Introduction
Gastric cancer is among the most common types of
cancer worldwide with an increasing incidence rate each year.
Approximately 650,000 patients succumb to gastric cancer, making
its mortality rate subsequent to that of lung cancer (1). Various factors attribute to the
occurrence of gastric cancer, including the genetic background of
patients and environmental factors (2). Although substantial efforts have been
made in the diagnosis and treatment of patients with gastric
cancer, little breakthrough has been made in previous decades due
to its high tendency for metastasis. Numerous patients are
diagnosed at such an advanced stage that even combined chemotherapy
or radiotherapy fail to yield a satisfactory outcome (3). Therefore, there is an urgency to develop
novel therapeutic targets for the treatment of gastric cancer,
particularly for those tolerant to traditional therapies.
It is known that only 2% of the mammalian genome is
able to be translated into protein; however, >85% of the genome
exhibits the potential to be transcribed into RNA, while the
majority of RNAs serve roles in regulation (4,5). Notably,
long non-coding RNAs (lncRNAs) are among the regulatory RNAs, which
have a length of >200 nucleotides (6). lncRNAs have been reported to interact
with DNAs, RNAs and proteins, and are involved in the processes of
DNA transcription, the cell cycle, apoptosis and autophagy
(7). Multiple lncRNAs have been
demonstrated to participate in the tumorigenesis of gastric cancer.
lncRNA AGAP2-AS1 was revealed to be activated by SP1, and promoted
cell proliferation and metastasis in patients with gastric cancer
(8). lncRNA PVT1 functions as a
competing endogenous RNA via sponging microRNA 186 in
gastric cancer (9).
Z38 was a newly-discovered lncRNA by Deng et
al in 2016 (10). Z38 was
demonstrated to be a protein coding isoform of claudin domain
containing 1 mRNA, which belongs to the claudin family, a family
that contains >26 members and is characterized by a common motif
in the para-cellular loop (11). Z38
was demonstrated to be an lncRNA by in vitro translation
experiments and was markedly upregulated in human breast cancer
(12). Knockdown of Z38 in breast
cancer cells inhibited cell proliferation and metastasis (10). However, the detailed mechanism of the
inhibitory roles of Z38 in breast cancer remains unknown.
Furthermore, the role of Z38 in other malignancies requires further
investigation.
In the present study, the relative transcript levels
of Z38 were examined in patients with gastric cancer and in
cultured cells. The roles of Z38 in cell proliferation and
metastasis were examined with cell viability assays, colony
formation assays and Transwell assays, as well as wound-healing
assays. A preliminary study focusing on the effects of Z38 on cell
apoptosis was also included. The results of the present study
indicated that Z38 may act as a potential therapeutic target for
the treatment of gastric cancer.
Materials and methods
Human samples
Gastric cancer tissues and matched adjacent
non-cancerous tissues from 100 patients (age range: 35–75 years,
average age: 62 years, males: 37, females: 63) who were admitted to
the Department of General Surgery, Weifang People's Hospital
(Weifang, China) between April 2014 and May 2016, were collected
following surgical resection and were immediately frozen in liquid
nitrogen. Clinical characteristics of these patients, including
age, sex, presenting symptoms and TNM stage were also assessed
(13). Written informed consent was
obtained from each patient and the present study was approved by
the Ethics Committee of Weifang People's Hospital.
Cell culture and antibodies
The human gastric cancer KATO III, SGC-7901 and AGS
cell lines, as well as the 293T cell line as a control, were
purchased from the cell bank of the Chinese Academy of Sciences
(Shanghai, China). The human gastric cancer MKN45 and MKN74 cell
lines were purchased from American Type Culture Collection
(Manassas, VA, USA). All cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.). Primary antibodies
against caspase-3 and caspase-9 were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The primary antibody against
GAPDH and the horseradish peroxidase-conjugated secondary
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA).
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from human tissues and
cultured gastric cancer cells using TRIzol® reagent
(Takara Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocols. The RNA quality and concentration were
determined by collecting the absorbance with the Nanodrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). Reverse
transcription (RT) of first-strand cDNAs (1 µg) was performed using
PrimeScript RT Master mix (Takara Biotechnology Co., Ltd.),
according to the manufacturer's protocol. All PCR amplifications
were performed in an ABI PRISM 7900 Real-Time system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the
SYBR® Premix Ex Taq™ kit (Takara
Biotechnology Co., Ltd.). The thermocycling conditions were as
follows: Initial denaturation at 95°C for 5 min, followed by 45
repeats of a three-step cycling program consisting of 10 sec at
95°C (denaturation), 10 sec at 60°C (primer annealing) and 10 sec
at 72°C (elongation), and a final extension step for 10 min at
72°C. The primer sequences used for qPCR are listed in Table I and GAPDH was used as the
internal control. Primers were synthesized by Shanghai Shenggong
Biology Engineering Technology Service, Ltd. (Shanghai, China). All
quantitative data were normalized to GAPDH using the
2−ΔΔCq method (14).
 | Table I.Primers sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer nucleotide
sequences |
|---|
| Z38 |
|
|
Forward |
5′-AGTGGGATTGTGGAGACGGTGT-3′ |
|
Reverse |
5′-AGGTAAAAGGAACTGGCAACGC-3′ |
| GAPDH |
|
|
Forward |
5′-GTGGACATCCGCAAAGAC-3′ |
|
Reverse |
5′-AAAGGGTGTAACGCAACTA-3′ |
Small interfering RNA (siRNA)
interference
For knockdown of Z38, specific siRNAs (siZ38-1 and
siZ38-2, 1uM) were designed and synthesized by Santa Cruz
Biotechnology, Inc. and diluted to a final concentration of 20 mM.
The transfection assay was performed using
Lipofectamine® 2000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. At 6 h after transfection, the medium was replaced with
fresh Dulbecco's modified Eagle's medium (DMEM) containing 10%
fetal bovine serum. The cells were subject to subsequent analysis
72 h later.
