Introduction
Hepatocellular carcinoma (HCC) is among the most
lethal types of cancer, characterized by diagnosis at an advanced
stage with regional vascular involvement, and distant metastasis
(1). Hepatitis B virus (HBV)
infection is one of the most common risk factors for HCC,
particularly in Asia (2).
Hepatitis B virus × (HBx), a protein encoded by HBV
virus, is essential for viral replication. Previous studies have
demonstrated that HBx contributes to the progression of HCC by
interacting with certain signal pathways, and promoting
proliferation and invasive potential of HCC cells (3–6). HBx
activates phosphatidylinositol 3-kinase (PI3K)/protein kinase B
(AKT), the Notch signal pathway, and various transcription factors,
including nuclear factor-kB (NF-kB) and activator protein 1 (AP-1)
(3–5).
However, the precise mechanisms by which HBx promotes the
epithelial-to-mesenchymal transition (EMT) and metastasis of HCC
remain unclear.
High mobility group AT-hook 2 (HMGA2), an
architectural transcription factor, is overexpressed during
embryogenesis, but not expressed in normal adult tissues (7,8). Previous
studies have reported HMGA2 is overexpressed in a variety of
tumors, including HCC, breast cancer, non-small-cell lung cancer
and gastric cancer (9–12). HMGA2 is involved in various essential
biological processes, including DNA repair, apoptosis, cell
proliferation, EMT and telomere restoration by regulating a wide
range of gene expression levels in HCC (13–16). Given
that HBx and HMGA2 serve important roles in HCC metastasis, the
effects of HBx overexpression on EMT markers, invasion and
metastasis of HCC cells were investigated in the present study.
Furthermore, the role of HMGA2 in HBx-mediated HCC metastasis was
explored.
Materials and methods
Cell culture
The HCCLM3 cell line was obtained from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in a humidified atmosphere of 5%
CO2 at 37°C in RPMI-1640 medium supplemented with 10%
fetal bovine serum (FBS) (both from Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA).
HBx-expression vector construct and
transfection
The construction of the HBx-expressing vector
(pcDNA3.1-HBx) and control vector (pCDNA3.1-EGFP Vector) has been
described in a previous study (5,17). A
full-length HBx gene was inserted into a pcDNA3.1 vector, and was
identified using polymerase chain reaction (PCR), restriction
endonuclease digestion and DNA sequencing methods. The PCR primers
were as follows: HBx forward, 5′-CCGCTCGAGATGGCTGCTAGGCTGTGCTG-3′
and reverse, 5′-CGGAATTCTTAGGCAGAGGTGAAAAAGTTG-3′. The PCR product
was digested with XhoI and BglII. The EGFP was
inserted into a pcDNA3.1 vector between EcoRI and NotI as a
control. The PCR primers for the control vector were as follows:
CMV-f 5′-CGCAAATGGGCGGTAGGCGTG-3′ and BGH-r
5′-TAGAAGGCACAGTCGAGG-3′. All ligated vectors were confirmed by DNA
sequence analysis. HCCLM3 cells were transfected with the
HBx-expressing vector, and then screened with puromycin to obtain
stable cell clones. The stable HBx-expressing cells were termed
HCCLM3-HBx cells.
RNA interference
The stable HBx-expressing HCCLM3 cell line was
further assigned into two subgroups: HBx group and HMGA2 knockdown
group [HBx + HMGA2 small interfering RNA (siRNA)]. The HMGA2 siRNA
and corresponding control siRNA were designed and synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China). The HMGA2 siRNA
target sequences was as follows: 5′-GGAAATGGCCACAACAAGTTG-3′. The
cells were transfected using Lipofectamine 2000 (cat. no. 11668019;
Invitrogen; Thermo Fisher Scientific, Inc, Waltham, MA, USA)
according to the manufacturer's protocol. Briefly, the cells were
seeded on 6-well plates at a density of 5×105
cells/well, and cultured for 48 h to reach 80% confluence. The
cells were washed twice with PBS. A mixture of individual HMGA2
siRNA (1 µg/ml), transfection medium and Lipofectamine 2000 was
added. After 30 min, 500 µl of Opti-MEM (cat. no. 31985062; Gibco;
Thermo Fisher Scientific, Inc.) was added. After 6 h, the
transfection medium was removed, and the cells were cultured in
RPMI-1640 medium with 10% fetal bovine serum. The gene silencing
efficiency was confirmed by western blotting and
immunocytochemistry 48 h after the transfection.
Proliferation assays
The cells were seeded at a density of
2×103 cells/well in 96-well plates and incubated with
RPMI-1640 medium at 37°C for 1–7 days. PBS (cat. no. SH30256.01;
Hyclone, Logan, UT, USA) was used to dissolve the purple formazan.
MTT was added to each well and incubated at 37°C for 4 h. The
absorbance was measured using a microplate reader with a wavelength
of 490 nm. Each experiment was repeated three times.
Transwell migration and invasion
assays
The invasion assay was performed as follows. At 48 h
after the transfection, 5×103 cells were seeded into the
upper compartments of a 24-well Transwell chamber, which was coated
with 100 µl Matrigel (200 µg/ml; cat. no. 356234; BD Biosciences,
Franklin Lakes, NJ, USA). The medium contained with 20% FBS was
added into the lower compartment. After 24 h incubation, the filter
membrane was washed with PBS. The cells were stained with 0.05%
crystal violet and fixed with 10% formaldehyde. The cells in five
random fields of the membrane were counted with microscope. Cell
migration assays were performed according to the same protocol,
except without Matrigel-coating.
