Introduction
Malignant ascites (MA) is a pathological condition
due to tumor cell invasion and metastasis to the abdominal cavity.
The appearance of MA indicates progression of cancer, poor quality
of life and decreased life expectancy for patients (1). Hepatocellular carcinoma (HCC), gastric
cancer and ovarian cancer typically lead to the occurrence of MA
(2). Similar to other types of immune
microenvironment, MA contains a large number of lymphocytes,
particularly cluster of differentiation (CD)4+ T helper
(Th) lymphocytes, tumor cells and other types of immune cell
(3). CD4+ T cells may
inhibit the T cell response to exogenous- and auto-antibodies to
maintain their own composition of immune tolerance in the tumor and
prevent the body mounting an immune response. In addition,
CD4+ T cells participate in tumor growth and metastasis
via the secretion of a variety of soluble inflammatory, and immune
factors (4).
Th22 cells have been identified as a novel
CD4+ T cell subset, and primarily secrete interleukin
(IL-)22, IL-6 and IL-1β, but not IL-17 or interferon (IFN-)γ
(5). IL-22 is the effector cytokine
of Th22 cells and belongs to the IL-10 cytokine family (6). Th22 cells and IL-22 has been implicated
in the pathogenesis of liver cancer (7). In addition, the overexpression of IL-22
in primary tumor tissue and malignant pleural effusion may be
involved in the occurrence, and progression of lung cancer
(8). Furthermore, Th17 cells are a
novel subset of CD4+ T cells and produce IL-17, but not
IFN-γ (9). Th17 cells serve a
dominant function in a variety of autoimmune diseases and cancers,
including rheumatoid arthritis and gastric cancer (10,11). An
increased number of Th17 cells have been observed in patients with
lung cancer exhibiting malignant pleural effusion (12,13). In a
previous study of multiple sclerosis lesions and lung inflammation,
it was demonstrated that IL-22 and IL-17 increased vascular
endothelial permeability (14,15).
However, studies regarding the function of Th22 and Th17 cells in
MA is limited, and the biological function of Th22 and Th17 cells
in MA remains unknown. Therefore, the aim of the present study was
to investigate the distribution and the phenotypic characteristics
of Th22 and Th17 cells in patients with HCC. The present study
additionally aimed to determine the molecular mechanism underlying
the recruitment of peritoneal Th22 and Th17 cells into MA in
patients with HCC. Finally, the prognostic value of peritoneal Th17
and Th22 cells was examined in patients with MA.
Patients and methods
Patients
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Guangxi Medical
University for human studies and all patients provided written
informed consent. MA samples were selected from 26 patients with
newly diagnosed HCC with MA (AJCC stage IV) at The First Affiliated
Hospital of Guangxi Medical University (Nanning, China) between
February 2013 and December 2015. The mean age of the patients was
53.7±7.6 years. In addition, 15 healthy volunteers as controls
(mean age, 50.9±5.5 years) during the same period were recruited in
the present study. A diagnosis of MA was established by the
demonstration of malignant cells in peritoneal effusion and/or in
closed peritoneal biopsy specimen. The patients were excluded if
they had received any invasive procedures directed into the
peritoneal cavity or if any abdominal trauma had occurred within 3
months prior to hospitalization. No patients received antitumor
treatment and or glucocorticoid or other non-steroidal
anti-inflammatory drugs prior to participating in the present
study. The clinical information for the MA patients and healthy
controls are summarized in Table
I.
 | Table I.Characteristics of the participants
included in the present study. |
Table I.
Characteristics of the participants
included in the present study.
| Index | Patients with MA,
n=26 | Healthy controls,
n=15 |
|---|
| Sex,
male/female | 15/11 | 8/7 |
| Age, years | 53.7±7.6 | 50.9±5.5 |
| CRP in plasma,
mg/l | 62.6±15.4 | 8.1±1.2 |
| AST, U/l | 78.7±11.3 | 20.6±6.1 |
| ALT, U/l | 68.9±9.4 | 18.5±7.4 |
| Protein, g/ml | 4.1±0.4 |
|
| LDH, U/l | 747.3±125.6 |
|
| AFP, µg/l | 81.7±10.6 |
|
| CEA,
µg/la | 43.5±2.4 |
|
Sample collection and processing
Within 24 h of hospitalization, between 400 and 800
ml MA samples were selected from each patient and placed in
heparinization tubes, using a standard abdominocentesis technique,
and 10 mm peripheral blood was drawn. The MA specimens were
immersed in ice immediately and subsequently centrifuged at 800 × g
for 10 min at 4°C. The supernatants of MA and plasma were frozen at
−80°C immediately for use in subsequent experiments. The cell
pellets of MA were resuspended in PBS and mononuclear cells were
isolated using Ficoll-Hypaque gradient centrifugation (Solarbio,
Beijing, China) at 4°C, 800 × g to determine the T cell subsets
within 1 h.
Flow cytometry
Following surface or intracellular staining with
anti-human-specific antibodies (Abs) conjugated with
Peridinin-Chlorophyll-protein (PerCP)-cysteine (cy)5.5,
allophycocyanin (APC), phycoerythrin (PE), Alexa Fluor (AF)647,
AF488 or fluorescein isothiocyanate (FITC). The expression of
markers on T cells from MA and peripheral blood were determined
using flow cytometry as previously described (12). The human Abs against anti-CD4, -IL-22,
-IL-17A and -IFN-γ were purchased from BD Biosciences (Franklin
Lakes, NJ, USA; cat. nos. 561844, 12-7229-41, 559502 and 551221,
respectively). Human antibodies against -C-C motif chemokine
receptor (CCR)4, -CCR6 and -CCR10 were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA; cat. nos. PA5-11866,
CSB-E04660h and 720308). The anti-CD4, -IL-22, -IL-17A and -IFN-γ
were diluted at 1:200, and the CCR4, -CCR6 and -CCR10 were diluted
at 1:500. Intracellular staining for IL-22 or IL-17 was performed
on T cells stimulated with phorbol myristate acetate (50 ng/ml;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and ionomycin (1
µg/ml; Sigma-Aldrich; Merck KGaA) in the presence of GolgiStop (1.7
ug/ml; BD Biosciences) at 4°C for 5 h, and subsequently stained
with PerCP-cy5.5-CD4, PE-CCR4, FITC-CCR6 or PE-CCR10 at room
temperature in the dark for 30 min. Cells were washed twice with
staining buffer (1 ml/wash for tubes) and pellet by centrifugation
(250 × g). Cells were resuspended in 250 µl tubes of
Fixation/Permeabilization solution (BD Biosciences) for 20 min at
4°C. Cells were washed two times in 1X BD Perm/Wash™ buffer (1
ml/wash for staining in tubes). The cells were stained with APC or
PE-IL-22, AF647-IL-17A and AF488-IFN-γ at 4°C for 30 min, following
fixation (100 µl of Fixation Buffer and pulse vortex) at 4°C in the
dark room for 20 min. The permeabilization was performed according
to the manufacturer's protocol. Mouse immunoglobulin G irrelevant
isotype control (BD Biosciences; cat. no. MAB0032; dilution 1:100)
served as isotype control. Stained cells were washed 2 times with
1× BD Perm/Wash™ buffer (1 ml/tubes), resuspend in Staining Buffer
and subsequently analyzed by flow cytometric analysis using BD
FCSDiva Software and FCS Epress 4 software (De Novo Software, Los
Angeles, CA).
