cases with a median
follow-up time of 21 months (IQR=12–30 months). Median overall
survival time of the study group was 48 months (95% CI=39.7 to 56.3
months). The demographics of patients in the study along with the
preoperative full blood count results were listed in Table I.
 | Table I.Demographics along with the
preoperative CA19-9 and full blood count results of Ampullary
carcinoma cases who received pancreaticoduodenectomy. |
Table I.
Demographics along with the
preoperative CA19-9 and full blood count results of Ampullary
carcinoma cases who received pancreaticoduodenectomy.
| Demographics | Number |
|---|
| No. of patients
identified | 94 |
| Male/female | 50:44 |
| Median age
(IQR) | 62 (54–68)
years |
| Overall median
survival time (95% CI) | 48 (39.7–56.3)
months |
| Surgery |
|
|
Whipple | 89 |
|
PPPD | 5 |
| Preoperative
intervention for biliary stenting |
|
| No | 47 |
|
Yes | 47 |
| Adjuvant therapy
received |
|
| No | 82 |
|
Yes | 12 |
| Preoperative CA19-9
results available | 76 |
| Median
preoperative | 76.2 |
| CA19-9
(IQR)a | (15 to 256)
U/ml |
| Number of jaundiced
cases at time of CA19-9 estimation (bilirubin >35 µmol/l) |
|
|
Jaundice | 51 |
| No
jaundice | 45 |
| Preoperative FBC
available | 94 |
| Within
7 days | 68 |
|
Following 7 days | 26 |
| Median
platelet-lymphocyte ratio (IQR) | 152.6 |
|
| (114.7 to
229.6) |
| Neutrophilia
present (>7.5×106/ml) | 8 |
| Lymphocytopaenia
present (<1.0×106/ml) | 13 |
| Thrombocytosis
present (>400×106/ml) | 0 |
Outcomes
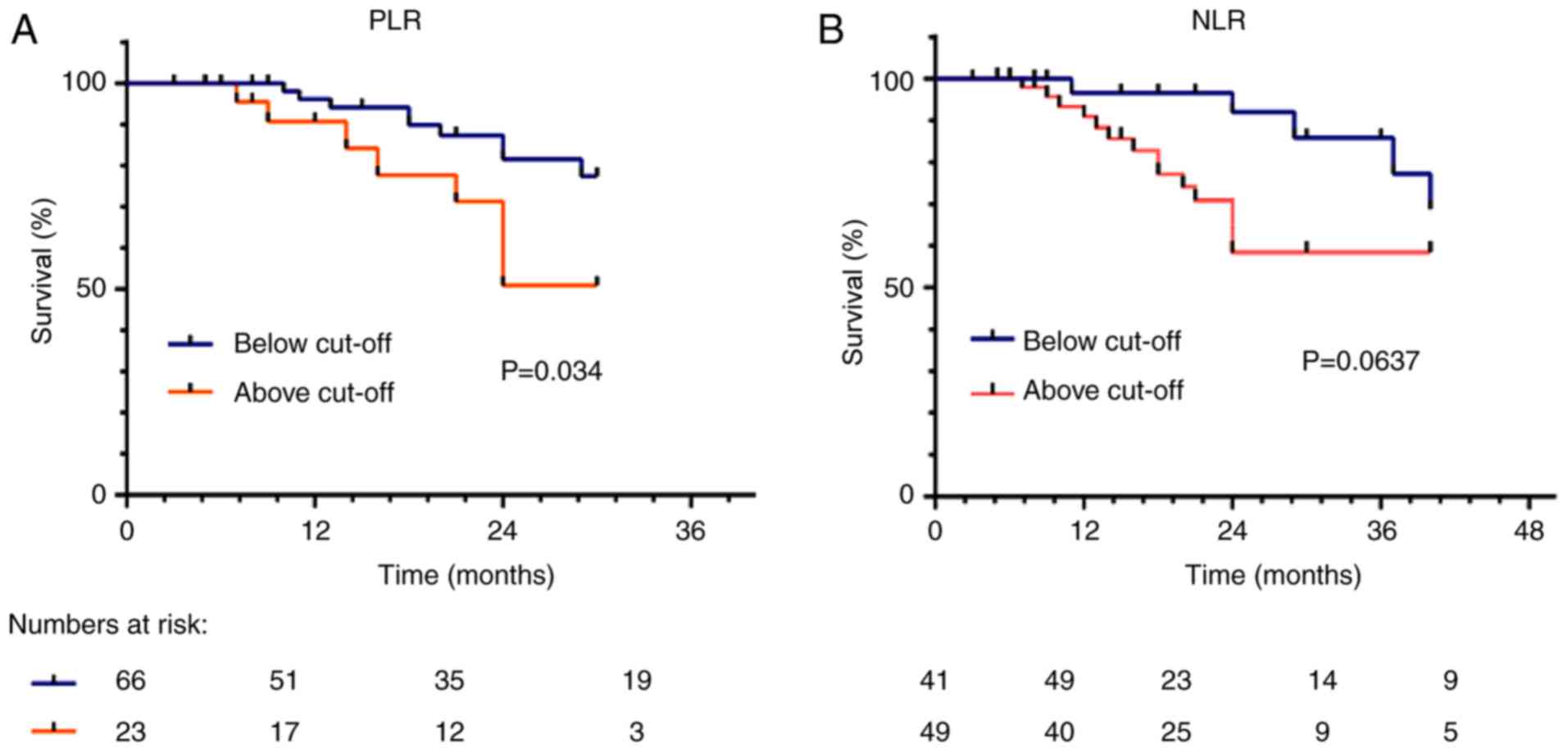
The median survival time stratified by preoperative
CA19-9 levels (at a cut-off value of ≤150 or ≤300 kU/l in jaundiced
cases, the cut-off value of 255.5 for all cases), PLR (at cut-off
value ≤226.83), NLR (at cut-off value ≤2.58) and total bilirubin
(Tbil) level (at the cut-off 12.6 µmol/l/211.7 µmol/l) as well as
the other various histological subgroups were shown in Table II.
 | Table II.Median overall survival times (log
rank) according to preoperative CA19-9, Tbil, platelet-lymphocyte
ratio and histological hub-groups in the overall study group. |
Table II.
