Introduction
Gastric cancer (GC) ranks fourth in males and fifth
in females for cancer incidence and ~9% cancer deaths in 2012
worldwide (1). Although systematic
therapeutic strategies such as trastuzumab, an anti-HER-2
monoclonal antibody, have already been used in clinical practice,
the prognosis of patients with late-stage GC remains poor (2–4).
Therefore, additional study into the molecular mechanisms
underlying the morbidity and metastasis of GC remain important.
Epithelial-mesenchymal transition (EMT) is a complex
molecular and cellular process involving the transformation of
cells from an epithelial to a mesenchymal phenotype. Cells
undergoing EMT are associated with increased cell mobility,
invasiveness and reinforced resistance to apoptosis, all of which
serve a crucial role in tumor progression (5,6).
Therefore, EMT has become an important topic of study with regard
to the progression and metastasis of epithelial derived tumors
(7). EMT is characterized by the
downregulation of the epithelial marker epithelial (E)-cadherin,
which promotes cell-cell contact, and the upregulation of
mesenchymal markers, including vimentin and neural (N)-cadherin
(6,8–10).
Furthermore, the EMT process allows epithelial cells to switch from
an immobile phenotype to a motile mesenchymal phenotype, leading to
increased cell migration and invasion (11,12). Liu
et al (13) revealed that the
expression of the mesenchymal markers N-cadherin and vimentin in GC
tissues were significantly increased compared with those in
adjacent normal tissues, which indicated the involvement of EMT in
GC oncogenesis. Zhang et al (12) also identified that C-C motif chemokine
receptor 7 promoted EMT in GC cells by regulating the expression of
zinc finger protein SNAI1, resulting in migration and invasion of
GC cells. Furthermore, previous studies have indicated that
microRNA also serve as important regulators of GC EMT (14–17).
Wilms' tumor on the X chromosome (WTX) was the first
tumor suppressor gene identified on the X chromosome, and has been
a topic of study since its identification in 2007 (18). WTX has been demonstrated to serve a
major role in tumor suppression in several somatic tumors, although
data concerning its expression and function in GC are limited
(19–22). Zhang et al (23) analyzed the expression of WTX in normal
and cancer tissues, and identified that WTX gene and mRNA
expression levels were decreased compared with those in normal
tissue, which indicated that the WTX gene may serve an important
role in tumor suppression of GC.
The present study aimed to investigate whether the
WTX gene inhibited gastric cancer morbidity, and the role of EMT
within this process. The results may provide novel insight for
gastric cancer cell metastasis, and identify possible clinical
targets for gastric cancer treatment.
Materials and methods
Cell lines and cell culture
The human gastric cancer AGS cell line and the 293T
cell line (only as transfection vectors) were obtained from the
American Type Culture Collection (Manassas, VA, USA) and maintained
in the Department of Pathology, Nanfang Hospital (Guangzhou,
China). The human gastric cancer AGS cell line was cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS;
both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C in a humidified atmosphere of 5% CO2. The 293T
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
medium supplemented with 10% FBS at 37°C in a humidified atmosphere
of 5% CO2.
Establishment of stable
WTX-overexpressing and control gastric cancer cell lines
The detailed methods of the establishment of stable
WTX-overexpressing colorectal cancer SW620 cell line were described
previously (24). The whole length of
WTX CDS region is 3405 bp in length (based on NCBI information on
APC membrane recruitment protein 1; NM_152424.3; http://www.ncbi.nlm.nih.gov/gene/139285). The region
was amplified from a WTX CDS clone vector (Shanghai GeneChem Co.,
Ltd., Shanghai, China), using a pair of primers as follows:
Forward, 5′-ACCGGTCGCCACCATGGAGACCCAAAAGGATGAAGCTGCTC-3 and
reverse, 5′-ACCCTTGGCTAGGTTTCCATTCATGGCAGTG-3. The polymerase chain
reaction (PCR) kit was provided by Takara Biotechnology, Co., Ltd.
(Dalian, China). Thermocycling conditions were as follows: 94°C for
5 min, followed by 30 cycles of 94°C for 30 sec, 55°C for 30 sec
and 72°C for 2 min, followed by 72°C for 10 min.
Subsequent to the PCR amplifying process, the PCR
product was subcloned into the GV287 lentivirus vector using
BamHI/AgeI restrictive enzymes. Agarose gel electrophoresis was
then used to evaluate the recombinant vector, followed by Sanger
sequencing to confirm the recombinant vector was successfully
constructed.
Subsequently, 293T cells were transfected
(Lipofectamine™ 2000 Transfection Reagent; Thermo Fisher
Scientific, Waltham, MA, USA) with packaging vectors and the WTX
recombinant lentivirus plasmid or control vector. The supernatant
containing Lenti-virus was collected and concentrated by Shanghai
GeneChem Co., Ltd. and the titer was measured.
AGS cells were seeded in a 24-well plate at a
density of 30–50% at 37°C for 24 h prior to being infected with WTX
CDS overexpression lentiviruses or control lenti-virus based on the
virus titer and multiplicity of infection (MOI) value of the AGS
cell line (MOI=50). Transfection efficiency was roughly evaluated
by fluorescence microscopy at magnification, ×100 48 h following
infection and was finally verified by reverse
transcription-quantitative PCR (RT-qPCR) and western blotting. The
pure WTX overexpression cells and control cells were isolated by
flow cytometry and named as AGS.W and AGS.veh cells.
