Introduction
Chronic obstructive pulmonary disease (COPD),
asthma, pulmonary fibrosis and acute lung injury are common
pulmonary diseases, which are acknowledged as public health
problems. COPD is reported to rank as the fifth leading contributor
to morbidity and mortality rates of chronic diseases, as a
worldwide burden, according to the World Band/World Health
Organization (1). The median survival
rate of patients with idiopathic pulmonary fibrosis is 4–5 years
(2). Airway hyper-responsiveness is
considered to be a main inducing factor of pulmonary diseases.
Epithelial cell injury is considered to initiate pulmonary
fibrosis. Human bronchial epithelial (HBE) cells form the first
protective barrier of the airway, secreting protective cytokines.
The balance of pro- and anti-apoptotic mechanisms is involved in
the occurrence and development of several pulmonary diseases,
including COPD, asthma, pulmonary fibrosis and acute lung
injury.
Previous studies have demonstrated that transforming
growth factor (TGF)-β1 is important in promoting the fibrotic
processes of pulmonary fibrosis (3).
It is an extracellular matrix inducer and a chemotactic factor of
fibroblasts (4). TGF-β1 is a
multifunctional cytokine, which is involved in cell growth,
differentiation, cell cycle and apoptosis. It is reported to
stimulate the apoptosis of pulmonary epithelial cells (5), gastric carcinoma cells (6) and hepatocytes (7). TGF-β1 can induce apoptosis through
different pathways, including the small mothers against
decapentaplegic (Smad)-dependent pathway and non-Smad dependent
pathway involving pathways which include the mitogen-activated
protein kinase (MAPK), Fas (8,9), or
extracellular signal-regulated kinase (ERK)/P38/c-Jun terminal
kinase (JNK) pathway (10–12).
Although corticosteroids remain the main treatment
option for pulmonary diseases, the cure rate is <30% (13). It is important to identify novel
treatments for pulmonary diseases. Heart and neural crest
derivatives expressed transcript 2 (HAND2), belonging to the basic
helix-loop-helix family, is a transcription factor which is
required for organ growth and development, including the heart,
limb buds and branchial arches, adjusting stem cell differentiation
(14). It has been found that a
missense mutation of HAND2 inpatients with congenital heart disease
significantly decreases the interactions of HAND2 with other key
developmental genes (15). Another
study showed that HAND2 was expressed in developing gut tissue and
was associated with enteric neuron formation in avian species
(16). Uterine tissue-specific
HAND2-knockout was found to maintain epithelial proliferation by
inducing paracrine mitogenic mediator function in the uterine
tissues of mice. However, whether and how HAND2 possess any
function on pulmonary disease remain to be elucidated.
The present study hypothesized that HAND2 is
associated with HBE cell proliferation, therefore, HAND2
interference was applied to investigate whether HAND2 was able to
repair TGF-β1-enhanced apoptosis in HBE cells. Variations in cell
proliferation, cell cycle, apoptosis and the ERK/P38/JNK pathway
were all examined in the present study. The results may provide a
novel gene target and treatment strategy for pulmonary
diseases.
Materials and methods
Cell culture
16HBE cells were purchased from the Cell Bank of the
Chinese Academy of Sciences (Shanghai, China) and cultured in
Dulbecco's modified Eagle's Medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C, in a humidified 5% CO2-containing
atmosphere. Cells of the logarithm phase were used in the following
experiments.
To examine the cell morphology of the TGF-β1-treated
HBE cells, 10 ng/ml TGF-β1 (R&D systems, Inc., Minneapolis, MN,
USA) was added to the culture media during cell growth. The cells
were observed under a light microscope following culture for 48
h.
Small interfering (si)RNA
transfection
siRNA transfection was used to validate the effect
of HAND2 on the TGF-β1-treated HBE cells. siRNA-HAND2
(5′-AAGATCAAGACACTGCGCCTG-3′) was designed and synthesized by
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). To perform siRNA
transfection, the cells were initially incubated in 6-well cell
culture plates at a density of 6×104 cells/well and
transfected with siRNA-HAND2 (siHAND2 group) and non-specific siRNA
(mock group) respectively for 2 days at 37°C, when the cells were
70% confluent, using Lipofectamine RNAi transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). HBE cells without any
treatment were included as a control.
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analyses were performed to
detect the levels of HAND2 in the different groups to measure the
interference efficiency of siHAND2 at 48 h-post transfection.
Cell treatment
For the following experiments, the cells were
divided into five groups: HBE cells transfected with siHAND2 and
treated with 10 µg/ml TGF-β1 (siHAND2 + TGF-β1 group), HBE cells
transfected with non-specific sequence and treated with 10 µg/ml
TGF-β1 (mock + TGF-β1 group), HBE cells only treated with 10 µg/ml
TGF-β1 (TGF-β1 group), HBE cells only transfected with non-specific
sequence (mock group), HBE cells without any treatment (control
group).
Cell viability assay
The effect of siHAND2 on the reduced HBE cell
viability induced by TGF-β1 was measured using a Cell Counting
kit-8 (CCK8) assay (Beyotime Institute of Biotechnology, Haimen,
China). In brief, the different groups of HBE cells were seeded in
96-well plates at a density of 5×103 cells/well and
incubated in a 5% CO2-containing atmosphere at 37°C for
determined times (12, 24, 48 and 72 h). Subsequently, 10 µl CCK8
was added to each well and the cells were incubated for another 4
h. The CCK8 assay is optimized from the traditional MTT assay, for
the production to be detected is dissolved formazan. Therefore, the
addition of organic solution to dissolve formazan is not required,
which can reduce errors. In addition, the toxicity of CCK8 is lower
than that of MTT. Therefore, CCK8 was selected in favor of MTT in
the present study. The optical density (OD) values were read at 450
nm using a microplate reader (Thermo Fisher Scientific, Inc.).
Cell cycle analysis
The cell cycle progression of the different cell
groups was measured using a propidium iodide (PI) staining assay
and detected by flow cytometry. Following washing twice with
phosphate buffered saline (PBS), the cells were fixed by 70%
ice-cold ethanol at 4°C and washed with PBS again. The cells were
then incubated in 400 µl PI (50 µg/ml) for 30 min at room
temperature, and analyzed immediately using a FACS flow cytometer
(BD Biosciences, San Diego, CA, USA). The cell proportions in the
G0/G1, S and G2/M phases were
detected.
