Introduction
Among various types of cancer, colon cancer is one
of the leading causes of cancer-associated mortalities worldwide
(1). Colon cancer involves the
formation of malignant tumors in the colon tissues and is one of
the most commonly diagnosed types of cancer (2). Currently, patients with colon cancer
undergo two main treatment options i.e., chemotherapy and surgery,
and among the two medical procedures the one that is used depends
upon the size of the tumor and stage of cancer in patients
(3,4).
Whereas in a protion of patients surgery is followed by
chemotherapy, there are other patients to whom chemotherapy is
initially administered to reduce the size of the tumor, which is
then typically followed by surgery (5–7). However
despite the advances made in medical sciences over the last decade,
drug resistance remains a principal reason for treatment failure
(8–10). In colon cancer, patients develop drug
resistance with the passage of treatment and ultimately stop
responding to the available treatment options, which culminates in
the failure of chemotherapy (11,12). Drug
resistance is defined as a decrease in the effect of drugs,
including chemotherapeutic agents or antibiotics, and targeting
drug resistance, primarily multidrug resistance, remains one a
major challenge (13,14).
Phosphatase and tensin homolog (PTEN) a tumor
suppressor gene, has been reported to be involved in various types
of cancer (15,16). Mutations in PTEN have been reported to
be involved in the development of cancer (17,18). In
various types of cancer the mutation frequency of PTEN is very high
(19). PTEN (phosphatase and tensin
homologue) is a phosphatase that dephosphorylates both protein and
phosphoinositide substrates that regulates longevity (20,21). The
intracellular levels of phosphatidylinositol are negatively
regulated by PTEN which acts as a tumor suppressor by negatively
regulating the Akt signaling pathway; a pathway that has been
demonstrated to be deregualted in the majority of cancers (22). Mutations in this gene contribute to
the failure of chemotherapy and therefore drug resistance (23).
Overproduction of interleukin (IL) 6 and 8 may be
caused due to decreased expression of PTEN as well as drug
resistance, and it has been hypothesized to be involved in the
expansion of cancer stem cell population in tumors (24). A positive feedback loop is generated
whereby IL6 activates the nuclear factor (NF)-κB signalling pathway
that further enhances the production of IL6 creating a positive
feedback loop, thus linking inflammation to the malignant
transformation of tumors (25). It
has also been reported that the levels of IL6 in patients directly
correlate with their overall survival rate (26). Therefore, studying drug resistance and
the underlying mechanisms is of great clinical importance.
Materials and methods
Drugs, reagents and chemicals
Doxorubicin was purchased from Selleck Chemicals
(Houston, TX, USA). RPMI-1640, radioimmunoprecipitation assay
(RIPA) buffer, Hanks buffer and MTT reagent were obtained from
Sigma-Aldrich (Merck KGaA; Darmstadt, Germany). Tocilizumab (an
anti-IL6R antibody) was purchased from Roche Diagnostics, Basel,
Switzerland. Primers, probes and cDNA kits for mRNA quantification
were obtained from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and foetal bovine serum (FBS), Lipofactamine 2000
and Antibiotic-Antimycotic were procured from Gibco (Thermo Fisher
Scientific, Inc). Lentiviral particles for knockdown of PTEN were
obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All
antibodies were obtained from CST (Cell Signaling Technology, Inc.,
Danvers, MA, USA).
Cell culture conditions
The LS180 cell line was obtained from American Type
Culture Collection (Manassas, VA, USA) and grown in RPMI-1640
supplemented with 1% antibiotics Antibiotic-Antimycotic and 10% FBS
at 37°C in a humidified atmosphere containing 5% CO2.
For the generation of doxorubicin-resistant cells, LS180 and LS180
short hairpin (sh)PTEN (shPTEN-treated LS180) cells were treated
with 1 µM of doxorubicin for a period of nine months. However,
resistant cells were grown in drug-free media for 48 h prior to
experimentation.
PTEN knockdown
shRNA PTEN lentiviral particles from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) were used for PTEN knockdown.
Briefly, LS180 and doxorubicin-resistant LS180 cells were grown in
6 well plates in an incubator at 37°C with 5% CO2 and
95% humidity for 24 h. shRNA PTEN Lentiviral particles Santa Cruz
Biotechnology Inc. at a concentration of 1 µg/ml with polybrene
[Sigma-Aldrich (5 µg/ml)] were transfected using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) to the cells for 24 h.