Colony formation assay
AGS and MKN74 cells were seeded onto 12-well plates
24 h prior to transfection, following which specific siRNA against
Z38 was transfected. Subsequently, a total of 500 cells were seeded
onto a 6-well plate in each treatment group. The plates were
incubated at 37°C for 2 weeks without changing the culture medium
and mixed with methanol at room temperature for 15 min. Finally,
the colonies were stained with 1% crystal violet for 10 min at room
temperature and images were captured (Leica DM IRB; Leica
Microsystems GmbH, Wetzlar, Germany) in 5 random fields of view.
The whole plates were counted under a Nikon light microscope (×200
magnification) and were statically analyzed.
Cell viability assay
AGS and MKN74 cells were seeded onto 96-well plates
(3,000 cells/well) and were cultured overnight. Cells were
subsequently transfected with siZ38-1 or siZ38-2 (10 µM), followed
by incubation in DMEM for another 72 h. Cell viabilities were
determined for 5 consecutive days using the MTT assay. For this, 2
mg/ml MTT solution was added to each well, followed by incubation
for 4 h at 37°C. Subsequently, the medium was removed and 200 µl
dimethyl sulfoxide was added to dissolve the purple formazan. The
plate was agitated for 5 min at room temperature and the optical
density was subsequently determined at 570 nm using a
spectrophotometer.
Transwell assays
AGS and MKN74 cells were cultured in 24-well plates
and transfected with specific Z38 siRNA or control siRNA, with
transfection protocols as stated previously. At 48 h
post-transfection, cells were harvested and single-cell suspensions
in serum-free DMEM were prepared, of which 150 µl (3×104
cells) was seeded into the upper chamber of an 8-mm Transwell plate
(Corning Life Sciences, Corning, NY, USA). The lower chamber was
filled with 600 µl DMEM, supplemented with 10% FBS. For the
invasion assay, the membrane was coated with Matrigel (Beyotime
Institute of Biotechnology) 6 h prior to seeding. Following
incubation at 37°C for 12 h, cells were fixed with ice-cold
methanol for 20 min and stained with 0.1% crystal violet for 5 min
at room temperature. Images were captured under an inverted light
microscope at a magnification of ×200.
Wound healing assay
AGS and MKN74 cells were seeded onto 6-well plates
(~5×105 cells/well) and transfected with siZ38 or
control siRNA. A sterile 10 µl pipette tip was used to scrape
across the center of each well at 48 h post-transfection three
times and immediately cultured with serum-free medium (Gibco;
Thermo Fisher Scientific, Inc.). Cells were allowed to migrate for
12 h, following which scratches were observed and images.
Subsequently, cells were rinsed with phosphate-buffered saline were
captured for each group using a light microscope (×200
magnification). Each assay was performed in triplicate and repeated
at least three times.
Flow cytometric analysis of cell
apoptosis
The Annexin V/propidium iodide (PI) assay was
performed according to the manufacturer's protocols (Invitrogen;
Thermo Fisher Scientific, Inc.). In brief, AGS and MKN74 cells were
plated onto 6-well plates and transfected with control or specific
siRNA against Z38. Subsequently, cells were washed with pre-cold
phosphate-buffered saline, trypsinized and re-suspended in 100 µl
binding buffer with 2.5 µl fluorescein isothiocyanate-conjugated
Annexin-V and 1 µl PI (100 µg/ml). Cells were subsequently
incubated at room temperature for 15 min in the dark. A total of
>10,000 cells were collected and calculated using a flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA) with FolwJo
7.6.1 software (FlowJo LLC, Ashland, OR, USA).
Determination of caspase
activities
The activities of caspase-3, caspase-8 and caspase-9
were determined using caspase activity kits (Beyotime Institute of
Biotechnology), according to the manufacturer's protocols. In
brief, cells were transfected with siRNAs for 72 h. Subsequently,
cell lysates were collected by low speed centrifugation (860 × g
for 5 min at 4°C). An equal amount of protein (10 µl) from each
sample were added to 96-well plates and mixed with an aliquot of 80
µl reaction buffer (Beyotime Institute of Biotechnology) supplied
with caspase substrates (2 mM). Following incubation at 37°C for 4
h, caspase activities were determined using a TECAN reader at an
absorbance of 450 nm.
Western blot analysis
Total protein was extracted from cultured cells.
Cell lines were permitted to grow until they reached 95%
confluence. Following two washes with phosphate-buffered saline,
cells were lysed with a general lysis buffer (NP40; Beyotime
Institute of Biotechnology) to generate the total protein lysate.
The protein was quantified with a Pierce BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). Protein was then subjected to 10%
SDS-PAGE by loading equal amounts of whole protein (50 µg) per
lane. PVDF membranes were blocked with 5% milk in TBST at room
temperature for 1 h and then incubated with the following
antibodies at 4°C for overnight: Anti-caspase-3 (cat. no. 9662;
dilution, 1:1,000), anti-caspase-9 (cat. no. 9508; dilution,
1:1,000), anti-GAPDH (cat. no. sc-47724; dilution, 1:2000), prior
to being incubated with a horseradish peroxidase-conjugated
secondary antibody (Santa Cruz Biotech., Santa Cruz, USA, dilution,
1:5,000 at room temperature for 1 h). GAPDH was synchronously
detected as a loading control. Immunoreactivity was determined
using enhanced chemiluminescence autoradiography (Thermo Fisher
Scientific., Inc.). Image J 2× software (National Institutes of
Health, Bethesda, MD, USA) was used to quantify the western
blotting data. Each experiment was repeated at least three
times.
Statistical analysis
Data are expressed as the mean ± standard deviation.
A two-tailed Student's t-test was used to compare the means of two
groups, while one-way analysis of variance was used for comparisons
among multiple groups (≥3 groups), followed by a least significant
difference post hoc test. P<0.05 was considered to indicate a
statistically significant difference. All experiments were repeated
at least three times unless otherwise stated.
