Introduction
Radiotherapy is the primary treatment approach for
patients with nasopharyngeal carcinoma (NPC) (1), during which at least a portion of the
thyroid gland is exposed to radiation. Previous studies reported
that the incidence of radiation-induced hypothyroidism (RIHT) was
significantly increased in radiotherapy-treated patients treated
with NPC, compared with those non-irradiated patients (9–53 and
3–8%, respectively) (2,3). The prognosis of patients with NPC is
primarily good, where the 5-year overall survival rate is ~70–80%
for stage I patients (4,5). Furthermore, a complication encountered
by patients with NPC is RIHT. Hypothyroidism may cause a range of
clinical symptoms, including chills, fatigue and hypomnesia.
Additionally, these patients may also experience hematological
changes, including hyperlipidemia, coagulation disorders and
abnormal levels of markers associated with cardiovascular diseases
(6). All of these changes increase
the risk of morbidity and mortality rates of cardiovascular
diseases (7), and notably affect the
quality of life (QoL) of the patients; therefore, RIHT has
attracted the attention of radiation oncologists and
endocrinologists. However, the association between dose-volume
parameters and the occurrence of hypothyroidism remains poorly
understood. Furthermore, follow-up procedures to ensure normal
thyroid function following radiotherapy have not been widely
adopted. To avoid potential confounding effects from the use of
surgery for the treatment of head and neck cancer types, patients
with NPC were selected to investigate the factors that affect RIHT
following intensity-modulated radiotherapy (IMRT), including
radiation dosage and volume. The present study aimed to identify an
effective way to reduce the incidence of RIHT in and improve the
QoL of patients with NPC.
Patients and methods
Patient selection
A total of 325 patients with primary NPC were
treated in The Department of Radiation Oncology of The Affiliated
Hospital of Xuzhou Medical University between May 2008 and December
2016 and 52 patients were enrolled in the present study. The
inclusion criteria were as follows: Pathologically confirmed NPC;
≥18 years old; no previous abnormalities or surgical history
involving the thyroid or the pituitary glands; treatment with
radical IMRT; no serious complications of the liver, kidneys or
heart; good compliance (periodic re-examination during the
follow-up period); complete clinical information; and no evidence
of distant metastasis or disease relapses. Exclusion criteria were
as follows: Prior radiotherapy in the head and neck areas; evidence
of malignant tumor types in other areas of the body; patients who
received immunotherapy or hormonotherapy concurrently; and patients
who are pregnant or lactating.
Based on the aforementioned criteria, 52 patients
were eligible for the present study, including 30 males and 22
females. The median age of the patients was 50 years (age range,
18–75 years, mean 49.9±12.9 years). According to the American Joint
Committee on Cancer staging system established in 2012 (8), 10 patients were in stage I–II, 24 in
stage III, and 18 in stage IVA or IVB (Table I).
 | Table I.Clinical features of the enrolled
patients. |
Table I.
Clinical features of the enrolled
patients.
| Clinical
features | Number of
cases | Percentage |
|---|
| Sex |
|
|
|
Male | 30 | 57.7 |
|
Female | 22 | 42.3 |
| Age (years) |
|
|
| <30
or >60 | 11 | 21.2 |
|
30–60 | 41 | 78.8 |
| Stage |
|
|
|
I–II | 10 | 19.2 |
|
III–IV | 42 | 80.8 |
| T stage |
|
|
|
T1–2 | 24 | 46.2 |
|
T3-4 | 28 | 53.8 |
| N stage |
|
|
|
N0–1 | 20 | 38.5 |
| N2 | 23 | 44.2 |
|
N3 | 9 | 17.3 |
| GTV simultaneous
integrated boost |
|
Yes | 45 | 86.5 |
| No | 7 | 13.5 |
| Dose
constraint |
|
|
|
Yes | 17 | 32.7 |
| No | 35 | 67.3 |
Radiotherapy
IMRT was delivered with Varian 23EX or UNIQUE
medical linear accelerator, and 6 MV X irradiation was
administered. The targeted regions were divided into high-risk and
low-risk regions. A low-risk region refers to a cervical lymphatic
drainage area without metastasis. The radiation dose used was
1.8–2.0 Gy/fx28f, 5f/w, amounting to 50.4–56 Gy in total. A
high-risk region refers to the entire nasopharynx, retropharyngeal
lymph nodes, clivus, cranial base, parapharyngeal space,
pterygopalatine fossa, sphenoid sinus, nasal cavity, the posterior
third of the maxillary sinus and cervical lymphatic drainage areas
with metastasis. The radiation dose used was 1.8–2.0 Gy/fx33f,
5f/w, amounting to 59.4–66 Gy in total. A simultaneously integrated
boost was delivered to the primary tumor and positive lymph nodes
were treated with a dose of 2.12–2.14 Gy/fx33f, 5f/w, and
69.96–70.62 Gy in total. Additionally, the radiation dose to the
nasopharynx of patients with locally advanced NPC ranged from 71.3
to 74.9 Gy in total.
