Introduction
Gallbladder carcinoma (GBC) is the fifth most common
neoplasm of the digestive tract and has an overall incidence of
3/100,000 (1). GBC is usually
diagnosed at an advanced stage due to a lack of specific symptoms
(1). According to epidemiological
investigation, it has been reported that the 5-year survival rate
for patients with GBC is <10% (1).
Despite advances in GBC diagnostic and therapeutic techniques and
management for consequent disease remission, prognosis for patients
with gallbladder cancer remains poor (2). It has been reported that numerous
post-surgery patients are likely to develop recurrence or even
metastasis following surgery (2).
Therefore, novel therapeutic strategies are required to enhance and
improve the response and survival rate of patients with GBC.
Ginseng is a long-living perennial plant that is
highly valued in herbal medicine. It has been used for over 2,000
years in various countries of East Asia, such as Korea, China,
Japan and Vietnam (3). Ginsenoside
Rg3, the most important biologically active compound of ginseng,
has been demonstrated to have antitumor effects against several
cancer cell lines, including breast cancer, non-small cell lung
cancer, melanoma cancer and leukemia (4–7). Zhang
et al (8) demonstrated that
20(S)-Rg3 inhibited proliferation and survival of GBC cells in
vitro and in vivo. However, the underlying molecular
mechanisms of Rg3 in human gallbladder cancer cell lines remain
unclear.
The endoplasmic reticulum (ER) serves a major role
in the synthesis, folding and the structural maturation of >33%
of the total proteins composed in the cell (9). The importance of the endoplasmic
reticulum (ER) in apoptotic processes has been recognized
previously (10). A range of
stressful cellular conditions, including aggregation of unfolded
and misfolded proteins in the lumen of the ER, may trigger ER
stress. During conditions of prolonged ER stress, pro-adaptive
responses have been reported to fail, resulting in apoptosis
(10). It has been demonstrated that
ER stress triggers different biochemical processes, including
leakage of calcium into the cytoplasm, leading to the activation of
death effectors (10), the unfolded
protein response (UPR), designating apoptosis (11) and reactive oxygen species (ROS)
production (12). A thorough
understanding of these responses may contribute to the development
of novel treatments for cancer. Several drugs that activate ER
stress have been approved for preclinical and clinical use,
including sorafenib, eeyarestatin, tanespimycin, radicicol and
MAL3-101 (13). ER stress has been
reported to trigger the activation of protein kinase R-like ER
kinase (PERK) and the subsequent phosphorylation of eukaryotic
translation-initiation factor 2α (eIF2α), promoting translation of
activating transcription factor 4 (ATF4) (14). In addition, ATF4 has been reported to
activate the expression of CCAAT/enhancer-binding protein
homologous protein (CHOP), as well as the growth arrest and DNA
damage inducible gene (GADD153) (14). Lipocalin 2 (Lcn2), also known as
neutrophil gelatinase-associated lipocalin, is a 25 kDa secretory
glycoprotein and a member of the lipocalin family. It has been
demonstrated that Lcn2 is a novel GADD153 target gene,
participating in ER stress-induced apoptosis (15).
In the present study, it was demonstrated that Rg3
induces gallbladder cancer apoptosis by activating the ER
stress-mediated apoptotic pathway in vitro and in
vivo.
Materials and methods
Reagents
Rg3 was obtained from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). An annexin V/fluorescein isothiocyanate
(FITC)/propidium iodide (PI)-phycoerythrin (PE) apoptosis detection
kit was purchased from Nanjing KeyGen Biotech. Co. Ltd. (Nanjing,
China). Anti-eIF2α was purchased from Affinity Biosciences
(Cincinnati, OH, USA), and the anti-rabbit secondary antibodies
(cat. no. A7016) were from Beyotime Institute of Biotechnology
(Haimen, China). ROS, glutathione peroxidase (GSH-PX) and total
superoxide dismutase (T-SOD) assay kits were purchased from Nanjing
Jiancheng Bioengineering Institute (Nanjing, China). SYBR Green PCR
Master mix was purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Lipofectamine® 2000 transfection
reagent was purchased from Thermo Fisher Scientific, Inc.
Cell culture and transfection
The human gallbladder cancer cell line GBC-SD was
purchased from Shanghai Xiang Biotech (Shanghai, China). The cells
were cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.) and incubated at 37°C
with 5% CO2. Transfection was performed using
Lipofectamine 2000 transfection reagent, according to the
manufacturer's protocols. GBC-SD cells were plated at a density of
5×105 cells/well in 1.5 ml medium and cultured for 24 h
prior to transfection. Control small interfering RNA (siRNA) (50
nM; siRNA-Scr) or targeted siRNAs (50 nM; eIF2α-siRNA and
ATF4-siRNA) were mixed with 2 µl Lipofectamine 2000 reagent and
incubated at room temperature for 20 min. The mixture was
subsequently added to the cells at 250 µl/well. The cells were
cultured for 6 h and the medium was subsequently replaced with
fresh RPMI-1640 medium supplemented with 10% fetal bovine
serum.
Cell Counting Kit-8 (CCK-8) assay
The anti-proliferative effect of Rg3 on GBC-SD cells
was detected using a CCK-8 assay (Dojindo Molecular Technologies,
Inc., Kumamoto, Japan). GBC-SD cells (5,000 cells/well) were seeded
into 96-well plates and cultured overnight. Subsequently, the cells
were treated with 1, 25, 50 and 100 µM Rg3, and DMSO (0.1%)
(Sigma-Aldrich; Merck KGaA) was added to the negative control (NC)
group at 37°C for 72 h. The medium was discarded and 10 µl CCK-8
solution was added to the wells. GBC-SD cells were then incubated
for another 4 h at 37°C with 5% CO2 and cell viability
was analyzed at a wavelength of 450 nm. The experiments were
performed in triplicate.
Western blotting
The cells or isolated tumor tissues (100 mg) were
lysed using radioimmunoprecipitation assay buffer lysis with
protease inhibitors (Sigma Aldrich; Merck KGaA). The protein
concentrations were determined using a bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). Equal amounts of total
protein (50 µg) were separated by SDS-PAGE (10% gel), transferred
onto a nitrocellulose membrane and blocked with 5% fat-free dried
milk in PBS for 2 h at room temperature. The membrane was
subsequently incubated at 4°C overnight with the following primary
antibodies: Anti-PERK (cat. no. AF5304; 1:1,000), anti-p-PERK (cat.
no. DF7576; 1:1,000), anti-eIF2α (cat. no. AF6087; 1:1,000),
anti-p-eIF2α (cat. no. AF3087; 1:1,000), anti-ATF4 (cat. no.
AF5416; 1:1,000), anti-Lcn2 (cat. no. DF6816; 1:1,000),
anti-β-actin (cat. no. AF7018; 1:1,000). Following washing three
times with Tris-buffered saline, membranes were incubated with
horseradish peroxidase-conjugated anti-rabbit secondary antibody
(cat. no. A7016; 1:5,000) (Beyotime Institute of Biotechnology) for
between 4 and 5 h at 4°C. The immunoreactive bands were visualized
using an enhanced chemiluminescence system (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and the signal was analyzed
using a Tanon-5200 imagine system (Tanon Science and Technology
Co., Ltd., Shanghai, China). All values were normalized to those of
β-actin.
