Introduction
A major drawback of cancer cell therapy is
chemoresistance of cancer stem cells, resulting in repopulation of
the tumor niche even following a period of prolonged dormancy.
Glioblastoma stem cells are responsible for the maintenance and
phenotype of glioblastoma (astrocytoma grade IV or glioblastoma
multiforme), the most common and aggressive tumor of the central
nervous system, which is characterized by rapid cell proliferation
and diffuse invasion into the healthy tissue (1). Following surgery, radiation and
chemotherapy, the prognosis for patients diagnosed with
glioblastoma remains poor. For instance, the postoperative median
survival time of patients with glioblastoma is 6 months;
radiotherapy increases the survival time of patients to 12 months,
and radiotherapy in combination with the standard chemotherapeutic
agent temozolomide (TMZ; Temodal®) increases the
survival time by a further 2.6 months (total, 14.6 months)
(2). Therefore, the elucidation of
novel and more effective chemotherapeutics that interfere with
glioblastoma stem cell proliferation, particularly invasion, is
required.
Isothiocyanates (ITCs) are natural components of the
Cruciferae family of plants (which includes radish, broccoli or
mustard) that have intrinsic antitumor capacity as previously
demonstrated (3,4). A major advantage of ITCs is that they
selectively elicit an accumulation of reactive oxygen species
(ROS), leading to apoptosis in transformed cells in contrast with
wild-type cells, which are more resistant to ROS (5). Recently, Grzywa et al (6) observed that the application of
diisothiocyanate-derived mercapturic acids was cytotoxic to a human
adenocarcinoma cell line with a drug concentration yielding
half-maximal response (EC50) of 2.02 µM. On the basis of
these data, in the present study, various diisothiocyanate-derived
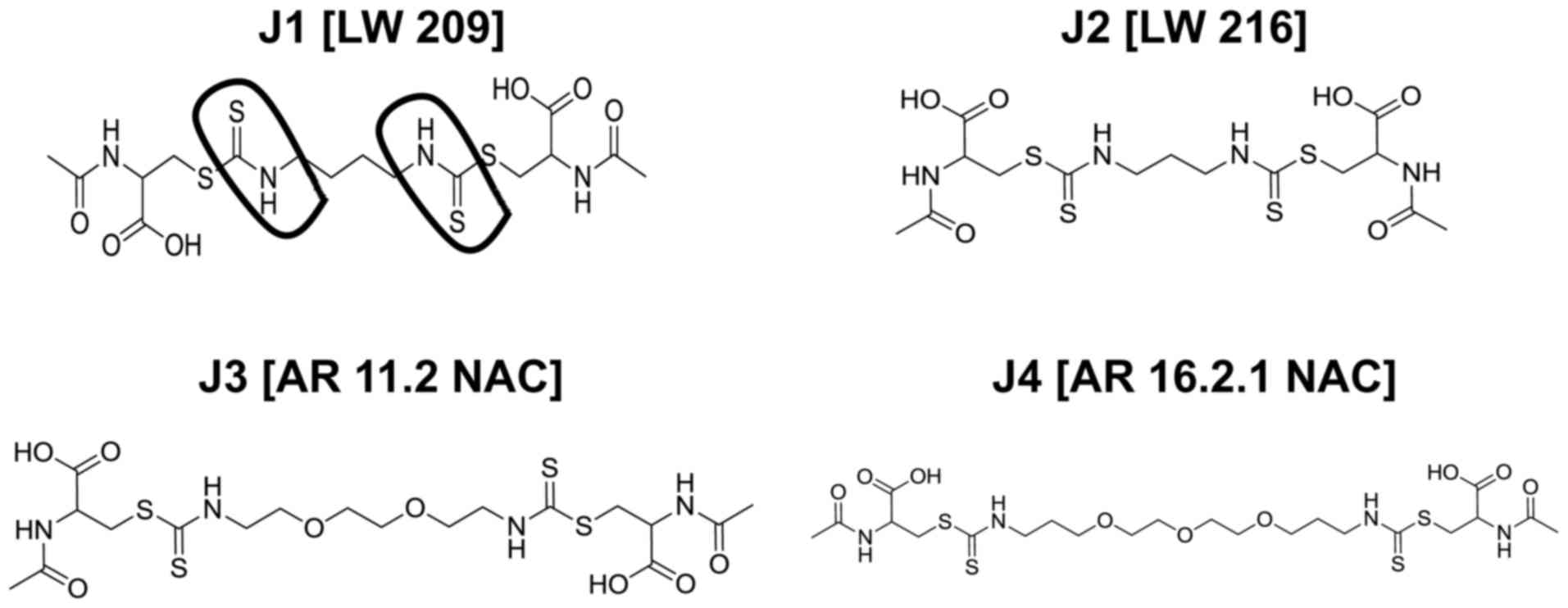
mercapturic acids (J1-J4; Fig. 1)
were investigated, and it was identified that J1-J4 selectively
inhibited cell viability in glioblastoma cells and glioblastoma
stem cells, indicating that these components are promising
antitumor drugs in glioblastoma research.
Materials and methods
Diisothiocyanate-derived mercapturic
acids
Diisothio-cyanate-derived mercapturic acids were
synthesized as described previously (6). The diisothiocyanates (for compounds J3
and J4) were prepared from appropriate diamine (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and carbon disulfide
(Sigma-Aldrich; Merck KGaA) using
2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium
hexa-fluorophosphate (Iris Biotech GmbH, Marktredwitz, Germany) in
the presence of triethylamine (Avantor Performance Materials Poland
S.A., Gliwice, Poland). The diisothiocyanates (for compounds J1 and
J2 commercially available from Sigma-Aldrich; Merck KGaA) and
N-acetyl-L-cysteine (Sigma-Aldrich; Merck KGaA) were mixed with
sodium hydrogen carbonate (Avantor Performance Materials Poland
S.A.) to yield the final product (diisothiocyanate-derived
mercapturic acid).