Measurement of thyroid function and
the diagnostic criteria of hypothyroidism
Morning fasting plasma was collected to determine
the levels of free triiodothyronine (FT3), free
tetraiodothyronine (FT4) and thyroid stimulating hormone
(TSH). The reference ranges were: FT3, 2.8–7.1 pmol/l;
FT4, 12–22 pmol/l; and TSH, 0.27–4.2 mIU/l. TSH and
FT4 were analyzed to distinguish central hypothyroidism
from primary hypothyroidism. Additionally, high TSH levels in the
presence of normal FT4 levels was referred to
subclinical hypothyroidism, high TSH levels in the presence of low
FT4 levels indicates the clinical subtype and low TSH
levels is indicative of central hypothyroidism.
Dose-volume parameters of the thyroid
and pituitary glands
The treatment plans for all patients were analyzed
retrospectively. In 17 cases, different degrees of dose constraints
were applied to the thyroid gland. Based on the computed topography
(CT)-based simulation images obtained prior to and during
radiotherapy, the thyroid and pituitary glands were delineated by a
senior radiation oncologist who were blind to the study conditions.
The relevant parameters, including mean dose (Dmean), maximum dose
(Dmax), minimum dose and V5, V10, V20, V30, V40, V50, V60 and V70
(percentage of organ receiving at least 5, 10, 20, 30, 40, 50, 60
and 70 Gy) were determined from dose-volume histograms.
Follow-up
Follow-up was initiated 3 months after the
completion of radiotherapy. The frequency of follow-up visits was
once every three months during the first 2 years after the
completion of radiotherapy, and once every 6 months for the next 3
years, followed by once a year in the following period. The
follow-up period was stopped when hypothyroidism occurred or no
hypothyroidism occurred until the end of the follow-up. During the
follow-up, peripheral FT3, FT4 and TSH levels
were evaluated in addition to the regular magnetic resonance
imaging/CT scan of the nasopharynx, chest CT scan, abdominal
ultrasonography and routine blood test. The interval time of
hypothyroidism was defined as the period between the end of
radiotherapy and the initial occurrence of hematologic
abnormality.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA) was used for all
statistical analyses. χ2 test was used to analyze
enumeration data, while independent or paired Student's t-tests
were applied for measurement data. The receiver operating
characteristic (ROC) curves were used to determine the possible
dose-volume threshold value. Kaplan-Meier survival curves were used
to evaluate the cumulative incidence of RIHT and the log rank test
was used to compare the survival curves. P<0.05 (two-sided) was
considered to indicate a statistically significant difference.
Results
Association between general clinical
characteristics and RIHT
The median follow-up period was 17 months (range,
3–95 months). A total of 52 patients with NPC were divided into the
euthyroid (24 patients) and hypothyroid groups (28 patients). In
the hypothyroid group, 3 cases (10.7%) had central hypothyroidism,
whereas the rest (89.3%) had primary hypothyroidism (14 as clinical
subtype and 11 as subclinical subtype). Additionally, 2 female
patients with clinical hypothyroidism experienced a concurrent
decrease in FT3 and FT4 levels, whereas the
TSH levels (56 and >100 mIU/l, respectively) were notably
elevated.
The incidence of RIHT was 72.7% (16/22) in female
patients and 40% (12/30) in male patients, and there was a
significant different between them (P=0.010). The majority of
female cases belonged to the clinical hypothyroidism subtype
(85.7%), while the majority of male patients belonged to the
subclinical subtype (81.8%). Although the difference in the median
age between female and male patients was not statistically
significant (48 years vs. 52 years; P=0.092), the age distribution
of the euthyroid and hypothyroid cohorts was distinct. A total of 9
patients in the hypothyroid group were <30 or >60 years
(32.1%), whereas only 2 patients in the euthyroid group (8.3%) were
these age groups, demonstrating a statistically significant
difference (P=0.036); however, other characteristics, including
clinical staging, T staging and N staging, were not significantly
associated with RIHT (Table II).
 | Table II.The clinical characteristics of the
euthyroid and hypothyroid groups. |
Table II.
The clinical characteristics of the
euthyroid and hypothyroid groups.
| Clinical
characteristics | Euthyroid
group | Hypothyroid
group | P-value |
|---|
| Case number | 24 | 28 |
|
| Sex |
|
| 0.010a |
|
Male | 18 | 12 |
|
|
Female | 6 | 16 |
|
| Age (years) |
|
|
|
|
Median | 52 | 48 | 0.092 |
|
Range | 35–69 | 18–75 |
|
| <30
or >60 | 2 | 9 | 0.036a |
|
30–60 | 22 | 19 |
|
| Clinical stage |
|
| 0.579 |
| Phase
I–II | 3 | 7 |
|
| Phase
III–IV | 21 | 21 |
|
| T stage |
|
| 0.500 |
|
T1–2 | 9 | 15 |
|
|
T3-4 | 15 | 13 |
|
| N stage |
|
| 0.410 |
|
N0–1 | 9 | 11 |
|
| N2 | 13 | 10 |
|
|
N3 | 2 | 7 |
|
Thyroid gland
The Dmean of euthyroid and hypothyroid groups were
4,834.9±676.1 and 5,326.3±718.4 cGy, respectively, and indicated
significant differences (P=0.017). The disparity in V50 of the two
groups (46.6±30.5 vs. 66.4±30.2%, respectively) was also
statistically significant (P=0.023), whereas no statistical
significance was observed for Dmax between the two groups
(6,176.9±571.7 vs. 6,490.5±569.4 cGy, respectively; P=0.060).
