Introduction
Lung cancer is one of the most common primary
malignant tumor types in numerous countries, including China and
North America (1). There are two
primary types of lung cancer: Small-cell lung cancer (SCLC) and
non-small-cell lung cancer (NSCLC) (1). NSCLC accounts for 75–85% of lung cancer
cases (2,3). Compared with SCLC, NSCLC cells grow and
divide more rapidly, resulting in diffusion and metastasis at an
earlier stage of disease (4).
However, only 15% of patients with NSCLC are diagnosed at an early
stage of the disease and current treatment modalities, including
surgical resection and chemoradiation are inadequate (5). Even if diagnosis occurs at stage I
(6), the 10-year survival rate for
patients with NSCLC is ~50% (7,8). The
5-year survival rate of patients with NSCLC is only 16% due to
early tumor metastasis and recurrence (9,10).
Therefore, the development of novel diagnostic strategies and
natural antineoplastic drugs for the treatment of lung cancer have
become the focus of clinical research.
Isoliquiritigenin (2′-4′-4-trihydroxychalcone, ISL),
a flavonoid identified in licorice, and is a potent antioxidant
with anti-inflammatory, antioxidant and antitumor capabilities
(11–13). ISL exhibits an inhibitory effect on
anti-proliferative activities in gastric cancer, prostate cancer,
hepatocellular carcinoma (HCC), breast cancer, melanoma, and lung
cancer cells (14–19). Furthermore, ISL has been demonstrated
to block the proliferation rate of HCC cells in a dose- and
time-dependent manner (20), and
induce apoptosis and autophagy in various cancer cells, including
drug-resistant breast cancer MCF-7/ADR cells, and endometrial
cancer Ishikawa and HEC-1A cells (21,22). In
recent years, the anticancer mechanism of ISL has been thoroughly
studied. In endometrial cancer cells, ISL was demonstrated to
induce cell cycle arrest in the sub-G1 or G2/M phase through the
p53/p21 pathway, and promote cell apoptosis and autophagy via
activation of the extracellular signal-regulated kinase pathway
(22). In addition, previous studies
have demonstrated that the phosphatidylinositol 3-kinase (PI3K)/AKT
serine/threonine kinase (AKT) signaling pathway is also involved in
ISL-induced cell apoptosis and proliferation (23,24);
however, the possible regulation of the PI3K/AKT signaling pathway
and its downstream pathways by ISL in the context of lung cancer
has received relatively little attention.
In the present study, the inhibitory effects of ISL
on cell proliferation, migration, invasion and apoptosis in A549
lung cancer cells were examined. Furthermore, in order to establish
the anticancer mechanism of ISL, the expression of B-cell lymphoma
2 (Bcl-2), Bcl-2-associated X protein (BAX), active caspase-3,
Cyclin D1, P70, AKT, phosphorylated (p)-AKT, mammalian target of
rapamycin (mTOR) and p-mTOR were assayed, due to their association
with cell apoptosis and the PI3K/AKT signaling pathway.
Materials and methods
Cell culture and treatment
A549 cells were obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
Cells were maintained in a monolayer culture with 5% CO2
at 37°C in RPMI-1640 (HyClone; GE Healthcare Life Sciences, Logan,
UT, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 10 U/ml
penicillin (Sigma-Aldrich Merck KGaA, Darmstadt, Germany) and 100
µg/ml streptomycin (Sigma-Aldrich; Merck KGaA). The cells in the
logarithmic phase were washed with PBS and detached with
Trypsin-EDTA (cat. no. 25200056; Thermo Fisher Scientific, Inc.,)
for 3 min at 37°C. Confluent A549 cells were then seeded into
6-well plates at a density of 5×104 cells/well with the
RPMI-1640 medium for subsequent experiments. ISL (MedChem Express,
Monmouth Junction, NJ, USA) was dissolved in DMSO (Amresco, LLC,
Solon, OH, USA) as the stock solution and final concentrations of
the compounds tested were prepared by diluting the stock solution
with RPMI-1640. A549 cells were treated with ISL (20 µM) for 24 h,
and the cells treated with 0.1% DMSO were designated the negative
control group (NC).
Cell proliferation assay
Inhibition of cell proliferation by ISL was measured
using the cell counting kit-8 (CCK8) assay. Cells were plated in
96-well plates (1×103 cells/well). Following a 24 h
incubation at 37°C, cells of the experimental group were treated
with ISL (20 µM) for 24, 48 and 72 h, and the negative control
group was treated with 0.1% DMSO. CCK8 (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China) test solution (10 µl)
was added to each well. Following a 90 min incubation, the
absorbance was measured on an ELISA reader at a wavelength of 450
nm. A curve was drawn for proliferation inhibition analysis. The
experiment was repeated in triplicate.