Cell apoptosis analysis
The apoptotic rates of the different cell groups
were measured using an Annexin V/PI double-stain assay (Roche
Diagnostics, Inc., Indianapolis, IN, USA), according to the
manufacturer's protocols. Briefly, the cells (1×106/ml)
were collected and reacted with 5 µl Annexin-V fluorescein
isothiocyanate and 10 µl PI. Following incubation in the dark for
15 min at room temperature, the cells were detected using a flow
cytometer (BD Biosciences) and analyzed using Cell Quest Pro
software version 5.1 (BD Biosciences).
RT-qPCR analysis
The cells were washed and collected, total RNA was
extracted respectively from the different groups using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and reverse
transcribed into cDNA using a cDNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. The reaction system involved: RNase
Free ddH2O 9.5 µl, Master Mix 12.5 µl, 10 µmol/l up
primer 1 µl, 10 µmol/l down primer 1 µl and cDNA template 1 µl. The
PCR amplification was performed at 95°C for 30 sec, followed by 40
cycles, including denaturation at 95°C for 5 sec, and
annealing/extension at 60°C for 30 sec using Fast SYBR Green Master
Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.), in an ABI
7300 Thermocycler (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The quantification was conducted according to the
2−ΔΔCq method (17). The
primer sequences are listed in Table
I.
 | Table I.Primers used in the present study. |
Table I.
Primers used in the present study.
| Name | Type | Sequence (5′-3′) | Size (bp) |
|---|
| GAPDH | Forward |
CCATCTTCCAGGAGCGAGAT | 222 |
|
| Reverse |
TGCTGATGATCTTGAGGCTG |
|
| HAND2 | Forward |
GAACCCCTACTTCCATGGCT | 236 |
|
| Reverse |
CGCTGTTGATGCTCTGAGTC |
|
| Caspase-3 | Forward |
TGAGCCATGGTGAAGAAGGA | 220 |
|
| Reverse |
TCGGCCTCCACTGGTATTTT |
|
| Caspase-8 | Forward |
GGAGGAGTTGTGTGGGGTAA | 207 |
|
| Reverse |
CCTGCATCCAAGTGTGTTCC |
|
| Caspase-9 | Forward |
AAAGTTGTCGAAGCCAACCC | 237 |
|
| Reverse |
GACTCACGGCAGAAGTTCAC |
|
| P21 | Forward |
GGATGTCCGTCAGAACCCAT | 222 |
|
| Reverse |
GTGGGAAGGTAGAGCTTGGG |
|
| Cyclin D1 | Forward |
CCCTCGGTGTCCTACTTCAA | 219 |
|
| Reverse |
CTTAGAGGCCACGAACATGC |
|
Western blot analysis
The cells were lysed using the protein lysis reagent
P0013 (Beyotime Institute of Biotechnology) and cell lysis was
centrifuged at 10,000 × g for 5 min at 4°C, and supernatants with
proteins were collected for western blot analysis. The
concentration of proteins was measured using a Bio-Rad protein
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Identical quantities of proteins (20 µg/well) were subjected to 15%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto a polyvinylidene fluoride membrane (GE Healthcare
Life Sciences, Chalfont, UK). Following being blocked with 5%
nonfat dried milk in PBS for 1.5 h, the blotting membranes were
incubated overnight respectively at 4°C in the presence of each of
the following primary antibodies: Rabbit anti-HAND2 (1:500; cat no.
ab10131), anti-Cleaved-caspase-3 (1:200; cat no. ab2302),
anti-caspase-8 (1:1,000; cat no. ab25901), anti-caspase-9 (1:500;
cat no. ab69514), anti-P21 (1:1,000; ab227443), anti-Cyclin D1
(1:2,000; cat no. ab226977) and anti-GAPDH (1:2,000; cat no.
ab9485; all Abcam, Cambridge, UK). The membranes were then washed
with 0.1 mol/l TBS with 0.2% Tween-20 (Wuhan Boster Biological
Technology, Ltd., Wuhan, China) 5 times, 10 min each time, and
incubated with appropriate secondary antibodies conjugated with HRP
(1:5,000; cat no. ab6721; Abcam) for 2 h at room temperature. The
PVDF membrane was exposed to X-ray and enhanced chemiluminescence
detection system reagents (GE Healthcare Life Sciences), which were
used to assist in visualizing bands. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was used as a loading control. Lab Works
Image Acquisition and VisionWorks®LS Analysis software
version 7.0 (UVP, Inc., Upland, CA, USA) were used to quantify band
intensities.
Detection of phosphorylation
The phosphorylation levels of ERK, P38 and JNK of
the MAPK pathway were measured using the above western blot
analysis protocol. The antibodies used were: Anti-JNK (1:1,000; cat
no. ab179461), anti-phospho-JNK (1:1,000; cat no. ab124956),
anti-ERK (1:1,000; cat no. ab17942), anti-phospho-ERK (1:1,000; cat
no. ab201015), anti-p38 (1:2,000; cat no. 170099) and
anti-phospho-p38 (1:1,000; cat no. ab47363; all Abcam).