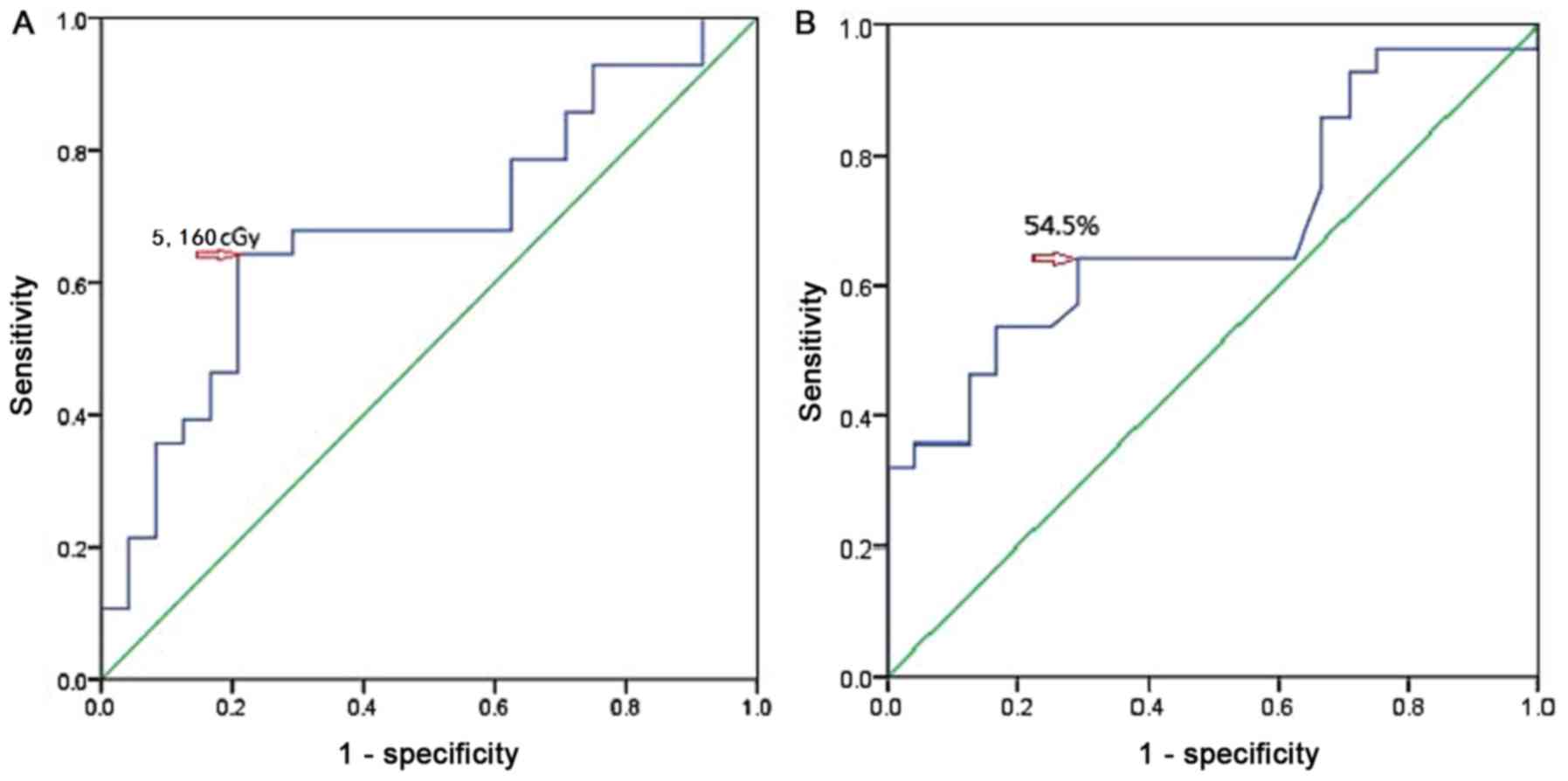
Analysis of the ROC curves indicated that the threshold value was
5,160 cGy (P=0.024) for the Dmean and 54.5% (P=0.007) for the V50
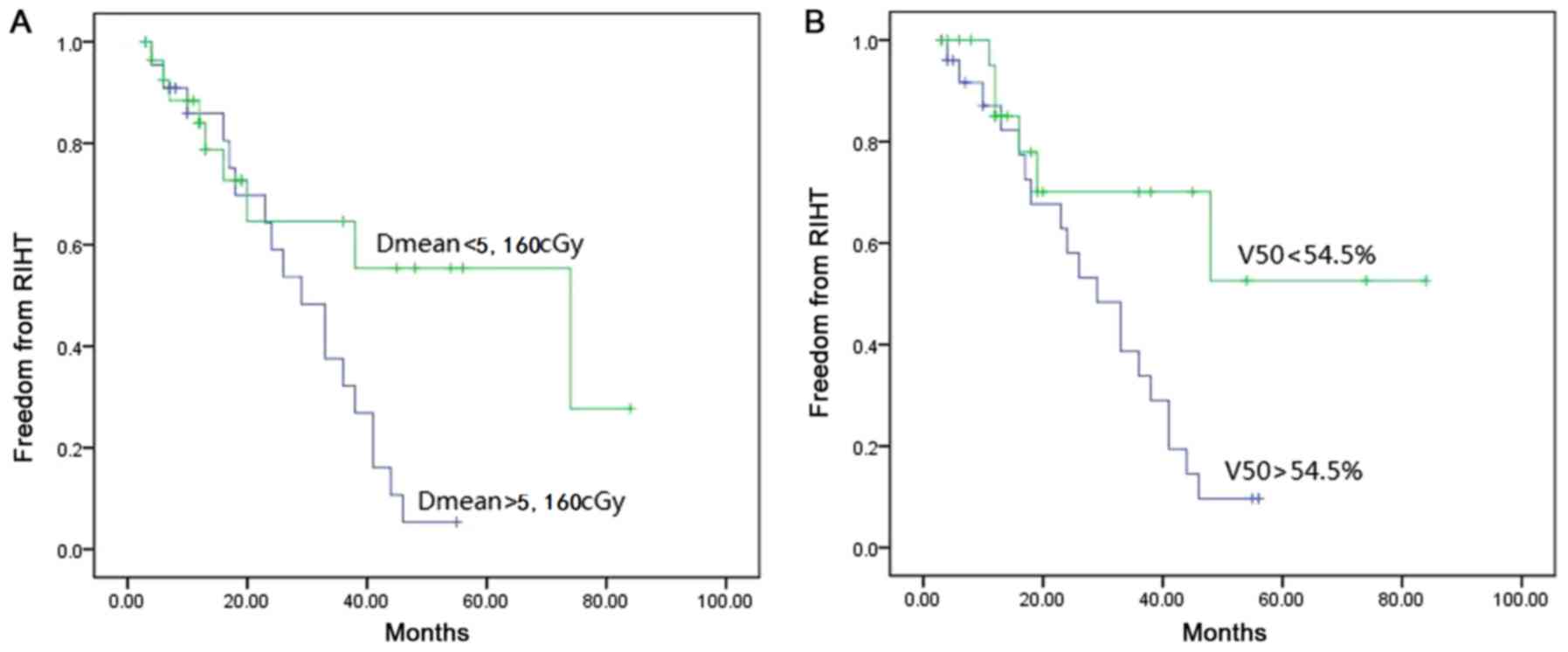
(Fig. 1). Kaplan-Meier survival
analysis demonstrated that the 3-year cumulative incidence of RIHT
was 67.8% when the Dmean was >5,160 cGy, which was significantly
increased, compared with the cohort with Dmean <5,160 cGy (log
rank test, P=0.036). Furthermore, the 3-year cumulative incidence
of RIHT in patients with V50 >54.5% was also significantly
increased, compared with patients with V50 <54.5% (66.1 vs.
29.9%, respectively; log rank test, P=0.025; Fig. 2). Regarding the thyroid volume,
comparison of CT localization images indicated a significantly
reduced thyroid volume (15.8±6.8 cm3 prior to treatment
vs. 14.7±6.6 cm3 during treatment; P=0.002).
Furthermore, subgroup evaluation demonstrated that the thyroid
volume in the euthyroid group was significantly increased, compared
with the hypothyroid group (17.85±5.89 cm3 vs. 13.9±7.48
cm3, respectively; P=0.048). Of the 52 patients, 45
(86.5%) patients had a simultaneously integrated boost of the gross
tumor volume (GTV), but this treatment was not associated with the
occurrence of RIHT (P=0.670); however, dose constraints were