Cell migration and invasion assay
For the invasion assay, a Transwell chamber (EMD
Millipore) was used to structure the cell invasion model. In
summary, 100 µl of Matrigel (BD Biosciences, San Jose, CA, USA),
melted overnight and diluted with serum-free RPMI-1640 medium in a
1:6 ratio, was added to the upper chamber of 24-well plates,
following incubation for 4–6 h at 37°C with 5% CO2. The
medium was removed, 500 µl of serum-free medium was added to the
lower chamber for 30 min at 37°C to hydrate the basement membrane.
A549 cells (1×105) treated with ISL (20 µM) or 0.1% DMSO
for 48 h at 37°C were seeded in the upper chamber in 100 µl of
serum-free medium. The lower chamber was filled with 500 µl of
medium supplemented with 10% FBS. Following a 24 h incubation at
37°C, non-invasive cells on the upper surface of the Matrigel were
gently scrubbed with a cotton swab and migrated cells on the lower
surface were washed with PBS, fixed with 4% paraformaldehyde for 30
min at room temperature, stained with 0.1% crystal violet for 20
min at room temperature and recorded for images under a light
microscope (Olympus Corporation, Tokyo, Japan; magnification,
magnification, ×100). For the migration assay, the same method as
the invasion assay was used, only without Matrigel. Each assay was
performed in triplicate.
Analysis of cell apoptosis
Apoptosis was assessed by flow cytometry analysis,
in which the percentage of apoptotic cells was determined using an
Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(Beijing 4A Biotech, Beijing, Co., Ltd., Beijing, China), according
to the manufacturer's protocol. The cells either treated with ISL
(20 µM) or 0.1% DMSO for 48 h at 37°C were cultured with serum-free
medium for 24 h at 37°C. The cells were subsequently collected and
washed twice with chilled PBS for 5 min at 0°C, resuspended in
binding buffer (1×106 cells/well) and incubated with
FITC-conjugated Annexin V (5 µl) in the dark for 30 min at room
temperature. Following the addition of 10 µl of propidium iodide
(PI) and 400 µl of PBS for 5 min at room temperature, the cells
were analyzed using a FACS caliber instrument. FlowJo software
(version 7.6.5; FlowJo LLC, Ashland, OR, USA) was used to analyze
the flow cytometry results. The experiment was performed in
triplicate.
Protein extraction and western blot
analysis
Protein expression was detected using a western blot
assay. The A549 cells treated with ISL (20 µM) or 0.1% DMSO for 48
h in 6-well plates were harvested and lysed in RIPA buffer (CWBio,
Inc., Beijing, China) at 4°C. Proteins were extracted and the
concentrations were measured using the bicinchoninic acid method. A
total of 20 µg of the total proteins were fractionated
electrophoretically by 10% SDS-PAGE gels (Beyotime Institute of
Biotechnology, Beijing, China) and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA), which were
then blocked with 5% nonfat milk for 1 h at room temperature, and
then cultured overnight at 4°C with the following primary
antibodies: E-cadherin (dilution, 1:1,000; cat. no. 20874-1-AP;
rabbit polyclonal), N-cadherin (dilution, 1:1,000; cat. no.
22018-1-AP; rabbit polyclonal), vimentin (dilution, 1:1,000; cat.
no. 10366-1-AP; rabbit polyclonal), Bcl-2 (dilution, 1:1,000; cat.
no. 12789-1-AP; rabbit polyclonal), Bax (dilution, 1:1,000; cat.
no. 50599-2-lg; rabbit polyclonal), active caspase-3 (dilution,
1:1,000; cat. no. 19677-1-AP; rabbit polyclonal), cyclin D1
(dilution, 1:1,000; cat. no. 60186-1-lg; mouse monoclonal), P70
(dilution, 1:1,000; cat. no. 14485-1-AP; rabbit polyclonal) and
β-tubulin (dilution, 1:5,000; cat. no. 10094-1-AP; rabbit
polyclonal; all from ProteinTech Group, Inc. Chicago, IL, USA), AKT
(dilution, 1:1,000; cat. no. 4691; rabbit polyclonal), p-AKT
(dilution, 1:1,000; cat. no. 4060; rabbit polyclonal), mTOR
(dilution, 1:1,000; cat. no. 2983; rabbit polyclonal) and p-mTOR
(dilution, 1:1,000; cat. no. 5536; rabbit polyclonal) (all from
Cell Signaling Technology, Inc., Danvers, MA, USA). β-tubulin was
used as a loading control. The membranes were washed with
Tris-buffered saline with 0.1% Tween-20 in triplicate and incubated
with horseradish peroxidase conjugated goat anti-rabbit or goat
anti-mouse IgG secondary antibodies (cat. nos. SA00001-2 and
SA00001-1, respectively; dilution, 1:5,000; ProteinTech Group,
Inc.) for 1 h at room temperature. Chemiluminescence detection was
performed using a standard enhanced chemiluminescence kit (cat. no.
W0049M; CWBio, Inc.), according to the manufacturer's protocol. The
gray values were obtained by Quantity One v4.6.2 software (BioRad,
Inc., California, USA) for calculating the protein relative
content. The experiment was repeated three times independently.