Statistical analysis
Data are expressed as the mean ± standard deviation
of at least three independent experiments. Statistical analysis was
performed by SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) and data were analyzed by one-way analysis of variance and
Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Function of TGF-β1 on HBE cell
morphologies and siHAND2-interfering efficiency
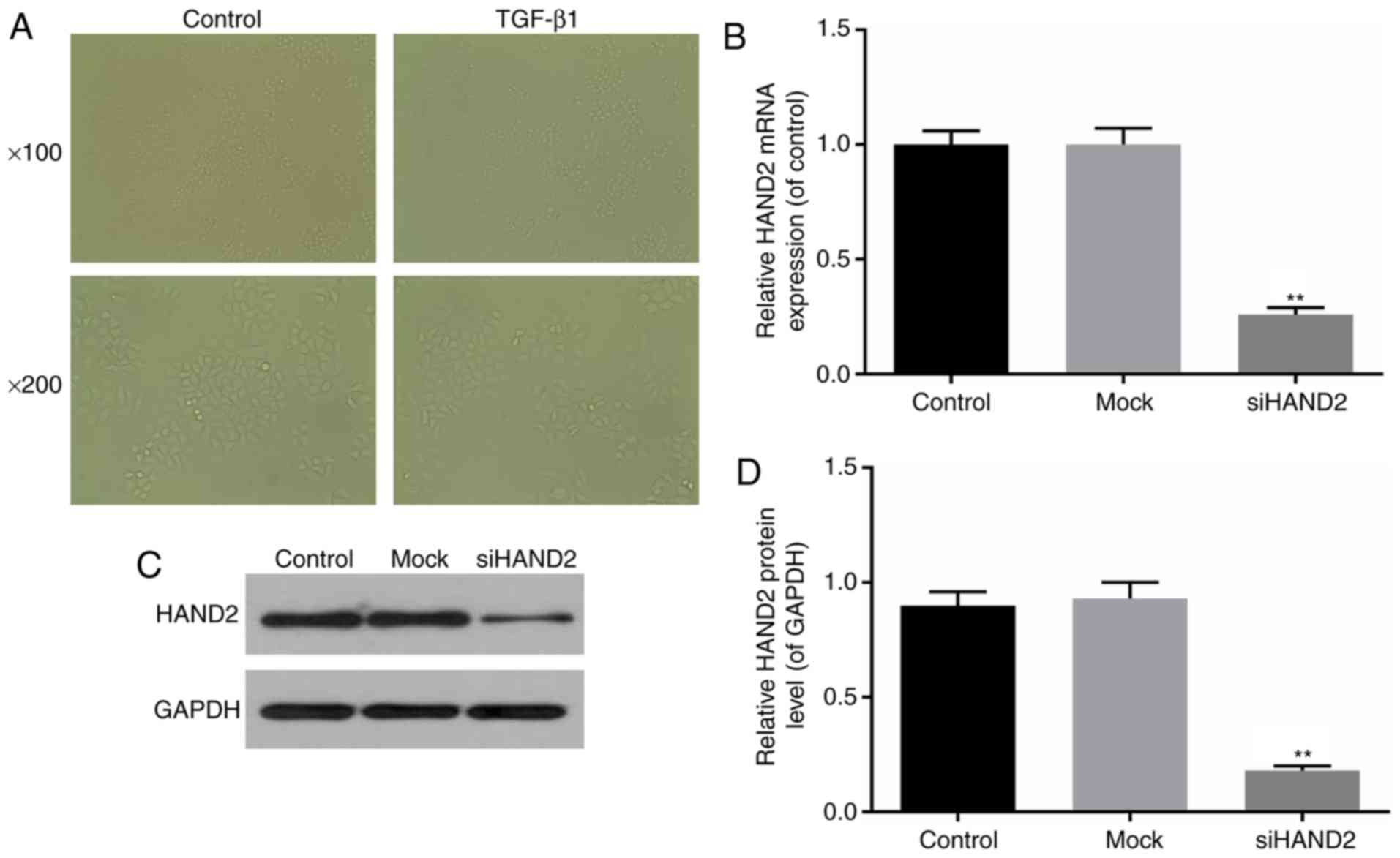
The effects of TGF-β1 on HBE cell morphologies were
observed under a light microscope, which showed that 10 µg/ml
TGF-β1 led to fewer cells and cell shrinkage (Fig. 1A). Interference of the mRNA expression
of HAND2 was induced in the HBE cells, as described above. The
interference efficiency was identified by RT-qPCR and western blot
analyses. Transfection with siRNA-HAND2 resulted in a significant
decline in the mRNA and protein levels of HAND2 in the siHAND2
group, compared with those in the control and mock group, which
suggested high interference efficiency (P<0.01; Fig. 1B-D).
siHAND2 interference recovers HBE cell
viabilities inhibited by TGF-β1
The cell viabilities in the siHAND2 + TGF-β1 group,
mock + TGF-β1 group, TGF-β1 group, mock group, and control group
were evaluated using a CCK8 assay. It was demonstrated that TGF-β1
significantly inhibited HBE cell viability in a time-dependent
manner (12, 24, 48 and 72 h), compared with that in the control
group, which was similar to that in the mock group (P<0.01).
siHAND2 significantly recovered the TGF-β1-inhibited viabilities of
HBE cells in the siHAND2 + TGF-β1 group, compared with that in the
TGF-β1 group, which was similar to that in the mock + TGF-β1 group
(P<0.05; Fig. 2).
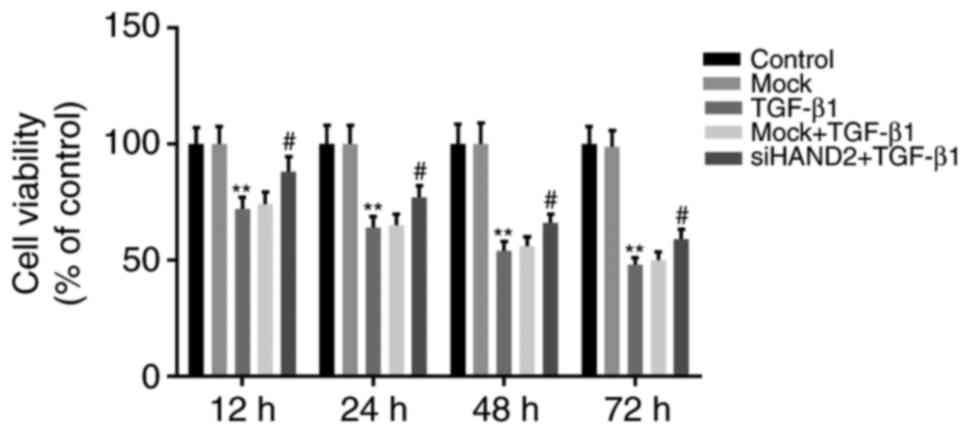 | Figure 2.siHAND2 interference recovers human
bronchial epithelial cell viabilities inhibited by TGF-β1. Cell
viabilities of cells in the siHAND2 + TGF-β1 group, mock + TGF-β1
group, TGF-β1 group, mock group and control group were evaluated
using a Cell Counting kit-8 assay. The viability of cells in the
TGF-β1 group decreased significantly in a time-dependent manner
(12, 24, 48 and 72 h), compared with that in the control group
(P<0.01). The viability of cells in the siHAND2 + TGFβ1 group
recovered significantly in a time-dependent manner, compared with
that in the TGF-β1 group (similar to that in the TGF-β1 + mock
group; P<0.01). Data are presented as the mean ± standard
deviation (n=3). **P<0.01 vs. control group;
#P<0.05 vs. TGF-β1 group. TGF-β1, transforming growth
factor-β1; HAND2, heart and neural crest derivatives expressed
transcript 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; si,
small interfering RNA. |
siHAND2 interference repairs the
promotion of cell cycle arrest and apoptosis induced by TGF-β1 in
HBE cells
As shown in Fig. 3A and
B, cell cycle status of the different groups was determined
using a PI assay and apoptotic status was detected using an
Annexin-V/PI assay, with the results of each analyzed using a flow
cytometer. The results showed that, in the TGF-β1-treated HBE cells
(TGF-β1 group), cell cycle was arrested at the G2 phase,
with the percentage of cells increased significantly in the
G2 phase and decreased significantly in the
G1 phase, compared with those in the control group
(P<0.01). The transfection of cells with siHAND2 in the siHAND2
+ TGF-β1 group significantly alleviated the cell cycle-inhibitory
effect of TGF-β1 (P<0.01). In addition, as shown in Fig. 3C and D, the apoptotic rate was
significantly promoted in the TGF-β1 group, compared with that in
the control group, and siHAND2 significantly inhibited the
apoptotic rate in the siHAND2 + TGF-β1 group (P<0.01).