Median overall survival times (log
rank) according to preoperative CA19-9, Tbil, platelet-lymphocyte
ratio and histological hub-groups in the overall study group.
|
Characteristics | Number of
patients | Median survival
(months) | P-value |
|---|
| Preoperative
CA19-9 |
|
| 0.499 |
| Below
cut-off | 50 | 48 (36.5–59.4) |
|
| Above
cut-off | 26 | 37 (33–63) |
|
| Preoperative
CA19-9 |
|
| 0.01a |
|
≤255.5 | 54 | NR (>60
months) |
|
|
>255.5 | 22 | 37 (16.1–57.9) |
|
| Preoperative
PLR |
|
| 0.255 |
|
≤160 | 49 | 48 (40–56) |
|
|
>160 | 45 | 40 (NR) |
|
| Preoperative
PLR |
|
| 0.0177a |
|
≤226.83 | 63 | 48 (NR) |
|
|
>226.83 | 31 | 24 (NR) |
|
| Preoperative
NLR |
|
| 0.0637 |
|
≤2.58 | 34 | 46 (NR) |
|
|
>2.58 | 60 | 48
(6.938–89.062) |
|
| Nodal
involvement |
|
| 0.0018a |
|
Negative | 59 | NR (66-NR) |
|
|
Positive | 35 | 33 (20.3–45.7) |
|
| Tumor
differentiation |
|
| 0.0187a |
|
Well/moderate | 69 | 48 (38.9–57.1) |
|
|
Poor | 25 | 24 (12.6–35.4) |
|
| Stage |
|
| 0.003a |
| 0 and
I | 32 | NR (30-NR) |
|
| II | 27 | 40 (NR-NR) | (0.0083) |
| III and
IV | 35 | 46 (36.8–55.2) |
|
| Nerve invasion |
|
| 0.4176 |
|
Positive | 12 | NR (24-NR) |
|
|
Negative | 82 | 48 (38.4–57.6) |
|
| ICE |
|
| 0.459 |
|
Positive | 20 | NR (33-NR) |
|
|
Negative | 74 | 37 (31.4–42.6) |
|
The corresponding survival curves when stratifying
by PLR and NLR are shown in Fig. 1A.
No significant associations between CA19-9 and PLR (Spearman,
rho=1, P=0.097) or between Tbil and platelet-lymphocyte ratio
(Spearman, rho=1, P=0.13) were identified. Significant correlation
was observed between Tbil and CA19-9 (Spearman, rho=0.487,
P=0.000), PLR and NLR (spearman, rho=0.727, P=0.000); NLR and tbil
(Spearman, rho=0.184, P=0.077) NLR and CA19-9 (Spearman, rho=0.107,
P=0.354).
Associations between PLR and
pathological characteristics
The correlation between preoperative indices and
postoperative histological characteristics using χ2 was
listed in Table III. The higher PLR
is, the higher proportion of positive lymph node is (P<0.01). No
significances were identified between CA19-9 and nodal status,
tumor differentiation or stage. Neither between Tbil or NLR and
those characteristics.
 | Table III.The association between preoperative
indices and postoperative histological characteristics using
χ2. |
Table III.
The association between preoperative
indices and postoperative histological characteristics using
χ2.
|
| CA19-9 (kU/l) | Tbil (µmol/l) | PLR | NLR |
|---|
|
|
|
|
|
|
|---|
|
Characteristics | ≤255.5 | >255.5 | P-value | ≤190 | >190 | P-value | ≤226.83 | >226.83 | P-value | ≤2.58 | >2.58 | P-value |
|---|
| LN |
|
| 0.361 |
|
| 0.733 |
|
|
<0.001a |
|
| 0.148 |
| LN
(+) | 22 | 7 |
| 30 | 5 |
| 12 | 23 |
| 13 | 22 |
|
| LN
(−) | 31 | 16 |
| 49 | 10 |
| 51 | 8 |
| 31 | 28 |
|
| Tumor |
|
| 0.128 |
|
| 0.215 |
|
| >0.100 |
|
| 0.889 |
|
Well/moderate | 39 | 16 |
| 60 | 9 |
| 54 | 15 |
| 32 | 37 |
|
|
Poor | 11 | 10 |
| 19 | 6 |
| 20 | 5 |
| 12 | 13 |
|
| Stage |
|
| 0.096 |
|
| 0.211 |
|
| 0.058 |
|
| 0.068 |
| 0 and
I | 20 | 5 |
| 29 | 3 |
| 28 | 4 |
| 20 | 12 |
|
| II | 11 | 11 |
| 20 | 7 |
| 23 | 4 |
| 11 | 16 |
|
| III and
IV | 19 | 10 |
| 30 | 5 |
| 23 | 12 |
| 13 | 22 |
|
Analysis of multiple prognostic
factors
The results of a multivariate survival analysis
using Cox Log rank proportional hazards with forward stepwise
regression are shown in Table IV. In
which preoperative indexes including CA19-9, Tbil, PLR and NLR
alongside with histological indexes including stage, tumor
differentiation and nodal status were presented. Nodal status
failed to emerge as a significant variable in forward stepwise
regression for it to demonstrate linear dependence with stage, and
was therefore omitted from the final multivariate model. The
supplementary Cox analysis including nodal status without stage
showed that it was also an independent index with Hazard risk (HR)
5.026 (95% CI 3.937–6.416, P<0.001). The Cox analysis suggested
that all the indexes included were independent prognostic variables
in this patient group. The results of a multivariate survival
analysis referring to NLR demonstrated NLR was not an independent
prognostic index (P>0.05) (data not shown).
 | Table IV.Multivariate (Cox proportional
hazards) survival analysis. |
Table IV.