Following this 24 h incubation, media was replaced with complete
DMEM. Puromycin (4 µg/ml) was used as a selection marker and three
rounds of selection were performed for 48 h each. Only the cells
resistant to puromycin were cultured for subsequent
experiments.
Cell proliferation assay
The LS180 cell line and its LS180 PTEN knockdown
model, as well as doxorubicin-resistant cells were seeded in
96-well plates at a density of 1.5×104 cells/well and
allowed to grow for 24 h at 37°C. At 24 h, parental and resistant
cells were treated with doxorubicin at a concentration dependent
manner (0.1, 0.2, 0.4, 0.8, 1, 2, 5, 10, 15 and 20 µM) for 48 h.
MTT solution was added into each well at a concentration of 2.5
mg/ml and cells were incubated for 4 h at 37°C. Finally, a total of
150 µl dimethylsulfoxide (DMSO) was added to each well to dissolve
the formazen crystals and absorbance was detected at 570 nm by a
synergy MX plate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Western blotting
LS180, LS180 shPTEN, doxorubicin LS180 and
doxorubicin LS180 shPTEN cells were seeded at a density of
1×106 in 60 mm dishes for 24 h. Cells were treated with
doxorubicin at a concentration of 1 µM for 24 h and were lysed
using RIPA buffer. Protein determination was performed by the
Bradford method, and proteins (70 µg) were separated on 10%
SDS-PAGE and then transferred onto a nitrocellulose membrane at 100
V for 2 h. To avoid non-specific binding, membranes were blocked in
5% fat-free milk for 1 h at room temperature and primary antibodies
p-AKT ser473 (10% SDS-PAGE; cat. no. 4060; dilution 1:1,000), AKT
(10% SDS-PAGE; cat. no. 4691; dilution 1:1,000), NF-κB p65 (10%
SDS-PAGE; cat. no. 8242; dilution 1:1,000), EpCAM (10% SDS-PAGE;
cat. no. 93790; dilution 1:1,000), claudin-3; 15% SDS-PAGE; cat.
no. 83609; dilution 1:1,000), PTEN; 10% SDS-PAGE; cat. no. 9188;
dilution 1:1,000), TGFR2 (10% SDS-PAGE; cat. no. 79424; dilution
1:1,000), vimentin (10% SDS-PAGE; cat. no. 5741; dilution 1:1,000),
β-actin (cat. no. 4970; dilution 1:2,000), TWIST (15% SDS-PAGE;
cat. no. 14472; dilution 1:1,000), E-cadherin (6% SDS-PAGE; cat.
no. 14472; dilution 1:1,000), HRP conjugated mouse anti-rabbit
secondary antibody (cat. no. 93702; dilution 1:2,500), anti-mouse
secondary antibody (cat. no. 14709; dilution 1:2,500) were obtained
from Cell Signaling Technology Inc., (Danvers, MA, USA) incubated
overnight at 4°C. Membranes were washed with Tris-buffered saline
containing 0.05% Tween-20 (TBST) two times for 5 min. The
horseradish peroxidase (HRP)-labeled antibody was added at room
temperature for 1 h, after which the protein blots were again
washed twice with TBST at room temperature for 5 min each. Finally,
protein bands were visualized using enhanced chemiluminescence
(ECL; GE Healthcare, Chicago, IL, USA) and an X-ray film.
CD44/CD24 assay
This assay was performed to detect the cancer stem
cell fraction within the colon cancer cell line LS180. Cells
(LS180, LS180 shPTEN, doxoLS180 and doxoLS180 shPTEN) were
incubated with anti-CD44-PE (cat. no. ab46793; dilution 1:200) and
anti-CD24-FITC (cat. no. ab30350; dilution 1:200) or stained with
their isotype controls IgG (cat. no. ab172730; dilution 1:200; all
Abcam) for 30 min on ice. Following this incubation, cells were
washed in Hanks' balanced salt solution (HBSS) supplemented with 2%
FCS and analysed by flow cytometry using FACSDiVa 6.2 software with
a (BD Accuri™ C6 Flow Cytometer (both BD
Bioscience).
ELISA
IL6, IL2 and IL8 levels were detected using ELISA.