Detection of the apoptotic rate by
flow cytometry
The apoptotic rate was detected using the annexin
V-FITC/PI-PE apoptosis detection kit. Following 100 µM Rg3 or/and
50 nM siRNA treatment for 72 h, GBC-SD cells were washed with
ice-cold PBS and dual-stained with 4 µl annexin V-FITC and 3 µl
PI-PE for 15 min, according to the manufacturer's protocols. Flow
cytometric analysis was performed using a FACSCalibur™ instrument
with Mac Pro CellQPro Dongle software (BD Biosciences, Franklin
Lakes, NJ, USA).
ROS detection assay
Following siRNA-Scr, Rg3 or/and siRNA treatment for
72 h, GBC-SD cells were incubated with RPMI-1640 medium containing
10 µM 2′,7′-dichlorofluorescein (DCF) diacetate for 1 h at 37°C,
following termination of the reaction with 0.25% trypsin and washed
twice with PBS. The intensity of DCF fluorescence was quantified
using a fluorescence analyzer (FLX800T; BioTek Instruments, Inc.,
Winooski, VT, USA) at a wavelength of 408 nm. The cells were
transfected with siRNA-Scr (50 nM) as negative control. The
experiments were performed in triplicate.
ELISA detection of GSH-PX and
superoxide dismutase (SOD)
Following Rg3 or/and siRNA treatment for 72 h,
GBC-SD cells were washed with PBS and harvested prior to GSH-PX and
SOD detection. The determination of cellular GSH-PX and SOD was
performed using GSH-PX (cat. no. A005) and SOD ELISA (cat. no.
A001-3) kits (Nanjing Jiancheng Bioengineering Institute),
according to the manufacturer's protocols. A microplate reader
(Thermo Fisher Scientific, Inc.) was used to determine the optical
density at 450 nm.
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The cells were treated with dimethylsulfoxide or 100
µM Rg3 for 72 h. Total RNA was subsequently extracted using
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. RNA was
reverse-transcribed into first-strand cDNA in the presence of a
poly(A) polymerase with an oligo(dT) adaptor. The cDNA template was
amplified by RT-qPCR using the SYBR Green PCR Master mix, according
to the manufacturer's protocol. Gene expression in each sample was
normalized to GAPDH expression. The primer sequences used were as
follows: GAPDH, forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′, reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′; long non-coding RNA-p21
(LincRNA-p21), forward, 5′-GGGTGGCTCACTCTTCTGGC-3′, reverse,
5′-TGGCCTTGCCCGGGCTTGTC-3′; metastasis-associated lung
adenocarcinoma transcript 1 (MALAT1), forward,
5′-TCTGCAGGGACTACAGCAAG-3′, reverse, 5′-TCACATTGGTGAATCCGTCT-3′;
glutathione transferase Alpha 7 pseudogene (GSTA7P), forward,
5′-ATGACCTATTTCACACTTAGC-3′, reverse, 5′-AGCATATACTTTGGAAAAC-3′;
long intergenic non-protein coding RNA 1093 (LINC01093), forward,
5′-AGTGAGCAATGCAATTCTGGGA-3′, reverse,
5′-CATCTTAAAACATGTTTATTTTTCCA-3′. RT-qPCRs were performed using the
ABI StepOne system with the StepOne™ software (version 2.0; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
PCR cycling procedures were as follows: 94°C for 5 min, and 40
cycles of 94°C for 30 sec, 57°C for 35 sec and 72°C for 40 sec and
72°C for 10 min. The relative expression fold change of mRNAs was
calculated using the 2−ΔΔCq method (16).
siRNA sequences synthesis
Using the tool from the Ambion website (http://www.thermofisher.com/cn/zh/home/brands/ambion.html/),
three siRNA sequences (sense strand and antisense strand) were
designed for the human eIF2α and ATF4 gene mRNAs with the following
sequences: eIF2α-siRNA1, sense, 5′-CAGCCUUACACUACUUCUATT-3′,
antisense, 5′-UAGAAGUAGUGUAAGGCUGTT-3′; eIF2α-siRNA2, sense,
5′-GGUAAUAGCUAGCACAGAUTT-3′, antisense,
5′-AUCUGUGCUAGCUAUUACCTT-3′; eIF2α-siRNA3, sense,
5′-CGGGUUAAUAAUGGAUACATT-3′, antisense 5′-UGUAUCCAUUAUUAACCCGTT-3′;
ATF4-siRNA1, sense, 5′-CAAGCTCCTTACACUACTTCG-3′, antisense,
5′-TACAAGCGAGTCCTAAAGGCTGTT-3′; ATF4-siRNA2, sense,
5′-AGGAGCAAAACAAGACAGCATTTT-3′, antisense,
5′-ATGCTGTCTTGTTTTGCTCCTTTT-3′; ATF4-siRNA3, sense,
5′-CTGGATCATGGTGGATAGACCTT-3′, antisense,
5′-CGTAACCAGCATTAACGCGTT-3′. The siRNA scrambled sequence (sense,
5′-CAGCTTACACUACAACtATT-3′; antisense, 5′-CAGTTACGUAATCGTT-3′) was
used as control. All single-stranded siRNAs were chemically
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
siRNAs, as aforementioned, at a concentration of 50 nM were
transfected into GBC-SD cells for 48 h using Lipofectamine 2000.
Western blot analysis was used to determine the transfection
efficiency.
Subcutaneous xenograft model
In the present study, 12 5-week-old BALB/c nude male
mice (18–20 g) were obtained from Cavens (Changzhou, China). The
study was performed in strict accordance with the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health. Animals were housed at a constant room temperature with a
12-h light/12-h dark cycle and fed on a standard rodent diet and
water. GBC-SD cells were harvested, washed and resuspended in PBS.
The animal tumor model was generated by subcutaneous injection with
0.2 ml cell suspension containing 5×106 cells into the
right flank of nude mice. Tumor sizes were measured every 3 days,
and tumor volumes were calculated using the formula: Tumor volume
(mm3)=0.5× length (mm)x width2
(mm2). When the tumor reached an average volume of 100
mm3, each mouse was administered orally with saline or
20 mg/kg Rg3 once daily for 21 days (n=6). The mice were sacrificed
at the end of the treatment period and the tumors were resected for
an immunoblotting assay. All animal procedures were approved by the
Ethics Committee of Zhejiang Chinese Medical University Animal
Center (Hangzhou, China; approval no. 201703345).