Results
lncRNA Z38 was overexpressed in
patients with gastric cancer and in gastric cancer cells
In order to investigate the role of Z38 in gastric
cancer, the relative transcript level of Z38 in 100 clinical
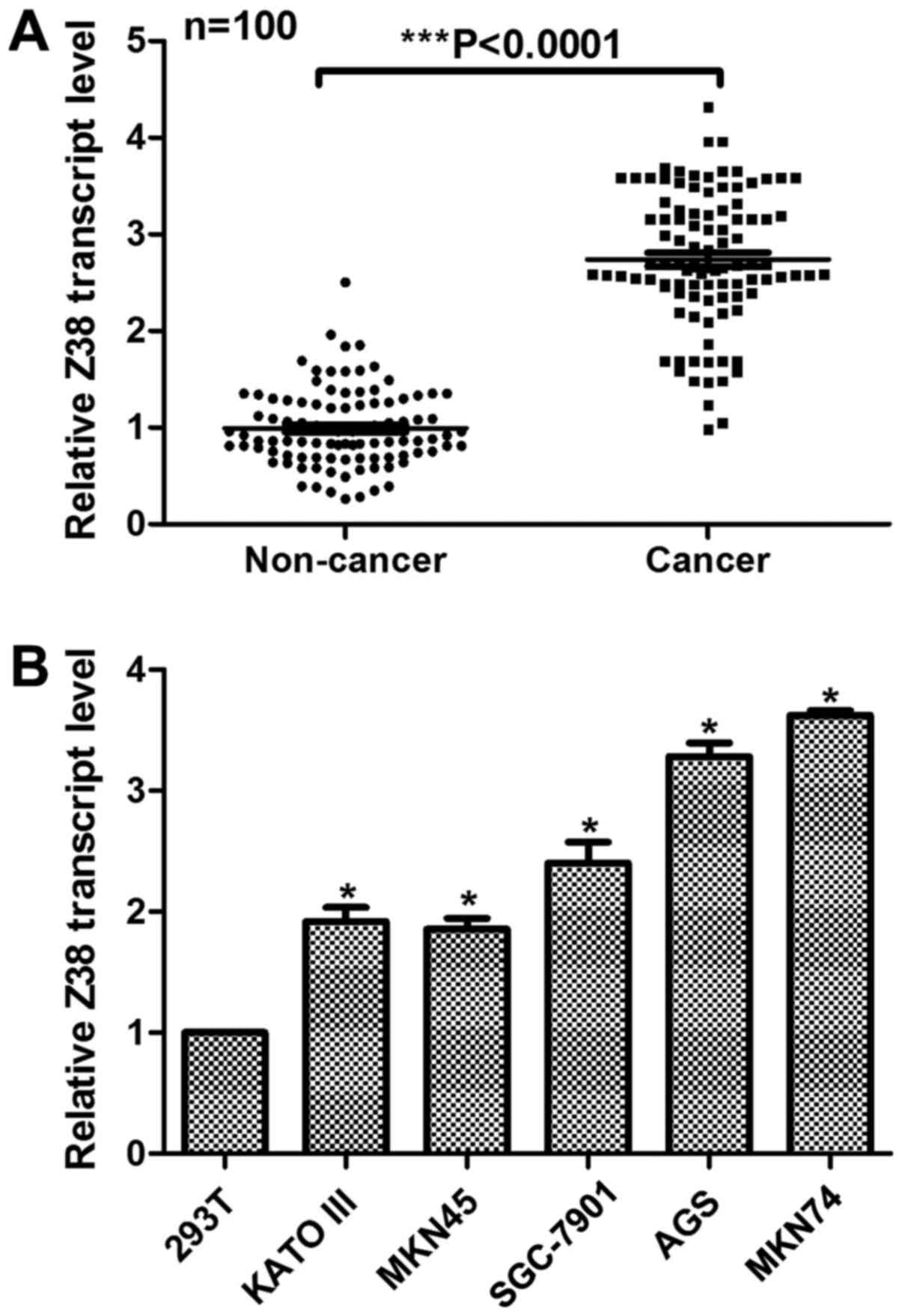
gastric cancer tissues was examined. As demonstrated in Fig. 1A, the relative expression of Z38 was
significantly increased in the clinical gastric cancer tissues
compared with expression in the adjacent non-cancerous tissues
(P<0.0001). Clinical characteristics of these patients were also
assessed. It is demonstrated in Table
II that the expression of Z38 was associated with tumor size,
lymph node metastasis, distant metastasis and Tumor-Node-Metastasis
(TNM) staging (13), but was not
associated with age, sex or presenting symptoms. Subsequently, the
expression of Z38 in gastric cancer cells was examined, using 293T
cells as a control. Compared with the control cells, all the
gastric cancer cells exhibited higher expression of Z38 (Fig. 1B). Of note, it was verified that AGS
and MKN74, the two most invasive cell lines, exhibited the highest
expression of Z38, indicating the potential role of Z38 in cell
metastasis. These data suggested that the transcript level of Z38
was upregulated in human gastric cancer.
 | Table II.Association between Z38 and clinical
variables among 100 gastric cancer patients. |
Table II.