applied in 17 patients (32.7%), which significantly affected the
incidence of RIHT (P=0.034; Table
III).
 | Table III.Thyroid-associated dose-volume
parameters of the euthyroid and hypothyroid groups. |
Table III.
Thyroid-associated dose-volume
parameters of the euthyroid and hypothyroid groups.
| Variable | Euthyroid
group | Hypothyroid
group | P-value |
|---|
| Dmean (cGy) | 4,834.9±676.1 | 5,326.3±718.4 | 0.017a |
| Dmax (cGy) | 6,176.9±571.7 | 6,490.5±569.4 | 0.060 |
| V50 (%) | 46.6±30.5 | 66.4±30.2 | 0.023a |
| Original volume of
thyroid gland (cm3) | 17.9±5.9 | 13.9±7.5 | 0.048a |
| GTV boost |
|
| 0.670 |
|
Yes | 20 | 25 |
|
| No | 4 | 3 |
|
| Dose
constraint |
|
| 0.034a |
|
Yes | 11 | 6 |
|
| No | 13 | 22 |
|
Pituitary
The hypothalamus-pituitary-thyroid axis along with
thyroid damage may impact the incidence of RIHT; therefore, in the
present study, the pituitary-associated dose-volume parameters were
analyzed. The present results demonstrated that the Dmean, V30,
V40, V50 and V55 of the pituitary gland indicated no significant
differences between the euthyroid and hypothyroid groups
(P>0.05); however, the exposure dose in the hypothyroid group
was notably increased, compared with the euthyroid group (Table IV).
 | Table IV.Pituitary-associated dose-volume
parameters of the euthyroid and hypothyroid groups. |
Table IV.