Briefly, cells (LS180, LS180 shPTEN, doxoLS180 and doxoLS180
shPTEN) were seeded at a density of 0.25×106 in a
24-well plate and allowed to grow in an incubator at 37°C with 5%
CO2 and 95% humidity for 3 days. The media from the
cultured cells was then removed and was analysed for the IL6, IL2
and IL8 levels using an antibody array 5 raybio human cytokine kit
according to the manufacturer's protocol.
3D sphere formation assay
Using mammocult medium (Stem Cell Technologies,
Inc., Vancouver, BC, Canada), cells (LS180, LS180 shPTEN, doxoLS180
and doxoLS180 shPTEN) were seeded in ultra-low attachment plates at
a density of 1×105 cells/well and allowed to grow for 7
days and were then treated with tocilizumab at a concentration of 1
mg/l for 48 h. Following treatment, the primary spheres were
dissociated by pippeting and single cells were reseeded in
ultra-low attachment 6-well plates at a density of 5×104
cells/well in mammary epithelial growth medium (MEGM, Lonza),
supplemented with B27 (Invitrogen; Thermo Fisher Scientific, Inc.),
20 ng/ml bFGF (Sigma-Alrich; Merck KGaA) and 30 ng/ml EGF were
incubator at 37°C with 5% CO2 and 95% humidity for 21
days of incubation. A total of 20 fields of view were randomly
selected, observed and secondary spheres were counted using a light
microscope at a magnification of ×30.
Reverse transcription-quantitative
polymerase chain reaction RT-qPCR
Primers were obtained from Invitrogen (Thermo Fisher
Scientific, Inc.) vimentin forward, 5′-GGCTCAGATTCAGGGGAACAGC-3′
and reverse, 5′-CAGGTTGTGCAGGTTGTTCTA-3′; TWIST forward,
5′-TGTAAAACGACGGCCAGT-3′ and reverse, 5′-CAGGAAACAGCTATGACC-3′;
N-cadherin forward, 5′-CACTGCTCAGGACCCAGAT-3′ and reverse,
5′-TAAGCCGAGTGATGGTCC-3′; TGFR forward, 5′-TGTAAAACGACGGCCAGT-3′
and reverse, 5′-CAGGAAACAGCTATGACC-3′; E-cadherin forward,
5′-GGGGTACCTGTCTCTCTACAAAAAGGCA-3′ and reverse,
5′-GGAAGATCTGGGCTGGAGCGGGCTGGAGT-3′; CLDN3 forward,
5′-CTGCTCTGCTGCTCGTGTCC-3′ and reverse,
5′-TTAGACGTAGTCCTTGCGGTCGTAG-3′; EpCAM forward,
5′-CGCAGCTCAGGAAGAATGTG-3′ and reverse,
5′-TGAAGTACACTGGCATTGACG-3′; and GAPDH forward,
5′-GGTGTGAACGGATTTGGCCGTATTG-3′; and reverse,
5′-CCGTTGAATTTGCCGTGAGTGGAGT-3′ Total RNA was isolated from
parental and doxorubicin-resistant cells using the TRIzol reagent
(Sigma-Aldrich; Merck KGaA). RNA was purified by using RNeasy mini
kit (Qiagen GmbH, Hilden, Germany). RNA was reverse-transcribed
into cDNA using M-MLV RT kit (Promega Corporation, Madison, WI,
USA). RT-qPCR was performed using a TaqMan universal PCR master mix
from Roche (Roche Diagnostics, Basel, Switzerland) with reverse
transcription involving denaturation at 94°C for 30 sec and
annealing and elongation at 72°C for 1 min followed by
aforementioned primers on an ABI PRISM sequencing detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) The relative
fold change of differential inducible expression of the genes vs.
control group was quantified by using the 2−∆∆Cq method
(27).