Association between Z38 and clinical
variables among 100 gastric cancer patients.
|
|
| Expression of
Z38 |
|
|---|
|
|
|
|
|
|---|
| Variable | No. | Low (n=40) | High (n=60) | P-value |
|---|
| Age, years |
|
|
| 0.526 |
|
<40 | 18 | 7 | 11 |
|
|
40–50 | 28 | 16 | 12 |
|
|
>50 | 54 | 27 | 27 |
|
| Sex |
|
|
| 0.094 |
|
Male | 62 | 29 | 33 |
|
|
Female | 38 | 11 | 27 |
|
| Presenting
symptoms |
|
|
|
|
|
Painless lump | 46 | 21 | 25 | 0.159 |
| Painful
lump | 48 | 15 | 33 |
|
|
Atypical symptoms | 6 | 4 | 2 |
|
| T, cm |
|
|
|
<0.001a |
| T1
(≤2) | 34 | 21 | 13 |
|
| T2
(>2 and <5) | 26 | 12 | 14 |
|
| T3
(≥5) | 22 | 6 | 16 |
|
| T4 (any
size with distant metastasis) | 18 | 1 | 17 |
|
| N |
|
|
|
<0.001a |
| N0 | 44 | 28 | 16 |
|
| N1 or
above | 56 | 12 | 44 |
|
| Distant metastasis
(M) |
|
|
| 0.023a |
| M0 | 45 | 24 | 21 |
|
| M1 | 55 | 16 | 39 |
|
| TNM stage |
|
|
| 0.008a |
|
I/II | 42 | 19 | 23 |
|
|
III/IV | 58 | 11 | 47 |
|
Knockdown of Z38 in gastric cancer
inhibited cell proliferation in vitro
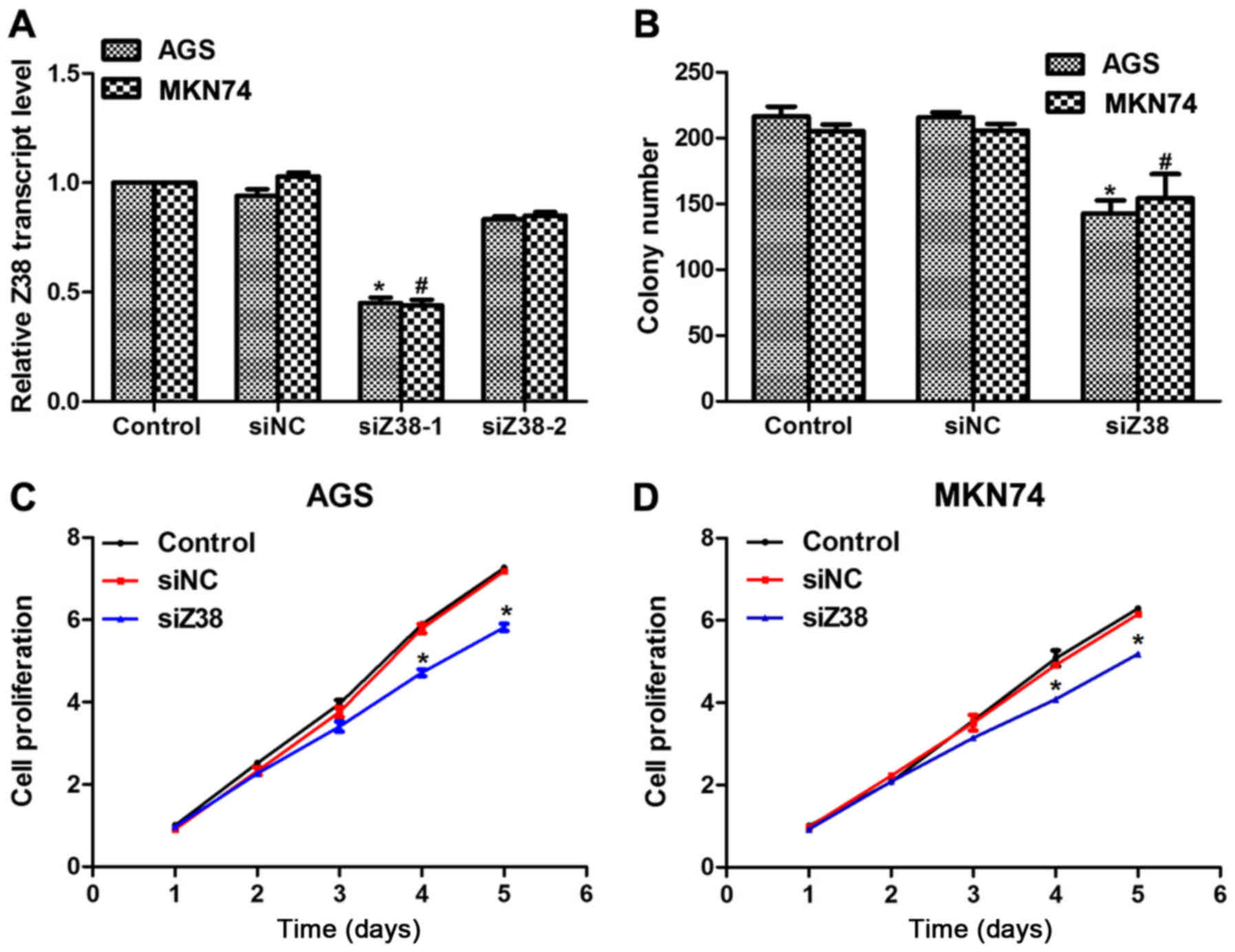
Next, two specific siRNAs against Z38, namely
siZ38-1 and siZ38-2, were designed. Subsequently, these two siRNAs
were transfected into AGS and MKN74 cells. It was revealed that the
relative transcript level of Z38 was significantly decreased by
siZ38-1, but not siZ38-2 (Fig. 2A);
therefore, only siZ38-1 was included in the subsequent analysis.
Colony formation and cell proliferation assays were performed to
investigate the role of Z38 in cell proliferation. Approximately
220 colonies were formed in control and siNC-treated AGS cells,
while only 150 colonies were observed in siZ38-transfected cells
(Fig. 2B). A similar phenomenon was
also observed in MKN74 cells. In the cell proliferation assays, no
significant difference was observed among the three groups in AGS
or MKN74 cells in the first three days; however, on the fourth day,
the proliferation rate was inhibited by 25 and 20% in AGS and MKN74
cells, respectively (Fig. 2C and D).