Pituitary-associated dose-volume
parameters of the euthyroid and hypothyroid groups.
| Variable | Euthyroid
group | Hypothyroid
group | P-value |
|---|
| Dmean (cGy) |
4,117.9±1,779.5 |
4,150.1±1,726.5 | 0.949 |
| V30 (%) | 75.7±37.9 | 76.5±39.7 | 0.949 |
| V40 (%) | 57.4±40.4 | 67.1±42.2 | 0.417 |
| V50 (%) | 34.4±40.1 | 39.6±41.3 | 0.650 |
| V55 (%) | 21.5±34.9 | 25.3±37.2 | 0.717 |
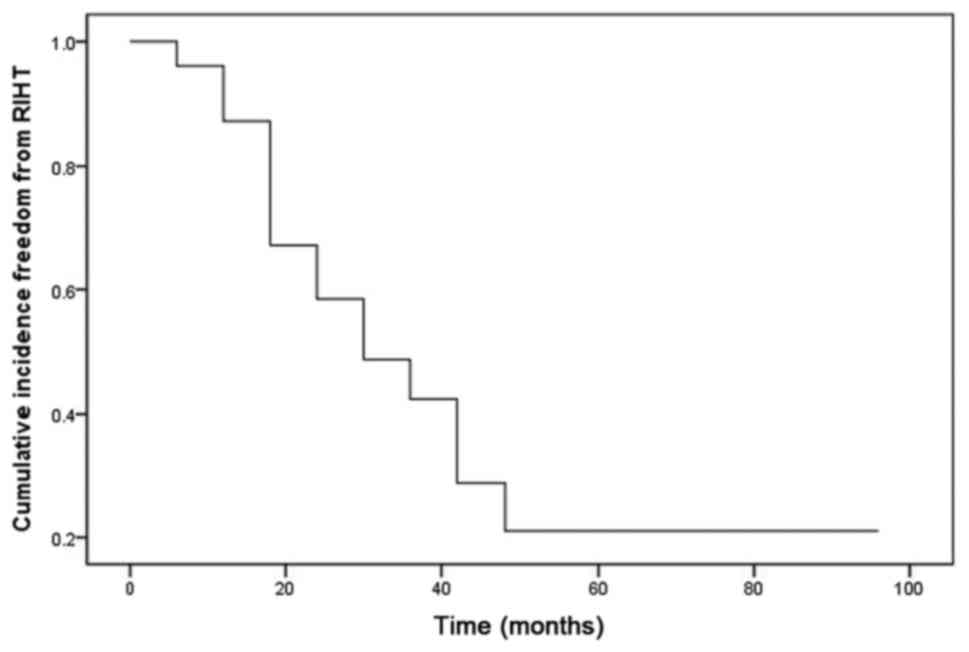
Cumulative incidence of RIHT
The incidence of RIHT was increased in a
time-dependent manner post treatment. The results demonstrated
cumulative incidence of 13, 33, 51, 71 and 79%, respectively, at 6
months, 1, 2, 3 and 4 years (Fig.
3).
Discussion
The incidence of RIHT occurs in numerous cancer
types, as demonstrated by a number of studies. Zohar et al
(9) reported that thyroid dysfunction
following radiotherapy occurred in 3% of patients with head and
neck malignancy types, whereas Hancock et al (10) reported that the proportion of thyroid
dysfunction was notable increased in the group who had received
more than 30 Gy to the thyroid (44%) compared with those who had
not undergone irradiation (2%) in patients with Hodgkin lymphoma.
Recently, the radiation dose to the target region has been notably
increased due to the usage of IMRT and intensity-modulated arc
therapy (11); however, the key issue
is the amount of radiation exposure to the organs at risk. RIHT is
one of the most common complications observed in patients with head
and neck neoplasms that underwent radiotherapy, which may occur due
to insufficient dose constraint during the treatment as well as
lack of follow-up care to ensure thorough thyroid function
following treatment.
The median follow-up time to RIHT was 16.8–21.6
months from the end of radiotherapy (range, 3.6–86.4 months)
(12,13). The median follow-up time for the
present study was 17 months (range, 3–95 months), and the incidence
of RIHT was 53.8%, and these results were consistent with the data
of other previous studies (12,13). In
the present study, female patients were more susceptible to RIHT
compared with males (72.7 vs. 40.0%; P=0.010), particularly for
those in the clinical subtype (85.7%). Furthermore, 2 female
patients who exhibited clinical hypothyroidism experienced
concurrent reduction of FT3 and FT4 levels
accompanied by notably increased TSH levels, indicating severe
thyroid dysfunction. The sex difference could be explained by the
smaller volume of the female thyroid glands, compared with male
thyroid glands. Previous studies have demonstrated that thyroid
gland size, particularly small thyroid glands, is a risk factor of
RIHT (14,15). Although the present study indicated no
significant difference in the thyroid volume between males and
females (16.25±5.7 and 15.09±8.85 cm3, respectively;
P=0.576), the thyroid volume prior to treatment was significantly
associated with the incidence of RIHT (P=0.048), which demonstrated
that patients with smaller thyroid glands have an increased
probability to experience hypothyroidism; therefore, female
patients who underwent radiation therapy may benefit from the extra
measures to protect their thyroid gland from radiation exposure.