RT-qPCR
AGS.W and AGS.veh cells were seeded in 6-well plates
at a density of 1×106 cells/well in advance for 24 h and
cells were washed 3 times with PBS. The cells were collected, and
cellular mRNA was extracted with RNAiso-Plus (Takara Bio, Dalian,
China), from which single-stranded cDNA was then synthesized at
37°C for 15 min, 85°C for 5 sec and stored at 4°C. Using the qPCR
cDNA synthesis kit (Takara Bio, Dalian, China) following the
manufacturer's protocol. qPCR was conducted with iQ™ SYBR-Green
Supermix (Takara Bio, Dalian, China) using Applied Biosystems 7500
Sequence Detection system. The thermo cycling condition were as
follows: 95°C at 5 min for 1 cycle, then 95°C for 5 sec, 60°C for
30 sec and 72°C for 34 sec for 40 cycles, followed by the melting
curve stage (95°C for 15 sec, 60°C for 1 min and 95°C for 30 sec).
The relative WTX mRNA level was calculated as 2−ΔΔCq
(25). The sense and anti-sense WTX
and GAPDH primers used are listed below: WTX forward,
5′-GACCCAAAAGGATGAAGCT-3′ and reverse, 5′-CCCCTCCAAAGAAACTAGGC-3′;
GAPDH forward, 5′-TGAAGGTCGGAGTCAACGGA-3′ and reverse,
5′-CCATTGATGACAAGCTTCCCG-3′.
Western blot analysis
AGS.W and AGS.veh cells were washed 3 times with PBS
prior to being lysed with Radioimmunoprecipitation Assay Lysis
buffer (KeyGen Biotech Co., Ltd., China). Cellular protein lysate
was separated from the residue through high-speed centrifugation
(12,000 × g; 30 min; 4°C) and mixed with loading buffer (Beyotime
Institute of Biotechnology, Haimen, China). The mixture was then
boiled for 5 min for protein denaturation. The total protein was
determined using a bicinchoninic acid assay. Samples (40 µg/lane)
were subjected to SDS-PAGE (10% gel), transferred to 0.45 µm
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA), blocked with 5% non-fat milk (diluted in PBS with 0.1% Tween
at 25°C for 1 h) and incubated with primary antibodies overnight at
4°C and secondary antibodies at 25°C for 1 h. An enhanced
chemiluminescence detection system (Fu De Biological Technology
Co., Ltd, Hangzhou, China) was used to visualize protein
expression. Anti-β-actin antibody was used as a loading control.
Protein bands were examined using the ChemiDoc™ Imaging system
(version. 5.2.1). Data were quantified using Image Lab (version.
5.2.1; both Bio-Rad Laboratories, Inc.).
Primary antibodies included rabbit monoclonal
antibodies anti-WTX (cat. no. ab91309; 1:500), anti-N-cadherin
(cat. no. ab76057; 1:500), anti-β-catenin (cat. no. ab27798; 1:500)
anti-Vimentin (cat. no. ab27608; 1:500), anti-E-cadherin (cat. no.
ab76319; 1:500; all Abcam, Cambridge, UK) and mouse monoclonal
antibody anti-β-actin (cat. no. TA-09; 1:500; OriGene Technologies,
Inc., Beijing, China).
Secondary antibodies included horseradish
peroxidase-conjugated goat anti-rabbit Immunoglobulin G (cat. no.
ZB-2301; 1:1,000) and peroxidase-conjugated goat anti-mouse
Immunoglobulin G (cat. no. ZB-2305; 1:1,000; both OriGene
Technologies, Inc.).
Cell morphology observation
AGS.W and AGS.veh cells were seeded in 6-well plate
(Corning Inc., Coring, NY USA) and grown to a density of 70–80%,
prior to being subjected to morphology observation with an Olympus
IX71 inverted light microscope (Olympus Corporation, Tokyo, Japan)
at magnification, ×200 in 5 random fields.
Immunofluorescence to detect changes
in EMT-associated proteins expression
AGS.W and AGS.veh cells were first seeded at a
density of 10% (300 cells/well) on confocal disks and then cultured
with full medium for 12 h. Cells were washed with PBS 3 times, and
then fixed in 4% formaldehyde for 30 min at room temperature,
followed by permeabilization with 0.2% Triton-100 for 15 min at
room temperature and incubation with specific primary antibodies
against the EMT-associated proteins [anti-N-cadherin (cat. no.
ab76057; 1:200), anti-β-catenin (cat. no. ab27798; 1:200),
anti-Vimentin (cat. no. ab27608; 1:200), anti-E-cadherin (cat. no.
ab76319; 1:200; all Abcam)] overnight at 4°C. Cells were incubated
with an Alexa Fluor-594 conjugated goat anti-rabbit Immunoglobulin
G secondary antibody (cat. no. SA00006-4; 1:500; ProteinTech Group,
Inc., Chicago, IL, USA) for 1 h at room temperature and finally
stained with DAPI (Beyotime Institute of Biotechnology, Haimen,
China) for 5 min at room temperature to prepare the samples for
immunofluorescence analysis. All immunofluorescence images were
captured using an Olympus confocal microscope (Fluoview FV1000;
Olympus Corporation, Tokyo, Japan) at magnification, ×1,200. The
mean intensity of indicated proteins was quantified using Image J
software (version 1.8.0; National Institutes of Health, Bethesda,
MA, USA).