Statistical analysis
Data are expressed as the mean ± standard deviation
from ≥3 independent experiments. For in vitro experiments
and in vivo mouse experiments, a two-sided Student's t-test
was applied for comparison of continuous variables between two
groups, and statistical differences among multiple groups were
analyzed by one-way ANOVA followed by Dunnett's test (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Rg3 activates the ER stress-mediated
PERK pathway in the gallbladder cancer cell line
CCK-8 assay was used to detect the effect of Rg3 on
GBC-SD proliferation. As presented in Fig. 1A, Rg3 dose-dependently inhibited
GBC-SD proliferation in vitro. To further demonstrate the
effect of Rg3 on the ER stress-mediated cell apoptotic pathway,
GBC-SD cells were stimulated with 1, 25, 50 and 100 µM Rg3 for 72 h
and harvested for western blot analysis. The results indicated that
1 µM Rg3 did not affect the protein expression of eIF2α, p-eIF2α,
ATF4 and Lcn2. However, 50 µM and 100 µM Rg3 significantly
increased the protein expression of p-eIF2α, ATF4 and Lcn2, but not
of eIF2α (Fig. 1B-F). These results
suggested that a higher concentration of Rg3 promoted the UPR-PERK
signaling pathway in the gallbladder cancer cell line.
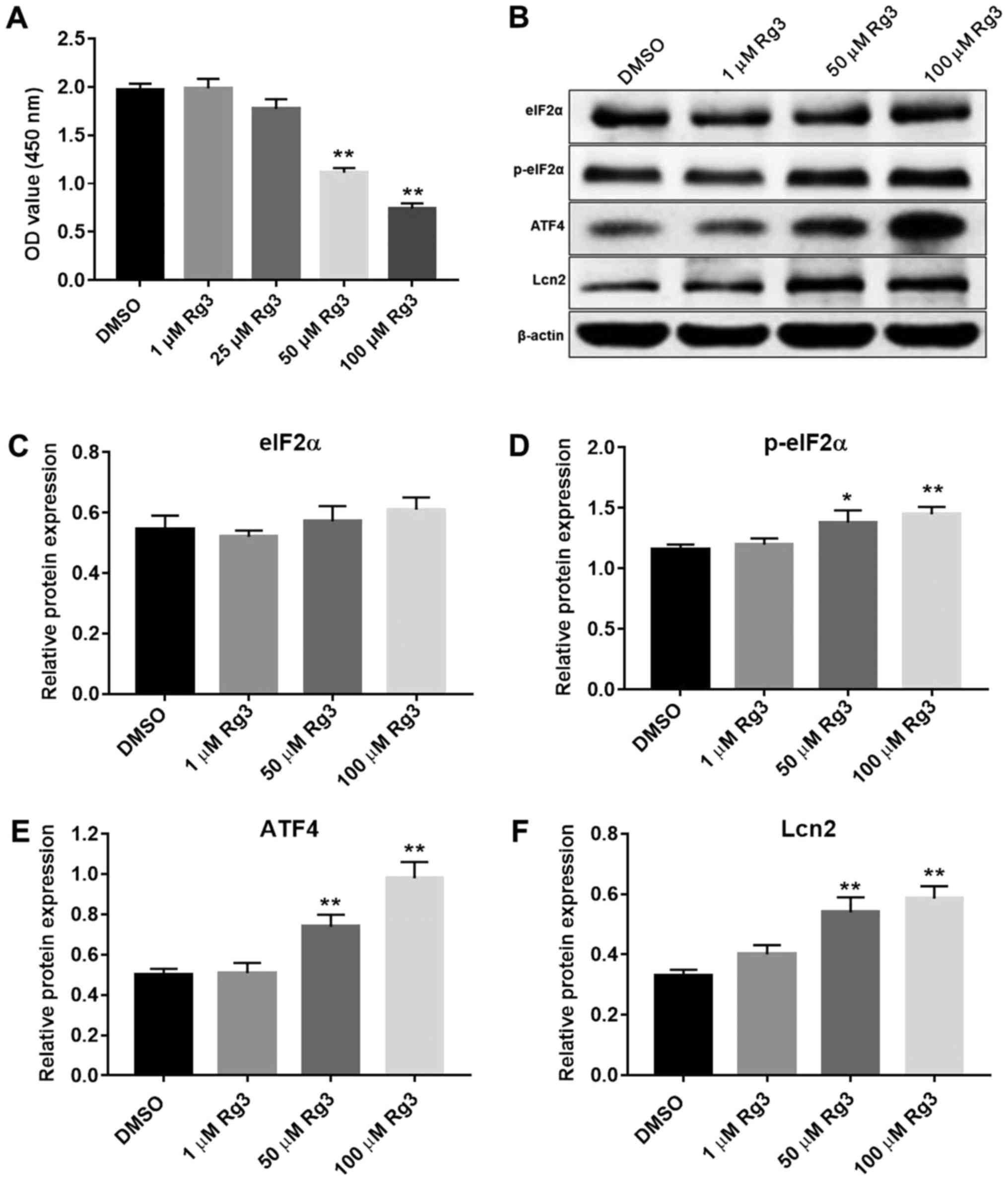 | Figure 1.Rg3 activates ER stress in GBC-SD
cells. (A) GBC-SD cells were treated with 1, 25, 50 and 100 µM Rg3
for 72 h, and proliferation was determined using a CCK-8 kit. (B)
Rg3 induced p-eIF2α, ATF4 and Lcn2 expression in GBC-SD cells.
Relative protein expression level of (C) eIF2α, (D) p-eIF2α, (E)
ATF4 and (F) Lcn2 compared with the DMSO group. Relative protein
expression was quantified by normalizing to the internal control
β-actin (n=3). *P<0.05, **P<0.01 vs. DMSO group. OD, optical
density; ER, endoplasmic reticulum; eIF2α, eukaryotic
translation-initiation factor 2α; ATF4, activating transcription
factor 4; p-, phospho-; DMSO, dimethylsulfoxide; Lcn2, lipocalin
2. |
eIF2α knockdown attenuates
Rg3-mediated gallbladder cancer cell apoptosis
To further detect the effect of Rg3 on the UPR-PERK
signaling pathway, an RNA interference-mediated method was used to
silence eIF2α expression to determine the biological effects in
GBC-SD cells. Western blot analysis revealed a decrease in eIF2α
protein expression levels in siRNA-transfected GBC-SD cells.
Compared with eIF2α-siRNA1 and eIF2α-siRNA2, eIF2α-siRNA3 resulted
in the most marked suppression of eIF2α protein expression, whereas
control siRNA vectors had no effect on eIF2α protein expression
(Fig. 2A and B). Therefore,
eIF2α-siRNA3 was selected to knock down eIF2α in subsequent
studies. As indicated in Fig. 2C and
D, eIF2α-siRNA3 significantly decreased the protein expression
levels of ATF4, Lcn2 and CHOP in the cells. In addition,
Rg3-induced ATF4, Lcn2 and CHOP upregulation was reversed by eIF2α
knockdown. These results indicated that Rg3 increased the
expression of ATF4, Lcn2 and CHOP, whereas eIF2α knockdown
attenuated the expression of these proteins in gallbladder cancer
cells.