Additionally, Lin et al (16)
determined that thyroid volume was notably decreased within 6
months after radiotherapy. This reduction in thyroid gland size was
also observed in the present study during treatment and indicated
that thyroid damage may occur in the early stages of
radiotherapy.
Age is an associated factor for the occurrence of
hypothyroidism (17,18). Colevas et al (19) and Wu et al (20) reported that the age of the patients
was associated with an increased risk for RIHT, particularly
patients <30 and >60 years. Murthy et al (21) also determined that young people were
more susceptible to hypothyroidism. Consistent with this, the
present study results demonstrated that patients aged >60 and
<30 years have an increased risk of hypothyroidism (P=0.036);
therefore, dose limitations to the thyroid gland should be
carefully applied in younger (aged <30 years) and older (aged
>60 years) patients with NPC who receive radiotherapy.
T stage is another risk factor associated with
hypothyroidism. Wu et al (20)
determined that patients in stages T1–2 have a
significantly increased probability to experience RIHT, compared
with patients in stages T3–4 (P=0.044). Furthermore, the
statistical analysis of clinical stage, T stage, N stage and GTV
boost did not determine any significant differences. In 52
patients, 10 cases were in stage I and II and RIHT occurred in
seven of them. Retrospective analysis of the treatment plan for
these 10 patients demonstrated that the lower bound of planning
target volume (PTV) reached the cricothyroid membrane in 3 cases,
and ~2 cm above the sternoclavicular joint in the rest of the
cases; therefore, a wide target region could contribute to the
development of RIHT. In contrast, GTV boost demonstrated less
impact on hypothyroidism, which may be explained by the distance
between the thyroid gland and the boost area.
IMRT is considered as a primary radiotherapy
approach and offers distinct dosimetric advantages, compared with
conventional and three-dimensional conformal radiotherapy (3D-CRT).
Diaz et al (18) demonstrated
that dosing limitations to the thyroid gland during IMRT
significantly reduced the Dmean, V30, V40 and V50 (P<0.005). In
the present study, dose constraint was conducted to various
degrees, but was conducive in the prevention of hypothyroidism
(P=0.034); therefore, dose constraint to the thyroid gland should
be applied in patients with NPC who are subjected to
radiotherapy.
Numerous studies have reported that RIHT is
associated with the dose-volume parameters of the thyroid gland
(10,22,23);
however, the nature of this association remains ambiguous. A
Hodgkin lymphoma study by Cella et al (24) reported that V30 was an independent
predictor for hypothyroidism. When V30 was >62.5%, the
occurrence of hypothyroidism was significantly increased, compared
with when V30 was <62.5% (11.5% vs. 70.8%; P<0.0001).
Similarly, studies on head and neck malignancy types conducted by
Kim et al (25) and Sachdev
et al (26) reported that V45
and V50 were independent predictors, the threshold values of which
were 50 and 60%, respectively. The present results demonstrated
that the threshold value of V50 was 54.5% according to the ROC
curve analysis. The 3-year cumulative incidence for patients with
V50 >54.5% was >2-fold increased, compared with the patients
with V50 <54.5% (66.1% vs. 29.9%; P=0.025), which was consistent
with the studies by Kim et al (25) and Sachdev et al (26); however, the threshold value may vary
in different diseases and radiation doses. For instance, the
prescribed dose for Hodgkin lymphoma ranged from 30–36 Gy, whereas
the recommended dose for head and neck tumor types was 54–70 Gy;
therefore, the volume parameters reported by Cella et al
(24) was notably reduced. The Dmean
has also been reported as an independent predictor for RIHT
(27,28); however, the associated threshold value
has rarely been studied. Fujiwara et al (29) estimated the threshold value as 30 Gy,
based on the incidence of RIHT being significantly reduced in
patients with Dmean <30 Gy compared with the other groups
(P<0.05). The present ROC curve analysis demonstrated that 5,160
cGy was a predictive threshold value, and the increased 3-year
cumulative incidence of RIHT in the Dmean >5,160 cGy cohort
confirmed this data (67.8% for Dmean >5,160 cGy and 44.6% for
Dmean <5,160 cGy; P=0.036). Notably, the threshold value in the
present study was >30 Gy reported by Fujiwara et al
(29). Possible explanations are as
follows: i) The dose in the target region was 54–60 Gy, and the
prescribed dose was 59.4–66 Gy for PTV and 70–74.9 Gy for GTV
boost, whereas the total dose in the Fujiwara et al
(29) study was 60–66 Gy; and ii) The
difference in the tumor types (NPC vs. head and neck malignancy
types) and physicians may result in variations in the target area
delineations. In the present study, 30.6% (range, 1–88.2%) of
thyroid tissues were exposed to radiation, resulting in the
exposure of increased radiation doses to the thyroid gland. In
clinical practice, the threshold value not only can be applied as a
reference to evaluate the risk of RIHT, but could also serve as an
indicator to take preventive care measures in high-risk patients as
early as possible.