ELISA
MA and peripheral blood specimens were collected
into heparinization tubes. The cell-free supernatants of MA and
plasma were obtained from all subjects by centrifugation 800 × g
for 10 min at room temperature and stored at −80°C immediately for
determination of cytokines. The concentration of cytokines IL-22
and IL-17, chemokines C-C motif chemokine ligand (CCL)20, CCL22 and
CCL27 in MA, and plasma were determined using an ELISA kit (cat.
no. CSB-E09125h; R&D Systems, Inc., minneapolis, MN, USA,) in
accordance with the manufacturer's protocol (lower detection limit
1 pg/ml; eBioscience; Thermo Fisher Scientific, Inc.). All samples
were assayed in duplicate.
Chemotaxis assay of Th22 and Th17
cells in vitro
The chemotaxis assay was observed using light
microscope with magnification of ×200. Briefly, the 8-µm pore
polycarbonate filters in 24-well Transwell chambers (Corning
Incorporated, Corning, NY, USA) were used. Transwell membranes were
coated with fibronectin (5 µg/ml; Chemicon International; EMD
Millipore, Billerica, MA, USA) for 30 min at 37°C. Purified
CD4+ T cells from blood (2×105) were isolated
using an EasySep™ Direct Human CD4+ T Cell Isolation kit (StemCell
Technologies Inc., Vancouver, BC, Canada), then added into the top
chamber and resuspended in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) with 0.5% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) (final volume, 100 µl). MA supernatants
were placed in the bottom chamber in a volume of 600 µl and the
chambers were incubated at 37°C in an atmosphere containing 5%
CO2 for 3 h. Finally, all the cells that migrated into
the bottom chamber were harvested, intracellularly stained for
IL-22 or IL-17 and analyzed using flow cytometry as aforementioned.
The chemotaxis index for Th22 and Th17 cells was calculated
individually as follows: (Number of cells that migrated in response
to MA or supernatants)/(number of cells that migrated in response
to medium alone). To investigate whether CCL20, CCL22 and CCL27
contributed to Th22 or Th17 cell migration, inhibition experiments
were performed by mixing the MA or supernatants with 100 ng/ml
anti-CCL20 mAb (cat. no. 500-M28), anti-CCL22 mAb (cat. no.
MAB336), anti-CCL27 mAb (cat. no. MAB376) or mouse immunoglobulin G
irrelevant isotype control (cat. no. MAB0032) (R&D systems,
Inc., Minneapolis, MN, USA), the anti-body was diluted for 1:100,
and incubated at 4°C for 20 min.
Survival analysis for MA patients
To estimate the survival time of MA patients with
different Th22 or Th17 cell, the patients were divided into cell
high group or cell low group based on the median value of cell. The
median value of Th17 cells and Th22 cells was 2.75, 3.02%,
respectively, and patient had the median value was considered in
high group in this study.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons of Th22, Th17, CCL20, CCL22, CCL27, IL-17 and IL-22
levels in MA, corresponding blood and peripheral blood from healthy
controls were determined using a one-way analysis of variance
(ANOVA). Newman-Keuls multiple comparison test (q test) was used to
determine the difference between two groups when the one-way ANOVA
analysis indicated a significant difference. Comparsion of Th22,
Th17, CCL20, CCL22, CCL27, IL-17 and IL-22 levels in MA and
corresponding blood was determined using Student's t-test. The
Pearson or Spearman correlation test was used for correlation
analysis, depending on the data distribution. Overall survival was
estimated using the Kaplan-Meier method and compared using the
log-rank test. Analysis was performed using SPSS version 16.0
statistical software (SPSS, Inc., Chicago, IL, USA) and P<0.05
was considered to indicate a statistically significant
difference.
Results
Th22, Th17 and Th1 cells are increased
in MA
It is reported that Th22 cells are associated with
Th17 (16) and Th1 cells (17); therefore, in the present study, the
distribution of Th22, Th17 and Th1 cells were studied in patients
with MA, and healthy controls. Representative flow cytometry
scatter plots of Th22, Th17 and Th1 cells in MA, and healthy
controls are presented in Fig. 1A.
The proportions of Th22 cells exhibited significantly increased
values in MA in comparison with the corresponding peripheral blood
and healthy controls. In addition, Th17 and Th1 cells in MA were
significantly increased compared with the corresponding peripheral
blood and healthy controls (Fig. 1B).
The proportions of peritoneal Th22 cells were positively associated
with Th17 or Th1 cells, and Th17 cells were positively associated
with Th1 cells in MA (all P<0.01; Fig.
1C).