Statistical analysis
The data were analyzed with the software SPSS 17.0
(SPSS Inc., Chicago, IL, USA). All values are expressed as the mean
± standard deviation. Statistical comparisons between two groups
were made using an independent sample two-tailed Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Inhibition of ISL on the proliferation
of A549 cells
The anti-proliferative effect of ISL on the lung
cancer A549 cells was examined using CCK8. Following exposure to
ISL (20 µM) for 24–72 h, cell viability of A549 cells was assessed.
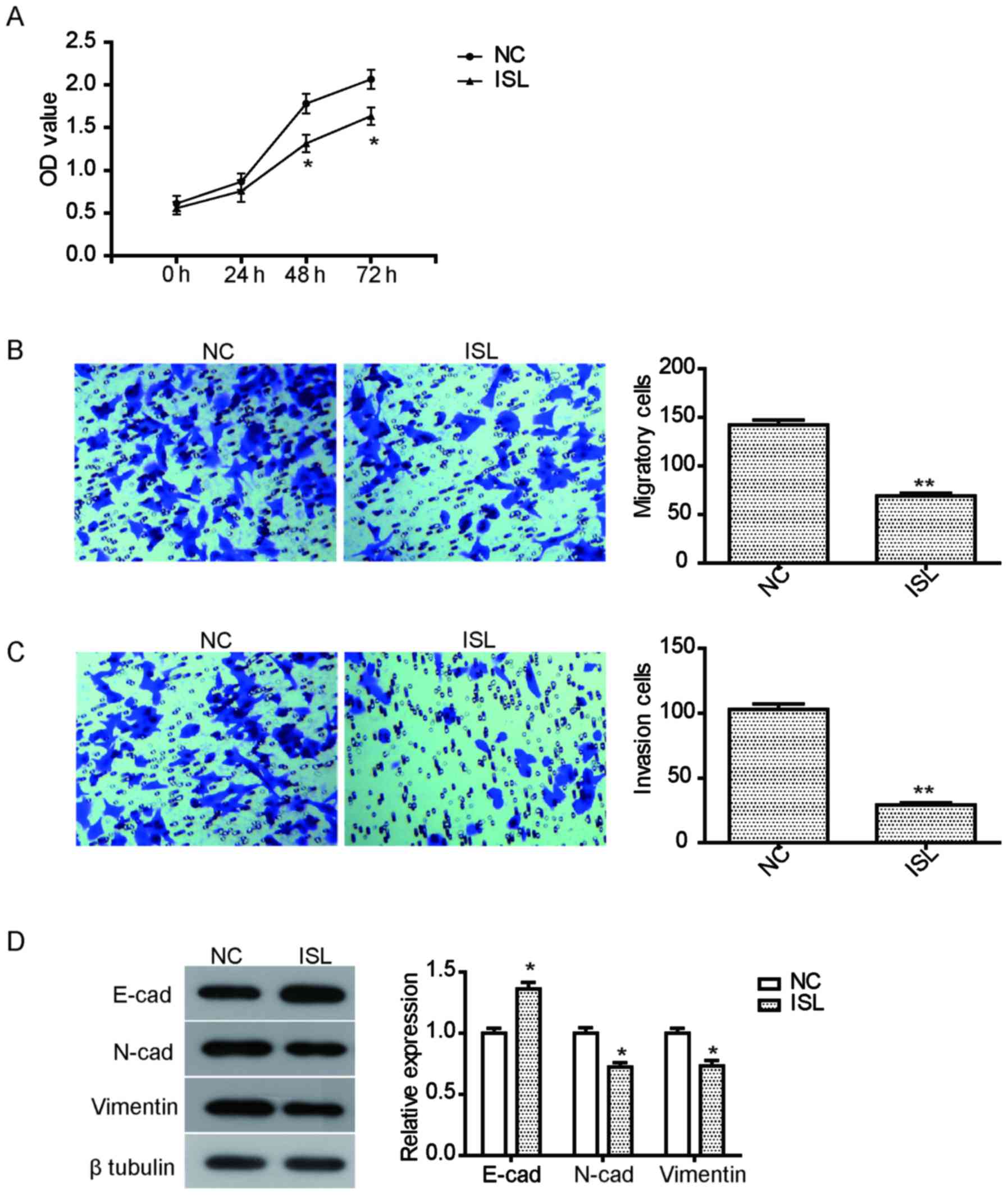
As illustrated in Fig. 1A, the
proliferation of A549 cells treated with ISL for 48 h or 72 h was
significantly suppressed compared with the NC group
(P<0.05).