Statistical analysis
For statistical analysis graphPad Instat3 software
(GraphPad Software Inc., La Jolla, CA, USA) was used. All the
experiments were performed three times. The relevant data are
expressed as the mean ± standard deviation (SD). One-way analysis
of variance by post-hoc analysis with Tukey's multiple-comparisons
test was performed was used to examine differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Development of a PTEN deficient and
chemoresistant cell line
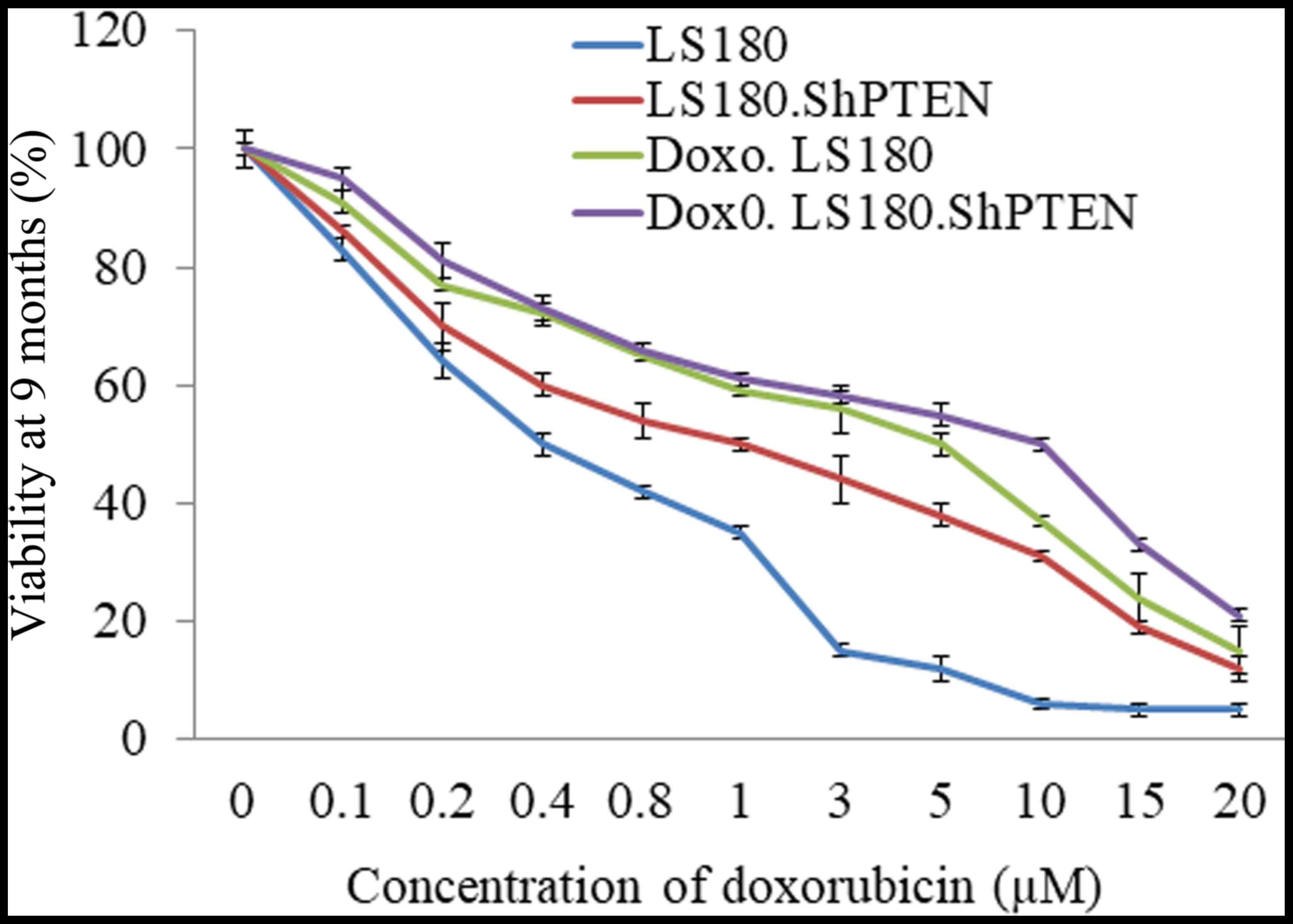
LS180, a colon cancer cell line was selected, and a
stable PTEN knockdown model of LS180 was generated. LS180 and LS180
shPTEN cells were treated with increasing concentrations of
doxorubicin for a period of over nine months. Resistance developed
by doxorubicin was calculated by cell viability. The
IC50 of doxorubicin had increased in the resistant cells
as compared with their parental cell lines. Furthermore, the
knockdown of PTEN also decreased the response of LS180 cells
towards doxorubicin (Fig. 1).