Peripheral blood mononuclear cells
(PBMCs)
Following Ficoll isolation of human PBMCs from
heparinized blood (buffy coat), PBMCs were resuspended in Roswell
Park Memorial Institute (RPMI-1,640 medium; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 1.5% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc.) and 1% penicillin (120
mg/ml)/streptomycin (120 mg/ml; Thermo Fisher Scientific, Inc.),
and were cultured with J1-J4, TMZ or dimethylsulfoxide (DMSO) for
72 h at 37°C and 5% CO2. The use of PBMCs for in
vitro studies was approved by the local Ethics committee of Ulm
University, Ulm, Germany (no. 327/14).
Glioblastoma cell line
The human glioblastoma cell line U87-MG (U87)
(American Type Culture Collection, Manassas, VA, USA), was cultured
in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and 1% penicillin (120
mg/ml) /streptomycin (120 mg/ml) at 37°C in a 5% CO2
atmosphere.
Sphere-cultured stem cell-enriched
glioblastoma cell populations (SCs)
Astrocytoma grade IV tissue from 3 patients was
obtained during surgery at the hospital in Ulm University Medical
Center in Günzburg, Germany [nos. 35 (44 years, male; sample
collected August 2009), 38 (75 years, male; sample collected, July
2010), and 40 (57 years, female; sample collected, July 2010)] was
minced separately, washed in PBS and incubated with TrypLE™ Express
(Gibco; Thermo Fisher Scientific, Inc.). Cells were filtered and
cultured at 37°C in a 5% CO2 atmosphere in DMEM/Ham's
F-12 medium (Gibco; Thermo Fisher Scientific, Inc.) containing
L-glutamine, 0.01% (v/v) epidermal growth factor (EGF; Biomol GmbH,
Hamburg, Germany), 0.04% (v/v) fibroblast growth factor (FGF;
Miltenyi Biotec GmbH, Bergisch Gladbach, Germany), 1% (v/v) B27
(Gibco; Thermo Fisher Scientific, Inc.), 2% fungizone (Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin (120 mg/ml)
/streptomycin (120 mg/ml; Thermo Fisher Scientific, Inc.) (7). These cells were defined as
sphere-cultured stem cell-enriched glioblastoma cell populations
(SCs; identified as SC35, SC38 and SC40 according to the patient
number from which they derived). Stem cell and differentiation
markers were expressed accordingly (8). To obtain adherent glioblastoma cells
(PCs; identified as PC35, PC38 and PC40 according to the patient
number from which they derived), SCs were kept at 37°C (5%
CO2 atmosphere) in DMEM supplemented with 10% FBS with 2
mM glutamine and 1% penicillin/streptomycin (120 mg/ml each; Thermo
Fisher Scientific, Inc.) as SCs differentiate into PCs when FBS is
present in the culture medium. Use of SCs and patient samples was
approved by the local ethics committee of Ulm University
(#162/10).
Determination of cellular metabolic
activity (cell viability)
An MTT test was performed to assay the metabolic
activity of the indicated cell populations as a measure of cell
viability. The method is based on the reduction of the yellow
tetrazolium compound MTT by metabolically active cells to an
intracellular purple formazan, which is spectrophotometrically
quantified. Adherent glioblastoma cells (U87, PC35, PC38 or PC40)
were seeded in 96-well flat-bottomed tissue culture plates at
1.5×104 cells/ml in 100 µl DMEM containing 10% FBS and
1% penicillin/streptomycin. SCs (SC35, SC38 or SC40) were seeded in
96-well flat-bottomed tissue culture plates at 1.5×104
cells/ml in 90 µl DMEM/Ham's F-12 containing 0.01% EGF, 0.04% FGF,
1% B27, 2% fungizone and 1% penicillin/streptomycin. Freshly
isolated PBMCs were seeded in 96-well flat-bottomed tissue culture
plates at 5×106 cells/ml in 90 µl RPMI-1,640 medium
containing 10% FBS, 1% penicillin/streptomycin, but lacking
L-glutamine and phenol red. After 24 h of incubation, the medium
was removed. Various concentrations (0.01, 0.1, 1, 10 and 100 µM)
of J1, J2, J3, J4 or TMZ (DMSO served as a control) were prepared
in DMEM containing 1.5% FBS and 1% penicillin/streptomycin, and
added to U87, PC35, PC38 and PC40 cells (final volume, 100 µl); in
the case of SCs or PBMCs, the medium was unchanged and the J1, J2,
J3, J4 or TMZ was added directly (final volume, 100 µl). The cells
were cultured for a further 3 days prior to removal of the medium.
U87, PC35, PC38 and PC40 cells were incubated with 100 µl MTT
working solution (Sigma-Aldrich; Merck KGaA), diluted 1:5 in
RPMI-1640 medium without L-glutamine and phenol red. SC plates were
centrifuged for 390 × g for 5 min at room temperature, the
supernatant was removed, and cells were resuspended in 100 µl MTT
working solution; 25 µl MTT working solution was added directly to
PBMCs. Cells were incubated for 3 h at 37°C. Following incubation,
formazan crystals were solubilized with 100 µl propan-2-ol. Cell
viability was determined by measuring the optical density at 550 nm
using a microplate spectrometer (Tecan Spectra Classic, Tecan Group
Ltd., Männedorf, Switzerland).
Microscopy images
Images were captured using a PrimoVert microscope
and AxioCam ICc1 camera (Zeiss AG, Oberkochen, Germany).