The thyroid gland is more susceptible to secondary
injuries during radiotherapy in patients with NPC due to a large
target area, including the proximity of the hypothalamus and
pituitary gland. Huang et al (30) demonstrated that the increase of Dmean
(P=0.009) and V55 (P=0.014) of the pituitary gland was
significantly associated with the increased TSH levels. The present
study did not determine an association between the pituitary
radiation dose and RIHT; however, the Dmean, V30, V40, V50 and V55
of the pituitary gland were increased in the hypothyroid group,
compared with the euthyroid group. This may be due to the thyroid
and pituitary gland concurrently influencing the thyroid function,
and hence it is necessary to limit the radiation dose to the
pituitary gland.
To conclude, the present study indicated that the
incidence of RIHT in patients with NPC was associated with sex and
age, as well as the Dmean and V50 of the thyroid gland. The
original gland volume prior to radiotherapy and dose constraints
associated with the gland demonstrated significant impact on the
incidence of RIHT; however, due to the lack of standardization of
dose constraints and a small cohort size, subgroup analysis was not
performed and the optimal radiation dose was not determined. Future
prospective studies should investigate threshold values with
increase accuracy to reduce RIHT-associated morbidity and to
improve the QoL of patients.
Acknowledgements
Not applicable.
Funding
The Project of Invigorating Health Care through
Science, Technology and Education Jiangsu Provincial Medical
Innovation Team, The Project of Science and Technology of Jiangsu
Provincial Commission Health and Family planning (grant no.
H201426), Xuzhou City Science and Technology Bureau issues (grant
nos. KC15SH024 and KC16SH065) and The Project or Invigorating
Health Care through Science, Technology and Education (grant no.
CXTDA2017034).
Availability of data and materials
The datasets analyzed for the current study are
available from the corresponding author upon request.
Authors' contributions
LZ and JW conceived the study. YX and ZS designed
the study. YX, TT, GL and YY made substantial contributions towards
the acquisition of data. YX conducted the statistical analysis. YX
and ZS drafted the manuscript. ZS, LZ and JW critically revised the
manuscript. All authors reviewed and approved the final paper.
Ethics approval and consent to
participate
This is a retrospective analysis of clinical data
without treatment intervention and personal identification.
Therefore, formal consent from our ethics committee is not
necessary.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
FT3
|
free triiodothyronine
|
|
FT4
|
free tetraiodothyronine
|
|
IMRT
|
intensity-modulated radiotherapy
|
|
NPC
|
nasopharyngeal carcinoma
|
|
PTV
|
planning target volume
|
|
QoL
|
quality of life
|
|
RIHT
|
radiation-induced hypothyroidism
|
|
ROC
|
receiver operating characteristic
|
|
TSH
|
thyroid stimulating hormone
|
References
|
1
|
Yi HM, Yi H, Zhu JF, Xiao T, Lu SS, Guan
YJ and Xiao ZQ: A five-variable signature predicts radioresistance
and prognosis in nasopharyngeal carcinoma patients receiving
radical radiotherapy. Tumour Biol. 37:2941–2949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boomsma MJ, Bijl HP and Langendijk JA:
Radiation-induced hypothyroidism in head and neck cancer patients:
A systematic review. Radiother Oncol. 99:1–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vanderpump MP and Tunbridge WM:
Epidemiology and prevention of clinical and subclinical
hypothyroidism. Thyroid. 12:839–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual. 7th.
Springer-Verlag; New York, NY: 2010
|
|
5
|
Tian YM, Xiao WW, Bai L, Liu XW, Zhao C,
Lu TX and Han F: Impact of primary tumor volume and location on the
prognosis of patients with locally recurrent nasopharyngeal
carcinoma. Chin J Cancer. 34:247–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duntas LH and Brenta G: The effect of
thyroid disorders on lipid levels and metabolism. Med Clin North
Am. 96:269–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laulund AS, Nybo M, Brix TH, Abrahamsen B,
Jørgensen HL and Hegedüs L: Duration of thyroid dysfunction
correlates with all-cause mortality. The OPENTHYRO register cohort.
PLoS One. 9:e1104372014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. Eigth edition.
Springer-Verlag; New York, NY: 2017, View Article : Google Scholar
|
|
9
|
Zohar Y, Tovim RB, Laurian N and Laurian
L: Thyroid function following radiation and surgical therapy in
head and neck malignancy. Head Neck Surg. 6:948–952. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hancock SL, Cox RS and McDougall IR:
Thyroid disease after treatment of Hodgkin'S disease. N Engl J Med.