Scratch wound-healing assays
A total of 1×106 cells were seeded into
6-well tissue culture plates and grown to reach 80% confluency. A
linear wound was created in the cell surface using a 10-µl
sterilized pipette tip. The wounded cell layers were washed twice
with PBS. The migrated distance at the wound space was measured at
0, 24 and 48 h in five random microscopic fields at magnification,
×40 under an Olympus BX53 microscope (Olympus Corporation, Tokyo,
Japan). Each independent experiment was repeated three times.
Immunofluorescence
A total of 2×105 cells were seeded and
cultured onto glass cover slips in 24-well plates. Then, they were
fixed in 4% paraformaldehyde for 20 min at 37°C, and incubated with
a primary antibody against HMGA2 (rabbit anti-human; 1:100; cat.
no. ab97276; Abcam, Cambridge, UK) overnight at 4°C. Next, the
slides were incubated with an Alexa Fluor 594-conjugated secondary
antibody (anti-rabbit immunoglobulin G; 1:500; cat. no. 7074; CST,
Boston, USA) for 1 h at room temperature. Lastly, the cells were
stained with DAPI at 37°C for 5 min and observed using fluorescence
microscopy at ×200 magnification.
Western blot analysis
The protein was extracted from the cells with 10%
MSDS (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and the
protein quantity was determined using a bicinchoninic acid assay.
The mass of the proteins loaded in per lane was 80 µg. The proteins
were separated using 10% SDS-PAGE, and then transferred onto
polyvinylidene fluoride membranes. The membranes were blocked in 5%
bovine serum albumin (cat. no. 10099141; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C for 1 h, and washed with TBST buffer