Analysis of DNA content
U87 cells were incubated with J1, J2, J3, J4 or TMZ,
or a combination of TMZ with J1, J2, J3 or J4 for 144 h at 37°C
(J1-J4 were used at their EC50 values of J1, 250 nM; J2,
290 nM; J3, 2200 nM; J4, 500 nM; TMZ was used at 100 µM). The cell
death readout used was DNA fragmentation (sub-G1
population), a hallmark of apoptosis, as assessed by
fluorescence-activated cell sorting using a FACScan instrument and
CellQuest software 5.1 (BD Biosciences, Franklin Lakes, NJ, USA)
analysis of DNA fragmentation of propidium iodide-stained nuclei as
previously described (9). The
specific (induced) DNA fragmentation was calculated as follows:
100× [experimental DNA fragmentation (%)-spontaneous DNA
fragmentation (%)]/[100%-spontaneous DNA fragmentation (%)]. For
cell cycle distribution of live cells, only populations in clearly
identifiable phases of the cell cycle, i.e., G0/1-, S-
or G2/M-phase, were considered. For analysis of
polyploidy, cells with DNA content exceeding that observed in the
G2/M-phase were compared with the total cell numbers
obtained from the cell cycle analysis. For each analysis, ~10,000
cells were assayed.
Alterations in cell number
U87 cells were seeded and allowed to proliferate
with or without J1, J2, J3, J4 or TMZ for the indicated times,
prior to treatment with a trypsin/EDTA solution (Biochrom GmbH,
Berlin, Germany) to suspend cells. The cell suspension was diluted
1:100 in CASYton solution (Innovatis AG, Reutlingen, Germany) and
cell numbers were determined using a CASY® 1 DT cell
counter (Innovatis AG).
Statistical analysis
Results are presented as the mean ± standard error
of the mean. Statistical analysis was assessed using an unpaired
two-tailed Student's t-test and EC50 values were
calculated using Prism (version 6; GraphPad Software, Inc., La
Jolla, CA, USA).
Results and Discussion
Diisothiocyanate-derived mercapturic
acids are cytotoxic to the glioblastoma cell line (U87)
To be viable for treatment of glioblastoma,