Multivariate (Cox proportional
hazards) survival analysis.
|
Characteristics | Coefficient | SE | Wald | HR | 95% CI | P-value |
|---|
| PLR | 1.099 | 0.144 | 58.412 | 3.001 | 2.264–3.978 | <0.001 |
| Tbil (µmol/l) |
|
|
|
|
| <0.001 |
|
Tb<17.1 | – | – | 35.548 | – | – |
|
|
Tb<190 | 1.634 | 0.279 | 34.310 | 5.125 | 2.966–8.854 |
|
|
Tb≤190 | 1.446 | 0.351 | 16.985 | 4.245 | 2.134–8.442 |
|
| CA19-9 | 0.416 | 0.137 | 9.185 | 1.516 | 1.158–1.984 | 0.002 |
|
Differentiation | 1.450 | 0.176 | 67.999 | 4.264 | 3.021–6.019 | <0.001 |
| Stage (21) |
|
|
|
|
| <0.001 |
| 0 and
I | – | – | – | – | – |
|
| II | 1.307 | 0.234 | 31.126 | 3.697 | 2.335–5.852 |
|
| III and
IV | 2.416 | 0.217 | 123.429 | 11.200 | 7.313–17.152 |
|
Risk stratification by Tbil and
PLR
CA19-9 and PLR risk stratification is less
satisfactory for the high-risk group only identified 1cases.
Consequently risk stratification by Tbil and PLR was conducted. The
high-risk group was defined as when Tbil and PLR values were above
the cut-off. Low-risk groups were determined when both of two
parameters were below the cut-off. and intermediate-risk group is
conformed that either PLR or Tbil was above the cut-off.
Table V demonstrates
that the only significant difference among the three risk groups
was adjuvant therapy with a trend toward an increased likelihood of
adjuvant therapy in the high-risk groups compared with the low-risk
group (Fisher's exact, P<0.001). No significant difference was
found in the proportion of patients undergoing preoperative biliary
drainage when comparing the three risk groups (P=0.650). There was
no difference in survival when comparing cases who did (n=12) or
did not (n=80) receive adjuvant therapy in the whole patient group
(median survival were >60 (95% CI=NR) months and 46, Log rank,
P=0.0956). Similarly, there was no significant difference in the
proportion of patients who undergoing preoperative biliary drainage
(median survival >60 (95% CI=NR) months and 40 (95% CI=23.891 to
56.109) months respectively-Log rank, P=0.138).
 | Table V.Tumor characteristics according to
total bilirubin/platelet-lymphocyte ratio risk stratification. |
Table V.
Tumor characteristics according to
total bilirubin/platelet-lymphocyte ratio risk stratification.
|
Characteristics | High | Intermediate | Low | P-value |
|---|
| Lymph node
status |
|
|
| 0.206 |
|
LN(+) | 3 | 16 | 16 |
|
|
LN(−) | 3 | 18 | 38 |
|
|
Differentiation |
|
|
| 0.682 |
|
Well/moderate | 6 | 21 | 42 |
|
|
Poor | 2 | 10 | 13 |
|
| Resection margin
status |
|
|
| 0.058 |
|
Positive | 1 | 0 | 0 |
|
|
Negative | 5 | 30 | 59 |
|
| Preoperative
biliary stenting |
|
|
| 0.702 |
| No | 2 | 13 | 29 |
|
|
Yes | 3 | 17 | 27 |
|
| Adjuvant therapy
received |
|
|
|
<0.01a |
| No | 2 | 26 | 54 |
|
|
Yes | 4 | 4 | 4 |
|
| Stage (21) |
|
|
| 0.086 |
| 0 and
I | 0 | 10 | 22 |
|
| II | 3 | 7 | 17 |
|
| III and
IV | 3 | 14 | 18 |
|
Analysis of risk model using cox
regression
Table VI demonstrates
that the risk model being an independent predictive value with a
hazard ratio 1.78 and 19.973 in intermediate-risk and high-risk
group respectively compared with low-risk group (P<0.0001).
Tumor-node-metastasis (TNM) was also an independent factor in the
final cox regression as well (P<0.0001). While nodal (df=0)
status and CA19-9 (P=0.078) failed to emerge as significant
covariates on forward stepwise regression and was excluded from the
final model.
 | Table VI.Cox regression Tbil/PLR risk
stratification and tumor histlogical characteristics. |
Table VI.