three times. The membranes were exposed to primary rabbit
anti-human HMGA2 antibodies (cat. no. ab97276; Abcam, San
Francisco, USA), HBx (cat. no. ab2741; Abcam, Cambridge, UK),
E-cadherin, Vimentin, N-cadherin (all 1:500; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and GAPDH (1:3,000,
ProteinTech Group, Inc., Chicago, IL, USA) overnight at 4°C. The
membranes were washed with TBST three times, and incubated with
secondary antibody (anti-rabbit Immunoglobulin G; 1:2,000; cat. no.
7074; CST, Boston, USA) for 2 h at room temperature, and washed
with TBST three times. The bands were visualized with the WEST ZOL
Plus system and quantified using ImageJ software (version 1.44P;
National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
Statistical analysis was performed using the SPSS
version 18.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation of three independent experiments, and
were analyzed using the Student's t-test or one-way analysis of
variance with the Least Significant Difference (LSD) post hoc test
to determine the different between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
HBx promotes proliferation, migration,
invasion, and EMT of HCC cells
In order to investigate the effect of HBx on the
proliferation of HCC cells, HCCLM3 cells were transfected with the
HBx-expressing (pcDNA3.1-HBx) and control vectors (NC). The
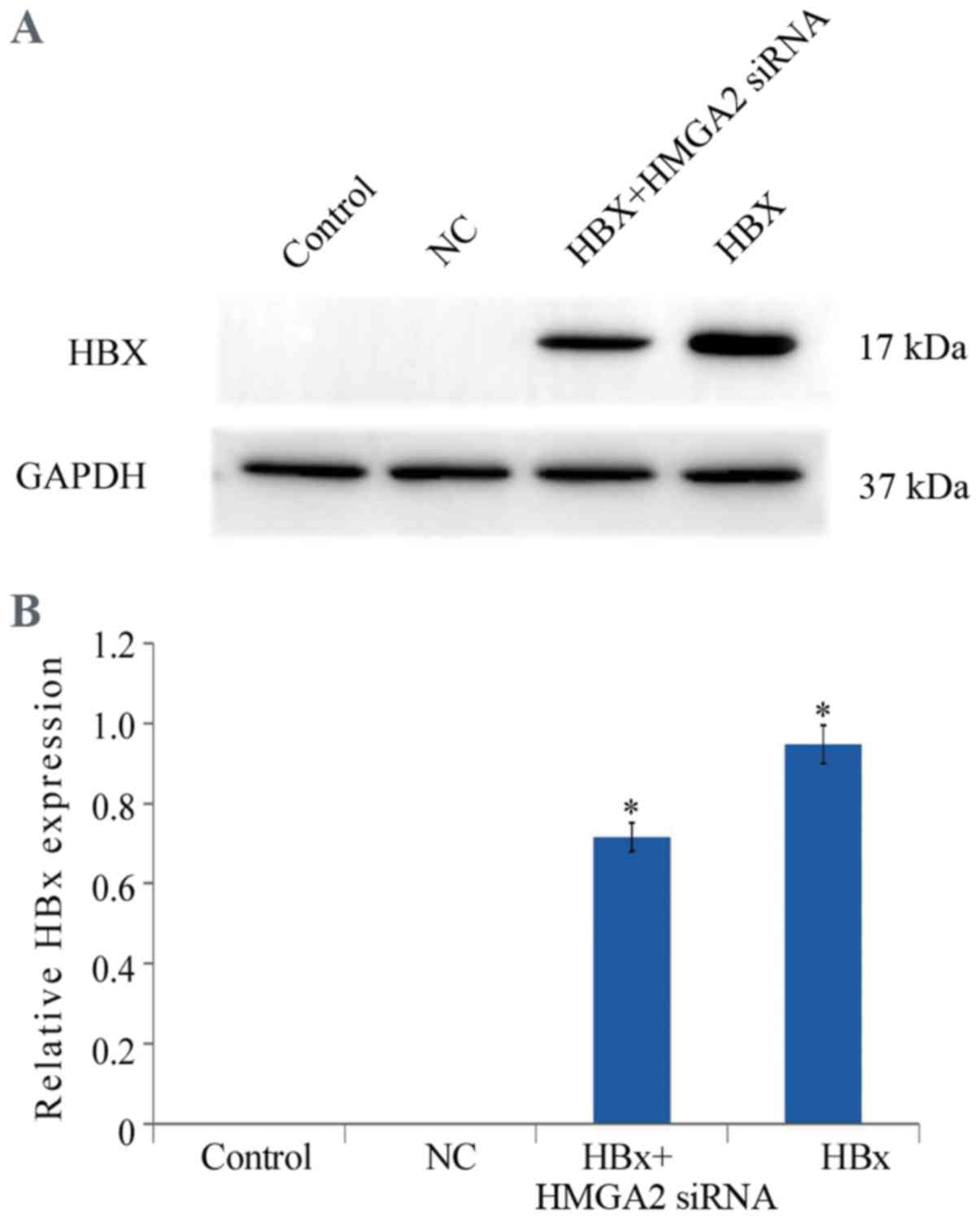
transfection efficiency was confirmed with western blotting
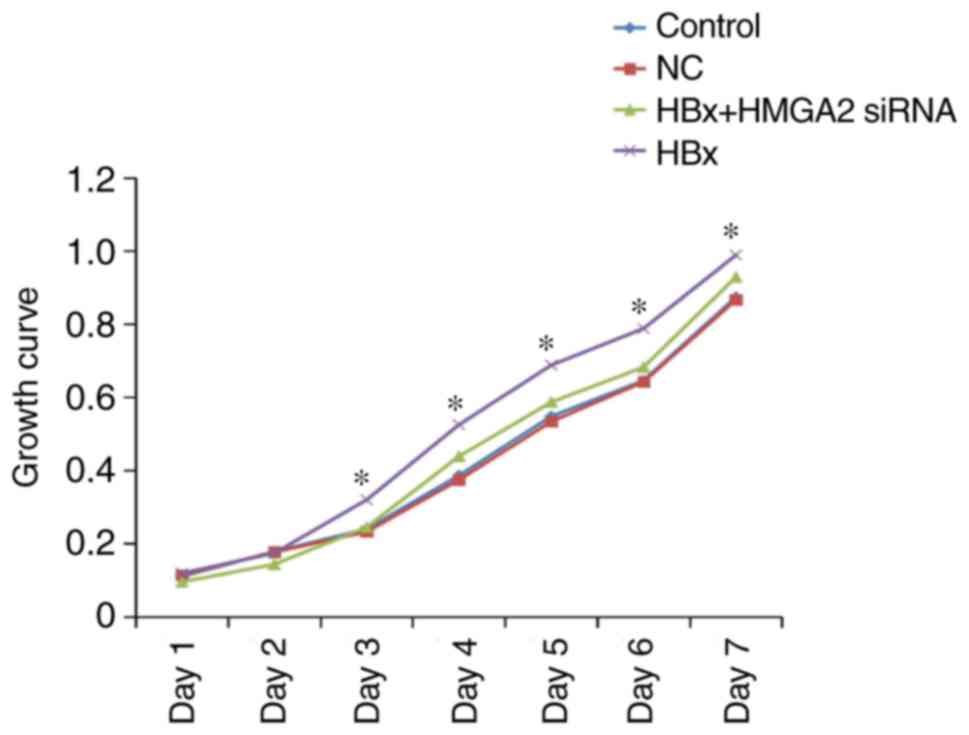
(Fig. 1). MTT analysis demonstrated
that the proliferation of HCCLM3 cells transfected with
pcDNA3.1-HBx vectors was significantly promoted when compared with
the control cells (Fig. 2).