diisothiocyanate-derived mercapturic acids in glioblastoma
treatment are required to be able to traverse the blood-brain
barrier (BBB). Therefore, compounds J1-J4 (Fig. 1) (6)
were analyzed using online BBB Predictor software admetSAR
(lmmd.ecust.edu.cn:8000) (10). J1-J4 were identified to be
theoretically able to cross the BBB, making delivery of such
diisothiocyanate-derived mercapturic acids to the site of
requirement possible.
It was determined whether diisothiocyanate-derived
mercapturic acids are a potential therapeutic option for treatment
of glioblastoma. U87 cells were incubated with various
concentrations of J1, J2, J3, J4 or TMZ, and the metabolic activity
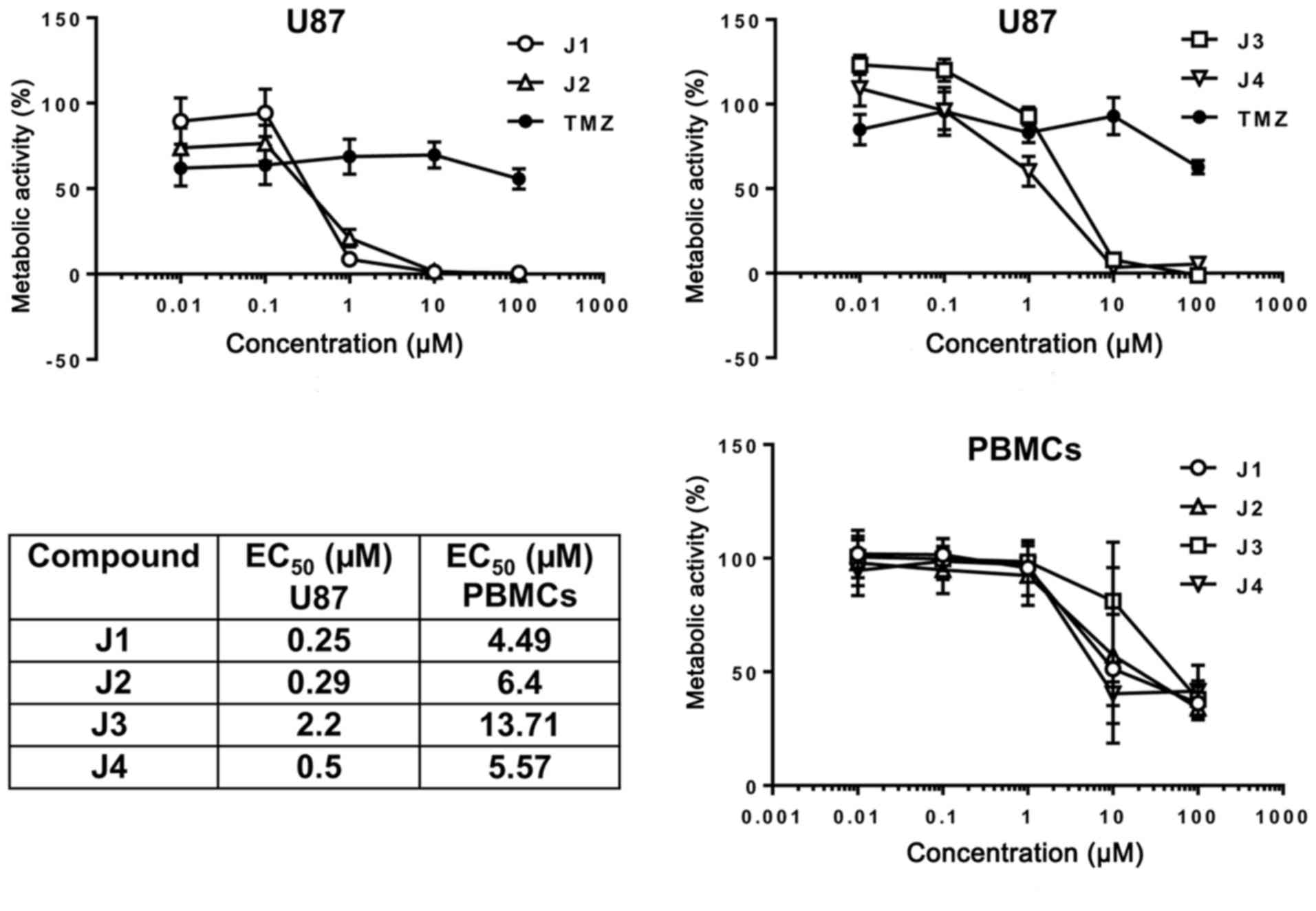
of U87 cells was determined using an MTT assay. J1 and J2 were
identified to markedly decreased U87 cell viability at a final
concentration of 1 µM with comparatively low EC50 values
of 250 and 290 nM, respectively, in contrast with J3 (2.2 µM) and
J4 (500 nM) (Fig. 2). At <1 µM J1,
J2, J3 or J4, there was no marked effect on PBMCs freshly isolated
from peripheral blood, and the EC50 values were ~10-fold
higher than for U87 cells. Furthermore, U87 cells exhibited
alterations in morphology when treated with J1, J2, J3 or J4; in
particular, J1 and J2 caused alterations in the size and cell
density of U87 cells at a final concentration of as low as 0.01 µM
(Fig. 3, microscopic images).
Notably, the high concentration of TMZ used did not elicit a
decrease in cell viability of <50 %, i.e., U87 cells exhibit
increased sensitivity to diisothiocyanate-derived mercapturic acids
than to the chemotherapeutic agent TMZ.
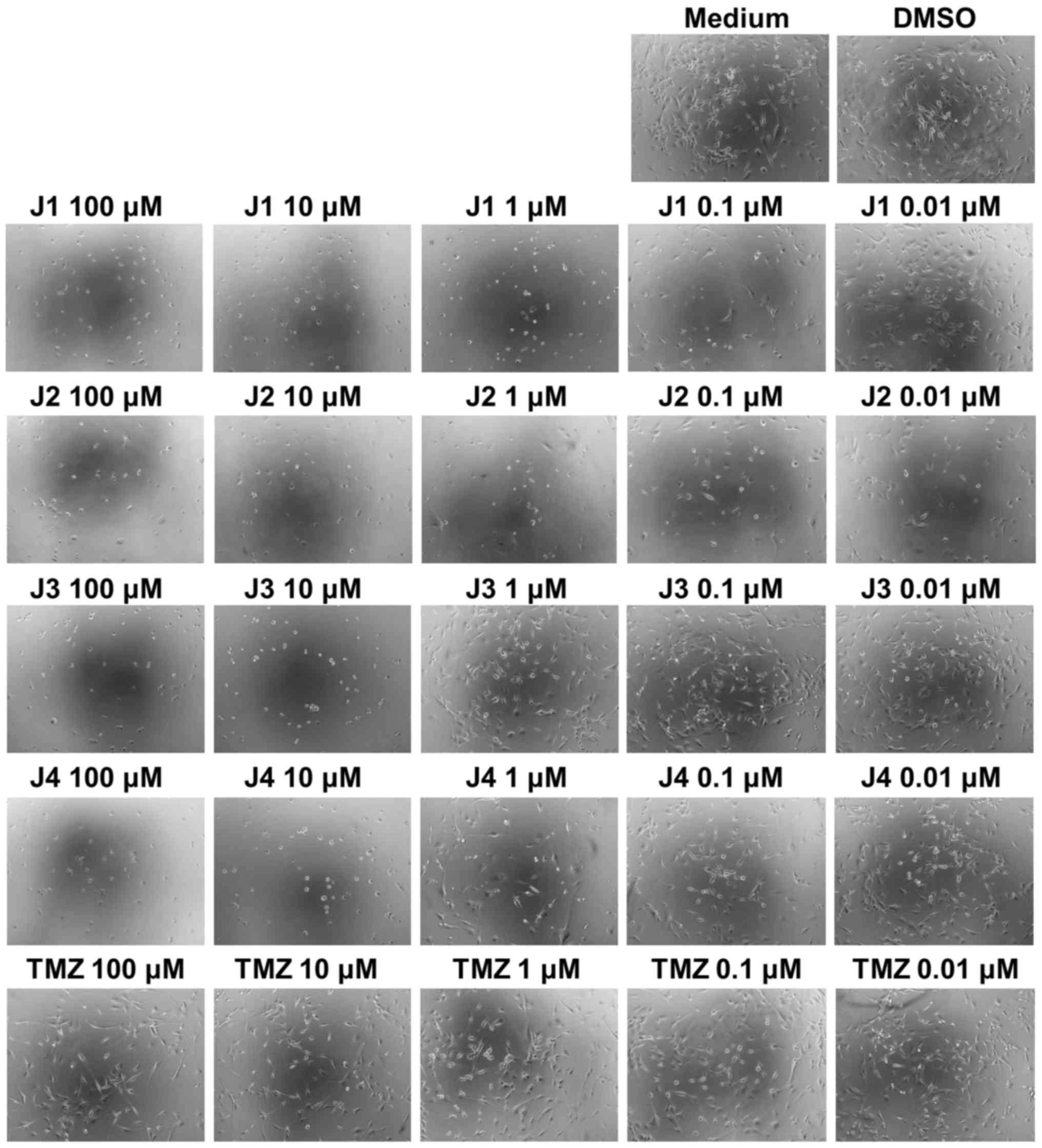 | Figure 3.Microscopic images of U87 cells
following treatment with medium, DMSO, J1, J2, J3, J4 or TMZ. U87
cells were treated with various concentrations of J1, J2, J3, J4 or
TMZ. DMSO and medium treatments served as controls. After 72 h,
images were captured under a light microscope. Magnification, ×10.