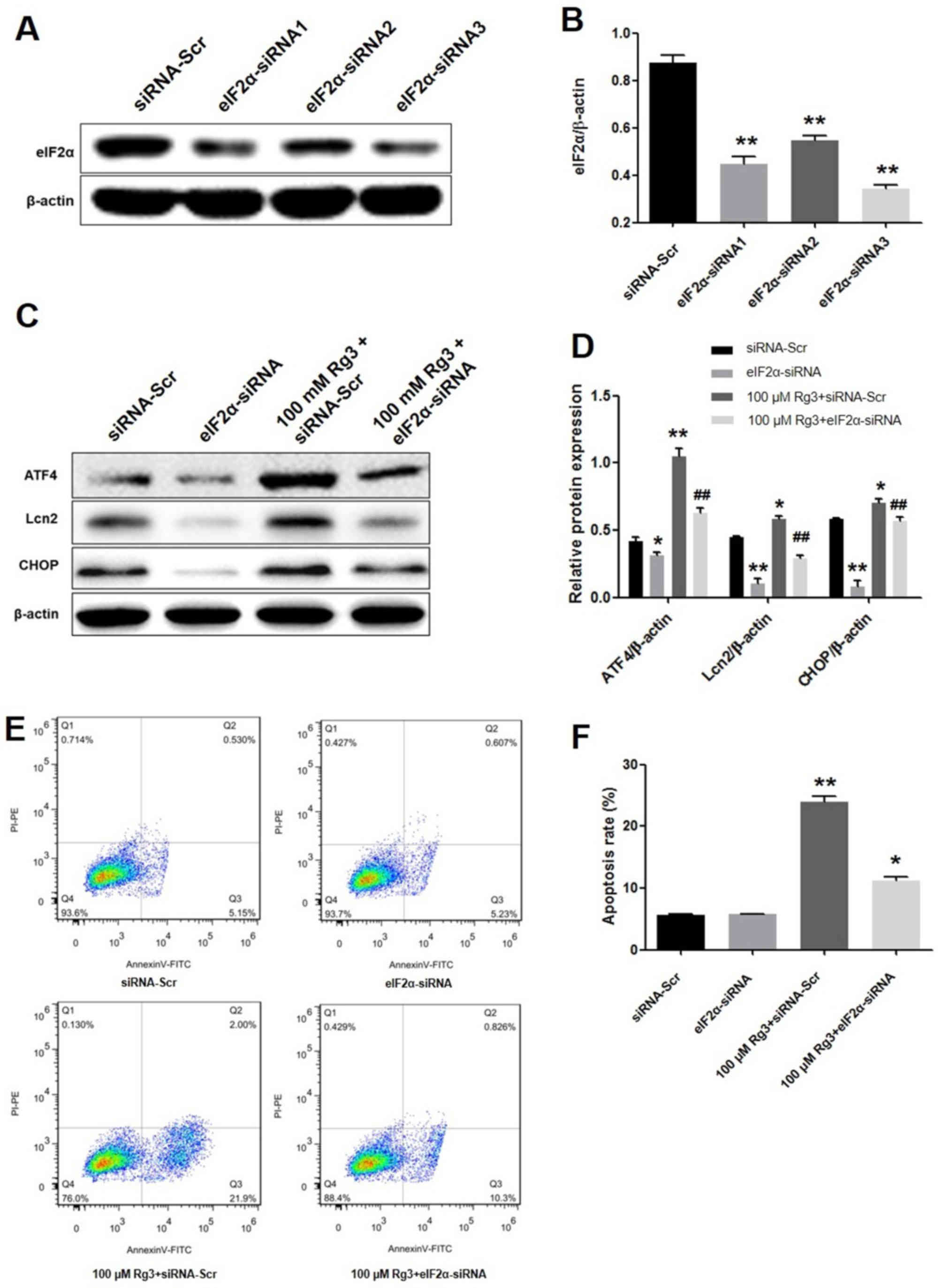 | Figure 2.eIF2α knockdown attenuates
Rg3-mediated gallbladder cancer cell apoptosis. (A) GBC-SD cells
were transfected with siRNA-Scr, eIF2α-siRNA1, eIF2α-siRNA2 and
eIF2α-siRNA3. eIF2α-siRNA3 resulted in the highest level of
suppression of eIF2α protein expression compared with eIF2α-siRNA1
and eIF2α-siRNA2, whereas siRNA-Scr vectors had no effect on the
levels of eIF2α protein expression. (B) Suppression of eIF2α
protein expression was significantly increased in the eIF2α-siRNA3
group. **P<0.01 vs. siRNA-Scr control group. Relative protein
expression levels were quantified by normalizing to the internal
control β-actin (n=3). (C) Effects of eIF2α-siRNA on the protein
levels of ATF4, Lcn2 and CHOP induced by Rg3. (D) Quantification of
the effects of eIF2α-siRNA induced by Rg3 on the relative protein
expression levels of ATF4, Lcn2 and CHOP by normalizing to the
internal control β-actin (n=3). *P<0.05, **P<0.01 vs.
siRNA-Scr group; ##P<0.01 vs. 100 µM Rg3 + siRNA-Scr
group. (E) The cells were treated with eIF2α-siRNA or/and 100 µM
Rg3 for 72 h and subsequently stained with annexin V and PI. (F)
The apoptotic rate of eIF2α-siRNA induced by Rg3, compared with
siRNA-Scr control group. *P<0.05, **P<0.01 vs. siRNA-Scr.
eIF2α, eukaryotic translation-initiation factor 2α; siRNA, small
interfering RNA; siRNA-Scr, scrambled siRNA; ATF4, activating
transcription factor 4; CHOP, CCAAT/enhancer-binding protein
homologous protein; DMSO, dimethylsulfoxide; Lcn2, lipocalin 2; PI,
propidium iodide; FITC, fluorescein isothiocyanate; PE,
phycoerythrin. |
Subsequently, the induction of GBC-SD cell apoptosis
by Rg3 was assessed. As indicated in Fig.
2E and F, 100 µM Rg3 significantly induced apoptosis (23.9%)
compared with the control (5.71%). However, the apoptotic rate
induced by Rg3 was decreased from 23.95 to 11.27% in the presence
of eIF2α-siRNA (P<0.01). The present data suggest that Rg3 may
induce gallbladder cancer cell apoptosis, which was significantly
reversed by eIF2α knockdown.
ATF4 knockdown attenuates Rg3-mediated
gallbladder cancer cell apoptosis
A total of three ATF4-siRNAs were constructed to
interfere with the expression of ATF4. Western blot analysis
revealed that ATF4-siRNA2 exhibited the highest inhibition of ATF4
expression compared with other vectors (Fig. 3A and B). Subsequently, GBC-SD cells
were transfected with ATF4-siRNA2 and the levels of PERK pathway
proteins were analyzed. As presented in Fig. 3C and D, Rg3-induced Lcn2 and CHOP
upregulation were reversed by ATF4 knockdown. Flow cytometric
analysis of the apoptosis assay indicated that ATF4 inhibition
significantly decreased apoptosis induced by Rg3 (P<0.01;
Fig. 3E and F).