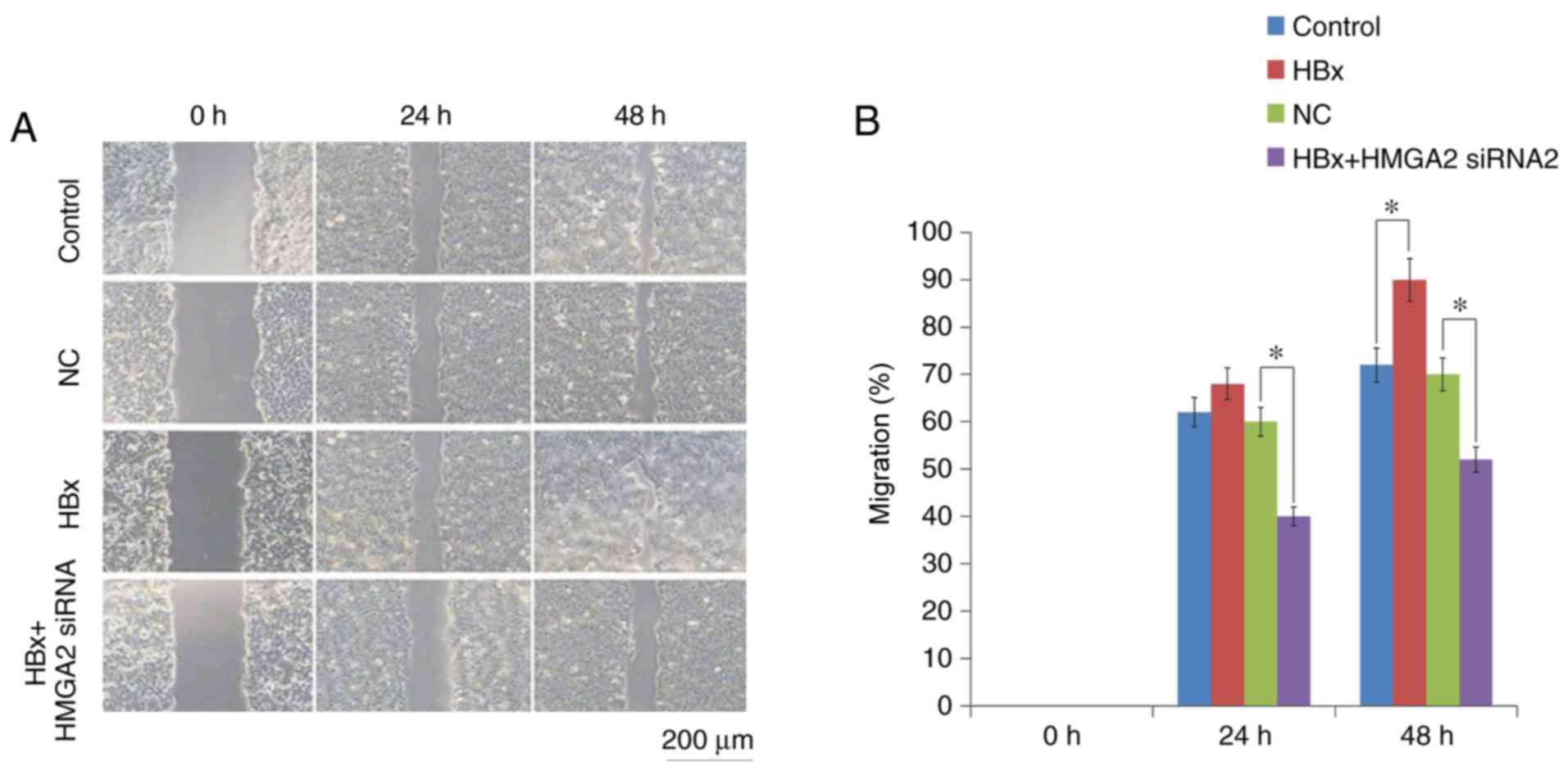
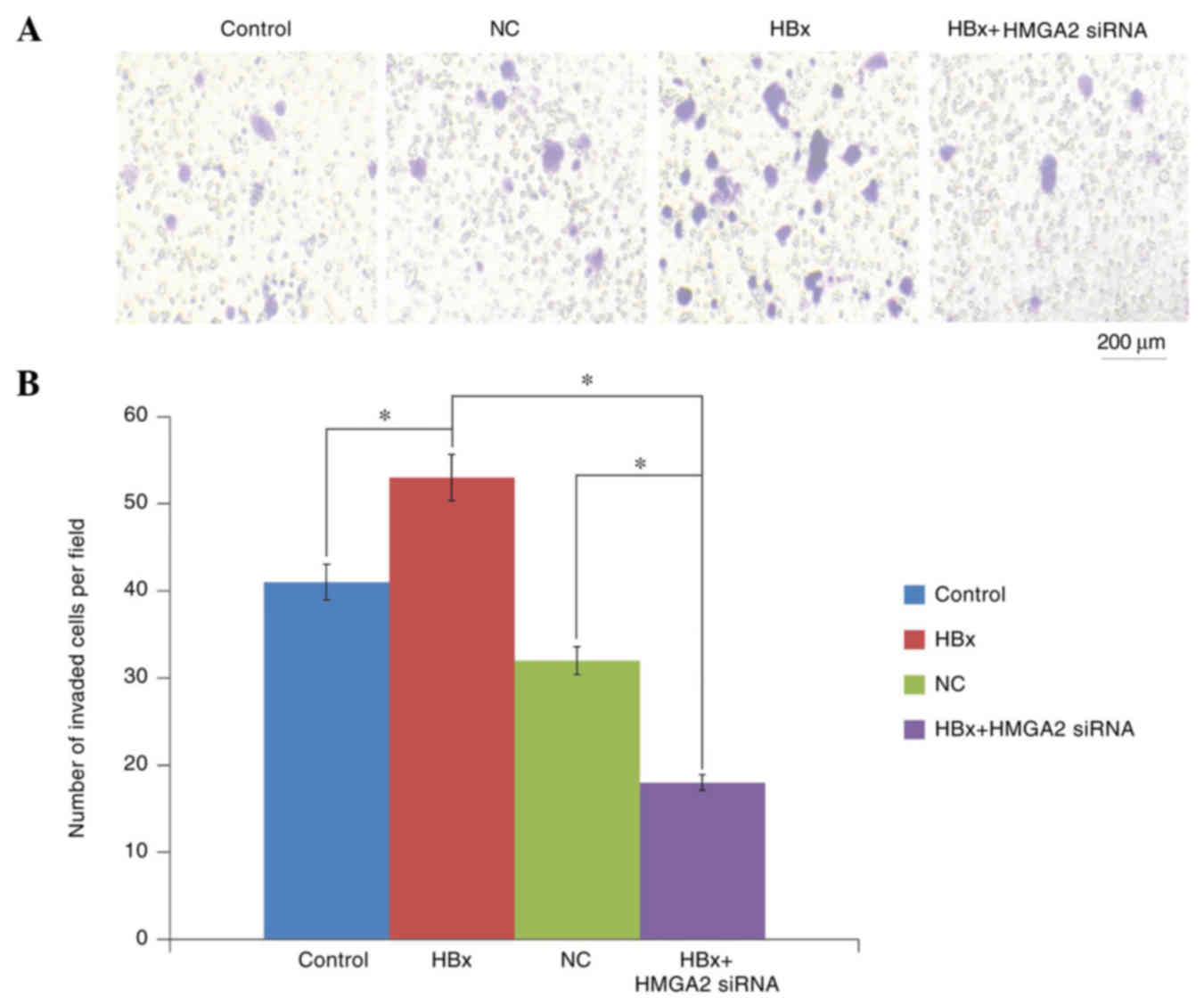
Then, Transwell migration and Matrigel invasion
assays were performed to determine the roles of HBx in HCC
metastasis. The results revealed that HCCLM3 cells transfected with
HBx-expressed vectors significantly increased the migratory and
invasive capacities compared with empty vector-transfected control