DMSO, dimethylsulfoxide; TMZ, temozolomide. |
Furthermore, it was assessed whether EC50
values of J1 (250 nM), J2 (290 nM), J3 (2.2 µM) or J4 (500 nM), or
the combination with TMZ (100 µM) were able to induce apoptosis in
U87 cells by investigating the cell cycle distribution. Treatment
with relatively low concentrations of diisothiocyanate-derived
mercapturic acids caused a significantly prolonged negative effect
on cell numbers for J1, J2 and J3 (Fig.
4A). Although only limited induction of apoptosis was observed
upon single treatment with J1, J2, J3 or J4, it is noteworthy that
robust cell death induced by TMZ, the standard chemotherapeutic
agent used to treat glioblastoma, was dependent on high
concentrations of the drug (Fig. 4B;
see also Fig. 2). Furthermore, single
treatment with J1, J2, J3 or J4 affects the cell cycle distribution
and the nuclear integrity of U87 cells (Fig. 4C and D), possibly indicating that the
cell cycle distribution of U87 cells is altered by J1. These
results demonstrate that diisothiocyanate-derived mercapturic acids
are cytotoxic to the glioblastoma cell line U87.
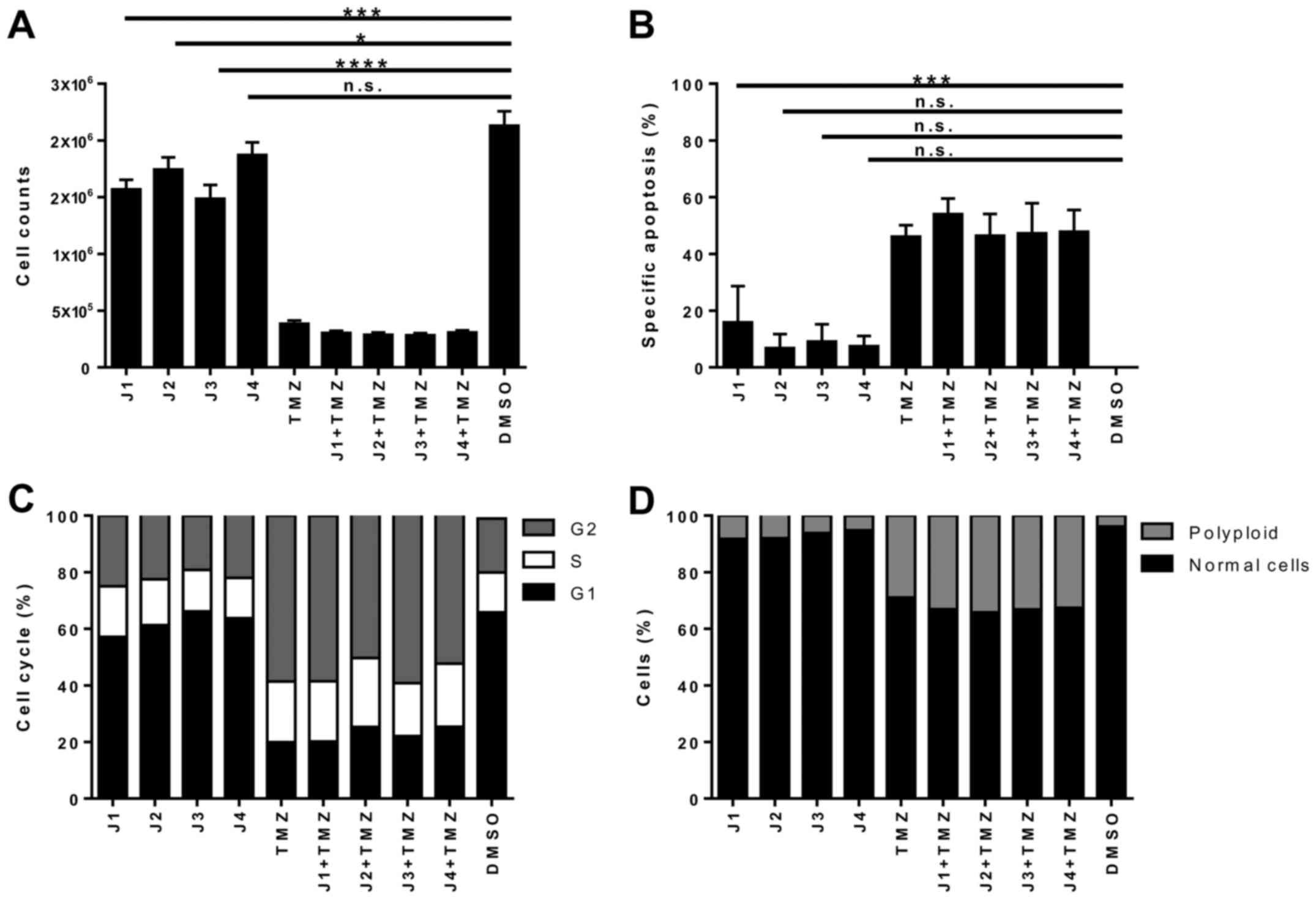 | Figure 4.Apoptosis and cell distribution in U87
cells treated with J1-J4. U87 cells were incubated with J1, J2, J3,
J4 or TMZ, or a combination of TMZ with J1, J2, J3 or J4, for 144
h. J1-J4 were used at their EC50 values (J1, 250 nM; J2,
290 nM; J3, 2,200 nM; J4, 500 nM), and TMZ was used at 100 µM. (A)
Cell numbers were determined using a CASY® 1 DT cell
counter. (B) DNA fragmentation, (C) cell cycle distribution of live
cells, and (D) analysis of polyploidy, determined using propidium
iodide staining and flow cytometry. Results are from three
independent experiments. *P≤0.05; ***P≤0.001; ****P≤0.0001. n.s.,
not significant; TMZ, temozolomide; DMSO, dimethylsulfoxide;
EC50, drug concentration yielding half-maximal
response. |
Viability of glioblastoma stem cells
is decreased by diisothiocyanate-derived mercapturic acids