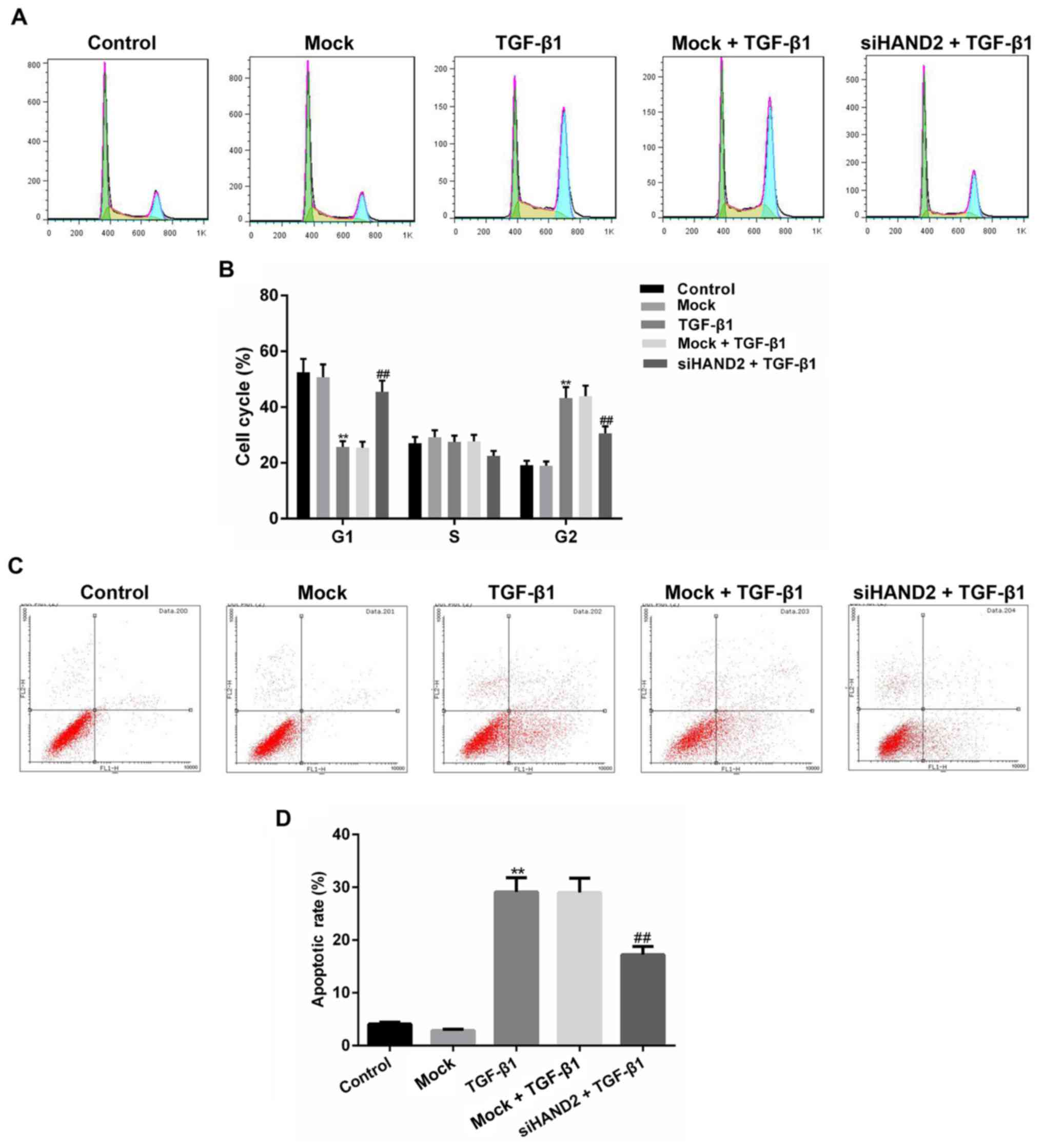 | Figure 3.siHAND2 interference reverses cell
cycle arrests and promotion of apoptosis induced by TGF-β1 in human
bronchial epithelial cells. (A) Cell cycle status of the siHAND2 +
TGF-β1 group, mock + TGF-β1 group, TGF-β1 group, mock group and
control group were determined using a PI assay and analyzed by flow
cytometry. (B) Cell cycle of TGF-β1 group was arrested at the
G2 phase, compared with that in the control group, and
siHAND2 interference in the siHAND2 + TGF-β1 group significantly
alleviated the inhibitory effect of TGF-β1 (P<0.01). (C) Cell
apoptosis status was detected using an Annexin-V/PI assay and
analyzed by flow cytometry. (D) Apoptotic rate was promoted in the
TGF-β1 group, compared with that in the control group, and siHAND2
interference significantly inhibited the apoptotic rate in the
siHAND2 + TGF-β1 group (P<0.01). Data are presented as the mean
± standard deviation (n=3). **P<0.01 vs. control group;
##P<0.01 vs. TGF-β1 group. TGF-β1, transforming
growth factor-β1; HAND2, heart and neural crest derivatives
expressed transcript 2; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase; si, small interfering RNA; PI, propidium iodide. |
siHAND2 restores the expression levels
of cell cycle- and apoptosis-related factors in HBE cells treated
with TGF-β1
RT-qPCR and western blot analyses were performed to
detect the expression levels of cell cycle- and apoptosis-related
factors in the different groups. As shown in Fig. 4A-C, the mRNA and protein levels of
cell cycle-related factors (Cyclin D1 and P21) were decreased
significantly in the TGF-β1 group, compared with those in the
control group. siHAND2 interference promoted the expression levels
of Cyclin D1 and P21 in the siHAND2 + TGF-β1 group, compared with
those in the TGF-β1 group (P<0.01). The mRNA and protein
expression levels of apoptosis-related factors (caspase-3,
caspase-8 and caspase-9) were increased markedly in the TGF-β1
group, compared with those in the control group. siHAND2
interference inhibited the expression levels of caspase-3,
caspase-8 and caspase-9 in the siHAND2 + TGF-β1 group, compared
with those in the TGF-β1 group (P<0.01).