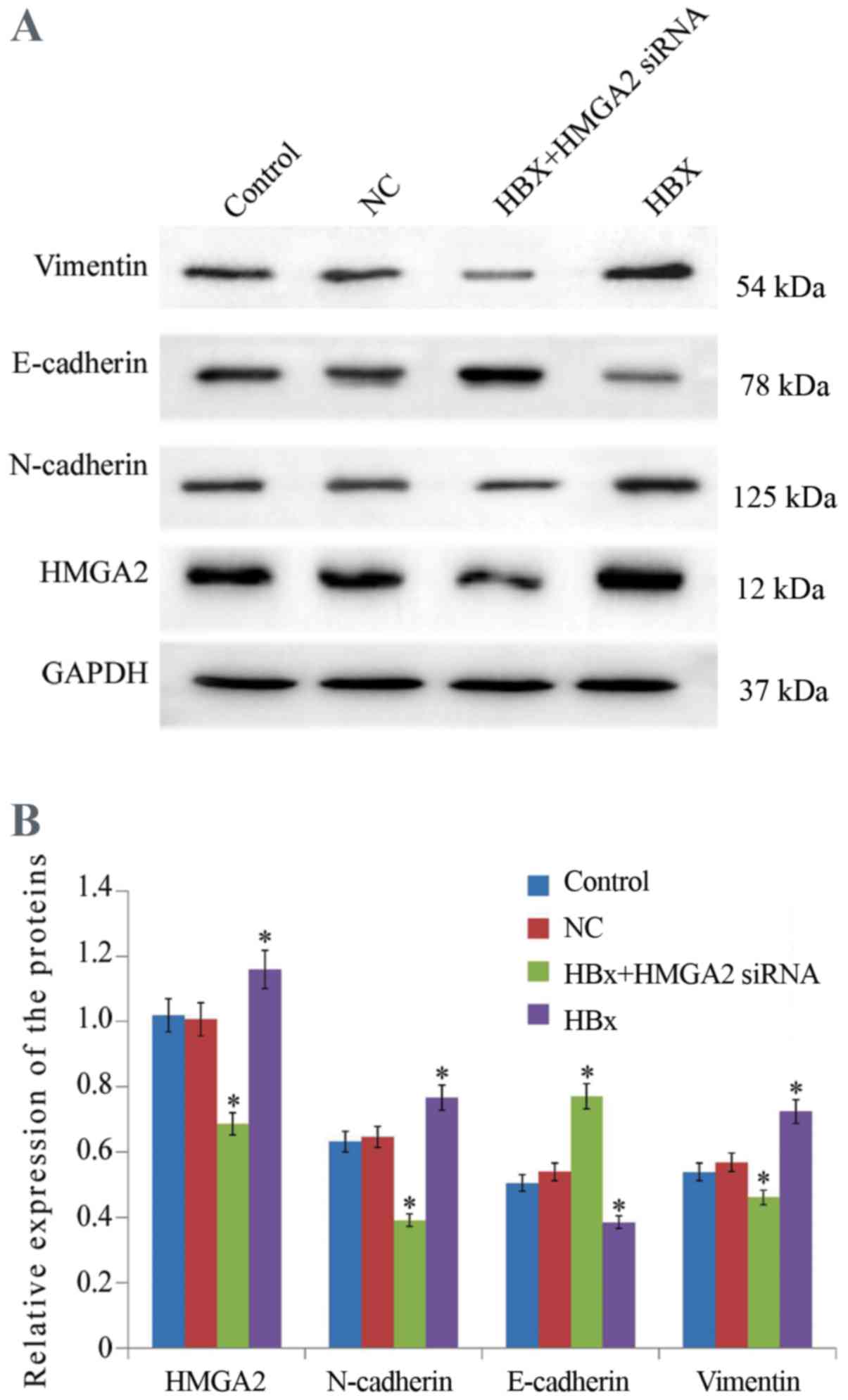
cells (Figs. 3 and 4). In addition, Western blot analysis
demonstrated that the upregulation of HBx resulted in a significant
increase in the epithelial cell marker E-cadherin expression and a
decrease in the mesenchymal cell marker Vimentin protein expression
in HCCLM3 cells (Fig. 5).