Doxorubicin resistance and PTEN
knockdown synergize to increase IL6 levels
The IL6 signalling pathway is a crucial cellular
pathway and its deregulation has been reported in various types of
cancer. Doxorubicin resistance in LS180 cells led to increased
level of IL6 which was further elevated in response to shPTEN
treatment (Fig. 2A). It was also
observed that shPTEN in doxorubicin resistance LS180 leads to an
increase in the level of IL2 and IL8 (Fig. 2B). Furthermore, tocilizzumab (an IL6
inhibitor) led to almost complete inhibition of IL6 production in
the parental cells as well as doxorubicin-resistant and PTEN
knockdown models and this effect was also observed in IL2 and IL8
(Fig. 2A and B). Furthermore, the
expression of p-AKT 473 has been increased in doxorubicin
resistance LS180 cells that leads to the upregulation of NF-kB as
compared with LS180 cells. However, the effect was further elevated
in response to shPTEN treatment (Fig. 2C
and D).
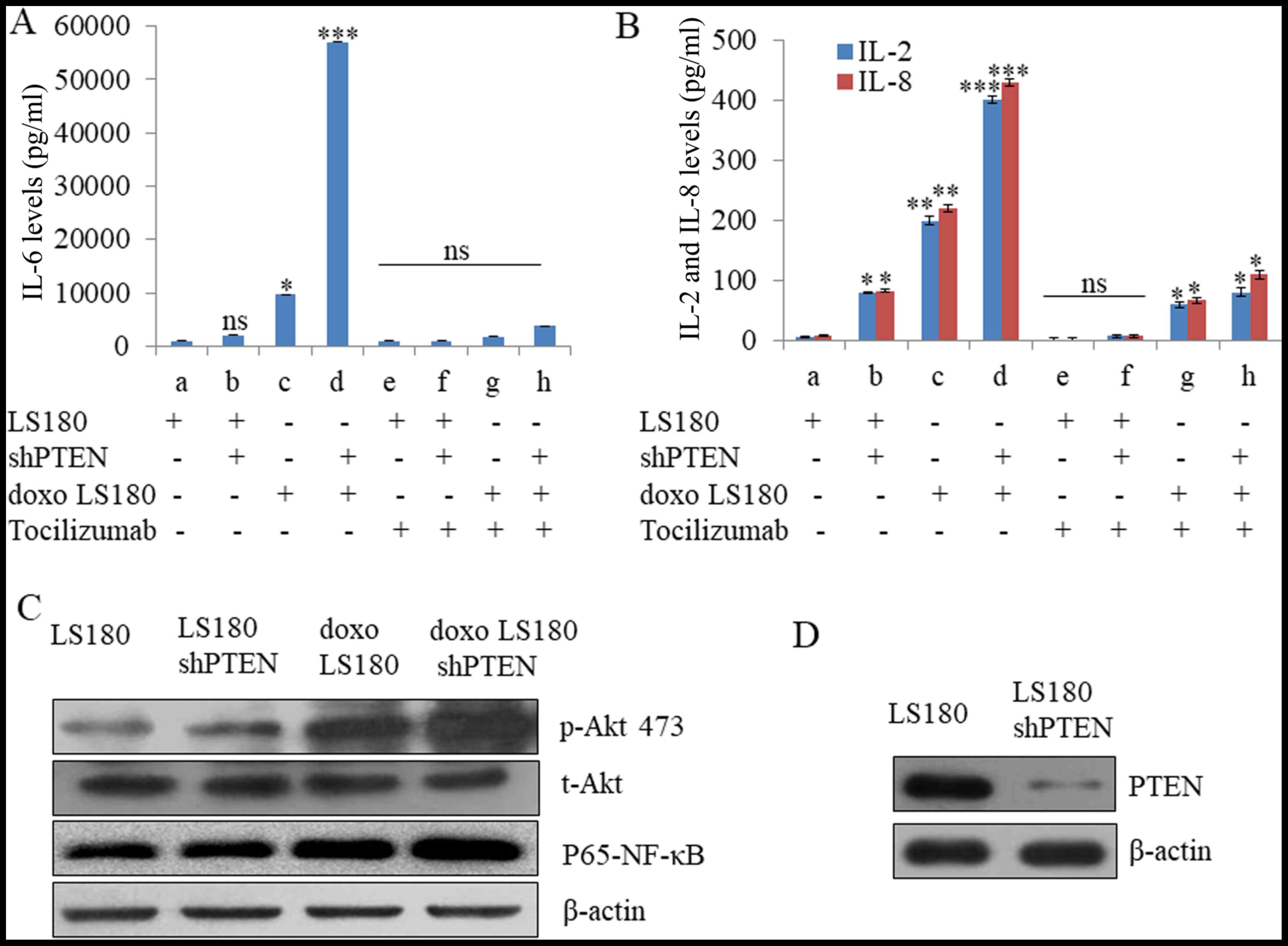 | Figure 2.Doxorubicin resistance enhances IL6
levels. (A) Doxorubicin resistance enhanced the production of IL6,
which was further increased in PTEN knockdown cells as determined
by Raybio human cytokine antibody array. However, Tocilizumab
antibody at a concentration of 5 µg/ml reduced the IL6 levels by
>90% in parental cells as well as doxorubicin-resistant models.