Transwell cell migration detection
assay
Transwell migration assay was performed using
Transwell inserts (BD Bioscience, SanJose, CA, USA) with a filter
of 8 µm pore. A total of 1×105 cells/chamber were seeded
in the upper chamber with serum-free medium, and the concentration
of FBS was increased to 20% in the lower chamber to serve as a
chemoattractant. Cells were cultured for 48 h, fixed with 4%
paraformaldehyde for 30 min at room temperature, and then stained
with hematoxylin for 30 min at room temperature. Cells invading to
the opposite side of membrane were observed with an inverted light
microscope at magnification, ×200 and counted by direct
visualization of nuclei in 5 different visual fields. A total of
three independent experiments were conducted for statistical
analysis.
Wound-healing assay
AGS.W and AGS.veh cells were seeded in a 6-well
plate at a density of 1×106 cells/well for 24 h, and
then scratched with a sharp 10-µl pipette tip. Floating cells were
removed with PBS three times. Cell migration was observed with an
Olympus IX71 inverted light microscope at ×100 magnification in 5
random fields every 6 h for 72 h, with images captured at each 6 h
interval.
Statistical analysis
Statistical analysis was performed with SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). A Student's independent t-test was
used to compare the WTX mRNA levels and Transwell migration cell
numbers between two groups. Data are presented as mean ± standard
deviation from three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
Establishment of AGS.W by lentivirus
infection combined with flow cytometry
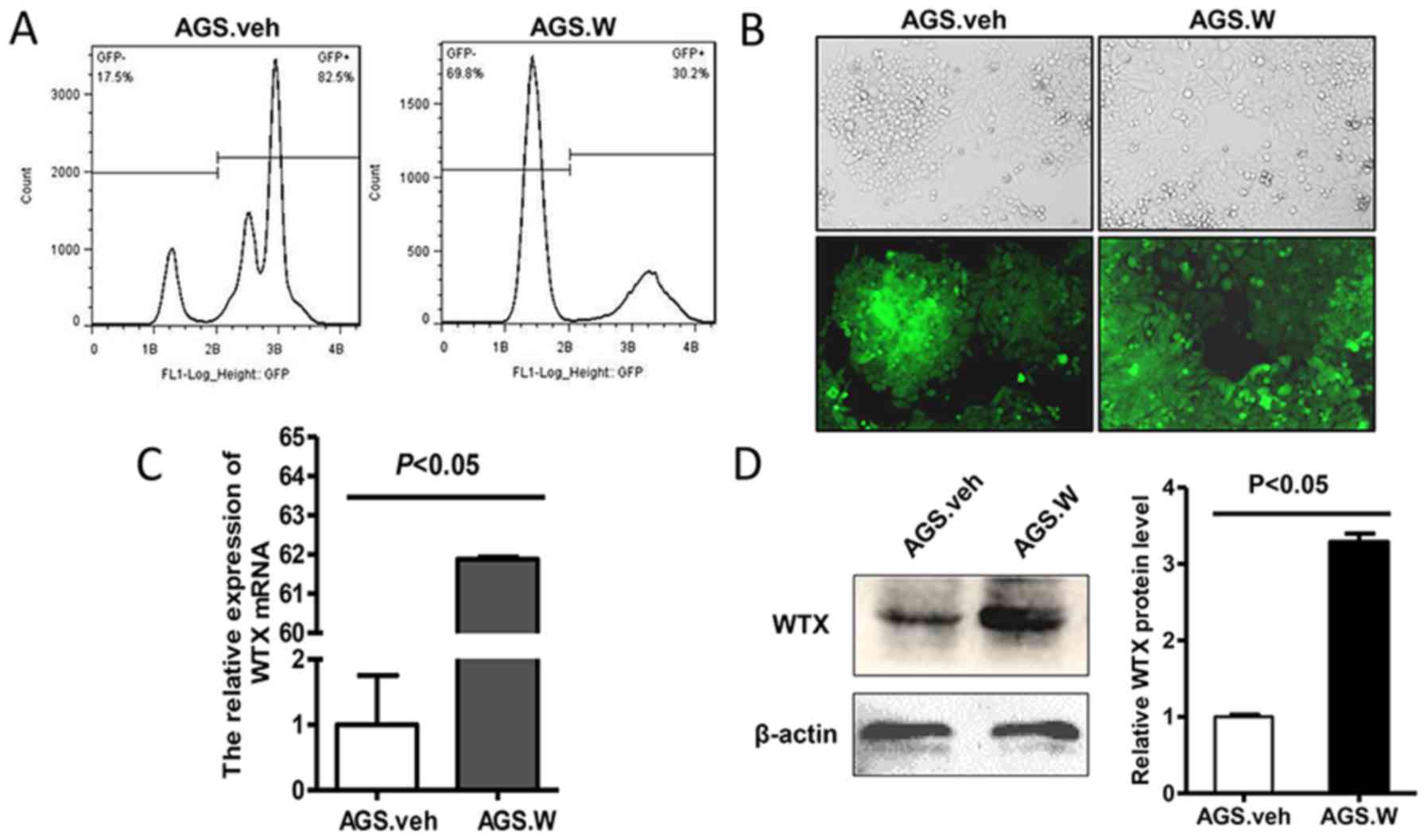
Flow cytometry results are presented in Fig. 1A. AGS.W and control AGS.veh cells
expressed green fluorescence under the fluorescence microscope
(Fig. 1B). Next, it was verified that
AGS.W expressed significantly increased WTX at mRNA (Fig. 1C) and protein (Fig. 1D) levels, compared with AGS.veh cells,
indicating that the recombinant AGS cell line with stable WTX
overexpression was successfully established.