Furthermore, the inhibitory effects were even pervasive on the
fifth day in the two cell lines. These results revealed that
knockdown of Z38 inhibited cell proliferation in the human gastric
cancer AGS and MKN74 cell lines.
Depletion of Z38 in human gastric
cancer cells suppressed cell metastasis in vitro
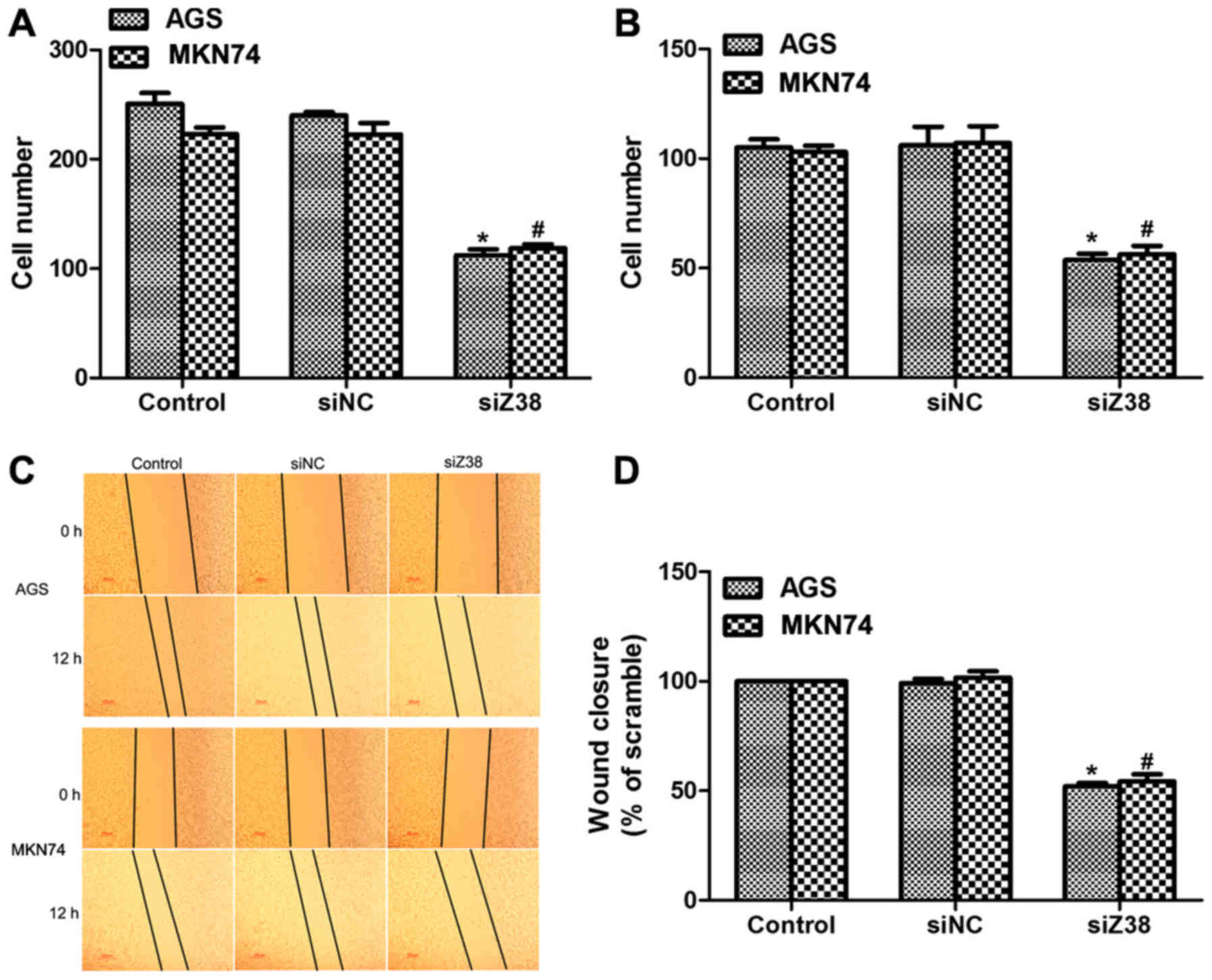
In order to further investigate the role of Z38,
Transwell and wound-healing assays were performed. It was revealed
that ~250 AGS and 220 MKN74 cells migrated through the membrane in
control and siNC-treated groups, while only 120 AGS and 125 MKN74
cells were observed on the lower surface of the membrane in the
migration assay (Fig. 3A). Similarly,
the invasive abilities of AGS and MKN74 cells were also suppressed
upon siZ38 transfection (Fig. 3B).
Furthermore, the wound-healing assay also revealed that the wound
closure area was decreased upon siZ38 transfection in the two cell
lines (Fig. 3C and D). All these data
suggested that knockdown of Z38 in human gastric cancer cells
suppressed cell metastasis in vitro.