HBx promotes the expression of HMGA2
in HCC cells
To explore the mechanism of HBx on HCC metastasis,
the influence of HBx on expression of HMGA2 in HCCLM3 cells was
evaluated. Western blotting analyzed indicated that HMGA2
expression was significantly upregulated in HCCLM3 cells
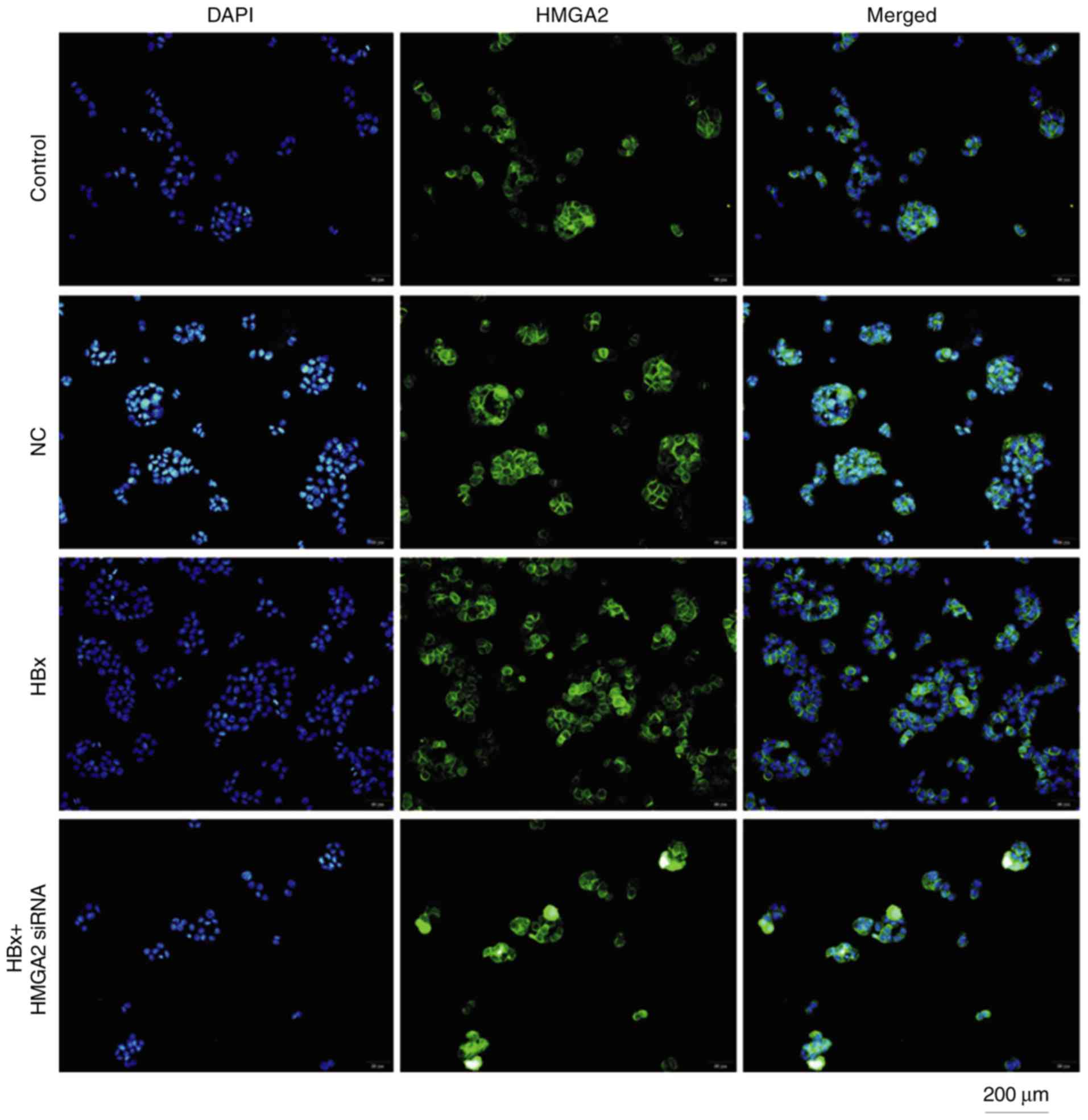
transfected with pcDNA3.1-HBx vectors at 48 h (Fig. 5). Fluorescent microscopy revealed that
HBx-transfected HCCLM3 cells had markedly increased HMGA2
expression in the nucleus (Fig. 6).
These results suggest that HBx may enhance the metastasis of HCCLM3
cells through HMGA2.
HMGA2 silencing restrains EMT and
metastasis of HCCLM3 cells induced by HBx
To further confirm that HMGA2 was a target of HBx,
the effect of HMGA2 knockdown on HBx-induced HCC metastasis was
examined. Transwell invasion and scratch wound-healing assays
showed that the migratory and invasive capacities of HBx-expressed
HCCLM3 cells were significantly inhibited by HMGA2 silencing
(Figs. 3 and 4). Furthermore, western blotting analysis
demonstrated that HMGA2-siRNA significantly inhibited the
expression of E-cadherin and increased in Vimentin protein
expression, reversing the EMT in HBx-expressed HCCLM3 cells
(Fig. 5). Collectively, these data
indicated that HBx enhanced the invasiveness and migration
capacities of HCC cells via HMGB2, HMGA2 knockdown was an effective
strategy to prevent HBx-induced HCC progression.