Inhibition of ISL on the migration and
invasion of A549 cells
To assay the inhibition of ISL on the migration and
invasion of A549 cells, a Transwell invasion experiment was
performed. Migration and invasion were assessed following treatment
ISL (20 µM) for 24 h. The results demonstrated that the migration
of A549 cells treated with ISL was effectively inhibited compared
with the NC (Fig. 1B). Furthermore,
ISL treatment also significantly suppressed the invasion ability of
A549 cells (Fig. 1C). To further
explore the relevant mechanism of inhibition on cell mobility
caused by ISL, the expression of cell metastasis-associated
proteins was examined. As illustrated in Fig. 1D, ISL treatment led to a significant
increase in the expression of E-cadherin, but reduced the
expression of N-cadherin and vimentin, suggesting that the
inhibition of migration and invasion by ISL may be mediated by
regulating the expression of these proteins.
ISL induces apoptosis in A549
cells
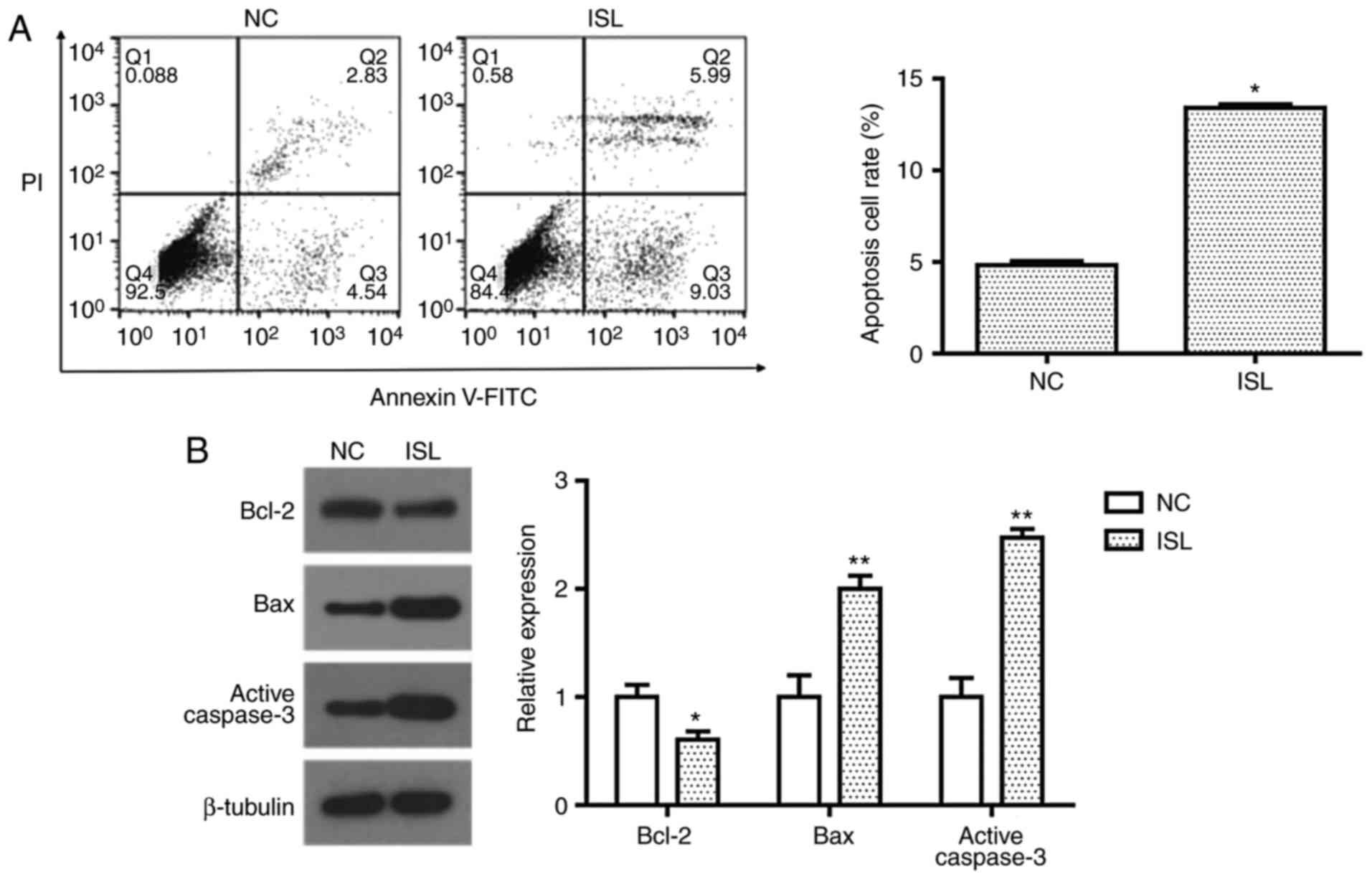
Cell apoptosis was evaluated by determining the
percentage of A549 cells undergoing apoptotic cell death following
treatment with ISL (20 µM) with Annexin V-FITC/PI staining. Flow
cytometric analysis demonstrated that ISL (20 µM) treatment
significantly promoted apoptosis in A549 cells compared with the NC
(Fig. 2A). To investigate the
apoptotic pathway induced by ISL, the expression levels of Bcl-2,
Bax and active caspase-3 were detected by western blot analysis.