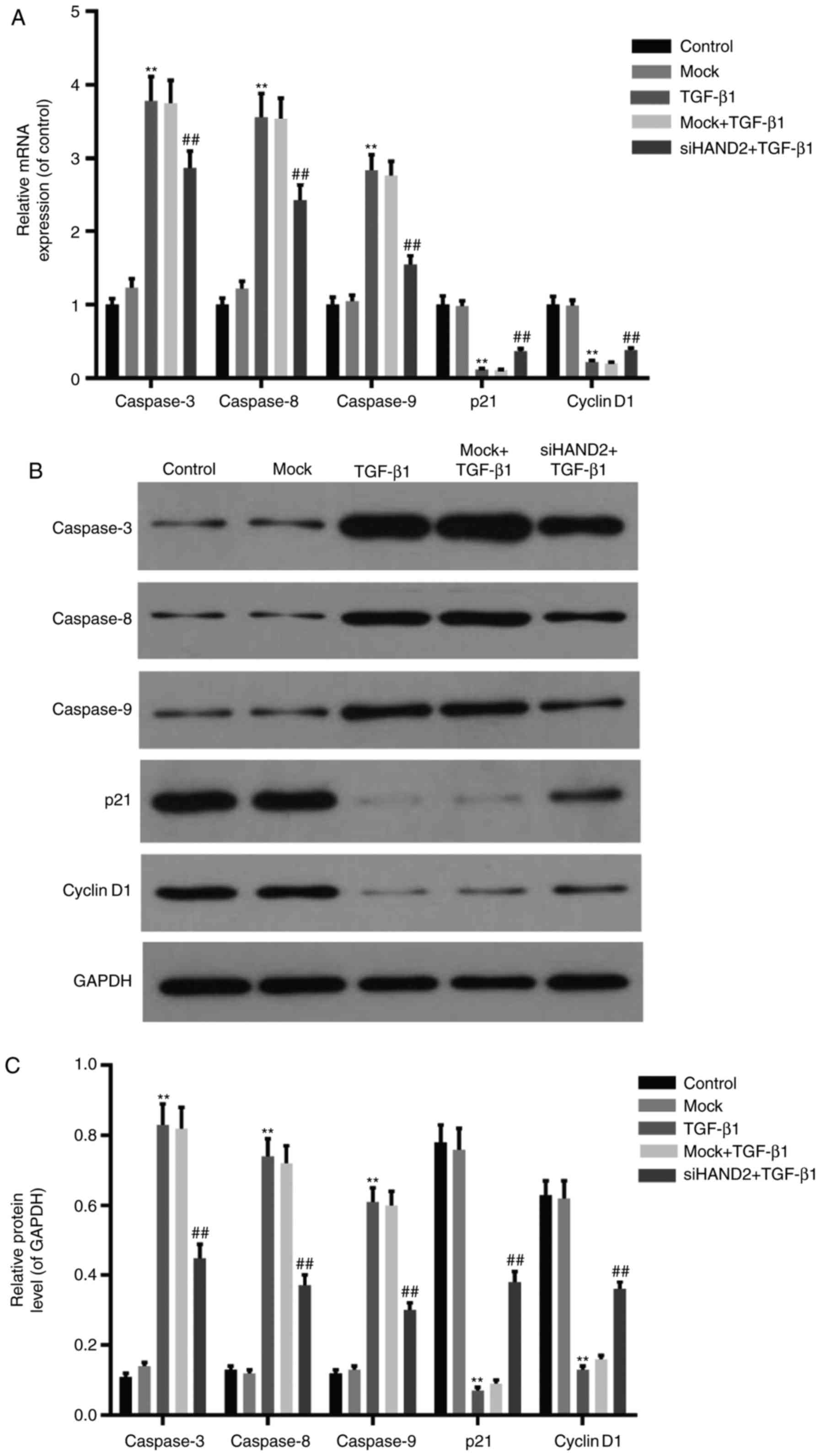 | Figure 4.siHAND2 restores the expression levels
of cell cycle- and apoptosis-related factors in HBE cells treated
with TGF-β1 (A) Reverse transcription-quantitative polymerase chain
reaction analysis was performed to detect mRNA expression of cell
cycle- and apoptosis-related factors in HBE cells of different
groups. The mRNA expression of cell cycle-related factors (Cyclin
D1 and P21) decreased, and apoptosis-related factors (caspase-3,
caspase-8 and caspase-9) increased significantly in the TGF-β1
group, compared with those in the control group. All expression
levels were recovered in the siHAND2 + TGF-β1 group, compared with
those in the TGF-β1 group (P<0.01). (B and C) Western blot
analysis was performed to detect protein levels of cell cycle- and
apoptosis-related factors in HBE cells of different groups. The
protein levels of Cyclin D1 and P21 decreased, and those of
caspase-3, caspase-8 and caspase-9 increased significantly in the
TGF-β1 group, compared with those in the control group. All levels
were recovered in the siHAND2 + TGF-β1 group, compared with those
in the TGF-β1 group (P<0.01). Data are presented as the mean ±
standard deviation (n=3). **P<0.01 vs. control group;
##P<0.01 vs. TGF-β1 group. HBE, human bronchial
epithelial; TGF-β1, transforming growth factor-β1; HAND2, heart and
neural crest derivatives expressed transcript 2; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; si, small interfering
RNA. |
siHAND2 inhibited the phosphorylation
of ERK/P38/JNK in the HBE cells treated with TGF-β1
Western blot analysis was performed to detect the
phosphorylation levels of ERK, P38 and JNK of the MAPK pathway. As
shown in Fig. 5A-C, the
phosphorylation levels of ERK, P38 and JNK were increased
significantly in the TGF-β1 group, compared with levels in the
control groups, and were decreased in the siHAND2 + TGF-β1 groups,
compared with levels in the TGF-β1 groups (P<0.01).