Morphological changes in AGS.W cells
compared with AGS.veh cells
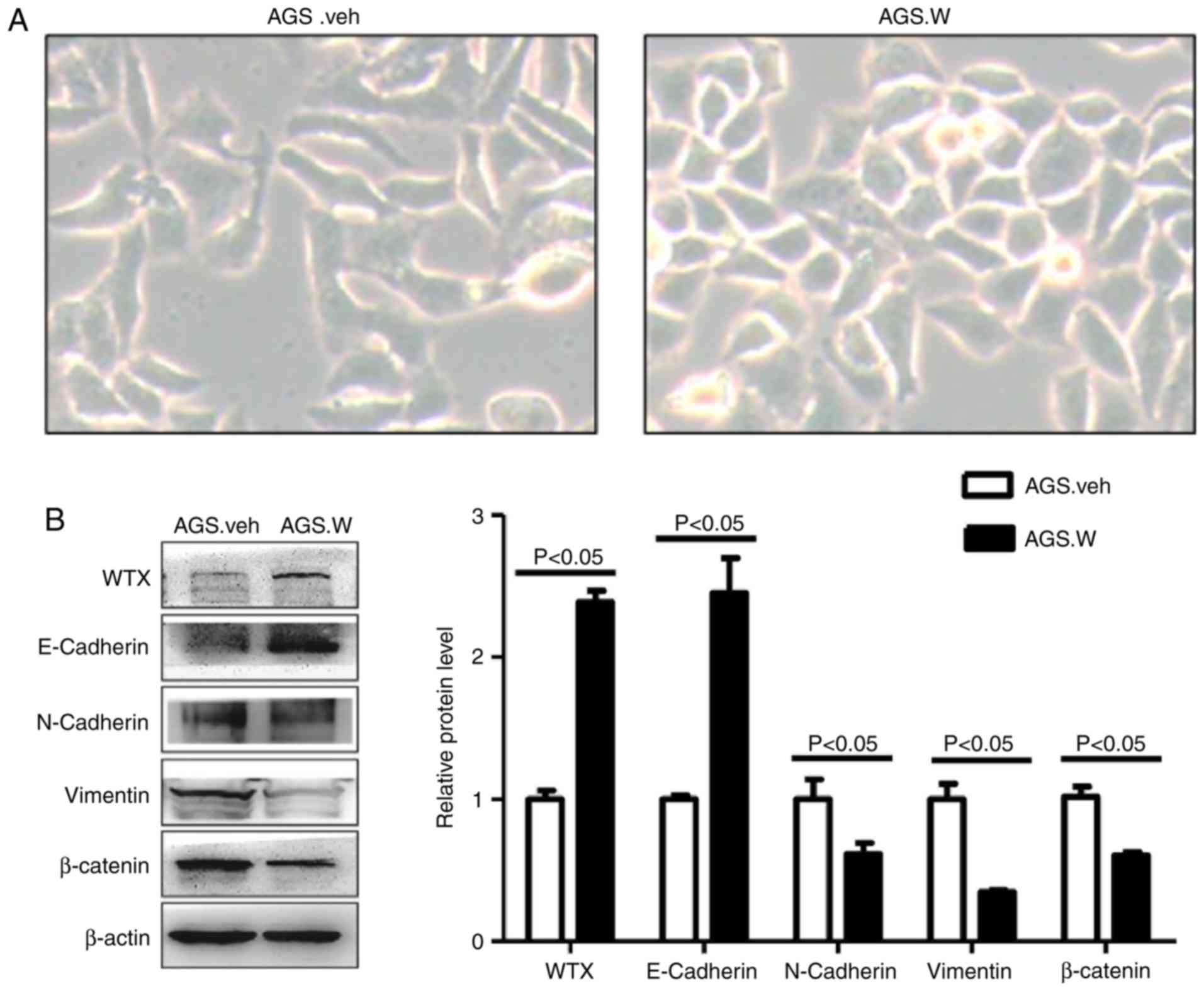
The morphology of AGS.W and AGS.veh cells was
observed using microscopy, and it was identified that the (26), whereas AGS.veh cells retained a
spindle shape and grew scattered (Fig.
2A). This morphological change is a phenotype of the tumor EMT
process (26,27).