Knockdown of Z38 in gastric cancer
cell lines promoted cell apoptosis in vitro
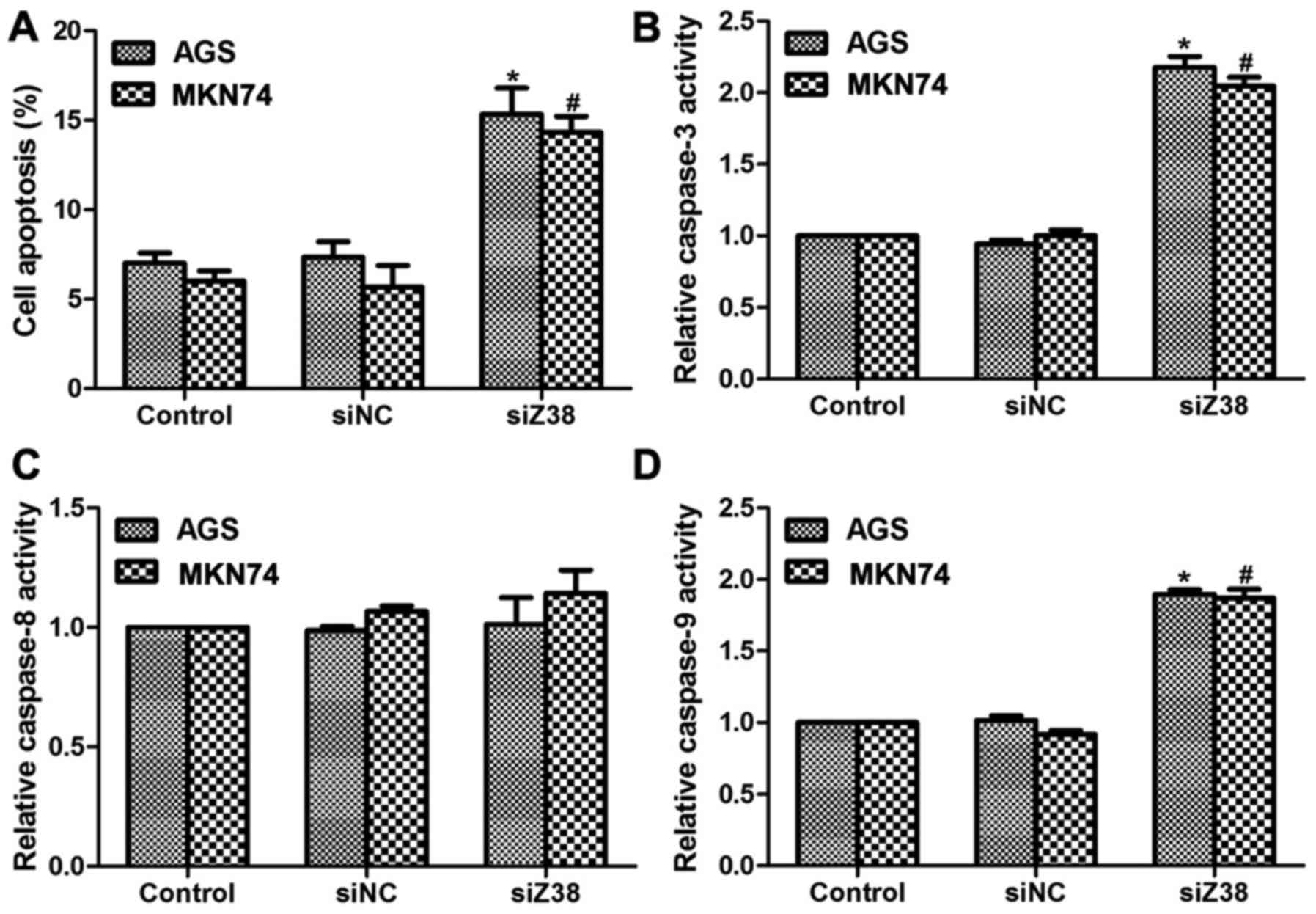
Increased cell proliferation rate, cell metastasis
potential and inhibited cell apoptotic capacity were the main
manifestations of the majority of malignancies (15); therefore, the present study also
investigated the effects of Z38 on cell apoptosis. As demonstrated
in Fig. 4A, transfection with siZ38
increased the cell apoptotic rate by 8 and 7% in AGS and MKN74
cells, respectively. Furthermore, the relative caspase activities
were also determined. The relative activities of caspase-3
(Fig. 4B) and caspase-9 (Fig. 4D) were increased ~2-fold upon siZ38
transfection compared with the control cells, while the activity of
caspase-8 remained stable (Fig. 4C),
indicating that the role of Z38 was associated with the intrinsic
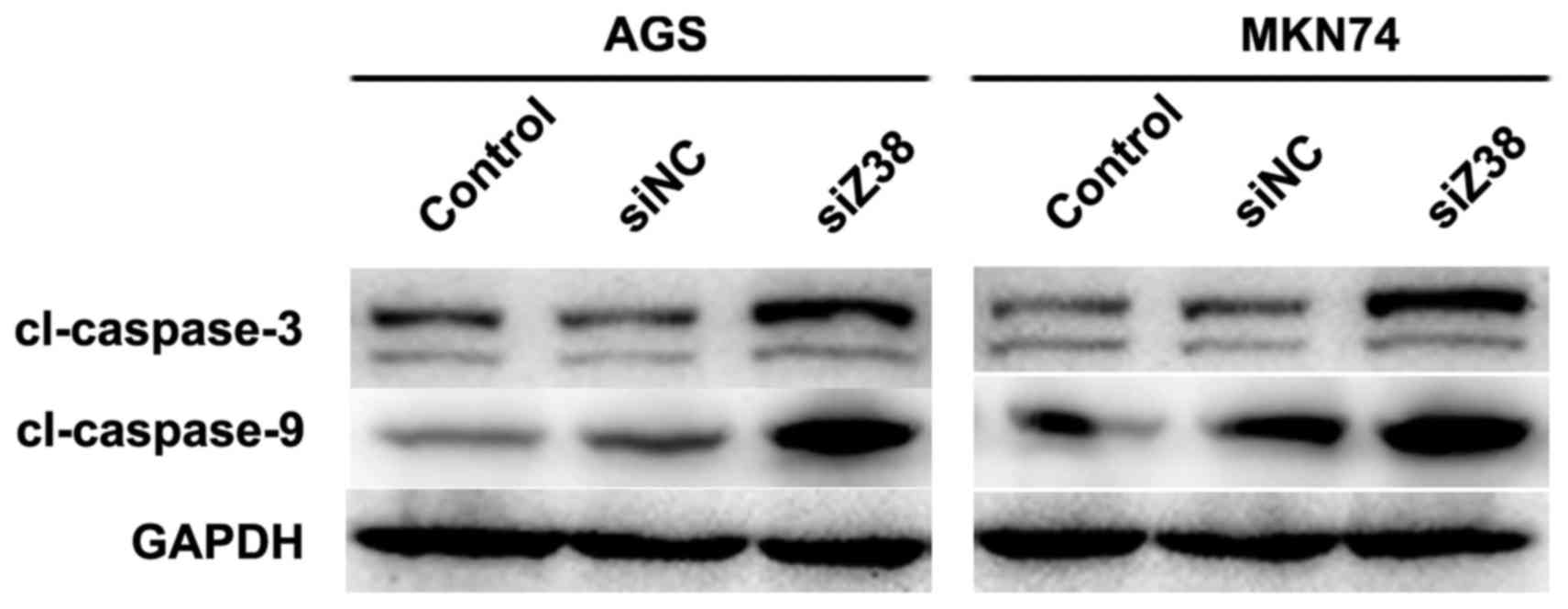
pathway of apoptosis. Subsequently, western blot analysis was
performed and it was revealed that in AGS and MKN74 cells,
knockdown of Z38 increased the protein levels of caspase-3 and
caspase-9 (Fig. 5), which was
consistent with the results demonstrated in Fig. 4. These results suggested that
depletion of Z38 in AGS and MKN74 cells increased cell apoptosis by
promoting the activities of caspase-3 and caspase-9.