When compared with the control group, the expression of
anti-apoptosis protein Bcl-2 was significantly decreased, while
pro-apoptosis proteins Bax and active caspase-3 were significantly
increased following treatment with ISL (Fig. 2B). These results suggest that the
induction of apoptosis in ISL-treated A549 cells may be associated
with the mitochondrial apoptosis pathway.
ISL inhibits the activation of the
PI3K/AKT signaling pathway
The PI3K/AKT signaling pathway is important in the
occurrence and development of cancer, as Akt and mTOR proteins
serve essential role in the proliferation and migration of tumor
cells (25). In the present study the
expression levels of these proteins were assessed by western blot
analysis. As illustrated in Fig. 3,
the phosphorylation levels of Akt and mTOR in the ISL-treated A549
cells were significantly decreased, and the expression levels of
their downstream proteins P70 and Cyclin D1 were decreased
accordingly.
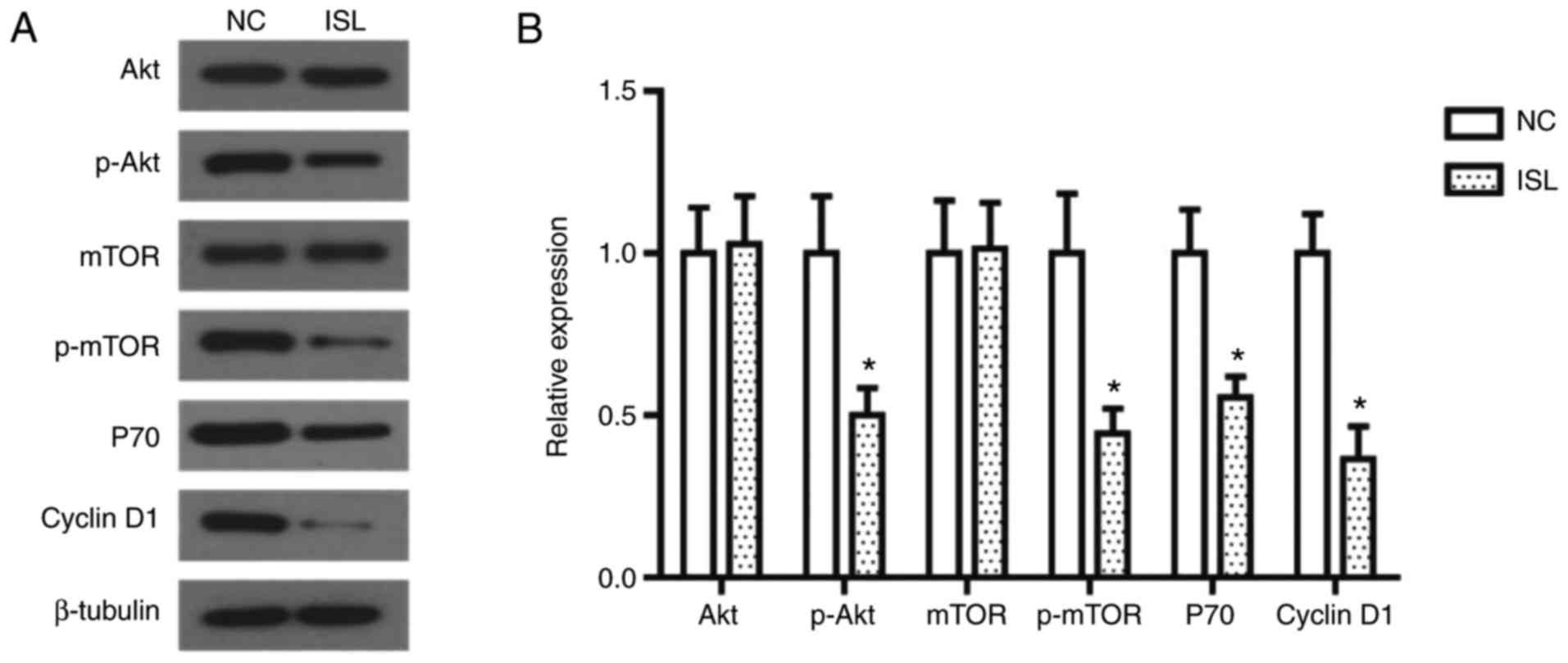 | Figure 3.ISL treatment inhibits the activation
of the PI3K/AKT signaling pathway in A549 cells. (A) Effects of ISL
on protein expression of AKT, mTOR, p-AKT, p-mTOR, P70 and Cyclin
D1 in A549 cells, determined using western blot analysis. β-tubulin
was used as the loading control. (B) Quantitative results of
protein expression. The data was calculated using mean ± standard
deviation of three repeats. *P<0.05 compared with NC. NC,
negative control; ISL, Isoliquiritigenin; PI3K,
phosphatidylinositol 3-kinase; AKT, AKT serine/threonine kinase;
mTOR, mammalian target of rapamycin; p, phosphorylated. |
Discussion
ISL belongs to the family of hydroxy chalcone
compounds and has a flavonoid composition extracted from licorice
roots (15). Results from several
studies indicate that ISL has antitumor, anti-virus and
anti-inflammatory effects, with treatment resulting in increased
vascular elasticity, inhibition of lipid peroxidation and numerous
biological effects, including proliferation, apoptosis and
autophagy (14–19). Its antitumor effects have attracted
much attention in recent years. A previous study revealed that the
active extract containing liquiritin, isoliquiritin and ISL may
inhibit cell proliferation, and induce cell cycle arrest and
apoptosis of A549 cells (26).