EMT-associated protein expression in
AGS.W and AGS.veh cells
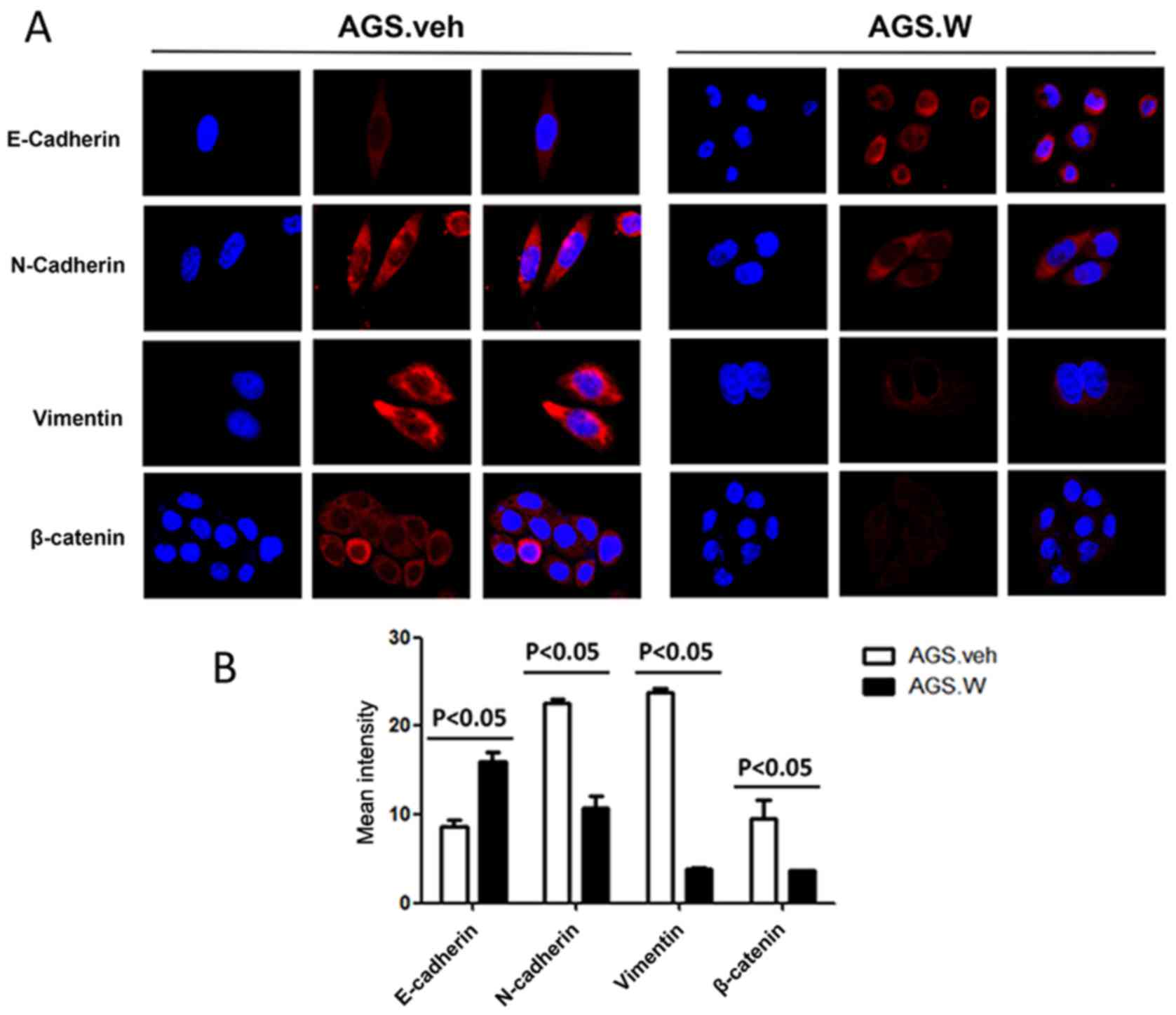
To detect the levels of EMT-associated protein
expression in AGS.W and AGS.veh cells, western blotting was
performed. The results indicated that the level of epithelial
marker E-cadherin was upregulated, while the levels of mesenchymal
markers including N-cadherin, vimentin and β-catenin were
downregulated in AGS.W cells (Fig.
2B). Similar trends were observed in immunofluorescence
(Fig. 3). These results validate the
inhibitory effect of WTX on EMT in the AGS gastric cancer cell
line.