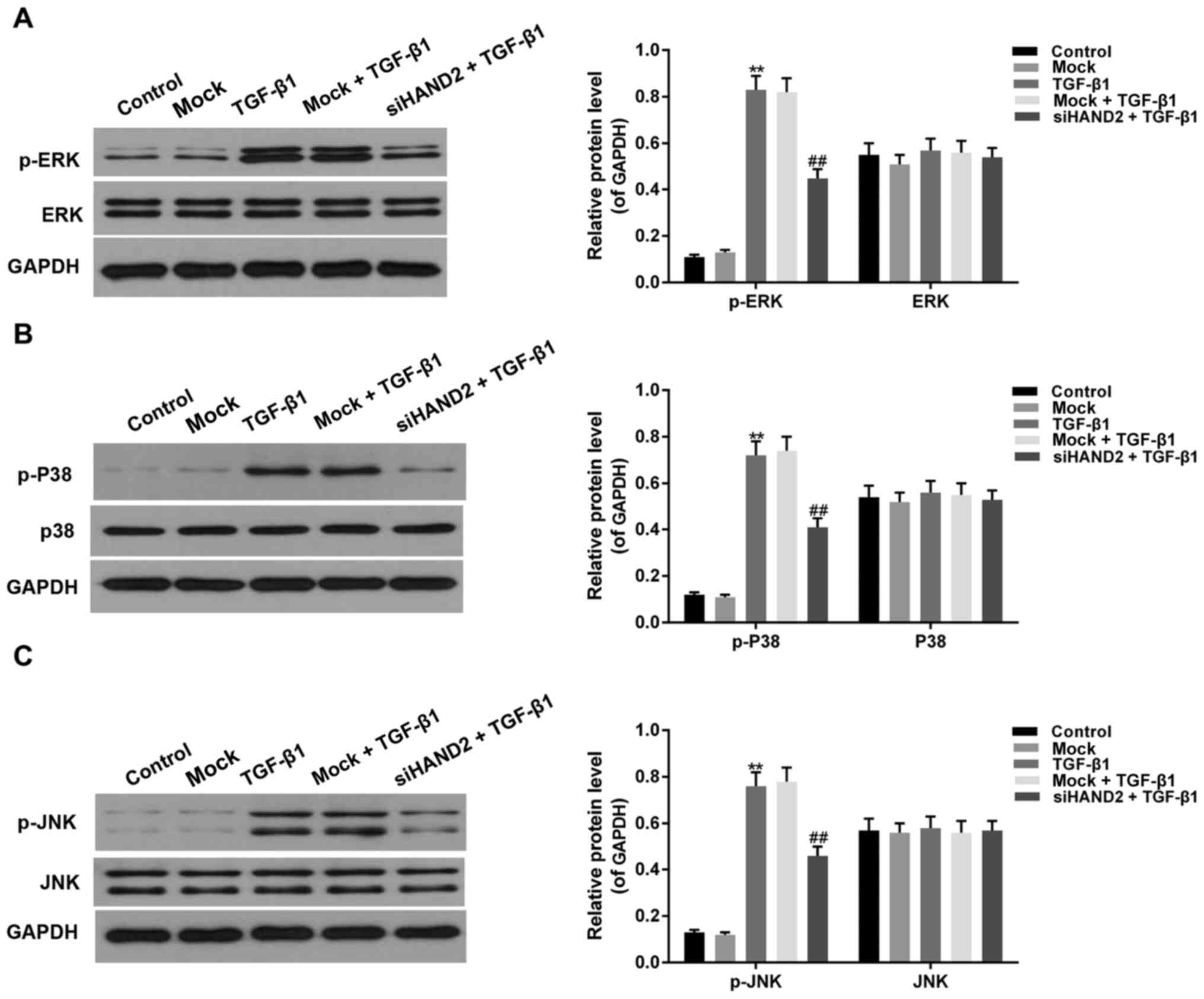 | Figure 5.siHAND2 inhibits ERK/P38/JNK
phosphorylation in human bronchial epithelial cells treated with
TGF-β1. Western blot analysis was performed to detect the
phosphorylation levels of ERK, P38 and JNK. The phosphorylation
levels of (A) ERK, (B) P38 and (C) JNK increased significantly in
the TGF-β1 group, compared with those in the control group, and
were decreased in the siHAND2 + TGF-β1 group, compared with those
in the TGF-β1 group (P<0.01). Data are presented as the mean ±
standard deviation (n=3). **P<0.01 vs. control group;
##P<0.01 vs. TGF-β1 group. TGF-β1, transforming
growth factor-β1; HAND2, heart and neural crest derivatives
expressed transcript 2; ERK, extracellular signal-regulated kinase;
JNK, c-Jun terminal kinase; p-, phosphorylated GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; si, small interfering
RNA. |
Discussion
Pulmonary diseases, including COPD and pulmonary
fibrosis, are common public health problems. HBE cells represent
the first protective barrier of the airway, and epithelial cell
injury is considered to initiate pulmonary fibrosis. Apoptosis,
also termed programmed cell death, is involved in the development
of several diseases, including pulmonary diseases. In the present
study, TGF-β1 was used to enhance apoptosis in HBE cells, on which
the function of HAND2 interference was detected.