However, it is unclear which molecule in the active extract is
responsible for causing this anti-cancer effect. Therefore, the
present study further investigated the effect of ISL on the
biological behaviors of NSCLC cells. In the present study, ISL was
demonstrated to significantly inhibit the proliferation, migration
and invasion of A549 cells, and induce cell apoptosis, suggesting
that ISL may have an anti-cancer effect on the growth and
metastasis of NSCLC cells. In addition, ISL significantly
upregulated the expression of E-cadherin, and downregulated the
expression of N-cadherin and vimentin in A549 cells. E-cadherin is
a marker expressed on epithelial cells, and a decrease in
expression of E-cadherin is one of the primary features of
epithelial-to-mesenchymal transition (EMT), which is a pivotal
mechanism involved in modulation of cell migration and invasion.
Furthermore, N-cadherin and vimentin are mesenchymal markers
involved in EMT process (27). Thus,
the results of the present study suggest that ISL suppresses
migration and invasion of A549 cells via inhibition of the EMT
process. Cell apoptosis, a tightly regulated process of cell death,
is associated to growth, organized stability, tumors, and
autoimmune and neurodegenerative disease (28,29). Since
Kerr established this concept in 1972 (30), the phenomenon of apoptosis has been
extensively researched. Cell apoptosis is currently one of the most
popular fields in life science research; however, the molecular and
biochemical mechanisms of apoptosis have yet to be completely
elucidated. The Bcl-2 and caspase protein families are of the most
well-studied among the multitude of apoptosis-regulating genes
(31). The Bcl-2 family has two
categories: Apoptosis inhibition and apoptosis promotion (32). The Bcl-2 family proteins are important
regulatory factors in response to apoptosis, and Bcl-2/Bax are
well-established as the most important pair of function
contradictory regulate genes in the process of apoptosis regulation
(33). Caspase-3 is an important
apoptosis execution protease, and the association between Bcl-2,
Bax and caspase-3 has become a principal focus in the study of
apoptosis (34). In the present
study, the contribution of Bcl-2, Bax and active caspase-3 proteins
to ISL-induced cell apoptosis was examined. The results
demonstrated that ISL significantly induced cell apoptosis in A549
cells via the mitochondrial apoptosis pathway. The anticancer role
of ISL in the progression of NSCLC will be investigated in animal
models in further studies.
The anti-cancer mechanism of ISL has also been
widely studied in recent years. ISL may cause glioma U87 cells to
enter the S phase and G2/M checkpoint of the cell cycle by
increasing P21/cyclin dependent kinase inhibitor 1A and P27
proteins (35). In prostate cancer,
ISL was demonstrated to downregulate the proliferation of DU145
cells through inhibition of AKT phosphorylation, ERB and PI3K/AKT
signaling pathways (36). Kang et
al (37) demonstrated that ISL
inhibits the formation of new blood vessels through blocking of the
c-Jun N-terminal kinase and p38/MAPK signaling pathways,
subsequently inhibiting the activation of matrix
metalloproteinases. The PI3K/AKT signaling pathway and its
downstream pathways have been demonstrated to serve essential roles
in growth regulation of lung cancer. For example, docosahexaenoic
acid induces cell death in human NSCLC cells by repressing mTOR via
PI3K/AKT inhibition (38). Plumbagin
induces apoptotic and autophagic cell death through inhibition of
the PI3K/Akt/mTOR pathway in human NSCLC cells (39). However, the contribution of the
PI3K/AKT signaling pathway to the anticancer effects of ISL in lung
cancer has received relatively little attention. The present study
demonstrated that ISL significantly reduced the expression of
p-AKT, p-mTOR, and the downstream proteins P70 and Cyclin D1,
suggesting that ISL inhibits the activation of the PI3K/AKT
signaling pathway in A549 cells. Taken together, ISL induces growth
inhibition and apoptosis through inhibiting the activation of the
PI3K/AKT/mTOR signaling pathway in A549 lung cancer cells. It is
suggested that the PI3K/AKT/mTOR signaling pathway serves an
important role in IS -mediated A549 cell apoptosis.