Cell migration and invasion ability in
AGS.W and AGS.veh cells
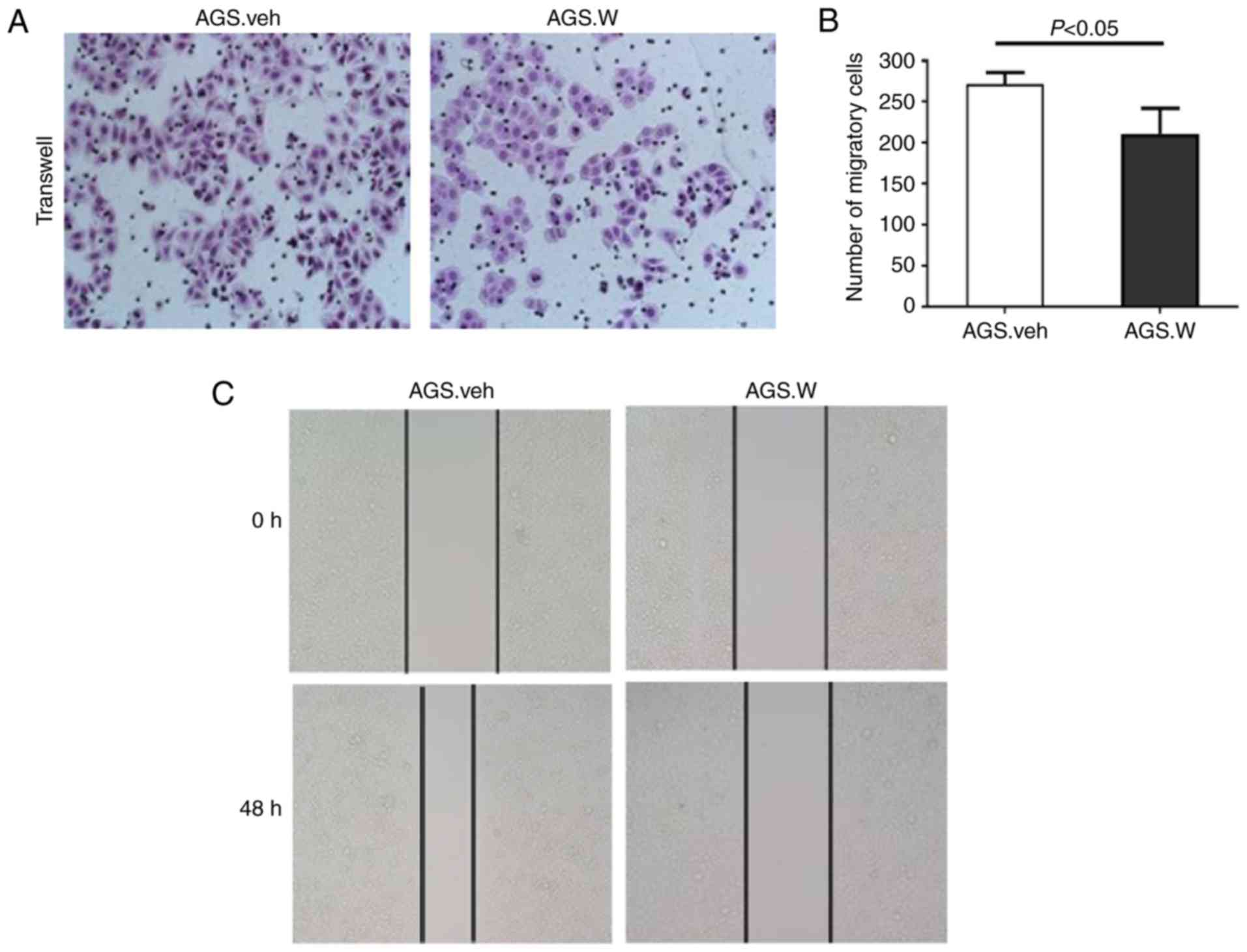
To determine whether WTX affects gastric cancer cell
invasion and migration, a Transwell Matrigel® invasion
assay and an in vitro wound-healing assay were performed in
AGS.W and AGS.veh cells. In the Matrigel® invasion
assay, the number of AGS.W cells invading to the opposite side of
chamber membrane was decreased compared with that of AGS.veh cells,
indicating that the invasive capabilities of the AGS.W cells was
decreased in comparison with those of the AGS.veh cells (Fig. 4A and B). The wound-healing assay also
indicated that WTX overexpression markedly decreased the migration
of AGS cells after 48 h (Fig. 4C).
The present study demonstrated that WTX overexpression prohibited
the invasive and migratory capabilities of the gastric cancer AGS
cell line.
Discussion
An estimated 951,600 new stomach cancer cases and
723,100 mortalities occurred in 2012 (1). Most newly diagnosed cases are identified
for cell migration and consequent metastasis (28). Due to early metastasis to distant
organs, particularly the liver, the prognosis of patients with
gastric cancer remains poor, despite the improving surgical
resection technologies for early stage tumors (29–31).
WTX has been demonstrated to serve as a tumor
suppressor in the oncogenesis of various tumors (18,32). To
investigate the exact mechanism of the WTX in GC,
WTX-overexpressing cells were established, and it was identified
that the overexpression of WTX may inhibit the invasive and
migratory capabilities of GC cells, indicating that the WTX gene
may serve a pivotal role in tumor metastasis.
Previous studies have indicated that the EMT process
is critical for the metastasis of GC by promoting cell migration
and invasion (33). Notably,
morphological changes were observed in the WTX-overexpressing
cells, which transformed from a spindle, mesenchyme-like shape into
an oval, epithelial shape. EMT is the physiological or pathological
conversion of epithelial cells to mesenchymal cells, in which cells
undergo phenotypic changes including the loss of cell polarity and
cell-cell adhesion, and the acquisition of migratory and invasive
properties, which are responsible for carcinoma progression. To
confirm this hypothesis, the expression levels of EMT-associated
proteins in WTX-overexpressing cells were analyzed, and it was
identified that epithelial cell adhesion molecules including
E-cadherin were upregulated, whereas mesenchymal markers, including
N-cadherin, vimentin and β-catenin, were downregulated by WTX
overexpression. These results indicated that WTX may inhibit the
EMT process, and consequently affect the migration of GC cells.