(B) The levels of IL8 and IL2 due to doxorubicin resistance were
reduced by using tocilizumab antibody at a concentration of 5
µg/ml. Data presented here are means of three similar experiments.
***P<0.001, **P<0.01, *P<0.05 vs. LS180. (C) Doxorubicin
resistance along with knockdown of PTEN led to activation of Akt
and P65-NF-κB. (D) Western blot analysis illustrating the knockdown
of PTEN in LS180 shPTEN cells. Data are presented the mean of three
independent experimental repeats. sh, short hairpin; PTEN,
phosphatase and tensin homolog; ns, not significant; doxo,
doxorubicin-resistant; NF, nuclear factor; IL, interleukin; R,
receptor. |
PTEN knockdown with doxorubicin
resistance increases the cancer stem cell population
To investigate the stem properties of different
subtype, the present study compared the expression of CD44, CD24 in
LS180, LS180 shPTEN, doxoLS180 and doxoLS180 shPTEN cell lines
using flow cytometry analysis. As expected doxorubicin resistance
led to an increase in the population of these cancer-like stem
cells whose numbers were further elevated by knockdown of PTEN by
analysing the markers of stem cells such as
CD44+/CD24− (Fig.
3A). Doxorubicin-resistant cells exhibited an increased ability
to form mammospheres. PTEN knockdown further increased the
formation of mammospheres. Notably, anti-IL6R has decreased the
formation of mammospheres in PTEN knockdown doxorubicin resistant
LS180 cells (Fig. 3B).
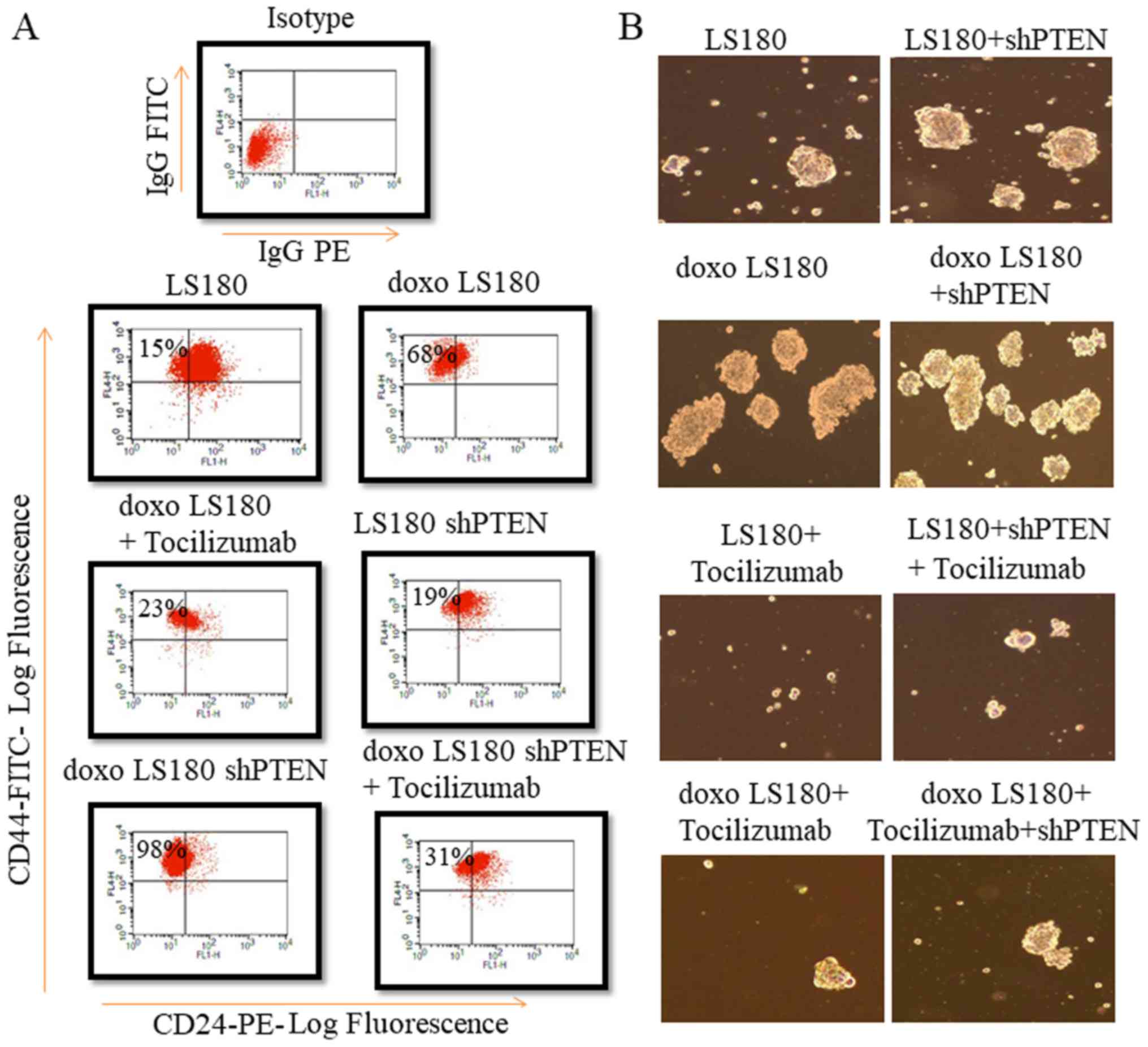 | Figure 3.PTEN knockdown along with doxorubicin
resistance increases fraction of cancer stem cells. (A) PTEN
knockdown increased the fraction of cells expressing
CD44+ CD24− markers in LS180 shPTEN cells,
which was further enhanced by doxorubicin resistance. However,
treatment with Tocilizumab antibody led to a decrease in cancer