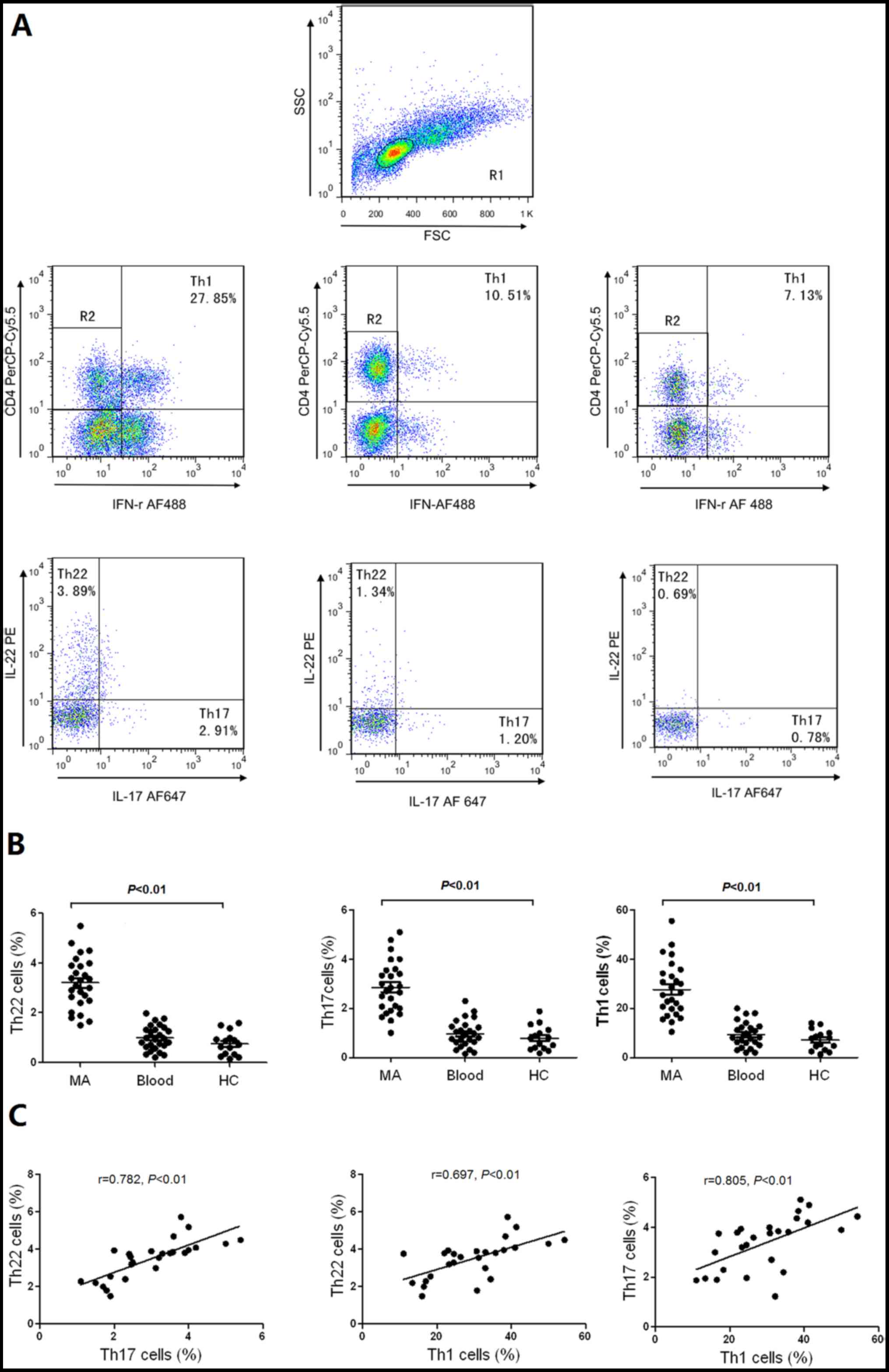 | Figure 1.Distribution of Th22, Th17 and Th1
cells in MA, corresponding peripheral blood and HC. (A) Anti-CD4
monoclonal antibodies were performed with gating on CD4+
T cells for further testing Th22, Th17 and Th1 cells. The typical
flow cytometry scatter plots of Th22 cells, Th17 cells and Th1
cells in MA, Blood and HC are presented. (B) Comparisons between
the proportion of Th22, Th17, and Th1 in MA, Blood and HC.
Horizontal line in the graph represents the mean. The proportion of
Th cells were determined using flow cytometry. (C) Th22 cells were
positively associated with Th17 cells or Th1 cells in MA. Th17
cells were positively associated with Th1 cells in MA. Th, T helper
cell; MA, malignant ascites; HC, healthy controls; CD, cluster of
differentiation; PE, phycoerythrin; PerCP cy5.5,
Peridinin-Chlorophyll-protein-cysteine 5.5; IFN, interferon; AF,