TGF-β1 is considered to be critical in promoting
pulmonary fibrosis development (18),
and to stimulate the apoptosis of pulmonary epithelial cells
through different signaling pathways, including the ERK/P38/JNK
pathway. The present study verified that treatment of HBE cells
with TGF-β1 (10 µg/ml) resulted in a decreased number of cells and
cell shrinkage, indicating that TGF-β1 caused damage to the HBE
cells. HAND2 is a transcription factor, which is associated with
the growth and development of organs, including the heart, limb
buds and branchial arches. HAND2-knockout has been previously been
reported to induce epithelial proliferation. In the present study,
thesiRNA-HAND2 sequence was used to establish a HAND2-silencing
model. RT-qPCR and western blot analyses were performed to detect
the efficiency of HAND2 interference, revealing high interference
efficiency.
TGF-β1 (10 µg/ml) was used to treat HBE cells, mock
cells and siHAND-treated HBE cells, following which cell
proliferation, cell cycle, apoptosis variations were measured. The
results showed that cell proliferation was decreased significantly
in the TGF-β1-treated HBE cells in a time-dependent manner,
compared with that in the control group. When HAND2 was silenced in
the HBE cells prior to TGF-β1 treatment, cell proliferation
increased significantly in a time-dependent manner, compared with
that in cells treated with TGF-β1 only. Therefore, HAND2
interference promoted HBE cell proliferation when treated with
TGF-β1. To identify the mechanism underlying the effect of HAND2
interference on the promotion of HBE cell proliferation, flow
cytometry was used to detect cell cycle progression and apoptotic
status. When treated with TGF-β1, the HBE cell cycle was arrested
at the G2 phase, with cells in the G2 phase
increased significantly, compared with those in the control group.
However, HAND2 interference almost recovered the condition. Higher
cell apoptotic rates were detected in the TGF-β1-treated HBE cells,
compared with the HBE cells without any treatment; whereas HAND2
interference markedly decreased the apoptotic rate. These results
indicated that TGF-β1 inhibited HBE cell proliferation, accompanied
by cell cycle arrest at the G2 phase and promotion of
cell apoptosis, whereas HAND2 interference in the HBE cells
repaired all these effects induced by TGF-β1.
To examine the molecular mechanisms underlying the
effect of HAND2 interference on the repair of TGF-β1-induced
inhibition of HBE cell proliferation and promotion of apoptosis,
the present study attempted to identify the expression levels of
cell cycle- and apoptosis-related factors. Cyclin D1 is the most
representative factor in promoting cells transferring from the
G1 phase to the S phase. Cyclin-dependent kinase (CDK)
inhibitor 1A (p21) is an inhibitor of cell cycle, known for its
extensive kinase inhibitory activity (19–22). It
has been reported that TGF-β1 can induce lung epithelial cell
apoptosis, through downregulating the expression of p21. The
decrease of p21 promoted TGF-β1-stimulated apoptosis (23,24), It is
possible that, in the presence of TGF-β1, p21 protects HBE cells
from apoptosis (25,26). Previous studies have found that the
activation of caspase-3 is regulated by p21, and the complex
formation of procaspase-3-p21 is an essential mechanism for cell
survival (27,28). Therefore, the downregulation of p21 by
TGF-β1 may activate caspase-3 to enhance apoptosis. The results of
the present study verified that TGF-β1 treatment inhibited the
expression of P21 and Cyclin D1 in HBE cells, whereas siHAND2
interference recovered the expression of P21 and Cyclin D1 in HBE
cells treated with TGF-β1, to inhibit cell apoptosis, recover cell
cycle progression and promote cell proliferation. Caspases
(cysteinyl aspartate-specific proteinases), are a family of
protease enzymes which are closely associated with cell apoptosis,
and are involved in cell development and differentiation (29). The activation of caspase-3 is a key
element in the TGF-β1-induced apoptotic signaling pathway.
Caspase-8 and caspase-9 are important initiator caspases, the
activation of which cleaves and stimulates caspase-3, functioning
on downstream factors. The results of the present study
demonstrated that TGF-β1 treatment promoted the expression of
apoptosis-activating factors, including caspase-3, caspase-8 and
caspase-9, to promote HBE cell apoptosis. In addition, siHAND2
effectively decreased the promotion of apoptosis induced by TGF-β1
in HBE cells. As Hagimoto et al reported, B-cell
lymphoma-2-related proteins were not affected by the addition of
TGF-β1 in small airway epithelial cells (23). In addition, there are several other
biomarkers involved in cell cycle and apoptosis, including CDKs
(CDK4 and CDK6). It was a potential limitation that these were not
investigated in the present study.
Subsequently, the present study performed western
blot analysis to determine whether the effect of HAND2 interference
on TGF-β1-enhanced apoptosis was mediated by the ERK/P38/JNK
signaling pathway. ERK, a critical factor of the MAPK family,
transmits signals from the cytoplasm to the nucleus, when it is
activated as a phosphorylated form, and functions in cell
development and differentiation (30,31). The
P38 and JNK pathways are activated to induce the release of
cytochrome c from mitochondria, and to activate caspase-9
and promote cell apoptosis. The phosphorylation levels of ERK, P38
and JNK were markedly increased in the TGF-β1-treated HBE cells,
and decreased significantly with siHAND2 interference in the HBE
cells. This indicated that siHAND2 interference promoted HBE cell
proliferation and inhibited apoptosis through the ERK/P38/JNK
signaling pathway in HBE cells treated with TGF-β1.
In conclusion, the novel function of HAND2
interference on repairing TGF-β1-inhibited cell proliferation,
arrested cell cycle and promoted apoptosis, through regulating cell
cycle- and apoptosis-related factors and the ERK/P38/JNK pathway,
maybe considered as a novel strategy in the treatment of pulmonary
diseases. To address the limitation of using only one cell line in
the present study, future investigations using other pulmonary cell
lines or in vivo may be performed to support these
conclusions.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XJ conducted all the experiments in the present
study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
All patients involved provided consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vestbo J, Hurd SS, Agusti AG, Jones PW,
Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ,
Nishimura M, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 187:347–365. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reynolds HY: Diagnostic and management
strategies for diffuse interstitial lung disease. Chest.
113:192–202. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khalil N, O'Connor RN, Unruh HW, Warren
PW, Flanders KC, Kemp A, Bereznay OH and Greenberg AH: Increased
production and immunohistochemical localization of transforming
growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir
Cell Mol Biol. 5:155–162. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Postlethwaite AE, Keski-Oja J, Moses HL
and Kang AH: Stimulation of the chemotactic migration of human
fibroblasts by transforming growth factor beta. J Exp Med.