Cox regression Tbil/PLR risk
stratification and tumor histlogical characteristics.
| Variable | Coefficient
(B) | SE | Wald | HR | 95% CI | P-value |
|---|
| Tbil/PLR risk
groups |
|
|
|
|
|
|
| Low
risk | – | – | – | – | – | – |
|
Intermediate risk | 0.58 | 0.111 | 27.325 | 1.786 | 1.437–2.220 | <0.001 |
| High
risk | 2.993 | 0.352 | 72.395 | 19.973 | 10.006–39.723 | <0.001 |
| Stage (21) |
|
|
|
|
|
|
| 0 and
I | – | – | – | – | – | – |
| II | 1.671 | 0.227 | 54.239 | 5.317 | 3.408–8.295 | <0.001 |
| II and
IV | 2.789 | 0.215 | 168.955 | 16.266 | 10.682–24.771 | <0.001 |
Analysis of predictive prognostic
value of PLR and NLR by subgroup analysis
subgroup survival analysis according to PLR and NLR
showed PLR was predictive marker in subgroup (male, CA19-9
below-cutoff, jaundice, Mediated/high Differentiation subgroup,
rather than other subgroups. While NLR show prognostic value in
subgroup (age ≤60 years, CA19-9 above cut-off, jaundice and
mediated/high differentiation subgroup) rather than the other
subgroups (data not shown). After adjusting for interfering factors
related to PLR/NLR and/or probability of tumor recurrence, low NLR
patients continued to display a better outcome with bordline
difference (P=0.0637) (Fig. 1B).
Particularly, significant differences were shown in postmenopausal
patients (HR=2.81, 95% CI 1.14 to 6.88, P=0.0243), node negative
cancers (HR=5.31, 95% CI 1.19 to 23.70, P=0.0292) (data not
shown).
Discussion
The present study identified preoperative PLR as an
independent prognostic factor for curative resectable ampullary
carcinoma. The results were in agreement with a previous report by
Smith et al (7); however, as
it was based on a larger number of cases, the present study
contained more detailed analyses, and was performed in accordance
with, at the time of analysis, the most up to date AJCC (21). The definition of tumor stage, which
was adjusted by evidence-based studies, demonstrated a better
guidance for the prognosis in ampullary cancer (1,21).
Furthermore, the present study extracted the first examination
results of all the preoperative indices to exclude potentially
influencing variables, including the preoperative bile drainage. In
terms of the optimum cut-off values of CA19-9, bilirubin and PLR,
various cut-off values were borrowed from previous ampullary
carcinomas and other solid carcinomas studies (24,25).
Firstly, the maximally selected log-rank statistics, the
statistical analysis designed for the optimum cut-off value of
continuous variable based on the Log-rank survival analysis were
applied, which was run in R software (26) (data not shown). To some extent, it
contributed to the conclusion that the preoperative PLR was related
to the lymph node involvement and prognosis in ampullary cancer
following surgical resection.
Furthermore, in the present study, a risk prediction
model was established using PLR and Tbil, which demonstrated a good
performance in univariate (P=0.0004) and multivariate (P<0.0001)
survival analysis. While the risk model using PLR and CA19-9
demonstrated a less satisfactory performance with (P=0.002) and
showed dependent predictive value at the expense of all the other
predictors (P<0.05). This maybe partly due to the correlation
between the CA19-9 and TNM notwithstanding the border significant
difference (P=0.053) and between PLR and nodal involvement
(P=0.025).
The present study demonstrates for the first time,
to the best of our knowledge, the prognostic role of NLR in
resectable ampullary cancer and the prognostic value of PLR/NLR in
specific patient subgroups, as the PLR exhibited a prognostic value
in a number of specific subgroups, including males, CA19-9 below
cutoff, jaundice and mediated/high differentiation subgroups. While
NLR exhibited prognostic value in subgroup (age ≤60 years, CA19-9
above cut-off, jaundice, Mediated/high Differentiation subgroup) in
spite of border significance in univariate analysis (P=0.0637;
Table II). Previous studies
(27,28) have indicated that NLR was a potential
independent and significant indicator of a positive outcome in
patients with carcinoma of the ampulla of Vater following a
pancreaticoduodenectomy. The present study was similar with the
conclusions of Haruki et al (27) with a larger study case 94 vs. 37
patients. An additional similar study, with a patient cohort of 87
patients concluded that high NLR had a significantly worse Eastern
Cooperative Oncology Group performance score as well as an
independent and significant predictive factor of prognosis in
patients with ampullary carcinoma (26). However, the present study demonstrated
that the predictive roles of NLR for LN metastasis. Two previous
meta-analysis regarding the prognostic role of NLR in cancer
concluded that a high-pretreatment blood NLR may serve as an
adverse prognostic indicator for patients with advanced tumors
(29) and predictor of survival in
patients with pancreatic cancer (30). However, a previous study reported that
a cut-off value of 5 indicated a worst progression free survival
(HR 2.23, 95% CI 1.54–3.23, P=0.019) (29). A latter study included a total of
2,035 patients in 9 cohorts and suggested that an elevated NLR was
a predictor of survival in patients with pancreatic cancer without
analyzing the cut-off values heterogeneity (28). More large-scale perspective studies,
which include a training cohort (the cohort used to calculate the
optimum cut-off values as an independent cohort) and validated
cohort (a cohort used to validate the efficiency and validity of
the optimum cut-off values based on the training cohort) are needed
in the future to determine the optimal cut-off values.
Additionally, concerning the clinical role of NLR, more detailed
studies included the subgroups analysis or propensity score
analysis are required to exclude confounding factors.
In 8th AJCC, the assessment of lymph node metastasis
was used to predict the prognosis (21). When the OS time was compared according
to the lymph node metastasis, the prognostic correlation for
patients with N1 (number of metastatic lymph nodes ≥1) or N2
(number of metastatic lymph nodes ≥4) was not clear (21). In addition, in the present study OS
time was compared according to the stage, and tumor
differentiation, there were no significant differences between
stage 0 and I or between well and moderate differentiation.
Furthermore, there were no significant effects of nerve or vessel
invasion. Following combined and the aforementioned variables were
adjusted and, the lymph status, TNM stage and tumor differentiation
were demonstrated as independent prognostic indices for the
ampullary cancers which were consistent with the AJCC stage based
evidences.