In conclusion, the results of the present study
demonstrate that ISL inhibits the proliferation, migration and
invasion of A549 lung cancer cells and induces cell apoptosis,
which may be associated with the downregulation of the
PI3K/AKT/mTOR signaling pathway proteins, and the downstream
proteins P70 and CyclinD1. And further studies should investigate
ISL as a therapeutic agent for inhibiting the growth and metastasis
of NSCLC.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed in the study are
included in the present published article.
Authors' contributions
TT and HL conceived and designed the study. The
experiments in the present study were performed by TT, JS and JW.
The statistical analysis was performed by YL and HL, and HL has
written the manuscript. All authors have read and approved this
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of Hebei Medical University Affiliated North China
Petroleum Bureau General Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoffman PC, Mauer AM and Vokes EE: Lung
cancer. Lancet. 355:479–485. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bilello KS, Murin S and Matthay RA:
Epidemiology, etiology, and prevention of lung cancer. Clin Chest
Med. 23:1–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sundar R, Soong R, Cho BC, Brahmer JR and
Soo RA: Immunotherapy in the treatment of non-small cell lung
cancer. Lung Cancer. 85:101–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao J, Qiao CR, Ding Z, Sheng YL, Li XN,
Yang Y, Zhu DY, Zhang CY, Liu DL, Wu K and Zhao S: A novel pathway
in NSCLC cells: miR-191, targeting NFIA, is induced by chronic
hypoxia, and promotes cell proliferation and migration. Mol Med
Rep. 15:1319–1325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Centers for Disease Control and Prevention
(CDC), . Recent trends in mortality rates for four major cancers,
by sex and race/ethnicity-united states, 1990–1998. MMWR Morb
Mortal Wkly Rep. 51:49–53. 2002.PubMed/NCBI
|
|
6
|
Scott WJ, Howington J, Feigenberg S,
Movsas B and Pisters K; American College of Chest Physicians, :
Treatment of non-small cell lung cancer stage I and stage II: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132 Suppl 3:S234–S242. 2007. View Article : Google Scholar
|
|
7
|
Rosell R and Karachaliou N: Lung cancer:
Maintenance therapy and precision medicine in NSCLC. Nat Rev Clin
Oncol. 10:549–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun SJ, Lin Q, Ma JX, Shi WW, Yang B and
Li F: Long non-coding RNA NEAT1 acts as oncogene in NSCLC by
regulating the Wnt signaling pathway. Eur Rev Med Pharmacol Sci.
21:504–510. 2017.PubMed/NCBI
|
|
9
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. Ca Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Li ZY, Hou XX, Wang X, Luo YH,
Ying YP and Chen G: Clinical significance and effect of AEG-1 on
the proliferation, invasion, and migration of NSCLC: A study based
on immunohistochemistry, TCGA, bioinformatics, in vitro and in vivo
verification. Oncotarget. 8:16531–16552. 2017.PubMed/NCBI
|
|
11
|
Vaya J, Belinky PA and Aviram M:
Antioxidant constituents from licorice roots: Isolation, structure
elucidation and antioxidative capacity toward LDL oxidation. Free
Radic Biol Med. 23:302–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan SC, Chang YS, Wang JP, Chen SC and
Kuo SC: Three new flavonoids and antiallergic, anti-inflammatory
constituents from the heartwood of dalbergia odorifera. Planta Med.
64:153–158. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto S, Aizu E, Jiang H, Nakadate T,
Kiyoto I, Wang JC and Kato R: The potent anti-tumor-promoting agent
isoliquiritigenin. Carcinogenesis. 12:317–323. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma J, Fu NY, Pang DB, Wu WY and Xu AL:
Apoptosis induced by isoliquiritigenin in human gastric cancer
MGC-803 cells. Planta Med. 67:754–757. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanazawa M, Satomi Y, Mizutani Y, Ukimura
O, Kawauchi A, Sakai T, Baba M, Okuyama T, Nishino H and Miki T:
Isoliquiritigenin inhibits the growth of prostate cancer. Eur Urol.
43:580–586. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsu YL, Kuo PL, Lin LT and Lin CC:
Isoliquiritigenin inhibits cell proliferation and induces apoptosis
in human hepatoma cells. Planta Med. 71:130–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang KL, Hsia SM, Chan CJ, Chang FY, Huang
CY, Bau DT and Wang PS: Inhibitory effects of isoliquiritigenin on
the migration and invasion of human breast cancer cells. Expert
Opin Ther Targets. 17:337–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen XY, Li DF, Han JC, Wang B, Dong ZP,
Yu LN, Pan ZH, Qu CJ, Chen Y, Sun SG and Zheng QS: Reprogramming
induced by isoliquiritigenin diminishes melanoma cachexia through
mTORC2-AKT-GSK3β signaling. Oncotarget. 8:34565–34575.