Discussion
Gastric cancer is the fourth most common type of
cancer among males and the sixth among females (16). Furthermore, it is the second cause of
cancer-associated mortality worldwide (17). In China, gastric cancer is the third
most common cause of cancer-associated mortality (18). In the year 2002, the age standardized
incidence rate was 22.0/100,000 males and 10.4/100,000 females and
the mortality rate was 16.3/100,000 males and 7.9/100,000 females,
according to the global estimation-GLOBOCAN 2002 (19). The property of easy distant metastasis
makes current therapeutics unable to treat gastric cancer in all
patients. Therefore, it is a priority to develop novel therapeutic
targets for the clinical treatment of gastric cancer.
Aberrant expression of certain regulatory RNAs
markedly influences cancer origination and progression (20). Therefore, investigating the role of
different regulatory RNAs in human tumorigenesis has attracted
attention worldwide. The present study examined the expression of
lncRNA Z38 in clinical gastric cancer tissues and cultured gastric
cancer cells. Of note, KATO III and MKN45 are two cell lines that
are poorly differentiated, while AGS and MKN74 are highly
differentiated and the SGC-7901 cell line is moderately
differentiated. Notably, the relative transcript level of Z38 was
highest in AGS and MKN74 cells, indicating the potential capacity
of Z38 involvement in cell metastasis. One of the limitations of
the present study was that only 293T cells were included as a
control cell line, but not normal gastric cells, as 293T cells are
widely used as control and tool cells in studies on gene expression
in healthy cells and cancer cells (21–23).
However, at the time of the present study, there was no access to
normal gastric cells and therefore, 293T cells were included as the
control cell line. In addition, expression of Z38 was associated
with gastric cancer aggressive parameters, including tumor size and
TNM staging. Therefore, it was hypothesized that Z38 may exert
critical roles in cell proliferation and migration. Cell viability,
Transwell and wound-healing assays were therefore performed to
confirm this hypothesis. The results of the present study supported
this aforementioned hypothesis and suggested the oncogenic property
of Z38 in gastric cancer.
The induction of apoptosis may be divided into two
categories: The intrinsic and extrinsic pathways. The initiation of
the intrinsic pathway is associated with the pro-apoptotic factors,
B cell lymphoma-associated X protein (Bax) and B cell
lymphoma-associated death promoter (Bad), which leads to increased
permeability of the mitochondrial membrane, loss of membrane
potential and release of cytochrome c (24,25).
Cytochrome c binds to apoptotic protease activating factor-1
and then pro-caspase-9 to form a protein complex known as
apoptosome, the role of which is to cleave pro-caspase to its
active form of caspase-9 and, in turn, to activate caspase-3
(2). The present study investigated
the apoptosis rate upon siZ38 transfection and revealed that Z38
may markedly inhibit cell apoptosis in AGS and MKN74 gastric cancer
cells. Knockdown of Z38 promoted the relative activities of
caspase-3 and caspase-9, but not that of caspase-8, which is
involved in the extrinsic pathway of apoptosis. To the best of our
knowledge, activation of caspase-3 requires proteolytic processing
of its inactive zymogen into activated p17 and p12 fragments.
Therefore, the lower bands of Fig. 5
were also from cleaved-caspase-3, which had a smaller molecular
weight (12KD). However, the top band was specific to
cleaved-caspase-3, the molecular weight of which was 17KD. At
present, the detailed mechanisms that underlie the biological
effects of Z38 remain to be elucidated, and further investigation
of the expression of other apoptosis-related proteins, including
Bax, Bad and B cell lymphoma 2, is warranted. For example, the
downstream targets of Z38 may be a useful area of further
investigation. At present, our group is working on the construction
of an expression plasmid of lncRNA Z38, since gain/loss of function
experiments are typical protocols for assessing molecular function.
The present study represents only a preliminary study reporting the
effects of knockdown of Z38 on gastric cancer proliferation and
metastasis. Future studies should aim to perform gain of function
experiments and to investigate the detailed molecular mechanisms
that contribute to Z38 functions in gastric cancer.
In conclusion, the present study revealed that the
expression of Z38 was upregulated in human gastric cancer.
Knockdown of Z38 in AGS and MKN74 cells inhibited cell
proliferation and metastasis, and promoted cell apoptosis by
upregulating the activities of caspase-3 and caspase-9. The results
of the present study indicated the oncogenic potential of Z38 in
human gastric cancer and provided evidence that Z38 may serve as a
potential therapeutic target for the treatment of gastric
cancer.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
YW and CZ performed the experiments. XW and TL
analyzed the data. RZ, YW and JZ analysed the data and revised the
manuscript and references. QH and ZS designed the project, analyzed
the data and reviewed the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient and the present study was approved by the Ethics Committee
of Weifang People's Hospital (Weifang, China).