Alexa Fluor; FSC, forward scatter; SSC, side scatter. |
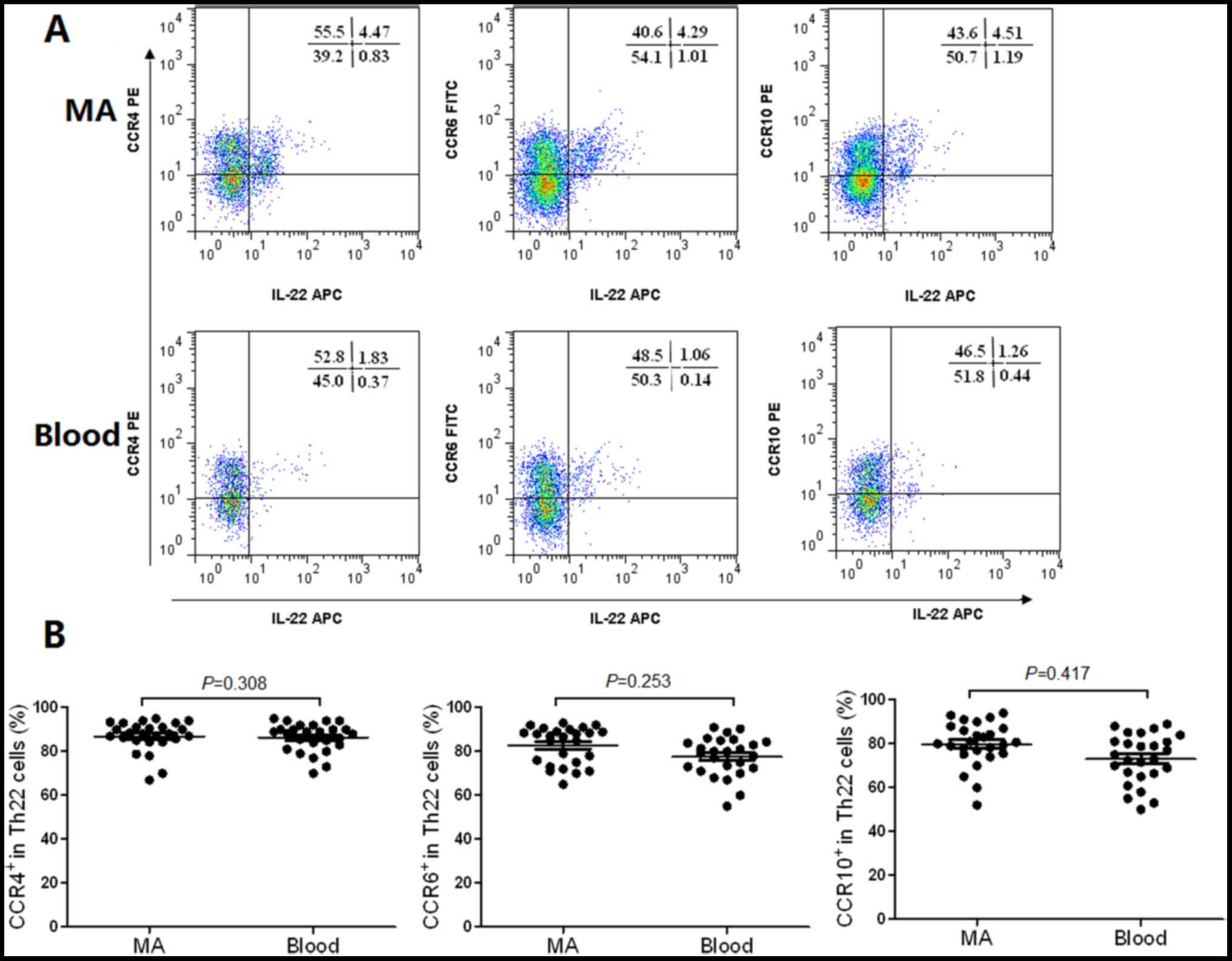
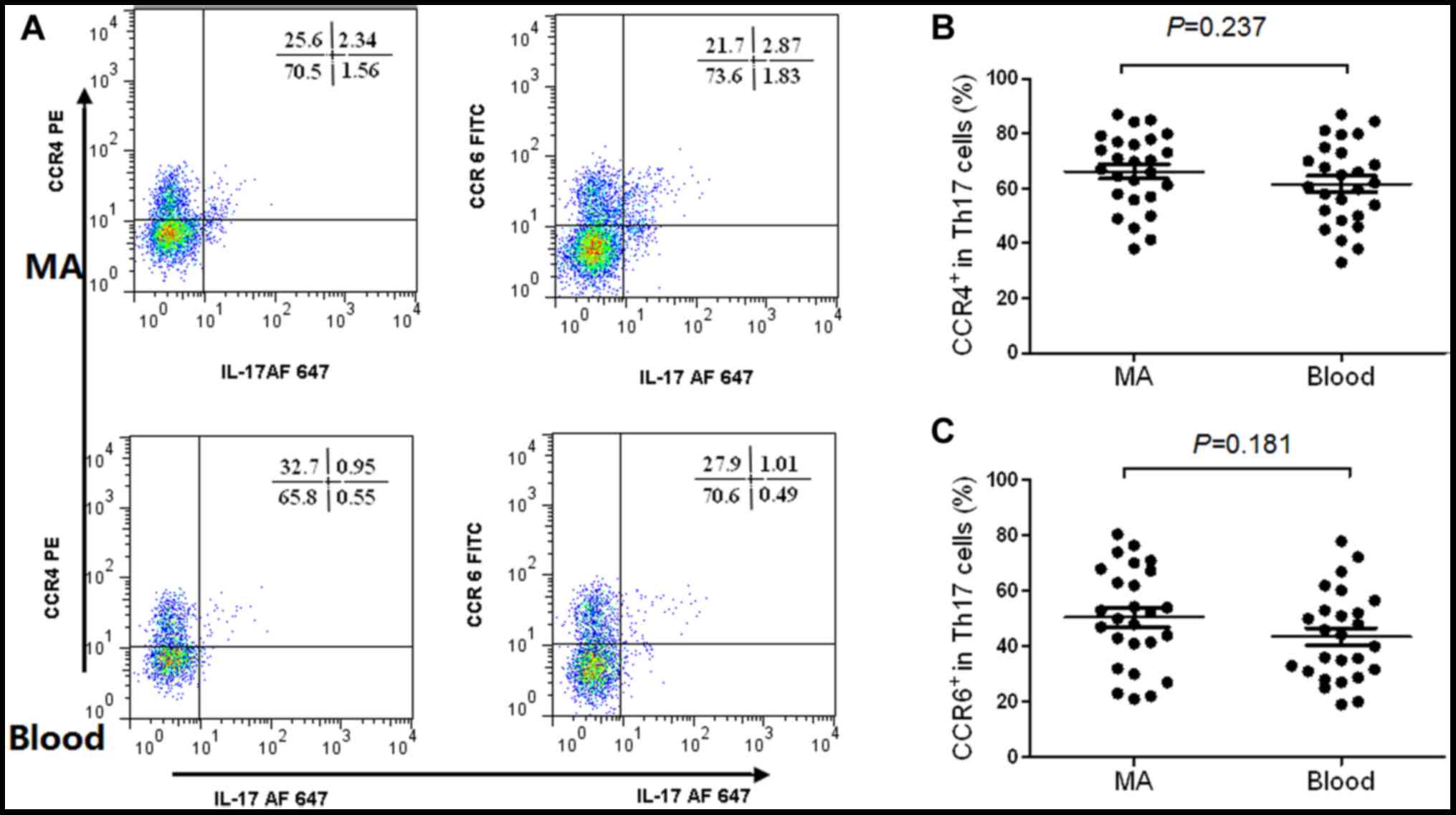
Th22 and Th17 cells exhibit similar
phenotypic characteristics in MA and peripheral blood samples
The flow cytometry scatter plots of the expression
levels of CCR4, CCR6 and CCR10 on Th22 cells in MA, and the
corresponding peripheral blood are presented in Fig. 2A. The phenotypic analysis revealed
that Th22 cells expressed high concentrations of CCR4, CCR6 and
CCR10 in MA compared with the corresponding peripheral blood
(Fig. 2B). The expression levels of
CCR4 and CCR6 in Th17 cells from MA, and the peripheral blood are
presented in Fig. 3A. The results
revealed that Th17 cells expressed high concentrations of CCR4
(Fig. 3B) and CCR6 (Fig. 3C) in MA compared with the
corresponding peripheral blood.