Further experiments were performed to address the
question of whether the viability of primary patient-derived
glioblastoma stem cells or primary glioblastoma cells is
susceptible to diisothiocyanate-derived mercapturic acids. To this
end, SCs from 3 different glioblastoma patients (SC35, SC38 and
SC40) or SCs differentiated using FBS (PCs; PC35, PC38 and PC40)
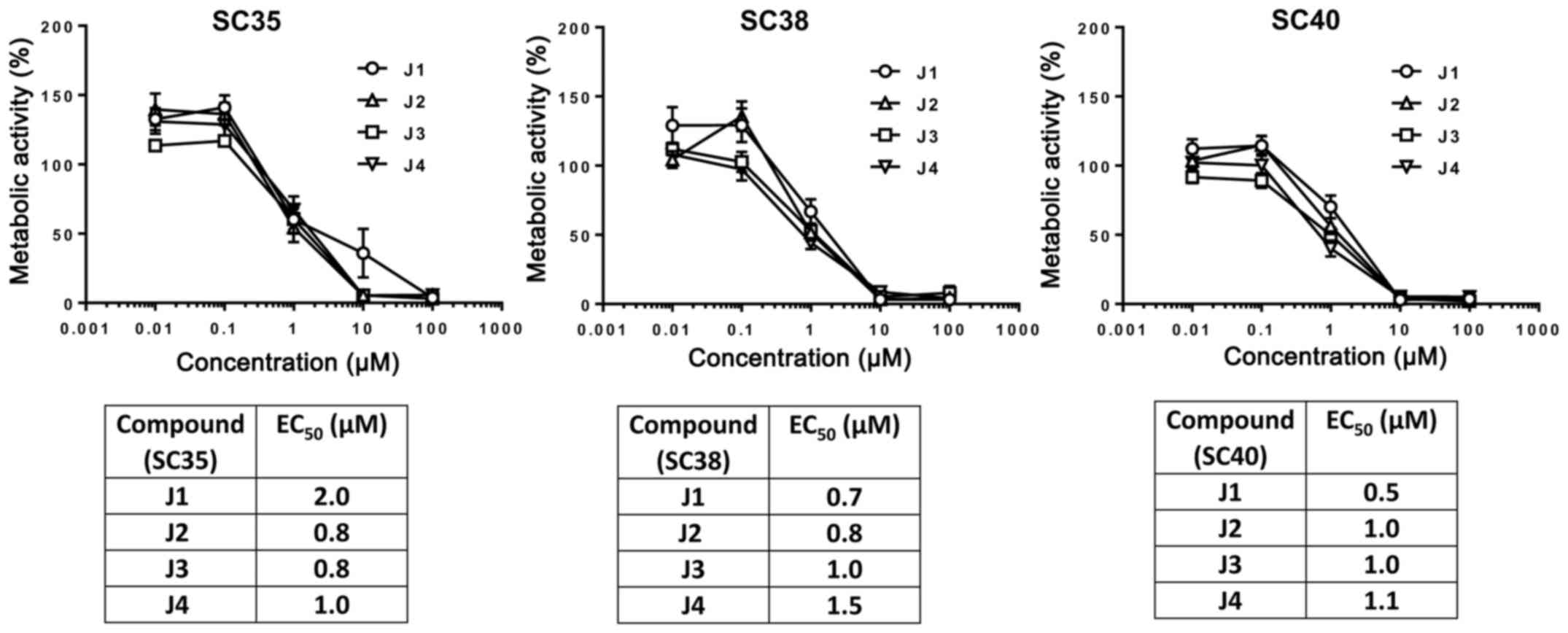
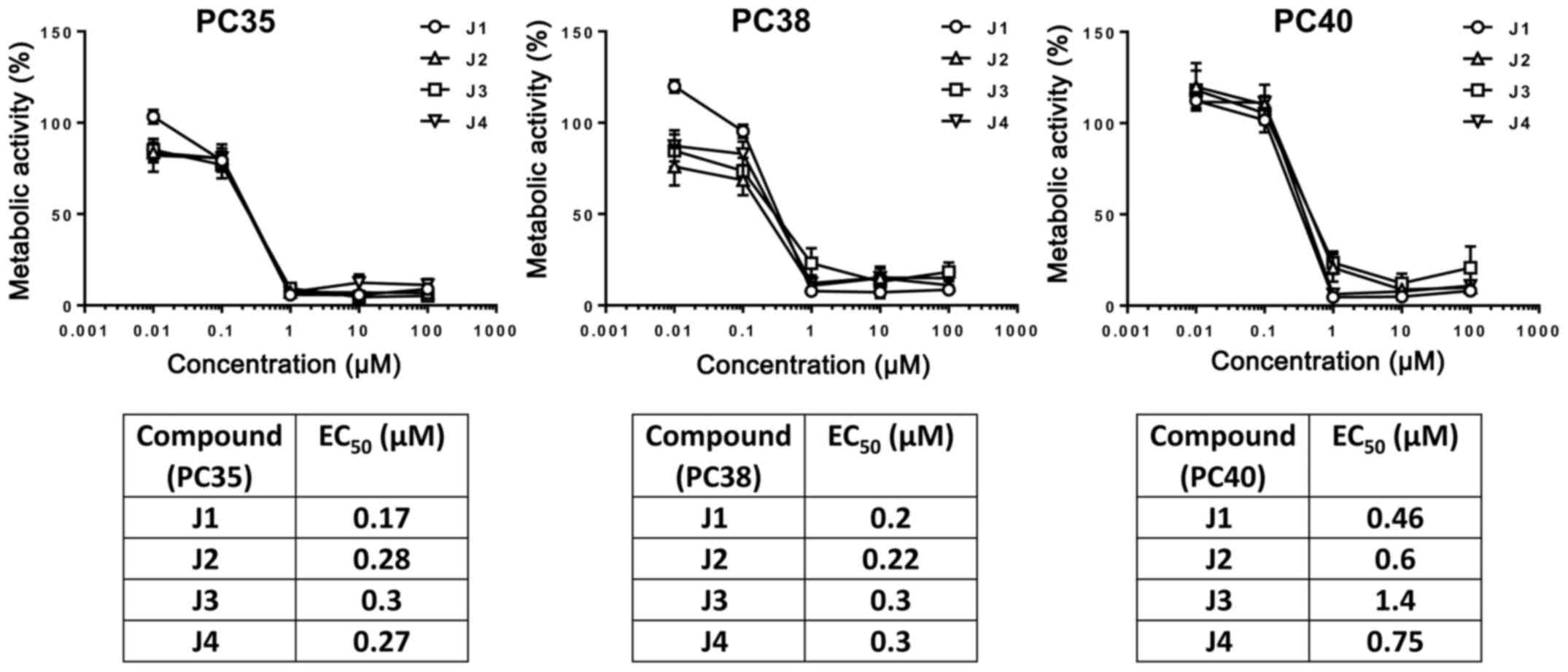
were incubated with J1, J2, J3 or J4, and cell viability was
measured using an MTT assay. The cell viability of SCs or PCs was
gradually decreased by increasing the concentration of J1, J2, J3
or J4 (Figs. 5 and 6). These compounds at a concentration of 1
µM decreased the cell viability of PCs markedly in contrast with
SCs which exhibited decreases in cell viability of 50% following
treatment with J1, J2, J3 or J4 which may be due to distinct
proliferation rates (11).
Importantly, the viability of PCs and SCs may be decreased by
diisothiocyanate-derived mercapturic acids; however, SCs are more
resistant to diisothiocyanate-derived mercapturic acids.
The presence of invasive glioblastoma stem cells in
glioblastoma (12) is thought to be
the reason for poor survival prognosis. These cells contribute to
recurrence and are highly resistant to typical treatments, which is
partially due to the increased expression of the multidrug
resistance of ATP-binding cassette transporter protein breakpoint
cluster region pseudogene 1, DNA repair protein
O-6-methylguanine-DNA methyltransferase and anti-apoptotic products
in cluster of differentiation 133-expressing glioblastoma stem
cells (13). This may explain, at
least in part, why in the present study PCs are more sensitive to
diisothiocyanate-derived mercapturic acids compared with SCs.
SCs exhibit increased therapy-resistance and are the
reason for tumor recurrence (14);
therefore, novel substances were sought which had been previously
been identified to be selectively toxic to tumor cells. ITCs have