However, the mechanism by which WTX inhibited the
EMT process and cell migration in gastric cancer cells was not
elucidated. The Wnt/β-catenin pathway has been demonstrated to
serve an important role in the proliferation, differentiation,
migration and adhesion of GC cells (34,35).
Heuberger and Birchmeier (36)
revealed that E-cadherin binds to β-catenin and suppresses its
nuclear localization by sequestering cytoplasmic β-catenin.
Furthermore, Howard et al (37) revealed that the combination between
β-catenin and E-cadherin is essential for the EMT process. Hlubek
et al (38) demonstrated that
β-catenin-dependent translation of target genes in the nuclei is
key player during EMT. additionally, WTX has been demonstrated to
negatively regulate the Wnt/β-catenin signaling pathway by forming
a complex with β-catenin, resulting in the promotion of complex
degradation (38,39). Therefore, we hypothesize that WTX may
inhibit the EMT process in AGS gastric cancer cells through the
suppression of the Wnt/β-catenin pathway directly and/or indirectly
associated with the upregulation of E-cadherin, which requires
additional study for confirmation.
In conclusion, the present study identified that WTX
may inhibit gastric cancer cell invasion and migration, and reverse
the EMT process. The results indicate that WTX may be an important
gene for future studies concerning the regulation of gastric cancer
cell metastasis, and may present a novel prospective for
understanding the molecular mechanisms that underlie this process.
The present study provides insight on the potential for
WTX-targeted therapy for patients with gastric cancer, with the aim
of minimizing cancer progression and metastasis.
Acknowledgements
The authors thank Dr Guangning Yan (General Hospital
of Guangzhou Military Command of PLA, Guangzhou, China) for
reviewing the manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81472712 and
81772918).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YD, QZ and DY designed the research. DY, JX, WM, CL,
GZ and DL conducted the experiments, performed the data analysis
and wrote the manuscript. DY and DL performed the assays. All
authors discussed the results and reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Zhen L, Zhang Y, Zhao L, Liu H, Cai
D, Chen H, Yu J, Qi X and Li G: Circ-104916 is downregulated in
gastric cancer and suppresses migration and invasion of gastric
cancer cells. Onco Targets Ther. 10:3521–3529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang P, Tang WM, Zhang H, Li YQ, Peng Y,
Wang J, Liu GN, Huang XT, Zhao JJ, Li G, et al: MiR-646 inhibited
cell proliferation and EMT-induced metastasis by targeting FOXK1 in
gastric cancer. Br J Cancer. 117:525–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Legras A, Pecuchet N, Imbeaud S, Pallier
K, Didelot A, Roussel H, Gibault L, Fabre E, Le Pimpec-Barthes F,
Laurent-Puig P and Blons H: Epithelial-to-mesenchymal transition
and MicroRNAs in lung cancer. Cancers (Basel). 9(pii): E1012017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
He SJ, Xiang CQ, Zhang Y, Lu XT, Chen HW
and Xiong LX: Recent progress on the effects of microRNAs and
natural products on tumor epithelial-mesenchymal transition. Onco
Targets Ther. 10:3435–3451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiao Y, Jiang X, Lee ST, Karuturi RK, Hooi
SC and Yu Q: FOXQ1 regulates epithelial-mesenchymal transition in
human cancers. Cancer Res. 71:3076–3086. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Zhou Y and Yang Y: CCR7 pathway
induces epithelial-mesenchymal transition through up-regulation of
Snail signaling in gastric cancer. Med Oncol. 32:4672015.PubMed/NCBI
|
|
13
|
Liu AN, Zhu ZH, Chang SJ and Hang XS:
Twist expression associated with the epithelial-mesenchymal
transition in gastric cancer. Mol Cell Biochem. 367:195–203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang L, Wu RL and Xu AM:
Epithelial-mesenchymal transition in gastric cancer. Am J Transl
Res. 7:2141–2158. 2015.PubMed/NCBI
|
|
15
|
Jia LT, Wu J, Zhang L, Chen J, Zhong D, Xu
S, Xie C and Cai J: Restoration of miR-1228* expression suppresses
epithelial-mesenchymal transition in gastric cancer. PLoS One.
8:e586372013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu S, Cui J, Liao G, Zhang Y, Ye K, Lu T,
Qi J and Wan G: miR-137 regulates epithelial-mesenchymal transition
in gastrointestinal stromal tumor. Tumor Biol. 35:9131–9138. 2014.