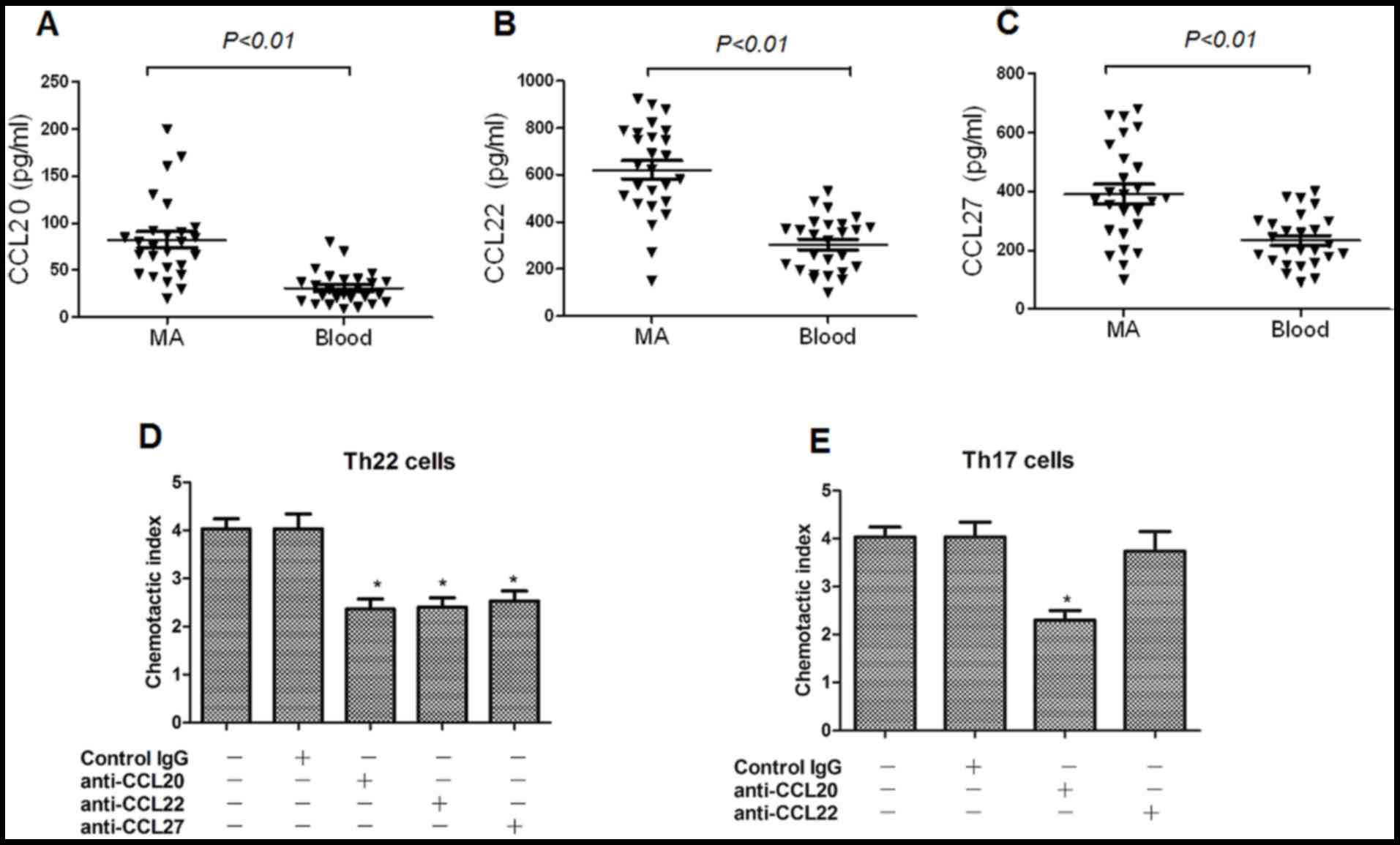
Chemokines CCL20, CCL22 and CCL27 are
increased in MA, and may be associated with the migration of Th17
and Th22 cells
The concentrations of chemokines CCL20, CCL22 and
CCL27 in MA were significantly increased, compared with that in the
corresponding peripheral blood, determined using ELISA (Fig. 4A-C), which are receptors for CCR4,
CCR6, and CCR10, respectively. On the basis of these results, it
was hypothesized that peripheral Th22 and Th17 cells may infiltrate
into the peritoneal cavity in response to these chemokines. In the
chemotaxis assays, it was revealed that the anti-CCL20, anti-CCL22
and anti-CCL27 mAbs significantly suppressed Th22 cell migration;
whereas, the anti-CCL20 mAb markedly inhibited Th17 cell migration
(Fig. 4D and E).
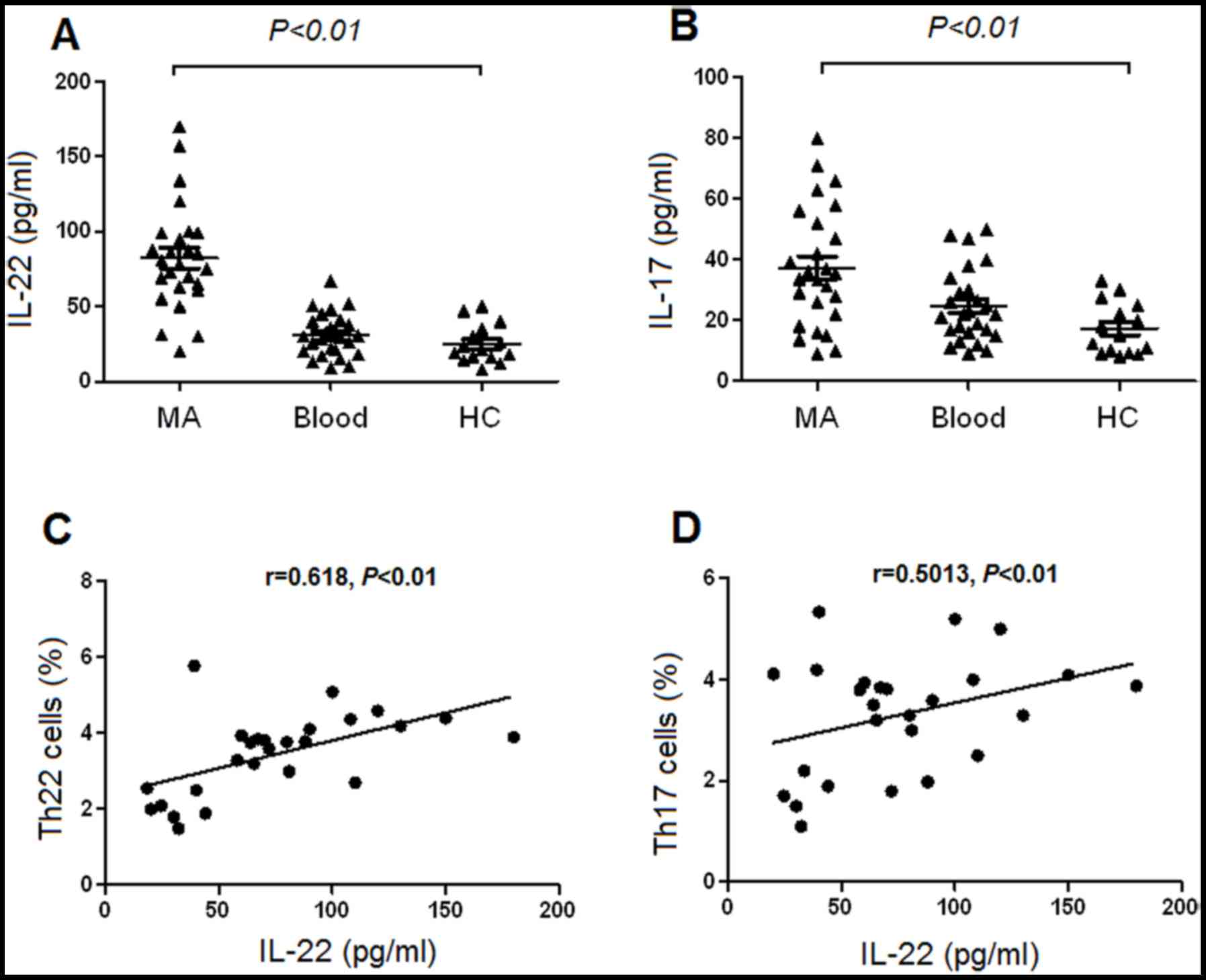
Increased concentrations of IL-22 and
IL-17 in MA
The concentration of IL-22 was significantly
increased in MA, compared with the corresponding peripheral blood
and healthy controls, determined using ELISA (Fig. 5A). Additionally, the concentration of
IL-17 was significantly increased in MA compared with that in the
corresponding peripheral blood and healthy controls (Fig. 5B). There was a positive association
identified between Th22 cells and the concentrations of IL-22 in MA
(Fig. 5C). Furthermore, there was a
positive association revealed between IL-22 and Th17 cells in MA
(P=0.001; Fig. 5D). However, no
association was identified between IL-22 and Th22 or Th17 cells in
either the peripheral blood or healthy controls (all P>0.05;
data not shown).