In addition, the present study demonstrated that PLR
could serve as a predictor for the lymph node (LN) metastasis in
the patient cohort of the present study by χ2 test
(shown in Table III). LN metastasis
is the strongest prognostic predictor for ampullary cancer, while
preoperative serum CA19-9 was not associated with the TNM stage,
pathologic differentiation or the tumor size (31–33) (shown
in Table III), which may serve as
an explanation for the prognostic role of PLR.
However, the cut-off values of PLR were not
consistent in different study backgrounds. Some studies have
reported that a PLR >300 could predict a worse survival in
ovarian cancers (34,35), whereas other studies have reported
different PLR values from 203 to 299 as prognostic factors in
ovarian cancer (36,37). In resectable gastric cancer, higher
pre-operative PLR at cut-off 208 showed decreased overall survival
time and disease-free survival (24).
Another study about advanced pancreatic cancer proved that patients
divided by PLR <200 vs. ≥200 with 9.1 vs. 4 months overall
survival time, respectively (25). In
present study, the optimum PLR cut-off value was calculated at
226.83 to predict overall survival following curative surgery.
The critical prognostic role of preoperative PLR in
ampullary cancers is well known; however, its mechanism of action
has not been completely elucidated in previous studies (7,38).
Previous research has demonstrated that tumor cells could increase
the peripheral blood platelet count via proinflammatory mediators
and other mechanisms such as CA19-9 induced inflammatory molecules
(39–43). Meanwhile, these inflammatory factors
could also decrease the lymphocyte counts such as IL-6 (43,44).
Furthermore, the role of platelets as a negative regulator in
immune system has been recently demonstrated, reporting that
platelet was the main regulator to decrease the adoptive T cell
count and obliterate its immunologic function in vitro by
producing transforming growth factor (TGF)-β (45). Therefore, the platelet to lymphocyte
ratio could be considered as a relative active inflammation or/and
inhibitive immune reaction (46).
Platelet count increase has been observed in numerous types of
tumors at earlier century and then proved to be a negative
predictor in some cancers latterly (47–49). As
critical roles in host immune response, lower levels of lymphocytes
were associated with a poor prognosis of a variety of tumor types,
including breast and colon cancer (50). Therefore, high level of PLR was
associated with poor prognosis of many various solid tumors
(51). These results have the
potential to provide evidence for the antitumor role of the
anti-platelet drugs.
Referring to the lymph node metastasis, studies have
previously reported that PLR may have the prospective potential to
predict nodal involvement in patients with gastric cancer (29), vulvular squamous cell sarcoma
(30), and endometrial adenocarcinoma
(31). The majority of these
demonstrated a higher PLR in nodal involvement group, which was
also observed in the present study (30,31);
however, a single hemolytic inflammatory index is not a practical
guide in clinical use for the accurate predication of lymph node
metastasis. Therefore, other studies concerning PLR should include
other preoperative indicators such as NLR (29) and contrast-enhanced computerized
tomography (52) to establish a model
with satisfactory specificity and sensitivity to predict nodal
involvement.
Previous studies demonstrated that CA19-9 and
bilirubin are independent prognostic factors in present study
despite different cut-off values (5,8).
Additionally, to the best of our knowledge, there is no existing
evidence suggesting that preoperative biliary stenting has an
influence on operative mortality or subsequent survival following a
pancreaticoduodenectomy (53–55), an adjusted cut-off value of bilirubin
to 190 µmol/l (elevated the best cut-off via maximally selected
log-rank statistics) was used for the survival benefit of
preoperative biliary drainge. Sex showed no significance in
univariate survival analysis of median survival time 42 months for
males and >60 months for females (P=0.7746). Age with median
survival time of 42 months vs. >60 months at a cut off 58 years
old (P=0.1749 demonstrated no significant impact in the overall
survival between the patients with and without preoperative biliary
drainage at cut-off 190 µmol/l (data not shown). Similarly, there
was no significant difference in survival with and without
postoperative adjuvant chemotherapy with a median survival >60
vs. 46 months in line with previous studies (56–58).
Hence, age, sex, preoperative biliary drainage and adjuvant therapy
were unlikely to act as a significant confounding factors when
interpreting the survival data.
In addition, it is well established that CA19-9 is
positively correlated with bilirubin (9–11).
Therefore, these two prognostic markers and the PLR alongside with
other univariates with statistics significance in Kaplan-Meier
analyses were analyzed using Cox Log-rank proportional hazards with
forward stepwise regression. Elevated preoperative CA19-9 level,
bilirubin level, PLR, poor differentiation, nodal metastases, and
advanced stage all exhibited the trend toward poorer survival in
univariate and multivariate analysis, which was consistent with the
results obtained by previous studies (6,11).
Additionally, a previous study demonstrated that preoperative
elevated serum CA19-9 (cut-off 36 kU/l) and total bilirubin
(cut-off 1 mg/ml) levels were prognostic factors in ampullary
adenocarcinoma and demonstrated an association with the
pathological pancreatobiliary type that which proved aggressively
invasive, which was closely associated with poor survival (2).
Jaundice was the most common symptom (50%, 47/94) in
the present study, which is similar to previous studies, which
accounted for 70–80% of the chief patient complaint (59,60). The
decline in component ratio of jaundice in the present study may be
attributed to the advanced economic and medical factors in China.
The remaining chief complaints consist of physical discomfort
(15.95%, 15/94) and other atypical digestive system complaints
including abdominal pain (34.04%, 32/94). Jaundice is a prognostic
factor in univariate Log-Rank analysis compared with a physical
discomfort group (P=0.00095, >60 vs. 40 months). This supports
the survival analysis of total bilirubin. So even compared with the
atypical digestive symptom (P=0.0034, >60 vs. 30 months).