Discussion
HBx is a risk factor for hepatocarcinogenesis and
has been implicated in HCC progression. Previous studies reported
HBx promoted the proliferation of HCC cells (5,13). HBx
also disrupts intercellular adhesion, and induces EMT, invasion,
migration and metastasis in HCC (13,14). In
the present study, it was demonstrated that HBx markedly
upregulated the expression of the epithelial cell marker E-cadherin
and downregulated the expression of the mesenchymal cell marker
Vimentin in HCCLM3 cells. In addition, the overexpression of HBx in
HCCLM3 cells resulted in significantly increased migratory and
invasive capacities compared with the control cells. These data
indicated that HBx promoted the EMT, migration and invasion
capabilities in HCC.
Previous studies have reported that HBx promotes HCC
metastasis through differing mechanisms. Jin et al (18) reported that HBx promoted HCC cell
metastasis and induced EMT by mediating long noncoding RNA, ZEB2
antisense RNA 1. Hou et al (19) reported that HBx promoted HCC cell
invasion and migration through the HBx-metastasis associated lung
adenocarcinoma transcript 1 (non-protein coding)/latent
transforming growth factor β binding protein 3 signaling axis. Chen
et al (6) demonstrated that
HBx overexpression induced the secretion of high-mobility group box
1 to promote the invasion and metastasis of HCC in an
autocrine/paracrine manner. In current study, it was revealed that
HMGA2 knockdown inhibited HBx-induced EMT and metastasis in HCCLM3
cells. The HMGA2 protein overexpression has been reported to be
associated with metastasis in HCC (20–22). Ou
et al (20) demonstrated that
HMGA2 promoted HCC cell proliferation and invasion via the
Wnt/β-catenin signaling pathway. Luo et al (21) reported that HMGA2 expression was
significantly associated with the expression of EMT markers,
whereby HMGA2 induced EMT by upregulating the expression of Twist
and Snail in HCC cell lines. The results suggest that HBx promotes
HCC progression at least in part through targeting HMGA2, HMGA2 is
an effective target to prevent HBx-induced HCC metastasis.
Previously, certain studies have reported that HBx
upregulated transforming growth factor-β1 (TGFβ1) expression
(23,24). TGF-β was also a vital cytokine to
activate HMGA2 and induce EMT in HCC (25). Increased TGF-β levels may upregulate
HMGA2 expression, which in turn promotes the EMT, invasion and
migration capabilities HCC. The data suggested that HBx activated
HMGA2 through TGF-β. However, further studies are required to
confirm this hypothesis.
There are certain limitations to the present study.
Firstly, the use of different experimental systems and HBx
expression levels may result in differences in the effects of HBx
on cellular signal transduction pathways and influence HCC
metastasis. Secondly, since HBx has been identified to serve a
vital function in the regulation of chronic liver inflammation and
the liver tumor microenvironment (26), further study is required to clarify
the interaction between HBx and HMGA2 under the specific liver
tumor microenvironment. Finally, it has been reported that
overexpression of HBx in hepatocellular carcinoma cells may induce
the secretion of high-mobility group box 1 (HMGB1) to promote
invasion and metastasis of HCC in an autocrine/paracrine manner
(6). However, the interactions
between HMGB1 and HMGB2 in HBx-induced HCC metastasis remain
largely unclear.
In conclusion, in spite of the aforementioned
limitations in the current study, an important role of the
HBx-induced HMGA2 signaling pathway in regulating EMT was
identified, which subsequently promotes the invasive and migratory
invasive capacities of HCC. The present study suggests that HMGA2
may serve as a potential therapeutic target for the treatment of
patients with HBV-associated HCC.
Acknowledgements
The authors would like to thank Dr Linbo Gao for
having reviewed the language of the manuscript prior to
submission.
Funding
The present study was supported in part by grants
from the Projects of National Natural Science Foundation of China
(grant no. 81560497) and Applied Basic Research Projects-Joint
special project of Yunnan Province (grant no. 2015FB073).
Availability of data and materials
All analyzed data and materials in this study are
included in this published article.