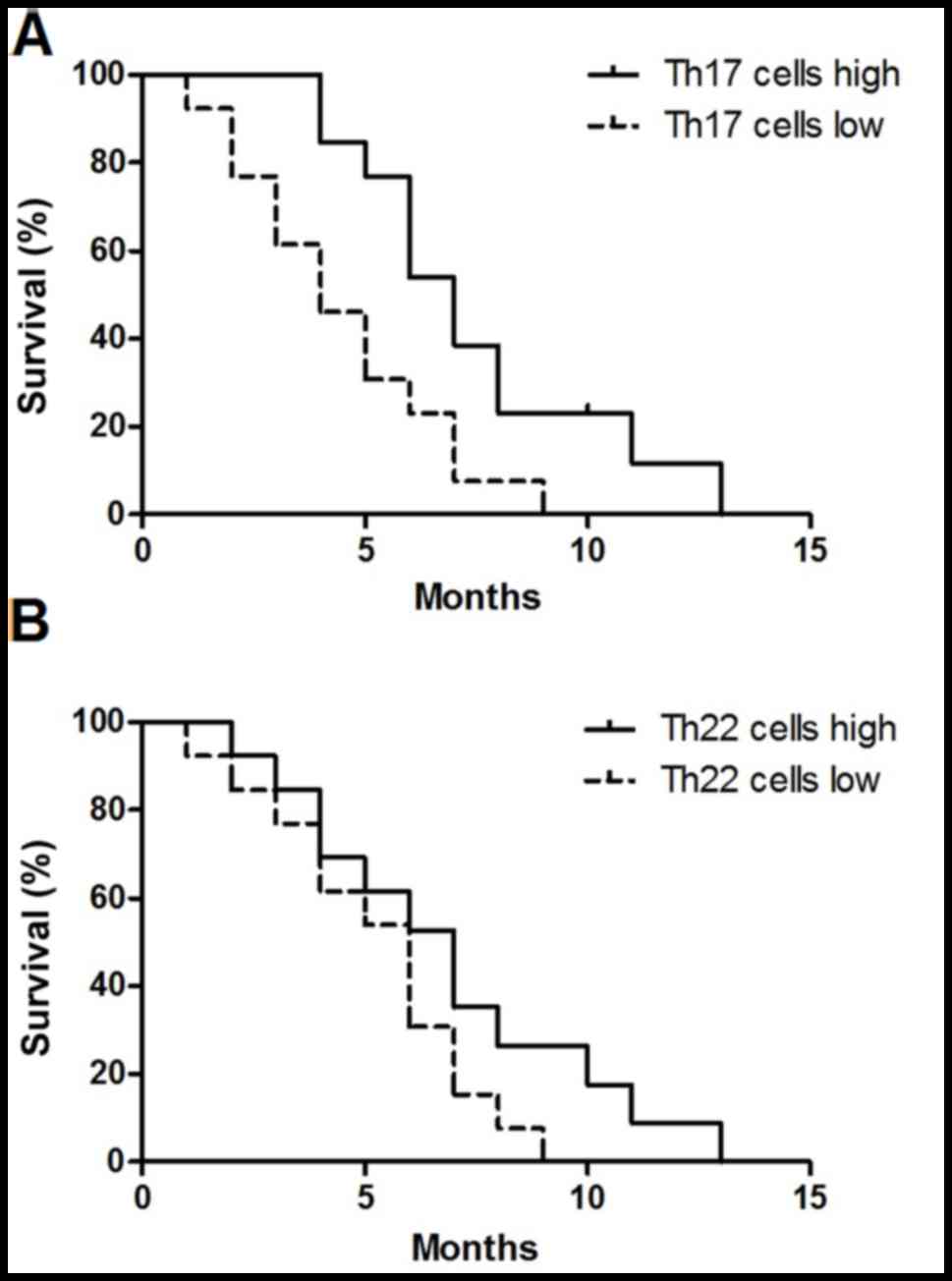
Increased number of Th17 cells
indicates an increased overall survival time
On the basis of the median value of Th17 cells
(2.75%), the 26 patients with MA were divided into either the Th17
cell high group (n=13) and Th17 cell low group (n=13). As presented
in Fig. 6A, patients with in the high
Th17 cell group exhibited an increased overall survival time,
compared with that in the low Th17 cell group [hazard ratio (HR),
1.75; 95% confidence interval (CI), 1.29–2.21; P=0.012]. The median
Th22 cells value was 3.02%; however, no significant difference was
identified between the Th22 cell high group (n=13) and the Th22
cells low group (n=13) (HR, 0.48; 95% CI, 0.19–1.23; P=0.127)
(Fig. 6B).
Discussion
To the best of our knowledge, the present study was
the first to investigate the distribution, the phenotypic
characteristics and the recruitment of Th22, and Th17 cells in
patients with HCC and MA. The results of the present study
demonstrated that the proportion of Th22 and Th17 cells were
significantly increased in MA compared with the corresponding
peripheral blood and healthy controls, indicating the involvement
of Th22 and Th17 cells in the development of MA. In addition, there
was a positive association identified between IL-22 and Th22 cells,
Th17 cells in MA, suggesting that Th22 and Th17 cells may have a
similar effect through the regulation of IL-22. However, the
association between Th22 and Th17 cells requires additional
study.