been identified to exhibit a selective inhibitory capacity towards
tumorigenesis (4). For instance, the
ITC iberin induced apoptosis, inhibited tumor cell growth and was
cytotoxic to the glioblastoma cell line SNB19 (15). Benzyl ITCs exhibited a decrease in
proliferation, invasion and cell viability of U87 cells with an
EC50 of 12.6 µM (16), and
a similar effect was identified by using the glioblastoma cell line
GBM 8401 (EC50, 6 µM) (17,18).
Furthermore, phenethyl ITC induced apoptosis in GBM 8401 cells at a
final concentration of 8 µM (19). In
the present study, it was demonstrated that the recently identified
diisothiocyanate-derived mercapturic acids (6) are selectively cytotoxic to the
glioblastoma cell line U87 (EC50 for J1, 250 nM),
differentiated glioblastoma cells and glioblastoma stem cells at
much lower concentrations compared with ITCs.
In a further set of experiments, it was investigated
whether J1-J4 may lower the threshold of intrinsic TMZ resistance
in SCs. To address this, J1, J2, J3 or J4 (at EC50) were
co-cultured (3 days) with 0.6 µg/ml (3.09 µM) TMZ which represents
the concentration in the brain following treatment with TMZ
(20). No sensitizing effect of J1-J4
for TMZ-induced apoptosis was identified when TMZ was used at
physiologically relevant concentrations (data not shown).
Diisothiocyanate-derived mercapturic acids exert a more potent
effect in comparison with TMZ, but do not exhibit a sensitizing
effect for TMZ-mediated apoptosis.
The cell viability of PBMCs was not impaired by low
concentrations of diisothiocyanate-derived mercapturic acids
(Fig. 2), which is important since a
chemopreventive mediator should activate an antitumor immune
response and not inhibit the function of immune cells (21). Therefore, diisothiocyanate-derived
mercapturic acids are potential therapeutic components to eliminate
glioblastoma stem cells and may be considered for novel therapeutic
treatments for glioblastoma.