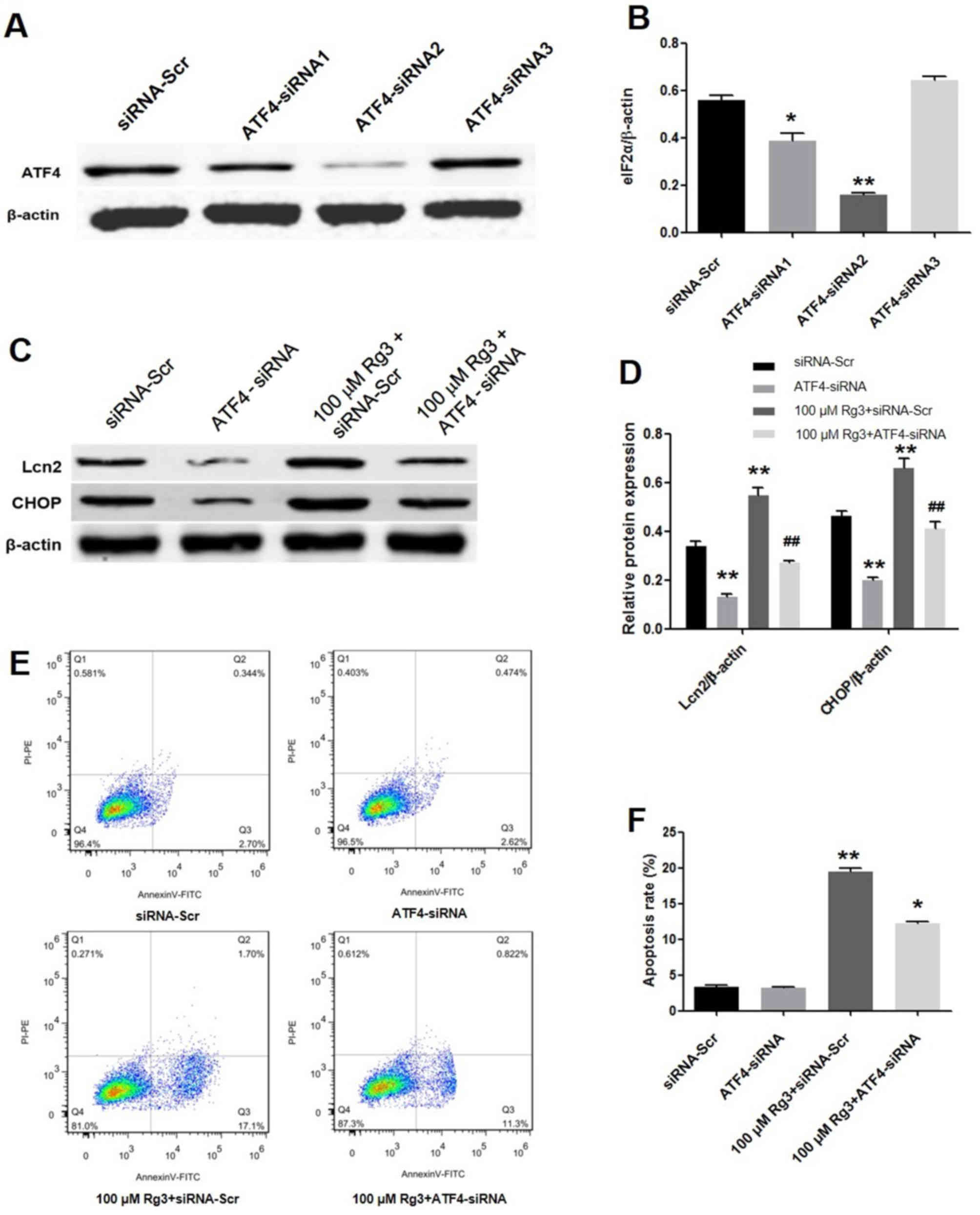 | Figure 3.ATF4 knockdown attenuates Rg3-mediated
gallbladder cancer cell apoptosis. (A) Western blot analysis of
GBC-SD cells transfected with siRNA-Scr control, ATF4-siRNA1,
ATF4-siRNA2 and ATF4-siRNA3. (B) Quantification of relative protein
expression levels by normalizing to the internal control β-actin
(n=3). *P<0.05, **P<0.01 vs. siRNA-Scr group. (C) Western
blot analysis of protein levels of cells treated with ATF4-siRNA2
or/and Rg3. (D) Quantification of the effects of ATF4-siRNA induced
by Rg3 on the relative protein expression levels of ATF4, Lcn2 and
CHOP by normalizing to the internal control β-actin (n=3).
**P<0.01 vs. siRNA-Scr group; ##P<0.01 vs. 100 µM
Rg3 + siRNA-Scr group. (E) Cells were treated with eIF2α-siRNA
or/and 100 µM Rg3 for 72 h and subsequently stained with annexin V
and PI. (F) The apoptotic rate of ATF4-siRNA induced by Rg3,
compared with siRNA-Scr group (n=3). *P<0.05, **P<0.01 vs.
siRNA-Scr. eIF2α, eukaryotic translation-initiation factor 2α;
siRNA, small interfering RNA; siRNA-Scr, scrambled siRNA; ATF4,
activating transcription factor 4; CHOP, CCAAT/enhancer-binding
protein homologous protein; DMSO, dimethylsulfoxide; Lcn2,
lipocalin 2; PI, propidium iodide; PE, phycoerythrin. |
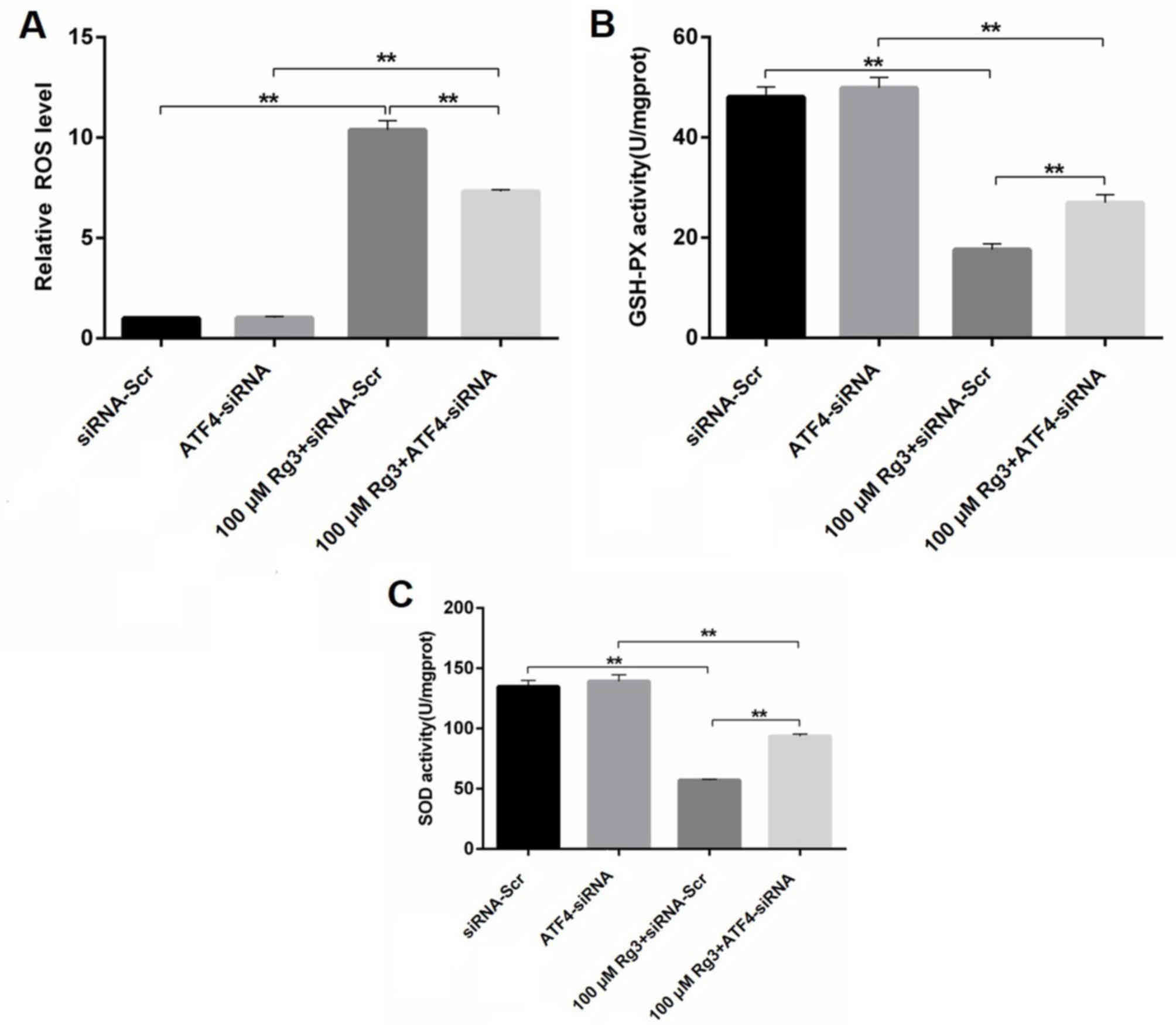
ATF4 knockdown attenuates Rg3-mediated
ROS generation in gallbladder cancer cells
ROS are small molecules that are highly reactive as
a result of the presence of unpaired electrons. An increase in the
protein-folding load in the ER can lead to the accumulation of ROS.
GSH-PX and SOD are the primary enzymes, which work synergistically
to neutralize ROS (17). In order to