Consent for publication
The study participants provided written informed
consent for the publication of the data included in the present
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shirahata A, Sakata M, Kitamura Y,
Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K,
Kigawa G, et al: MACC 1 as a marker for peritoneal-disseminated
gastric carcinoma. Anticancer Res. 30:3441–3444. 2010.PubMed/NCBI
|
|
2
|
Li JH, Shen WZ, Gu XQ, Hong WK and Wang
ZQ: Prognostic value of EUS combined with MSCT in predicting the
recurrence and metastasis of patients with gastric cancer. Jpn J
Clin Oncol. 47:487–493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bai T, Yokobori T, Altan B, Ide M, Mochiki
E, Yanai M, Kimura A, Kogure N, Yanoma T, Suzuki M, et al: High
STMN1 level is associated with chemo-resistance and poor prognosis
in gastric cancer patients. Br J Cancer. 116:1177–1185. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun KY, Peng T, Chen Z, Song P and Zhou
XH: Long non-coding RNA LOC100129148 functions as an oncogene in
human nasopharyngeal carcinoma by targeting miR-539-5p. Aging
(Albany NY). 9:999–1011. 2017.PubMed/NCBI
|
|
5
|
Qian Y, Liu D, Cao S, Tao Y, Wei D, Li W,
Li G, Pan X and Lei D: Upregulation of the long noncoding RNA UCA1
affects the proliferation, invasion, and survival of hypopharyngeal
carcinoma. Mol Cancer. 16:682017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu M, Liu J, Xiao J, Yang L, Cai M, Shen
H, Chen X, Ma Y, Hu S, Wang Z, et al: Lnc-mg is a long non-coding
RNA that promotes myogenesis. Nat Commun. 8:147182017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palmieri G, Paliogiannis P, Sini MC, Manca
A, Palomba G, Doneddu V, Tanda F, Pascale MR and Cossu A: Long
non-coding RNA CASC2 in human cancer. Crit Rev Oncol Hematol.
111:31–38. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi F, Liu X, Wu H, Yu X, Wei C, Huang X,
Ji G, Nie F and Wang K: Long noncoding AGAP2-AS1 is activated by
SP1 and promotes cell proliferation and invasion in gastric cancer.
J Hematol Oncol. 10:482017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang T, Liu HW, Chen JQ, Wang SH, Hao LQ,
Liu M and Wang B: The long noncoding RNA PVT1 functions as a
competing endogenous RNA by sponging miR-186 in gastric cancer.
Biomed Pharmacother. 88:302–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deng R, Liu B, Wang Y, Yan F, Hu S, Wang
H, Wang T, Li B, Deng X, Xiang S, et al: High expression of the
newly found long noncoding RNA Z38 promotes cell proliferation and
oncogenic activity in breast cancer. J Cancer. 7:576–586. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Starovasnik MA, Braisted AC and Wells JA:
Structural mimicry of a native protein by a minimized binding
domain. Proc Natl Acad Sci USA. 94:pp. 10080–10085. 1997;
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nie ZL, Wang YS, Mei YP, Lin X, Zhang GX,
Sun HL, Wang YL, Xia YX and Wang SK: Prognostic significance of
long noncoding RNA Z38 as a candidate biomarker in breast cancer. J
Clin Lab Anal. 32:e221932018. View Article : Google Scholar
|
|
13
|
Guo P, Huang ZL, Yu P and Li K: Trends in
cancer mortality in china: An update. Ann Oncol. 23:2755–2762.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ribeiro RX, Nascimento CILL and Silva
AMTC: Genotype association gstm1 null and gastric cancer:
Evidence-based meta-analysis. Arq Gastroenterol. 54:101–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lopez-Ramirez MA, Lever-Rosas CD,
Motta-Ramirez GA, Rebollo-Hurtado V, Guzman-Barcenas J,
Fonseca-Morales JV and Carreno-Lomeli MA: Correlation between
preoperative tomographic staging and definitive histopathologic
results in gastric cancer at the Hospital Central Militar. Rev
Gastroenterol Mex. 82:210–216. 2017.(In English, Spanish).
PubMed/NCBI
|
|
17
|
Pan Y, Zhou F, He C, Hui L, Huang T and
Wei Y: Leptin-leprb expressed in gastric cancer patients and
related to cancer-related depression. Biomed Res Int.
2017:64828422017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tian Q, Xiao Y, Wu Y, Liu Y, Song Z, Gao
W, Zhang J, Yang J, Zhang Y, Guo T, et al: MicroRNA-33b suppresses
the proliferation and metastasis of hepatocellular carcinoma cells
through the inhibition of Sal-like protein 4 expression. Int J Mol
Med. 38:1587–1595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The gencode v7 catalog of human long noncoding rnas:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai Y, Yi M, Chen D, Liu J, Guleng B, Ren
J and Shi H: Trefoil factor family 2 expression inhibits gastric
cancer cell growth and invasion in vitro via interactions with the
transcription factor sp3. Int J Mol Med. 38:1474–1480. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang S, Chen R, Yu J, Li N, Ke R, Luo L,
Zou J, Zhang J, Zhang K, Lu N and Huang D: Clinical significance
and role of LKB1 in gastric cancer. Mol Med Rep. 13:249–256. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spencer SL and Sorger PK: Measuring and
modeling apoptosis in single cells. Cell. 144:926–939. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen SD, Wu CL, Hwang WC and Yang DI: More
insight into bdnf against neurodegeneration: Anti-apoptosis,
anti-oxidation, and suppression of autophagy. Int J Mol Sci.
18:E5452017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dejean LM, Martinez-Caballero S and
Kinnally KW: Is MAC the knife that cuts cytochrome c from
mitochondria during apoptosis? Cell Death Differ. 13:1387–1395.
2006. View Article : Google Scholar : PubMed/NCBI
|