However there was no significance between the jaundice and other
atypical digestive symptoms (P=0.3291, 40 vs. 30 months).
The present study also had some limitations that
should not be ignored. Firstly, as a respective study in a single
center, all the indexes and pathological were collected from a
computerized database, consequently the study cohort may have some
unintentional selection bias. Secondly, the optimum cut-off was
specifically suitable for the current patients cohort of 94 cases
regarding the survival analysis. Finally, the relationship between
certain peripheral hematological inflammatory components and the
tumor histological items were not assessed in the present study,
such as the novel pathological type neuroendocrine tumor. Thus,
further large-scale prospective studies are required to verify the
results obtained. Furthermore, other specific markers associated
with ampullary carcinoma require investigation in future study.
Preoperative PLR and NLR possesses further
evaluation as prognostic indices in curative resected ampullary
carcinoma. Preoperative PLR is a candidate predictor for lymph node
metastasis.
Acknowledgements
Not applicable.
Funding
The present study was supported by National Natural
Science Foundation of China (grant no. 81372578).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
XH contributed to the conception and design of the
study. WL and NZ contributed to the analysis data for the study. WW
drafted the study and analyzed the data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Peking Union Medical College Hospital, and all the
participants have included the consent forms.
Patient consent for publication
All the patients provided consent for
publication.
Competing interests
All the authors declare that they have no conflict
of interests.
References
|
1
|
Eeson G, Cleary S, Moulton CAE and Ridgway
PF: Ampullary cancer. Springer International Publishing; 2016
|
|
2
|
Okano K, Oshima M, Yachida S, Kushida Y,
Kato K, Kamada H, Wato M, Nishihira T, Fukuda Y, Maeba T, et al:
Factors predicting survival and pathological subtype in patients
with ampullary adenocarcinoma. J Surg Oncol. 110:56–162. 2014.
View Article : Google Scholar
|
|
3
|
Tian Y, Wu SD, Chen YS and Chen CC:
Transumbilical single-incision laparoscopic cholecystojejunostomy
using conventional instruments: The first two cases. J Gastrointest
Surg. 14:1429–1433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo S, Takada T, Miyazaki M, Miyakawa S,
Tsukada K, Nagino M, Furuse J, Saito H, Tsuyuguchi T, Yamamoto M,
et al: Guidelines for the management of biliary tract and ampullary
carcinomas: Surgical treatment. J Hepatobiliary Pancreat Surg.
15:41–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yokoyama N, Shirai Y, Wakai T, Nagakura S,
Akazawa K and Hatakeyama K: Jaundice at presentation heralds
advanced disease and poor prognosis in patients with ampullary
carcinoma. World J Surg. 29:519–523. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robert PE, Leux C, Ouaissi M, Miguet M,
Paye F, Merdrignac A, Delpero JR, Schwarz L, Carrere N, Muscari F,
et al: Predictors of long-term survival following resection for
ampullary carcinoma: A large retrospective French multicentric
study. Pancreas. 43:692–697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith RA, Ghaneh P, Sutton R, Raraty M,
Campbell F and Neoptolemos JP: Prognosis of resected ampullary
adenocarcinoma by preoperative serum CA19-9 levels and
platelet-lymphocyte ratio. J Gastrointest Surg. 12:1422–1428. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao X, Dong J, Huang X, Zhang W and Jiang
K: Prognostic factors for survival of patients with ampullary
carcinoma after local resection. ANZ J Surg. 85:567–571. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mann DV, Edwards R, Ho S, Lau WY and
Glazer G: Elevated tumour marker CA19-9: Clinical interpretation
and influence of obstructive jaundice. Eur J Surg Oncol.
26:474–479. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ker CG, Chen JS, Lee KT, Sheen PC and Wu
CC: Assessment of serum and bile levels of CA19-9 and CA125 in
cholangitis and bile duct carcinoma. J Gastroenterol Hepatol.
6:505–508. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kau SY, Shyr YM, Su CH, Wu CW and Lui WY:
Diagnostic and prognostic values of CA 19-9 and CEA in
periampullary cancers. J Am Coll Surg. 188:415–420. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Au KK, Josahkian JA, Francis JA, Squire JA
and Koti M: Current state of biomarkers in ovarian cancer
prognosis. Future Oncol. 11:3187–3195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu L, Li X, Shen Y, Ying C, Fang X, Chen
J and Ying Y: A new prognostic score based on the systemic
inflammatory response in patients with inoperable non-small-cell
lung cancer. Onco Targets Ther. 9:4879–4886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Namikawa T, Munekage E, Munekage M, Maeda
H, Yatabe T, Kitagawa H, Kobayashi M and Hanazaki K: Evaluation of
systemic inflammatory response biomarkers in patients receiving
chemotherapy for unresectable and recurrent advanced gastric
cancer. Oncology. 90:338–326. 2016. View Article : Google Scholar
|
|
15
|
Dreyer SB, Powell AG, Mcsorley ST,
Waterston A, Going JJ, Edwards J, Mcmillan DC and Horgan PG: The
pretreatment systemic inflammatory response is an important
determinant of poor pathologic response for patients undergoing
neoadjuvant therapy for rectal cancer. Ann Surg Oncol.
24:1295–1303. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Geng Y, Qi Q, Sun M, Chen H, Wang P and
Chen Z: Prognostic nutritional index predicts survival and
correlates with systemic inflammatory response in advanced
pancreatic cancer. Eur J Surg Oncol. 41:1508–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asher V, Lee J, Innamaa A and Bali A:
Preoperative platelet lymphocyte ratio as an independent prognostic
marker in ovarian cancer. Clin Transl Oncol. 13:499–503. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, Xu L, Huang Z, Zhang L, Zhang H,
Zhu W and Liu P: The hematologic markers as prognostic factors in
patients with resectable gastric cancer. Cancer Biomark.