Acknowledgements
K-MD and M-AW were partially supported by the
Förderkreis für Tumor-und Leukämiekranke Kinder Ulm e.V., T.B. was
supported by Alexander von Humboldt Polish Honorary Research
Scholarship (grant no. DPK-422-1658/2013), and J.O. was supported
by the National Science Center Poland (grant no.
2011/03/B/ST5/01058).
Glossary
Abbreviations
Abbreviations:
|
ITCs
|
isothiocyanates
|
|
PCs
|
SC-derived differentiated/adherent
glioblastoma cells
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
ROS
|
reactive oxygen species
|
|
SCs
|
sphere-cultured stem cell-enriched
glioblastoma cell populations
|
|
TMZ
|
temozolomide
|
References
|
1
|
Jue TR and McDonald KL: The challenges
associated with molecular targeted therapies for glioblastoma. J
Neurooncol. 127:427–434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koukourakis MI, Mitrakas AG and
Giatromanolaki A: Therapeutic interactions of autophagy with
radiation and temozolomide in glioblastoma: Evidence and issues to
resolve. Br J Cancer. 114:485–496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dinkova-Kostova AT and Kostov RV:
Glucosinolates and isothiocyanates in health and disease. Trends
Mol Med. 18:337–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SV and Singh K: Cancer
chemoprevention with dietary isothiocyanates mature for clinical
translational research. Carcinogenesis. 33:1833–1842. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Trachootham D, Zhou Y, Zhang H, Demizu Y,
Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J and
Huang P: Selective killing of oncogenically transformed cells
through a ROS-mediated mechanism by beta-phenylethyl
isothiocyanate. Cancer Cell. 10:241–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grzywa R, Winiarski Ł, Psurski M, Rudnicka
A, Wietrzyk J, Gajda T and Oleksyszyn J: Synthesis and biological
activity of diisothiocyanate-derived mercapturic acids. Bioorg Med
Chem Lett. 26:667–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schneider M, Ströbele S, Nonnenmacher L,
Siegelin MD, Tepper M, Stroh S, Hasslacher S, Enzenmüller S,
Strauss G, Baumann B, et al: A paired comparison between
glioblastoma ‘stem cells’ and differentiated cells. Int J Cancer.
138:1709–1718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ströbele S, Schneider M, Schneele L,
Siegelin MD, Nonnenmacher L, Zhou S, Karpel-Massler G, Westhoff MA,
Halatsch ME and Debatin KM: A potential role for the inhibition of
PI3K signaling in glioblastoma therapy. PLoS One. 10:e01316702015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Westhoff MA, Zhou S, Bachem MG, Debatin KM
and Fulda S: Identification of a novel switch in the dominant forms
of cell adhesion-mediated drug resistance in glioblastoma cells.
Oncogene. 27:5169–5181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng F, Li W, Zhou Y, Shen J, Wu Z, Liu
G, Lee PW and Tang Y: admetSAR: A comprehensive source and free
tool for evaluating chemical ADMET properties. J Chem Inf Model.
52:3099–3105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider M, Ströbele S, Nonnenmacher L,
Siegelin MD, Tepper M, Stroh S, Hasslacher S, Enzenmüller S,
Strauss G, Baumann B, et al: A paired comparison between
glioblastoma ‘stem cells’ and differentiated cells. Int J Cancer.
138:1709–1718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
13
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem
cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jordan CT, Guzman ML and Noble M: Cancer
stem cells. N Engl J Med. 355:1253–1261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jadhav U, Ezhilarasan R, Vaughn SF, Berhow
MA and Mohanam S: Dietary isothiocyanate iberin inhibits growth and
induces apoptosis in human glioblastoma cells. J Pharmacol Sci.
103:247–251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Y, Liu A, Zhang X, Qi L, Zhang L, Xue
J, Liu Y and Yang P: The effect of benzyl isothiocyanate and its
computer-aided design derivants targeting alkylglycerone phosphate
synthase on the inhibition of human glioma U87MG cell line. Tumour
Biol. 36:3499–3509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang NY, Chueh FS, Yu CC, Liao CL, Lin JJ,
Hsia TC, Wu KC, Liu HC, Lu KW and Chung JG: Benzyl isothiocyanate
alters the gene expression with cell cycle regulation and cell
death in human brain glioblastoma GBM 8401 cells. Oncol Rep.
35:2089–2096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shang HS, Shih YL, Lu TJ, Lee CH, Hsueh
SC, Chou YC, Lu HF, Liao NC and Chung JG: Benzyl isothiocyanate
(BITC) induces apoptosis of GBM 8401 human brain glioblastoma
multiforms cells via activation of caspase-8/bid and the reactive
oxygen species-dependent mitochondrial pathway. Environmental
Toxicology. 31:1751–1760. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chou YC, Chang MY, Wang MJ, Harnod T, Hung
CH, Lee HT, Shen CC and Chung JG: PEITC induces apoptosis of human
brain glioblastoma GBM8401 cells through the extrinsic- and
intrinsic -signaling pathways. Neurochem Int. 81:32–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Portnow J, Badie B, Chen M, Liu A,
Blanchard S and Synold TW: The neuropharmacokinetics of
temozolomide in patients with resectable brain tumors: Potential
implications for the current approach to chemoradiation. Clin
Cancer Res. 15:7092–7098. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel MA, Kim JE, Ruzevick J, Li G and Lim
M: The future of glioblastoma therapy: Synergism of standard of
care and immunotherapy. Cancers (Basel). 6:1953–1985. 2014.
View Article : Google Scholar : PubMed/NCBI
|