further confirm the role of ER stress during Rg3-induced GBC-SD
apoptosis, the generation of ROS, GSH-PX and SOD were determined.
As presented in Fig. 4A, 100 µM Rg3
significantly increased the ROS level compared with the scrambled
control group, whereas ATF4 knockdown diminished this effect. The
ELISA data also indicated that the enzyme activities of GSH-PX and
SOD were significantly decreased by Rg3 treatment, which were
partly recovered in the presence of ATF4-siRNA (P<0.01; Fig. 4B and C). These results suggest that
Rg3 induced ER stress-mediated cell death due to increased ROS
generation, which was partly reversed by ATF4 knockdown.
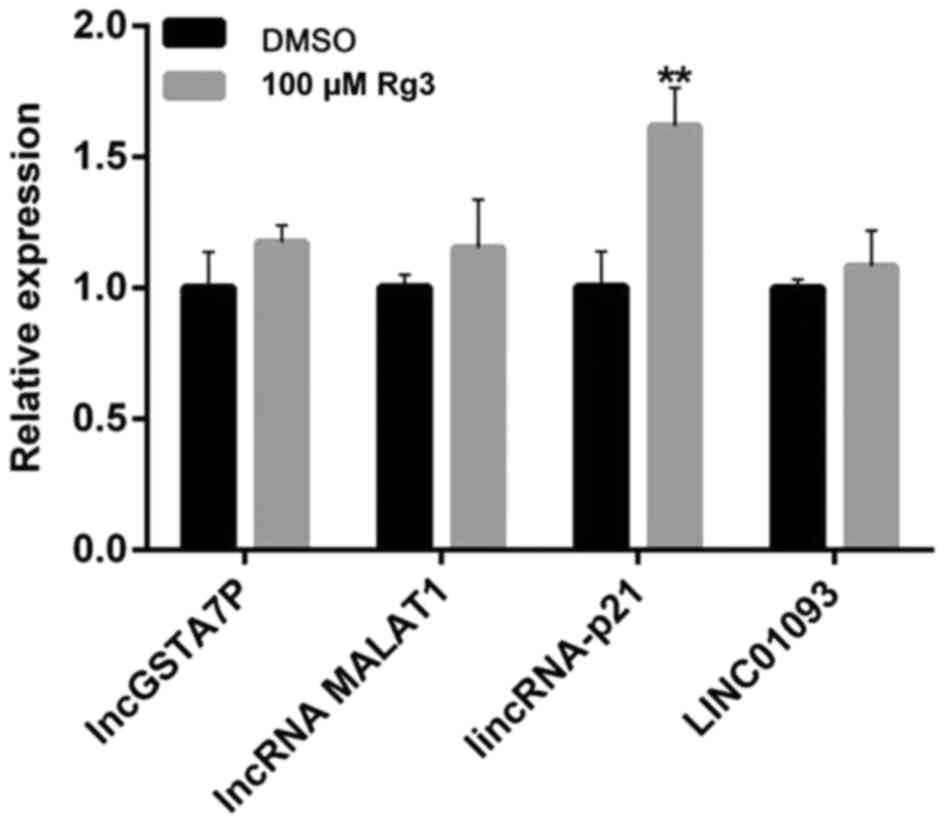
Effects of Rg3 on the expression of
long non-coding RNA (lncRNA) in gallbladder cancer cells
lncRNAs are transcribed RNA molecules of >200
nucleotides and with no significant protein-coding capacity
(18). Important research into the
role of lncRNAs in the diagnosis and prognosis of various types of
cancer, such as gallbladder carcinoma, is being conducted (18). In order to investigate the role of
lncRNA in the Rg3-induced ER stress activation, lincRNA-p21, lncRNA
MALAT1, lncGSTA7P and LINC01093 were evaluated. As presented in
Fig. 5, Rg3 significantly increased
the expression of lincRNA-p21 (P<0.01), but had no effect on the
other three lncRNAs. LincRNA-p21 is a downstream lncRNA transcript
of p53, which participates in diverse biological processes,
including apoptosis, cell cycle, metabolism and pluripotency.
LincRNA-p21 is implicated in the development and progression of a
number of human diseases, particularly cancer (19). The results of the present study
revealed that lincRNA-p21 was involved in Rg3-induced ER stress
activation.