17:359–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schlieman MG, Ho HS and Bold RJ: Utility
of tumor markers in determining resectability of pancreatic cancer.
Arch Surg. 138:951–956. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Connor S, Bosonnet L, Alexakis N, Raraty
M, Ghaneh P, Sutton R and Neoptolemos JP: Serum CA19-9 measurement
increases the effectiveness of staging laparoscopy in patients with
suspected pancreatic malignancy. Dig Surg. 22:80–85. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahul BA, Stephen E, Frederick G, David
RB, Robert KB, Mary KW, Jeffrey EG, Carolyn CC, Kenneth RH, Daniel
CS, et al: AJCC cancer staging manual. 8th. New York: Springer;
2017
|
|
22
|
Yuan J, Zhou J, Dong Z, Tandon S, Kuk D,
Panageas KS, Wong P, Wu X, Naidoo J, Page DB, et al: Pretreatment
serum VEGF is associated with clinical response and overall
survival in advanced melanoma patients treated with ipilimumab.
Cancer Immunol Res. 2:127–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hothorn T and Lausen B: On the exact
distribution of maximally selected rank statistics. Comput Stat
Data Anal. 43:121–137. 2003. View Article : Google Scholar
|
|
24
|
Lian L, Xia YY, Zhou C, Shen XM, Li XL,
Han SG, Zheng Y, Mao ZQ, Gong FR, Wu MY, et al: Application of
platelet/lymphocyte and neutrophil/lymphocyte ratios in early
diagnosis and prognostic prediction in patients with resectable
gastric cancer. Cancer Biomark. 15:899–907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin HL, Ohara K, Kiberu A, Van Hagen T,
Davidson A and Khattak MA: Prognostic value of systemic
inflammation-based markers in advanced pancreatic cancer. Intern
Med J. 44:676–682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lausen B and Schumacher M: Maximally
selected rank statistics. Biometrics. 48:3–85. 1992. View Article : Google Scholar
|
|
27
|
Haruki K, Shiba H, Horiuchi T, Shirai Y,
Iwase R, Fujiwara Y, Furukawa K, Misawa T and Yanaga K: Neutrophil
to lymphocyte ratio predicts therapeutic outcome after
pancreaticoduodenectomy for carcinoma of the ampulla of vater.
Anticancer Res. 36:403–408. 2016.PubMed/NCBI
|
|
28
|
Demirci NS and Erdem GU: Prognostic role
of the neutrophil-to-lymphocyte ratio (NLR) in patients with
operable ampullary carcinoma. Bosn J Basic Med Sci. Nov
13–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du
P, Wang Q and Yang W: Prognostic role of pretreatment blood
neutrophil-to-lymphocyte ratio in advanced cancer survivors: A
systematic review and meta-analysis of 66 cohort studies. Cancer
Treat Rev. 58:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheng H, Long F, Jaiswar M, Yang L, Wang C
and Zhou Z: Prognostic role of the neutrophil-to-lymphocyte ratio
in pancreatic cancer: A meta-analysis. Sci Rep. 5:110262015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pang W, Lou N, Jin C, Hu C, Arvine C, Zhu
G and Shen X: Combination of preoperative platelet/lymphocyte and
neutrophil/lymphocyte rates and tumor-related factors to predict
lymph node metastasis in patients with gastric cancer. Eur J
Gastroenterol Hepatol. 28:493–502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ertas IE, Gungorduk K, Akman L, Ozdemir A,
Terek MC, Ozsaran A, Sanci M and Dikmen Y: Can preoperative
neutrophil:lymphocyte and platelet:lymphocyte ratios be used as
predictive markers for lymph node metastasis in squamous cell
carcinoma of the vulva? Eur J Obstet Gynecol Reprod Biol.
171:138–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suh DH, Kim HS, Chung HH, Kim JW, Park NH,
Song YS and Kang SB: Pre-operative systemic inflammatory response
markers in predicting lymph node metastasis in endometrioid
endometrial adenocarcinoma. Eur J Obstet Gynecol Reprod Biol.
162:206–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Asher V, Lee J and Bali A: Preoperative
serum albumin is an independent prognostic predictor of survival in
ovarian cancer. Med Oncol. 29:2005–2009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Supoken A, Kleebkaow P, Chumworathayi B,
Luanratanakorn S and Kietpeerakool C: Elevated preoperative
platelet to lymphocyte ratio associated with decreased survival of
women with ovarian clear cell carcinoma. Asian Pac J Cancer Prev.
15:10831–10836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang WW, Liu KJ, Hu GL and Liang WJ:
Preoperative platelet/lymphocyte ratio is a superior prognostic
factor compared to other systemic inflammatory response markers in
ovarian cancer patients. Tumor Biol. 36:8831–8837. 2015. View Article : Google Scholar
|
|
37
|
Nakamura K, Nagasaka T, Nishida T, Haruma
T, Ogawa C, Kusumoto T, Seki N and Hiramatsu Y: Neutrophil to
lymphocyte ratio in the pre-treatment phase of final-line
chemotherapy predicts the outcome of patients with recurrent
ovarian cancer. Oncol Lett. 11:3975–3981. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fan F, Tian Y, Liu S, Zheng G, Zhen L, Xu
G, Man G, Xiao L, Fan D and Zhang H: Combination of PLR, MLR, MWR,
and tumor size could significantly increase the prognostic value
for gastrointestinal stromal tumors. Medicine (Baltimore).