IL-22 and IL-17 may induce Th cell differentiation,
and IL-22 may be more widely involved in IFN-γ-producing
CD4+ T cell (Th1 cell)-mediated immune responses
(15). Furthermore, it is IL-22, not
IL-17, that may induce wound-healing responses, and tissue-repair
protecting from tuberculosis (13,18). IL-22
is predominantly secreted by Th22, Th17 and Th1 cells (19). Additionally, of the IL-22-producing T
cells, the proportion of Th22 cells accounts for between 37 and
63%, Th17 cells accounts for between 10 and 18%, and Th1 cells
accounts for ~35% (20). It has been
demonstrated that IL-6 promotes the expression of IL-22, and is
required for IL-17 induction from naïve T cells (21,22); this
may explain the positive association between the concentration of
peritoneal IL-22 and the number of peritoneal Th22 cells, and the
positive association between IL-22 and Th17 cells identified in the
present study. In addition, the results of the present study
indicated that proinflammatory cytokines in MA may promote the
secretion of IL-22.
Some mechanism has been identified to increase the
number of Th22 and Th17 cells. In addition to the local generation
and differentiation, the reason for the increase in peritoneal Th22
and Th17 cells may have been in response to recruitment from the
peripheral blood (13). A previous
study demonstrated that the migration of lymphocytes is regulated
by the chemokine/chemokine receptor axis (23). CCL20/CCR6, CCL22/CCR4 and CCL27/CCR10
chemotaxis axes have been identified to serve functions in the
recruitment of lymphocytes to local tissues (24). Previously studies have demonstrated
that the recruitment of Th9 cells into malignant pleural effusion
may be induced by pleural CCL20 (25), and that CCL20 and CCL22 were
responsible for the recruitment of IL-27-producing CD4+T
cells to tuberculous pleural effusion (13,18).
However, the function of the chemokine/chemokine receptor
interaction in the recruitment of peripheral Th22 cells and Th17
cells into MA remain unknown.
In the phenotypic analysis of the present study, the
results revealed that Th22 cells expressed increased concentrations
of CCR4, CCR6 and CCR10, whereas Th17 cells expressed increased
concentrations of CCR4, and CCR6 in MA compared with the
corresponding peripheral blood. In addition, the concentrations of
CCL20, CCL22 and CCL27 in MA were markedly increased, compared with
those in the plasma from corresponding peripheral blood. These
results indicated that the axes of CCL20/CCR6, CCL22/CCR4 and
CCL27/CCR10 may be involved in the recruitment of Th22, and Th17
cells to the peritoneal cavity from the peripheral blood. Using
chemotaxis assays, it was observed that, by inhibiting the
CCL20/CCR6, CCL22/CCR4 and CCL27/CCR10 axes, the migration rate of
Th22 cells was significantly suppressed, and inhibition of
CCL20/CCR6 prevented Th17 cell migration, indicating that these
chemotaxis axes may be responsible for the recruitment of
peripheral Th22 and Th17 cells into MA.
Previously, it was reported that high expression of
IL-17 was associated with poor prognosis, whereas high Th17 cell
frequencies were associated with improved prognosis (26). Th17 cells are a subpopulation of
IL-17+ cells and exhibit a distinct association with
prognosis compared with total IL-17 (27). Th17 cells serve an antitumor function
in antitumor immunity and promote the immune response of
tumor-specific cytotoxic T cells in the pulmonary melanoma model
(28). Accumulation of Th17 cells in
malignant pleural effusion indicates the improvement of patient
survival (12) and a decrease in Th17
cells in ascites from patients with ovarian cancer indicated a poor
prognosis (29). Similar to these
aforementioned studies, the present study demonstrated that the
accumulation of Th17 cells in MA indicated the improvement of
patient survival.
Th22 cells accumulate in a number of different types
of tumor, compared with healthy tissues, and the patient outcome
may differ from one tumor type to another (30). Previous studies have demonstrated that
increased Th22 cells were associated with poor prognosis of
patients with pancreatic cancer (31), multiple myeloma (32) and colorectal cancer (33); however, Th22 cells are protective in
colorectal cancer (34). In the
present study, no significant different was identified in the
survival time between the Th22 cell high and Th22 cell low groups
of patients. Therefore, it was hypothesized that the different
prognostic functions of Th22 and Th17 cells may be due to the
cytokines secreted by the different cell types or the tumor type,
including HCC or ovarian cancer at stage III or IV; however, the
underlying molecular mechanisms require additional study.
The results of the present study demonstrated that
Th22 and Th17 cells may serve functions in the pathogenesis of MA,
and accumulation of Th22 cells and Th17 cells in MA may be due to
locally increased proinflammatory cytokines and chemokines.
Furthermore, increased numbers of Th17 cells in MA indicates an
improved survival time for patients with HCC. However, due to the
limited number of samples included, a study with larger umber
samples is warranted, and an in vivo animal experiment to
further validate these results is needed.
Acknowledgements
Not applicable.
Funding
The present study was partially supported by the
Guangxi Natural Science Foundation (grant no. 2014GXNSFAA118203).
The funders had no role in study design, data collection and
analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SYQ and HXJ designed the study, interpreted the
experimental results and modified the manuscript. XWY, RL and WSL
collected the samples and performed the experiment. XWY and SHT
performed statistical analysis. All authors have read and approved
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Guangxi Medical
University for human studies and all patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith EM and Jayson GC: The current and
future management of malignant ascites. Clin Oncol (R Coll Radiol).
15:59–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adam RA and Adam YG: Malignant ascites:
Past, present, and future. J Am Coll Surg. 198:999–1011. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hao C, Shi Y, Yu J, Wei X, Li S and Tong
Z: The therapeutic function of the chemokine RANTES on the H22
hepatoma ascites model. Mol Cell Biochem. 367:93–102. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang N, Pan HF and Ye DQ: Th22 in
inflammatory and autoimmune disease: Prospects for therapeutic
intervention. Mol Cell Biochem. 353:41–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eyerich K, Pennino D, Scarponi C, Foerster
S, Nasorri F, Behrendt H, Ring J, Traidl-Hoffmann C, Albanesi C and
Cavani A: IL-17 in atopic eczema: Linking allergen-specific
adaptive and microbial-triggered innate immune response. J Allergy
Clin Immunol. 123:59–66.e4. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dumoutier L, Louahed J and Renauld JC:
Cloning and characterization of IL-10-related T cell-derived
inducible factor (IL-TIF), a novel cytokine structurally related to
IL-10 and inducible by IL-9. J Immunol. 164:1814–1819. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin S, Ma S, Huang X, Lu D, Zhou Y and
Jiang H: Th22 cells are associated with hepatocellular carcinoma
development and progression. Chin J Cancer Res. 26:135–141.