165:251–256. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yanagisawa K, Osada H, Masuda A, Kondo M,
Saito T, Yatabe Y, Takagi K and Takahashi T and Takahashi T:
Induction of apoptosis by Smad3 and down-regulation of Smad3
expression in response to TGF-beta in human normal lung epithelial
cells. Oncogene. 17:1743–1747. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yanagihara K and Tsumuraya M: Transforming
growth factor beta 1 induces apoptotic cell death in cultured human
gastric carcinoma cells. Cancer Res. 52:4042–4045. 1992.PubMed/NCBI
|
|
7
|
Teramoto T, Kiss A and Thorgeirsson SS:
Induction of p53 and Bax during TGF-beta 1 initiated apoptosis in
rat liver epithelial cells. Biochem Biophys Res Commun. 251:56–60.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bai L, Yu Z, Wang C, Qian G and Wang G:
Dual role of TGF-β1 on Fas-induced apoptosis in lung epithelial
cells. Respir Physiol Neurobiol. 177:241–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zanetti D, Poli G, Vizio B, Zingaro B,
Chiarpotto E and Biasi F: 4-hydroxynonenal and transforming growth
factor-beta1 expression in colon cancer. Mol Aspects Med.
24:273–280. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Attisano L and Wrana JL: Smads as
transcriptional co-modulators. Curr Opin Cell Biol. 12:235–243.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massagué J: How cells read TGF-beta
signals. Nat Rev Mol Cell Biol. 1:169–178. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyazono K: TGF-beta/SMAD signaling and
its involvement in tumor progression. Biol Pharm Bull.
23:1125–1130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ryu JH, Colby TV and Hartman TE:
Idiopathic pulmonary fibrosis: Current concepts. Mayo Clin Proc.
73:1085–1101. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu N, Barbosa AC, Chapman SL,
Bezprozvannaya S, Qi X, Richardson JA, Yanagisawa H and Olson EN:
DNA binding-dependent and -independent functions of the Hand2
transcription factor during mouse embryogenesis. Development.
136:933–942. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu CX, Gong HR, Liu XY, Wang J, Zhao CM,
Huang RT, Xue S and Yang YQ: A novel HAND2 loss-of-function
mutation responsible for tetralogy of Fallot. Int J Mol Med.
37:445–451. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu X and Howard MJ: Transcripts encoding
HAND genes are differentially expressed and regulated by BMP4 and
GDNF in developing avian gut. Gene Expr. 10:279–293. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cipolla E, Fisher AJ, Gu H, Mickler EA,
Agarwal M, Wilke CA, Kim KK, Moore BB and Vittal R: IL-17A
deficiency mitigates bleomycin-induced complement activation during
lung fibrosis. FASEB J. 13:5543–5556. 2017. View Article : Google Scholar
|
|
19
|
Zhang T, Jiang T, Zhang F, Li C, Zhou YA,
Zhu YF and Li XF: Involvement of p21Waf1/Cip1 cleavage during
roscovitine-induced apoptosis in non-small cell lung cancer cells.
Oncol Rep. 23:239–245. 2010.PubMed/NCBI
|
|
20
|
Ii T, Satomi Y, Katoh D, Shimada J, Baba
M, Okuyama T, Nishino H and Kitamura N: Induction of cell cycle
arrest and p21(CIP1/WAF1) expression in human lung cancer cells by
isoliquiritigenin. Cancer Lett. 207:27–35. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmider-Ross A, Pirsig O, Gottschalk E,
Denkert C, Lichtenegger W and Reles A: Cyclin-dependent kinase
inhibitors CIP1 (p21) and KIP1 (p27) in ovarian cancer. J Cancer
Res Clin Oncol. 132:163–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neto AG, McCutcheon IE, Vang R, Spencer
ML, Zhang W and Fuller GN: Elevated expression of p21 (WAF1/Cip1)
in hormonally active pituitary adenomas. Ann Diagn Pathol. 9:6–10.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hagimoto N, Kuwano K, Inoshima I, Yoshimi
M, Nakamura N, Fujita M, Maeyama T and Hara N: TGF-beta 1 as an
enhancer of Fas-mediated apoptosis of lung epithelial cells. J
Immunol. 168:6470–6478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Betts-Obregon BS, Mondragon AA, Mendiola
AS, LeBaron RG, Asmis R, Zou T, Gonzalez-Fernandez F and Tsin AT:
TGFβ induces BIGH3 expression and human retinal pericyte apoptosis:
A novel pathway of diabetic retinopathy. Eye (Lond). 30:1639–1647.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poluha W, Poluha DK, Chang B, Crosbie NE,
Schonhoff CM, Kilpatrick DL and Ross AH: The cyclin-dependent
kinase inhibitor p21 (WAF1) is required for survival of
differentiating neuroblastoma cells. Mol Cell Biol. 16:1335–1341.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gorospe M, Wang X, Guyton KZ and Holbrook
NJ: Protective role of p21(Waf1/Cip1) against prostaglandin
A2-mediated apoptosis of human colorectal carcinoma cells. Mol Cell
Biol. 16:6654–6660. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suzuki A, Tsutomi Y, Akahane K, Araki T
and Miura M: Resistance to Fas-mediated apoptosis: Activation of
caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene
family ILP. Oncogene. 17:931–939. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki A, Tsutomi Y, Miura M and Akahane
K: Caspase 3 inactivation to suppress Fas-mediated apoptosis:
Identification of binding domain with p21 and ILP and inactivation
machinery by p21. Oncogene. 18:1239–1244. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y, Bei Y, Li Y and Chu H: Phenotypic
and functional transformation in smooth muscle cells derived from
varicose veins. J Vasc Surg Venous Lymphat Disord. 5:723–733. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsieh HL, Wang HH, Wu WB, Chu PJ and Yang
CM: Transforming growth factor-beta1 induces matrix
metalloproteinase-9 and cell migration in astrocytes: Roles of
ROS-dependent ERK- and JNK-NF-kappaB pathways. J Neuroinflammation.
7:882010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu HW, Liu QF and Liu GN: Positive
regulation of the Egr-1/osteopontin positive feedback loop in rat
vascular smooth muscle cells by TGF-beta, ERK, JNK, and p38 MAPK
signaling. Biochem Biophys Res Commun. 396:451–456. 2010.
View Article : Google Scholar : PubMed/NCBI
|