Rg3 inhibits tumor growth through the
ER stress-mediated pathway in a xenograft model
To assess the antitumor effect of Rg3 on gallbladder
cancer in vivo, a GBC-SD subcutaneous xenograft model was
used. As presented in Fig. 6A and B,
tumor growth was significantly inhibited with Rg3 treatment from
day 12, compared with the vehicle group. In addition, western blot
analysis of tumor samples identified that the expression of p-PERK,
p-eIF2α, ATF4 and Lcn2 was increased by Rg3 treatment compared with
the vehicle group (Fig. 6C and D).
Furthermore, treatment with Rg3 did not reveal any adverse effect
on the body weight of the mice, in comparison with the vehicle
group, during the experimental period (data not shown), indicating
the safety of Rg3. The aforementioned data were consistent with the
in vitro study, which further demonstrated Rg3 induced
gallbladder cancer apoptosis through the ER stress pathway.
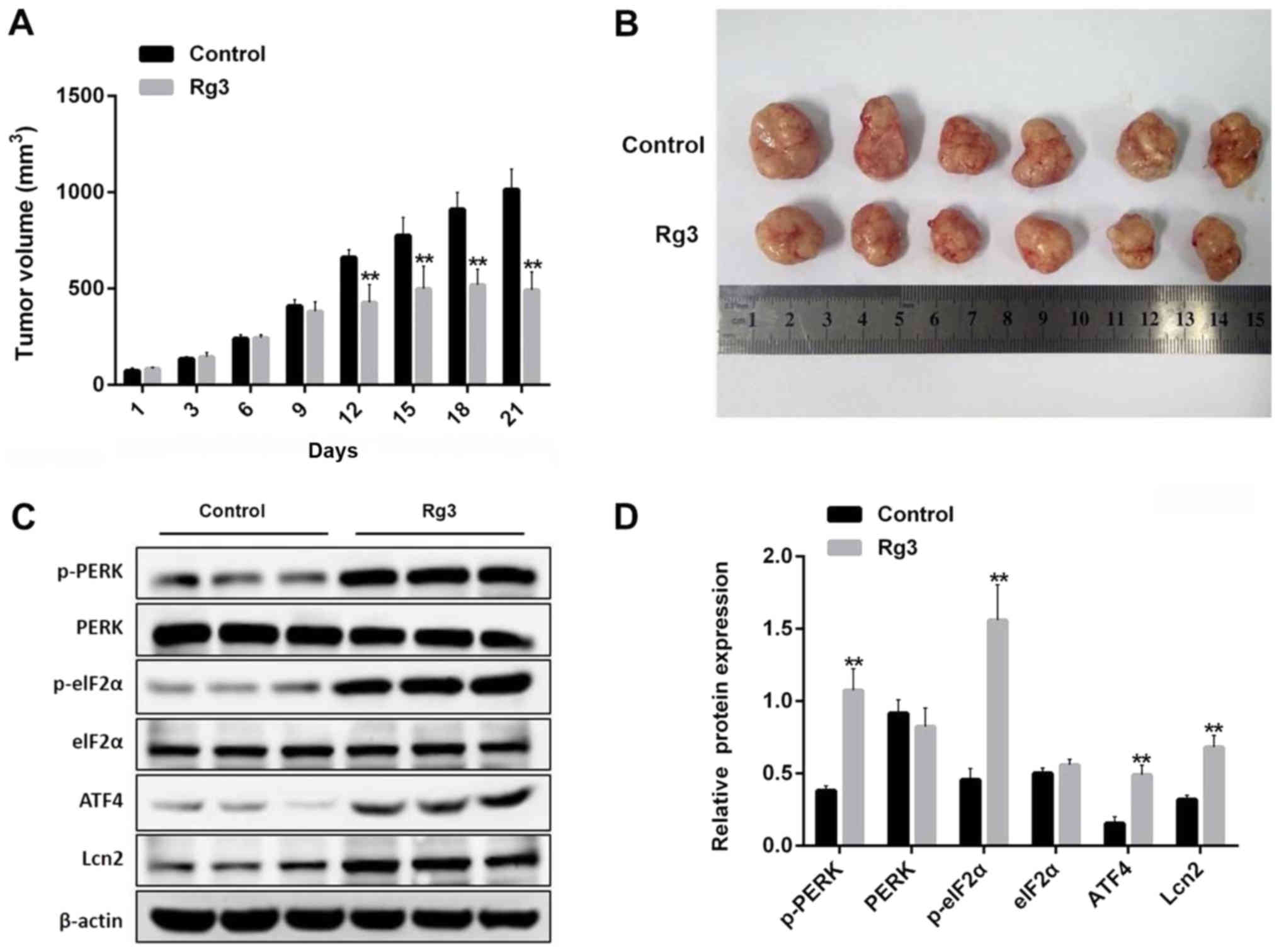 | Figure 6.Rg3 inhibits tumor growth through the
ER stress-mediated pathway in a xenograft model. (A) The GBC-SD
xenograft mice were treated once a day with 0.2 ml saline or 0.2 ml
Rg3 (20 mg/kg) over a period of 21 days and tumor volumes were
determined. **P<0.01 vs. control group. (B) The mice were
sacrificed on day 21 and the tumors were isolated and measured with
a caliper (n=6). (C) Tumor tissues from each group were processed
for the proteins p-PERK, PERK, p-eIF2α, eIF2α, ATF4 and Lcn2
detection. (D) Relative protein expression levels of p-PERK, PERK,
p-eIF2α, eIF2α, ATF4 and Lcn2 were quantified by normalizing to
internal control β-actin (n=3). **P<0.01 vs. control group.
ATF4, activating transcription factor 4; ER, endoplasmic reticulum;
PERK, eukaryotic translation initiation factor 2 α kinase 3; p-,
phospho-; Lcn2, lipocalin 2; eIF2α, eukaryotic
translation-initiation factor 2α. |
Discussion
Ginsenoside Rg3 is a bioactive ginseng constituent
that has been reported to inhibit proliferation of numerous cancer
cell lines; however, the underlying mechanism remains unclear
(5–7).
In the present study, it was demonstrated using flow cytometry
analysis with annexin V-FITC/PI staining that Rg3 treatment led to
significant GBC-SD human gallbladder cancer cell apoptosis. Zhang
et al (8) also revealed that
Rg3 induced a dose-dependent increase in GBC-SD cell apoptosis,
which is consistent with the results of the present study.
Furthermore, it was demonstrated that Rg3 inhibited tumor growth in
a GBC-SD xenograft nude mice model, in accordance with the results
of Zhang et al (8), who used
NOZ cells to construct the animal model.
At the molecular level, it was revealed that
pathological ER stress activation could be the key signaling
mechanism in gallbladder cancer cells. The hallmarks of ER stress,
p-eIF2α and ATF4, were upregulated in Rg3-treated GBC-SD cell lines
and xenograft mice models. Wang and colleagues indicated that Rg3
induced anti-gallbladder cancer cell activity in vitro and
in vivo was mediated by ER stress activation (8). The activation of CHOP, PERK and
inositol-requiring enzyme 1 (IRE1) were additionally involved. It
has been reported that, under ER stress, binding immunoglobulin
protein chaperone dissociates from the luminal domain of PERK,
which leads to the activation of three sensors, PERK, IRE1 and ATF6
(20). One of the mechanisms of ER
stress-induced apoptosis involves sequential steps of PERK-mediated
eIF2α phosphorylation, preferential translation of ATF4/cyclic
AMP-response element-binding protein 2 mRNA and induction of
CHOP/GADD153. In the present study, Rg3 was identified to inhibit
GBC-SD cell apoptosis through the PERK/p-eIF2α/ATF4/CHOP/Lcn2
signaling pathway in vitro and in vivo, which was
further validated by knockdown of eIF2α and ATF4 in GBC-SD cells.