2014.PubMed/NCBI
|
|
8
|
Zhang W, Chen Y, Wei H, Zheng C, Sun R,
Zhang J and Tian Z: Antiapoptotic activity of autocrine
interleukin-22 and therapeutic effects of interleukin-22-small
interfering RNA on human lung cancer xenografts. Clin Cancer Res.
14:6432–6439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bettelli E, Korn T, Oukka M and Kuchroo
VK: Induction and effector functions of T(H)17 cells. Nature.
453:1051–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bettelli E, Korn T and Kuchroo VK: Th17:
The third member of the effector T cell trilogy. Curr Opin Immunol.
19:652–657. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guéry L and Hugues S: Th17 cell plasticity
and functions in cancer immunity. Biomed Res Int. 2015:3146202015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ye ZJ, Zhou Q, Gu YY, Qin SM, Ma WL, Xin
JB, Tao XN and Shi HZ: Generation and differentiation of
IL-17-producing CD4+ T cells in malignant pleural effusion. J
Immunol. 185:6348–6354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye ZJ, Zhou Q, Yuan ML, Du RH, Yang WB,
Xiong XZ, Huang B and Shi HZ: Differentiation and recruitment of
IL-22-producing helper T cells stimulated by pleural mesothelial
cells in tuberculous pleurisy. Am J Respir Crit Care Med.
185:660–669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kebir H, Kreymborg K, Ifergan I,
Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N,
Becher B and Prat A: Human TH17 lymphocytes promote blood-brain
barrier disruption and central nervous system inflammation. Nat
Med. 13:1173–1175. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
You QH, Sun GY, Wang N, Shen JL and Wang
Y: Interleukin-17F-induced pulmonary microvascular endothelial
monolayer hyperpermeability via the protein kinase C pathway. J
Surg Res. 162:110–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liang SC, Tan XY, Luxenberg DP, Karim R,
Dunussi-Joannopoulos K, Collins M and Fouser LA: Interleukin
(IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively
enhance expression of antimicrobial peptides. J Exp Med.
203:2271–2279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Volpe E, Touzot M, Servant N,
Marloie-Provost MA, Hupé P, Barillot E and Soumelis V:
Multiparametric analysis of cytokine-driven human Th17
differentiation reveals a differential regulation of IL-17 and
IL-22 production. Blood. 114:3610–3614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye ZJ, Xu LL, Zhou Q, Cui A, Wang XJ, Zhai
K, Wang Z, Tong ZH and Shi HZ: Recruitment of IL-27-producing
CD4(+) T cells and effect of IL-27 on pleural mesothelial cells in
tuberculous pleurisy. Lung. 193:539–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trifari S, Kaplan CD, Tran EH, Crellin NK
and Spits H: Identification of a human helper T cell population
that has abundant production of interleukin 22 and is distinct from
T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 10:864–871. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duhen T, Geiger R, Jarrossay D,
Lanzavecchia A and Sallusto F: Production of interleukin 22 but not
interleukin 17 by a subset of human skin-homing memory T cells. Nat
Immunol. 10:857–863. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng Y, Danilenko DM, Valdez P, Kasman I,
Eastham-Anderson J, Wu J and Ouyang W: Interleukin-22, a T(H)17
cytokine, mediates IL-23-induced dermal inflammation and
acanthosis. Nature. 445:648–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nishihara M, Ogura H, Ueda N, Tsuruoka M,
Kitabayashi C, Tsuji F, Aono H, Ishihara K, Huseby E, Betz UA, et
al: IL-6-gp130-STAT3 in T cells directs the development of IL-17+
Th with a minimum effect on that of Treg in the steady state. Int
Immunol. 19:695–702. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moser B and Loetscher P: Lymphocyte
traffic control by chemokines. Nat Immunol. 2:123–128. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao C, Zhou Q, Li X, Li H, Meng T, Zhong
Y, Pu J, Zhu M, Xu Y, Gan L, et al: Differentiation and recruitment
of IL-22-producing helper T cells in lgA nephropathy. Am J Transl
Res. 8:3872–3882. 2016.PubMed/NCBI
|
|
25
|
Bu XN, Zhou Q, Zhang JC, Ye ZJ, Tong ZH
and Shi HZ: Recruitment and phenotypic characteristics of
interleukin 9-producing CD4+ T cells in malignant pleural effusion.
Lung. 191:385–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW,
Cai XY, Zhou J, Cheng YF, Fan J and Qiu SJ: High expression of
IL-17 and IL-17RE associate with poor prognosis of hepatocellular
carcinoma. J Exp Clin Cancer Res. 32:32013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Punt S, Langenhoff JM, Putter H, Fleuren
GJ, Gorter A and Jordanova ES: The correlations between IL-17 vs.
Th17 cells and cancer patient survival: A systematic review.
Oncoimmunology. 4:e9845472015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin-Orozco N, Muranski P, Chung Y, Yang
XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW and Dong C: T
helper 17 cells promote cytotoxic T cell activation in tumor
immunity. Immunity. 31:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kryczek I, Banerjee M, Cheng P, Vatan L,
Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et
al: Phenotype, distribution, generation, and functional and
clinical relevance of Th17 cells in the human tumor environments.
Blood. 114:1141–1149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia L and Wu C: The biology and functions
of Th22 cells. Adv Exp Med Biol. 841:209–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Niccolai E, Taddei A, Ricci F, Rolla S,
D'Elios MM, Benagiano M, Bechi P, Bencini L, Ringressi MN, Pini A,
et al: Intra-tumoral IFN-γ-producing Th22 cells correlate with TNM
staging and the worst outcomes in pancreatic cancer. Clin Sci
(Lond). 130:247–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Lullo G, Marcatti M, Heltai S, Brunetto
E, Tresoldi C, Bondanza A, Bonini C, Ponzoni M, Tonon G, Ciceri F,
et al: Th22 cells increase in poor prognosis multiple myeloma and
promote tumor cell growth and survival. Oncoimmunology.
4:e10054602015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang YH, Cao YF, Jiang ZY, Zhang S and
Gao F: Th22 cell accumulation is associated with colorectal cancer
development. World J Gastroenterol. 21:4216–4224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ling L, Zhao P, Yan G, Chen M, Zhang T,
Wang L and Jiang Y: The frequency of Th17 and Th22 cells in
patients with colorectal cancer at pre-operation and
post-operation. Immunol Invest. 44:56–69. 2015. View Article : Google Scholar : PubMed/NCBI
|