2017.PubMed/NCBI
|
|
19
|
Hsu YL, Kuo PL, Chiang LC and Lin CC:
Isoliquiritigenin inhibits the proliferation and induces the
apoptosis of human non-small cell lung cancer a549 cells. Clin Exp
Pharmacol Physiol. 31:414–418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cao LJ, Li HD, Yan M, Li ZH, Gong H, Jiang
P, Deng Y, Fang PF and Zhang BK: The protective effects of
isoliquiritigenin and glycyrrhetinic acid against
triptolide-induced oxidative stress in hepG2 cells involve Nrf2
activation. Evid Based Complement Alternat Med. 2016:89121842016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Z, Wang N, Liu P, Chen Q, Situ H, Xie
T, Zhang J, Peng C, Lin Y and Chen J: MicroRNA-25 regulates
chemoresistance-associated autophagy in breast cancer cells, a
process modulated by the natural autophagy inducer
isoliquiritigenin. Oncotarget. 5:7013–7026. 2014.PubMed/NCBI
|
|
22
|
Wu CH, Chen HY, Wang CW, Shieh TM, Huang
TC, Lin LC, Wang KL and Hsia SM: Isoliquiritigenin induces
apoptosis and autophagy and inhibits endometrial cancer growth in
mice. Oncotarget. 7:73432–73447. 2016.PubMed/NCBI
|
|
23
|
Li Y, Zhao H, Wang Y, Zheng H, Yu W, Chai
H, Zhang J, Falck JR, Guo AM, Yue J, et al: Isoliquiritigenin
induces growth inhibition and apoptosis through downregulating
arachidonic acid metabolic network and the deactivation of PI3K/Akt
in human breast cancer. Toxicol Appl Pharmacol. 272:37–48. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu S, Xue J, Yang Y, Zhu H, Chen F, Wang
J, Lou G, Liu Y, Shi Y, Yu Y, et al: Isoliquiritigenin inhibits
interferon-γ-inducible genes expression in hepatocytes through
down-regulating activation of JAK1/STAT1, IRF3/MyD88, ERK/MAPK,
JNK/MAPK and PI3K/Akt signaling pathways. Cell Physiol Biochem.
37:501–514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu G, Zhang W, Bertram P, Zheng XF and
Mcleod H: Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR
pathway in common human tumors. Int J Oncol. 24:893–900.
2004.PubMed/NCBI
|
|
26
|
Zhou Y and Ho WS: Combination of
liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell
death through upregulating p53 and p21 in the A549 non-small cell
lung cancer cells. Oncol Rep. 31:298–304. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao C, Wu CH and Hu HZ: LncRNA UCA1
promotes epithelial-mesenchymal transition (EMT) of breast cancer
cells via enhancing Wnt/beta-catenin signaling pathway. Eur Rev Med
Pharmacol Sci. 20:2819–2824. 2016.PubMed/NCBI
|
|
28
|
Magnaldo T and Sarasin A: Xeroderma
pigmentosum: From symptoms and genetics to gene-based skin therapy.
Cells Tissues Organs. 177:189–198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meier P, Finch A and Evan G: Apoptosis in
development. Nature. 407:796–801. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Borner C: The Bcl-2 protein family:
Sensors and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brown R: The bcl-2 family of proteins. Br
Med Bull. 53:466–477. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao H, Yenari MA, Cheng D, Sapolsky RM
and Steinberg GK: Bcl-2 overexpression protects against neuron loss
within the ischemic margin following experimental stroke and
inhibits cytochrome c translocation and caspase-3 activity. J
Neurochem. 85:1026–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou GS, Song LJ and Yang B:
Isoliquiritigenin inhibits proliferation and induces apoptosis of
U87 human glioma cells in vitro. Mol Med Rep. 7:531–536. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jung JI, Chung E, Mi RS, Seon MR, Shin HK,
Kim EJ, Lim SS, Chung WY, Park KK and Park JH: Isoliquiritigenin
(ISL) inhibits ErbB3 signaling in prostate cancer cells.
Biofactors. 28:159–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kang SW, Choi JS, Choi YJ, Bae JY, Li J,
Kim DS, Kim JL, Shin SY, Lee YJ, Kwun IS and Kang YH: Licorice
isoliquiritigenin dampens angiogenic activity via inhibition of
MAPK-responsive signaling pathways leading to induction of matrix
metalloproteinases. J Nutr Biochem. 21:55–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim N, Jeong S, Jing K, Shin S, Kim S, Heo
JY, Kweon GR, Park SK, Wu T, Park JI and Lim K: Docosahexaenoic
acid induces cell death in human non-small cell lung cancer cells
by repressing mTOR via AMPK activation and PI3K/akt inhibition.
Biomed Res Int. 2015:2397642015.PubMed/NCBI
|
|
39
|
Li YC, He SM, He ZX, Li M, Yang Y, Pang
JX, Zhang X, Chow K, Zhou Q, Duan W, et al: Plumbagin induces
apoptotic and autophagic cell death through inhibition of the
PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells.
Cancer Lett. 344:239–259. 2014. View Article : Google Scholar : PubMed/NCBI
|