95:e32482016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yokoigawa N, Takeuchi N, Toda M, Inoue M,
Kaibori M, Yanagida H, Tanaka H, Ogura T, Takada H, Okumura T, et
al: Enhanced production of interleukin 6 in peripheral blood
monocytes stimulated with mucins secreted into the bloodstream.
Clin Cancer Res. 11:6127–6132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yokoigawa N, Takeuchi N, Toda M, Inoue M,
Kaibori M, Yanagida H, Inaba T, Tanaka H, Ogura T, Takada H, et al:
Overproduction of PGE2 in peripheral blood monocytes of
gastrointestinal cancer patients with mucins in their bloodstream.
Cancer Lett. 245:149–155. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kannagi R, Izawa M, Koike T, Miyazaki K
and Kimura N: Carbohydrate-mediated cell adhesion in cancer
metastasis and angiogenesis. Cancer Sci. 95:377–384. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Honn KV, Tang DG and Crissman JD:
Platelets and cancer metastasis: A causal relationship? Cancer
Metastasis Rev. 11:325–351. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hussain SP and Harris CC: Inflammation and
cancer: An ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sotomayor EM, Fu YX, Lopezcepero M,
Herbert L, Jimenez JJ, Albarracin C and Lopez DM: Role of
tumor-derived cytokines on the immune system of mice bearing a
mammary adenocarcinoma. II. Down-regulation of macrophage-mediated
cytotoxicity by tumor-derived granulocyte-macrophage
colony-stimulating factor. J Immunol. 147:2816–2823.
1991.PubMed/NCBI
|
|
45
|
Rachidi S, Metelli A, Riesenberg B, Wu BX,
Nelson MH, Wallace C, Paulos CM, Rubinstein MP, Garrettmayer E,
Hennig M, et al: Platelets subvert T cell immunity against cancer
via GARP-TGFβ axis. Sci Immunol. 2:pii: eaai79112017. View Article : Google Scholar
|
|
46
|
Kim EY, Lee JW, Yoo HM, Park CH and Song
KY: The platelet-to-lymphocyte ratio versus
neutrophil-to-lymphocyte ratio: Which is better as a prognostic
factor in gastric cancer? Ann Surg Oncol. 22:4363–4370. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang X and Ran Y: Prognostic role of
elevated platelet count in patients with lung cancer: A systematic
review and meta-analysis. Int J Clin Exp Med. 8:5379–5387.
2015.PubMed/NCBI
|
|
48
|
Ishizuka M, Nagata H, Takagi K, Iwasaki Y
and Kubota K: Combination of platelet count and neutrophil to
lymphocyte ratio is a useful predictor of postoperative survival in
patients with colorectal cancer. Br J Cancer. 109:401–407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
O'Byrne KJ, Dobbs N, Propper D, Smith K
and Harris AL: Vascular endothelial growth factor platelet counts,
and prognosis in renal cancer. Lancet. 353:1494–1495. 1999.
View Article : Google Scholar
|
|
50
|
Quigley DA and Kristensen V: Predicting
prognosis and therapeutic response from interactions between
lymphocytes and tumor cells. Mol Oncol. 9:2054–2062. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Templeton AJ, Mcnamara MG, Šeruga B,
Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G,
Knox JJ, Tran B, et al: Prognostic role of neutrophil-to-lymphocyte
ratio in solid tumors: a systematic review and meta-analysis. J
Natl Cancer Inst. 106:dju1242014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huang C, Jie Y, Li Z, Na L, Zhao J and Qi
D: Usefulness of the neutrophil-to-lymphocyte ratio in predicting
lymph node metastasis in patients with non-small cell lung cancer.
Tumor Biol. 36:7581–7589. 2015. View Article : Google Scholar
|
|
53
|
Sewnath ME, Karsten TM, Prins MH, Rauws
EJ, Obertop H and Gouma DJ: A meta-analysis on the efficacy of
preoperative biliary drainage for tumors causing obstructive
jaundice. Ann Surg. 236:17–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Povoski SP, Karpeh MS Jr, Conlon KC,
Blumgart LH and Brennan MF: Preoperative biliary drainage: Impact
on intraoperative bile cultures and infectious morbidity and
mortality after pancreaticoduodenectomy. J Gastrointest Surg.
3:496–505. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Barauskas G, Urbonas K, Smailyte G, Pranys
D, Pundzius J and Gulbinas A: Preoperative endoscopic biliary
drainage may negatively impact survival following
pancreatoduodenectomy for ampullary cancer. Dig Surg. 33:462–469.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tank AK, Singh RK, Prakash A, Behari A,
Kumar A, Saxena R and Kapoor VK: Adjuvant therapy does not improve
survival of ampullary carcinoma patients. Gastrointest Cancer Res.
Suppl 1:S312010.
|
|
57
|
Evans DB, Wolff RA and Hess KR: Adjuvant
radiotherapy and 5-fluorouracil after curative resection of cancer
of the pancreas and periampullary region. Ann Surg. 230:776–784.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Furuse J, Takada T, Miyazaki M, Miyakawa
S, Tsukada K, Nagino M, Kondo S, Saito H, Tsuyuguchi T, Hirata K,
et al: Guidelines for chemotherapy of biliary tract and ampullary
carcinomas. J Hepatobiliary Pancreat Surg. 15:55–62. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yamaguchi K and Enjoji M: Carcinoma of the
ampulla of vater. A clinicopathologic study and pathologic staging
of 109 cases of carcinoma and 5 cases of adenoma. Cancer.
59:506–515. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Knox RA and Kingston RD: Carcinoma of the
ampulla of Vater. Br J Surg. 73:72–73. 1986. View Article : Google Scholar : PubMed/NCBI
|