Increased expression of ATF4 and CHOP results in increased protein
synthesis, which produces ROS, a necessary signal to induce
apoptosis in response to ER stress. Increased ROS expression was
also detected along with decreased GSH-PX and SOD activity. These
results indicated that Rg3-induced gallbladder cancer cell
apoptosis was mediated through ER stress signal pathway.
An important result of the present study is that
lincRNA-p21 was significantly increased by Rg3 treatment in GBC-SD
cells. lincRNA-p21 interacts with a number of RNA-binding proteins,
microRNAs and mRNA targets, and regulates the expression of the
targets (21). lincRNA-p21 has been
identified to be a novel regulator of proliferation, apoptosis and
DNA damage response (21). The
results of the present study revealed that p21 is involved in
Rg3-induced ER stress activation in gallbladder cancer cells.
Prognosis, preoperative diagnosis, surgical
management and systemic treatment of gallbladder cancer remains a
problem. In the present study, the traditional herbal medicine Rg3
was used to treat gallbladder cancer in vitro and in
vivo. The results revealed pathological ER stress activation
mediated the antitumor effect of Rg3 on gallbladder cancer in
vitro and in vivo, providing a novel strategy for
anticancer drug design and development based on Rg3.
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang
Provincial Natural Science Foundation of China (grant no.
LY17H290008; Hangzhou, China) and Zhejiang Medical and Health
Science and Technology Program (grant no. 2018KY558; Hangzhou,
China).
Availability of data and materials
All datasets used in this study are available from
the corresponding author upon reasonable request.
Authors' contributions
KW, JH, NL and TX performed experiments, analyzed
data and were the major contributors in writing the manuscript. WC
and ZY performed experiments. KW and JH collected tissues,
interpreted the patient data and reviewed the final version of the
manuscript.
Ethics approval and consent to
participate
All animal procedures were approved by the Ethics
Committee of The First Affiliated Hospital of Zhejiang Chinese
Medical University (Hangzhou, China; approval no. 201703345).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horgan AM, Amir E, Walter T and Knox JJ:
Adjuvant therapy in the treatment of biliary tract cancer: A
systematic review and meta-analysis. J Clin Oncol. 30:1934–1940.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
An YE, Ahn SC, Yang DC, Park SJ, Kim BY
and Baik MY: Chemical conversion of ginsenosides in puffed red
ginseng. LWT-Food Sci Technol. 44:370–374. 2011. View Article : Google Scholar
|
|
4
|
Wang CZ, Aung HH, Zhang B, Sun S, Li XL,
He H, Xie JT, He TC, Du W and Yuan CS: Chemopreventive effects of
heat-processed Panax quinquefolius root on human breast cancer
cells. Anticancer Res. 28:2545–2552. 2008.PubMed/NCBI
|
|
5
|
Wang L, Li X, Song YM, Wang B, Zhang FR,
Yang R, Wang HQ and Zhang GJ: Ginsenoside Rg3 sensitizes human
non-small cell lung cancer cells to γ-radiation by targeting the
nuclear factor-κB pathway. Mol Med Rep. 12:609–614. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SG, Kang YJ and Nam JO:
Anti-metastasis effects of ginsenoside Rg3 in B16F10 cells. J
Microbiol Biotechnol. 25:1–2006. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng D, Wang J, Kong P, Chang C and Li J
and Li J: Ginsenoside Rg3 inhibits HIF-1α and VEGF expression in
patient with acute leukemia via inhibiting the activation of
PI3K/Akt and ERK1/2 pathways. Int J Clin Exp Pathol. 7:2172–2178.
2014.PubMed/NCBI
|
|
8
|
Zhang F, Li M, Wu X, Hu Y, Cao Y, Wang X,
Xiang S, Li H, Jiang L, Tan Z, et al: 20(S)-ginsenoside Rg3
promotes senescence and apoptosis in gallbladder cancer cells via
the p53 pathway. Drug Des Devel Ther. 9:3969–3987. 2015.PubMed/NCBI
|
|
9
|
Anelli T and Sitia R: Protein quality
control in the early secretory pathway. EMBO J. 27:315–327. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malhotra JD and Kaufman RJ: ER stress and
its functional link to mitochondria: Role in cell survival and
death. Cold Spring Harb Perspect Biol. 3:a0044242011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han J, Back SH, Hur J, Lin YH,
Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M,
et al: ER-stress-induced transcriptional regulation increases
protein synthesis leading to cell death. Nat Cell Biol. 15:481–490.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu L, Wise DR, Diehl JA and Simon MC:
Hypoxic reactive oxygen species regulate the integrated stress
response and cell survival. J Biol Chem. 283:31153–31162. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hetz C, Chevet E and Harding HP: Targeting
the unfolded protein response in disease. Nat Rev Drug Discov.
12:703–719. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rozpedek W, Pytel D, Mucha B, Leszczynska
H, Diehl JA and Majsterek I: The role of the PERK/eIF2α/ATF4/CHOP
signaling pathway in tumor progression during endoplasmic reticulum
stress. Curr Mol Med. 16:533–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsin IL, Hsiao YC, Wu MF, Jan MS, Tang SC,
Lin YW, Hsu CP and Ko JL: Lipocalin 2, a new GADD153 target gene,
as an apoptosis inducer of endoplasmic reticulum stress in lung
cancer cells. Toxicol Appl Pharmacol. 263:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Albuquerque YM, Lima AL, Lins AK,
Magalhães M and Magalhães V: Quantitative real-time PCR (q-PCR) for
sputum smear diagnosis of pulmonary tuberculosis among people with
HIV/AIDS. Rev Inst Med Trop Sao Paulo. 56:139–142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwiecien S, Jasnos K, Magierowski M,
Sliwowski Z, Pajdo R, Brzozowski B, Mach T and Wojcik D: Lipid
peroxidation, reactive oxygen species and antioxidative factors in
the pathogenesis of gastric mucosal lesions and mechanism of
protection against oxidative stress-induced gastric injury. J
Physiol Pharmacol. 65:613–622. 2014.PubMed/NCBI
|
|
18
|
Tekcham DS and Tiwari PK: Non-coding RNAs
as emerging molecular targets of gallbladder cancer. Gene.
588:79–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang SS, Zheng BY and Xiong XD:
LincRNA-p21: Implications in human diseases. Int J Mol Sci.
16:18732–18740. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clarke HJ, Chambers JE, Liniker E and
Marciniak SJ: Endoplasmic reticulum stress in malignancy. Cancer
Cell. 25:563–5763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|