Authors' contributions
YZ designed the study. QY, JSL, YYW and WMS
completed the experiment. YZ drafted the manuscript. All authors
have approved the final version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Akamatsu N, Cillo U, Cucchetti A, Donadon
M, Pinna AD, Torzilli G and Kokudo N: Surgery and hepatocellular
carcinoma. Liver Cancer. 6:44–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tseng TC and Kao JH: HBV markers for HCC
prediction: Three heads are better than two? J Hepatol. 67:203–204.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou SJ, Deng YL, Liang HF, Jaoude JC and
Liu FY: Hepatitis B virus X protein promotes CREB-mediated
activation of miR-3188 and Notch signaling in hepatocellular
carcinoma. Cell Death Differ. 24:1577–1587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang ST, Yen CJ, Lai CH, Lin YJ, Chang KC,
Lee JC, Liu YW, Chang-Liao PY, Hsu LS, Chang WC, et al: SUMOylated
CPAP is required for IKK-mediated NF-κB activation and enhances
HBx-induced NF-κB signaling in HCC. J Hepatol. 58:1157–1164. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu M, Li W, Lu Y, Dong X, Lin B, Chen Y,
Zhang X, Guo J and Li M: HBx drives alpha fetoprotein expression to
promote initiation of liver cancer stem cells through activating
PI3K/AKT signal pathway. Int J Cancer. 140:1346–1355. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen S, Dong Z, Yang P, Wang X, Jin G, Yu
H, Chen L, Li L, Tang L, Bai S, et al: Hepatitis B virus X protein
stimulates high mobility group box 1 secretion and enhances
hepatocellular carcinoma metastasis. Cancer Lett. 394:22–32. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fusco A and Fedele M: Roles of HMGA
proteins in cancer. Nat Rev Cancer. 7:899–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Franco R, Esposito F, Fedele M, Liguori G,
Pierantoni GM, Botti G, Tramontano D, Fusco A and Chieffi P:
Detection of high-mobility group proteins A1 and A2 represents a
valid diagnostic marker in post-pubertal testicular germ cell
tumours. J Pathol. 214:58–64. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun M, Gomes S, Chen P, Frankenberger CA,
Sankarasharma D, Chung CH, Chada KK and Rosner MR: RKIP and HMGA2
regulate breast tumor survival and metastasis through lysyl oxidase
and syndecan-2. Oncogene. 33:3528–3537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piton N, Angot É, Marguet F and Sabourin
JC: HMGA2 immunostaining is a straightforward technique which helps
to distinguish pulmonary fat-forming lesions from normal adipose
tissue in small biopsies: A retrospective observational study about
a series of 13 lung biopsies. Diagn Pathol. 12:212017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu J, Zhang S, Shan J, Hu Z, Liu X, Chen
L, Ren X, Yao L, Sheng H, Li L, et al: Elevated HMGA2 expression is
associated with cancer aggressiveness and predicts poor outcome in
breast cancer. Cancer Lett. 376:284–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong J, Wang R, Ren G, Li X, Wang J, Sun
Y, Liang J, Nie Y, Wu K, Feng B, et al: HMGA2-FOXL2 axis regulates
metastases and epithelial-to-mesenchymal transition of
chemoresistant gastric cancer. Clin Cancer Res. 23:3461–3473. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen Z, Tang J, Cai X, Huang Y, Gao Q,
Liang L, Tian L, Yang Y, Zheng Y, Hu Y and Tang N: HBx mutations
promote hepatoma cell migration through the Wnt/β-catenin signaling
pathway. Cancer Sci. 107:1380–1389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ha HL, Kwon T, Bak IS, Erikson RL, Kim BY
and Yu DY: IGF-II induced by hepatitis B virus X protein regulates
EMT via SUMO mediated loss of E-cadherin in mice. Oncotarget.
7:56944–56957. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Agostini A, Panagopoulos I, Andersen HK,
Johannesen LE, Davidson B, Tropé CG, Heim S and Micci F: HMGA2
expression pattern and TERT mutations in tumors of the vulva. Oncol
Rep. 33:2675–2680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Zhao Z, Xu C, Zhou Z, Zhu Z and You
T: HMGA2 induces transcription factor Slug expression to promote
epithelial-to-mesenchymal transition and contributes to colon
cancer progression. Cancer Lett. 355:130–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo SP, Wang WL, Zhai YQ and Zhao YL:
Expression of nuclear factor-kappa B in hepatocellular carcinoma
and its relation with the X protein of hepatitis B virus. World J
Gastroenterol. 7:340–344. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin Y, Wu D, Yang W, Weng M, Li Y, Wang X,
Zhang X, Jin X and Wang T: Hepatitis B virus × protein induces
epithelial-mesenchymal transition of hepatocellular carcinoma cells
by regulating long non-coding RNA. Virol J. 14:2382017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou Z, Xu X, Fu X, Tao S, Zhou J, Liu S
and Tan D: HBx-related long non-coding RNA MALAT1 promotes cell
metastasis via up-regulating LTBP3 in hepatocellular carcinoma. Am
J Cancer Res. 7:845–856. 2017.PubMed/NCBI
|
|
20
|
Ou W, Lv J, Zou X, Yao Y, Wu J, Yang J,
Wang Z and Ma Y: Propofol inhibits hepatocellular carcinoma growth
and invasion through the HMGA2-mediated Wnt/β-catenin pathway. Exp
Ther Med. 13:2501–2506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo Y, Li W and Liao H: HMGA2 induces
epithelial-to-mesenchymal transition in human hepatocellular
carcinoma cells. Oncol Lett. 5:1353–1356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Chen F, Zhao M, Yang Z, Li J,
Zhang S, Zhang W, Ye L and Zhang X: The long noncoding RNA HULC
promotes liver cancer by increasing the expression of the HMGA2
oncogene via sequestration of the microRNA-186. J Biol Chem.
292:15395–15407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Xu Y, Ma H, Wang B, Xu L, Zhang H,
Song X, Gao L, Liang X and Ma C: Hepatitis B virus X protein
amplifies TGF-β promotion on HCC motility through down-regulating
PPM1a. Oncotarget. 7:33125–33123. 2016.PubMed/NCBI
|
|
24
|
Murata M, Matsuzaki K, Yoshida K, Sekimoto
G, Tahashi Y, Mori S, Uemura Y, Sakaida N, Fujisawa J, Seki T, et
al: Hepatitis B virus X protein shifts human hepatic transforming
growth factor (TGF)-beta signaling from tumor suppression to
oncogenesis in early chronic hepatitis B. Hepatology. 49:1203–1217.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morishita A, Zaidi MR, Mitoro A,
Sankarasharma D, Szabolcs M, Okada Y, D'Armiento J and Chada K:
HMGA2 is a driver of tumor metastasis. Cancer Res. 73:4289–4299.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu S, Zhou RR, Li N, Huang Y and Fan XG:
Hepatitis B virus X protein in liver tumor microenvironment. Tumour
Biol. Sep 23–2016.(Epub ahead of print). View Article : Google Scholar
|