325:599–605. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng YL, Chen L, Shen GZ, Li YN, Yao JJ,
Xiao WW, Yang L, Zhou S, Li JX, Cheng WQ, et al: Interobserver
variations in the delineation of target volumes and organs at risk
and their impact on dose distribution in intensity-modulated
radiation therapy for nasopharyngeal carcinoma. Oral Oncol. 82:1–7.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mercado G, Adelstein DJ, Saxton JP, Secic
M, Larto MA and Lavertu P: Hypothyroidism: A frequent event after
radiotherapy and after radiotherapy with chemotherapy for patients
with head and neck carcinoma. Cancer. 92:2892–2897. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tell R, Lundell G, Nilsson B, Sjödin H,
Lewin F and Lewensohn R: Long-term incidence of hypothyroidism
after radiotherapy in patients with head-and-neck cancer. Int J
Radiat Oncol Biol Phys. 60:395–400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cella L, Liuzzi R, Conson M, D'Avino V,
Salvatore M and Pacelli R: Development of multivariate NTCP models
for radiation-induced hypothyroidism: A comparative analysis.
Radiat Oncol. 7:2242012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Feen Rønjom M: Radiation-induced
hypothyroidism after treatment of head and neck cancer. Dan Med J.
63:2016.
|
|
16
|
Lin Z, Wu VW, Lin J, Feng H and Chen L: A
longitudinal study on the radiation-induced thyroid gland changes
after external beam radiotherapy of nasopharyngeal carcinoma.
Thyroid. 21:19–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tell R, Sjödin H, Lundell G, Lewin F and
Lewensohn R: Hypothyroidism after external radiotherapy for head
and neck cancer. Int J Radiat Oncol Biol Phys. 39:303–308. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Diaz R, Jaboin JJ, Morales-Paliza M,
Koehler E, Phillips JG, Stinson S, Gilbert J, Chung CH, Murphy BA,
Yarbrough WG, et al: Hypothyroidism as a consequence of
intensity-modulated radiotherapy with concurrent taxane-based
chemotherapy for locally advanced head-and-neck cancer. Int J
Radiat Oncol Biol Phys. 77:468–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colevas AD, Read R, Thornhill J, Adak S,
Tishler R, Busse P, Li Y and Posner M: Hypothyroidism incidence
after multimodality treatment for stage III and IV squamous cell
carcinomas of the head and neck. Int J Radiat Oncol Biol Phys.
51:599–604. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu YH, Wang HM, Chen HH, Lin CY, Chen EY,
Fan KH, Huang SF, Chen IH, Liao CT, Cheng AJ and Chang JT:
Hypothyroidism after radiotherapy for nasopharyngeal cancer
patients. Int J Radiat Oncol Biol Phys. 76:1133–1139. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murthy V, Narang K, Ghosh-Laskar S, Gupta
T, Budrukkar A and Agrawal JP: Hypothyroidism after 3-dimensional
conformal radiotherapy and intensity-modulated radiotherapy for
head and neck cancers: Prospective data from 2 randomized
controlled trials. Head Neck. 36:1573–1580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
August M, Wang J, Plante D and Wang CC:
Complications associated with therapeutic neck radiation. J Oral
Maxillofac Surg. 54:1409–1416. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grande C: Hypothyroidism following
radiotherapy for head and neck cancer: Multivariate analysis of
risk factors. Radiother Oncol. 25:31–36. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cella L, Conson M, Caterino M, De Rosa N,
Liuzzi R, Picardi M, Grimaldi F, Solla R, Farella A, Salvatore M
and Pacelli R: Thyroid V30 predicts radiation-induced
hypothyroidism in patients treated with sequential
chemo-radiotherapy for Hodgkin's lymphoma. Int J Radiat Oncol Biol
Phys. 82:1802–1808. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim MY, Yu T and Wu HG: Dose-volumetric
parameters for predicting hypothyroidism after radiotherapy for
head and neck cancer. Jpn J Clin Oncol. 44:331–337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sachdev S, Refaat T, Bacchus ID,
Sathiaseelan V and Mittal BB: Thyroid V50 highly predictive of
hypothyroidism in head-and-neck cancer patients treated with
intensity-modulated radiotherapy (IMRT). Am J Clin Oncol.
40:413–417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boomsma MJ, Bijl HP, Christianen ME, Beetz
I, Chouvalova O, Steenbakkers RJ, van der Laan BF, Wolffenbuttel
BH, Oosting SF, Schilstra C and Langendijk JA: A prospective cohort
study on radiation-induced hypothyroidism: Development of an NTCP
model. Int J Radiat Oncol Biol Phys. 84:e351–e363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akgun Z, Atasoy BM, Ozen Z, Yavuz D,
Gulluoglu B, Sengoz M and Abacioglu U: V30 as a predictor for
radiation-induced hypothyroidism: A dosimetric analysis in patients
who received radiotherapy to the neck. Radiat Oncol. 9:1042014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fujiwara M, Kamikonya N, Odawara S, Suzuki
H, Niwa Y, Takada Y, Doi H, Terada T, Uwa N, Sagawa K and Hirota S:
The threshold of hypothyroidism after radiation therapy for head
and neck cancer: A retrospective analysis of 116 cases. J Radiat
Res. 56:577–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang S, Wang X, Hu C and Ying H:
Hypothalamic-pituitary-thyroid dysfunction induced by
intensity-modulated radiotherapy (IMRT) for adult patients with
nasopharyngeal carcinoma. Med Oncol. 30:7102013. View Article : Google Scholar : PubMed/NCBI
|