View Article : Google Scholar
|
|
17
|
Sakimura S, Kurashige J, Sugimachi K, Ueda
M, Hirata H, Shinden Y, Sakimura E, Matsumura T, Takano Y, Uchi R,
et al: Decreased expression of miR-506 induced
epithelial-mesenchymal transition and poor prognosis in gastric
cancer patients. Cancer Res. 74:2014. View Article : Google Scholar
|
|
18
|
Rivera MN, Kim WJ, Wells J, Driscoll DR,
Brannigan BW, Han M, Kim JC, Feinberg AP, Gerald WL, Vargas SO, et
al: An X chromosome gene, WTX, is commonly inactivated in Wilms
tumor. Science. 315:642–645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akhavanfard S, Vargas SO, Han M, Nitta M,
Chang CB, Le LP, Fazlollahi L, Nguyen Q, Ma Y, Cosper A, et al:
Inactivation of the tumor suppressor WTX in a subset of pediatric
tumors. Genes Chromosomes Cancer. 53:67–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim WJ, Wittner BS, Amzallag A, Brannigan
BW, Ting DT, Ramaswamy S, Maheswaran S and Haber DA: The WTX tumor
suppressor interacts with the transcriptional corepressor TRIM28. J
Biol Chem. 290:14381–14390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qing Ling Z, LiNa Y, Li L, Shuang W,
YuFang Y, Yi D, Divakaran J, Xin L and YanQing D: LMP1 antagonizes
WNT/β-catenin signalling through inhibition of WTX and promotes
nasopharyngeal dysplasia but not tumourigenesis in LMP1(B95-8)
transgenic mice. J Pathol. 223:574–583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujita A, Ochi N, Fujimaki H, Muramatsu H,
Takahashi Y, Natsume J, Kojima S, Nakashima M, Tsurusaki Y, Saitsu
H, et al: A novel WTX mutation in a female patient with osteopathia
striata with cranial sclerosis and hepatoblastoma. Am J Med Genet
A. 164A:1–1002. 2014.PubMed/NCBI
|
|
23
|
Zhang YY, Wang QM, Niu HL, Liu X and Zhang
QL: The general expression analysis of WTX gene in normal and
cancer tissues. Pathol Oncol Res. 23:439–446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma W, He L, Liu C, Zhang Q and Ding Y:
Establishment of a colorectal cancer SW620 cell line stably
over-expressing Wilm's tumor on X chromosome using a recombinant
lentivirus vector. Nan Fang Yi Ke Da Xue Xue Bao. 35:1122–1127.
2015.(In Chinese). PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wendt MK, Allington TM and Schiemann WP:
Mechanisms of the epithelial-mesenchymal transition by TGF-beta.
Future Oncol. 5:1145–1168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu C, Zhuang Y, Jiang S, Liu S, Zhou J, Wu
J, Teng Y, Xia B, Wang R and Zou X: Interaction between
Wnt/β-catenin pathway and microRNAs regulates
epithelial-mesenchymal transition in gastric cancer (Review). Int J
Oncol. 48:2236–2246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Camargo MC, Kim WH, Chiaravalli AM, Kim
KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R,
Meneses-Gonzalez F, et al: Improved survival of gastric cancer with
tumour Epstein-Barr virus positivity: An international pooled
analysis. Gut. 63:236–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng JY and Liang H: Clinical significance
of lymph node metastasis in gastric cancer. World J Gastroenterol.
20:3967–3975. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martinez Mier G, Alvarez-Tostado Fernandez
JF, Romero Hernandez T, Martinez Mier EA and Blanco Benavides R:
Morbidity and mortality in surgery for gastric cancer. Rev
Gastroenterol Mex. 64:78–84. 1999.(In Spanish). PubMed/NCBI
|
|
32
|
Comai G, Boutet A, Neirijnck Y and Schedl
A: Expression patterns of the Wtx/Amer gene family during mouse
embryonic development. Dev Dyn. 239:1867–1878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xia P and Xu XY: Epithelial-mesenchymal
transition and gastric cancer stem cell. Tumour Biol. 39:2017.
View Article : Google Scholar
|
|
34
|
Huang J, Xiao D, Li G, Ma J, Chen P, Yuan
W, Hou F, Ge J, Zhong M, Tang Y, et al: EphA2 promotes
epithelial-mesenchymal transition through the Wnt/β-catenin pathway
in gastric cancer cells. Oncogene. 33:2737–2747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li K and Dan Z: Research progress of
Wnt/β-catenin signaling pathway in prevention and treatment of
gastric cancer. Nan Fang Yi Ke Da Xue Xue Bao. 34:1852–1856.
2014.(In Chinese). PubMed/NCBI
|
|
36
|
Heuberger J and Birchmeier W: Interplay of
cadherin-mediated cell adhesion and canonical Wnt signaling. Cold
Spring Harb Perspect Biol. 2:a0029152010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Howard S, Deroo T, Fujita Y and Itasaki N:
A positive role of cadherin in Wnt/β-catenin signalling during
epithelial-mesenchymal transition. PLoS One. 6:e238992011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hlubek F, Jung A, Kotzor N, Kirchner T and
Brabletz T: Expression of the invasion factor laminin gamma2 in
colorectal carcinomas is regulated by beta-catenin. Cancer Res.
61:8089–8093. 2001.PubMed/NCBI
|
|
39
|
Major MB, Camp ND, Berndt JD, Yi X,
Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss
MJ, et al: Wilms tumor suppressor WTX negatively regulates
WNT/beta-catenin signaling. Science. 316:1043–1046. 2007.
View Article : Google Scholar : PubMed/NCBI
|