stem cell fraction. (B) PTEN knockdown increased mammosphere
formation, which was further enhanced by doxorubicin resistance.
However, a marked decrease in mammosphere formation was observed in
samples treated with Tocilizumab antibody. Mammospheres were
counted using a light microscope at a magnification of ×30. sh,
short hairpin; PTEN, phosphatase and tensin homolog; doxo,
doxorubicin-resistant; FITC, fluorescein isothiocyanate; PE,
phycoerythrin; R, receptor. |
PTEN knockdown with doxorubicin
resistance induces epithelial-mesenchymal transition (EMT)
Cancer cells undergo reprogramming and change
morphologically when treated with chemoresistant drugs over a
period of time (28). The results of
the present study demonstrated that RT-qPCR analysis of the
epithelial to mesenchymal responsive genes including neural
(N)-cadherin, vimentin, TWIST and TGFR2 was markedly increased in
doxorubicin-resistant cells which was further enhanced by shPTEN
doxorubicin-resistant cells as compared with LS180 (Fig. 4A). Furthermore, the expression of
E-cadherin, epithelial cell adhesion molecule (EpCAM) and claudin-3
was decreased in both doxorubicin-resistant and shPTEN
doxorubicin-resistant cells compared with LS180 cells (Fig. 4A). It was further confirmed by western
blotting that there was a increase in the expression of N-cadherin,
vimentin, TWIST and TGFR2 in doxorubicin-resistant cells as
compared to parental cells (Fig. 4C)
whereas an increase in the expression of mesenchymal proteins,
including E-cadherin, epithelial cell adhesion molecule (EpCAM) and
claudin-3 in the doxorubicin-resistant cells compared with parental
cells (Fig. 4D. Taken together, these
results demonstrate that doxorubicin leads to resistance in colon
cancer and induces EMT-like phenotype which is further elevated by
the knockdown of PTEN.
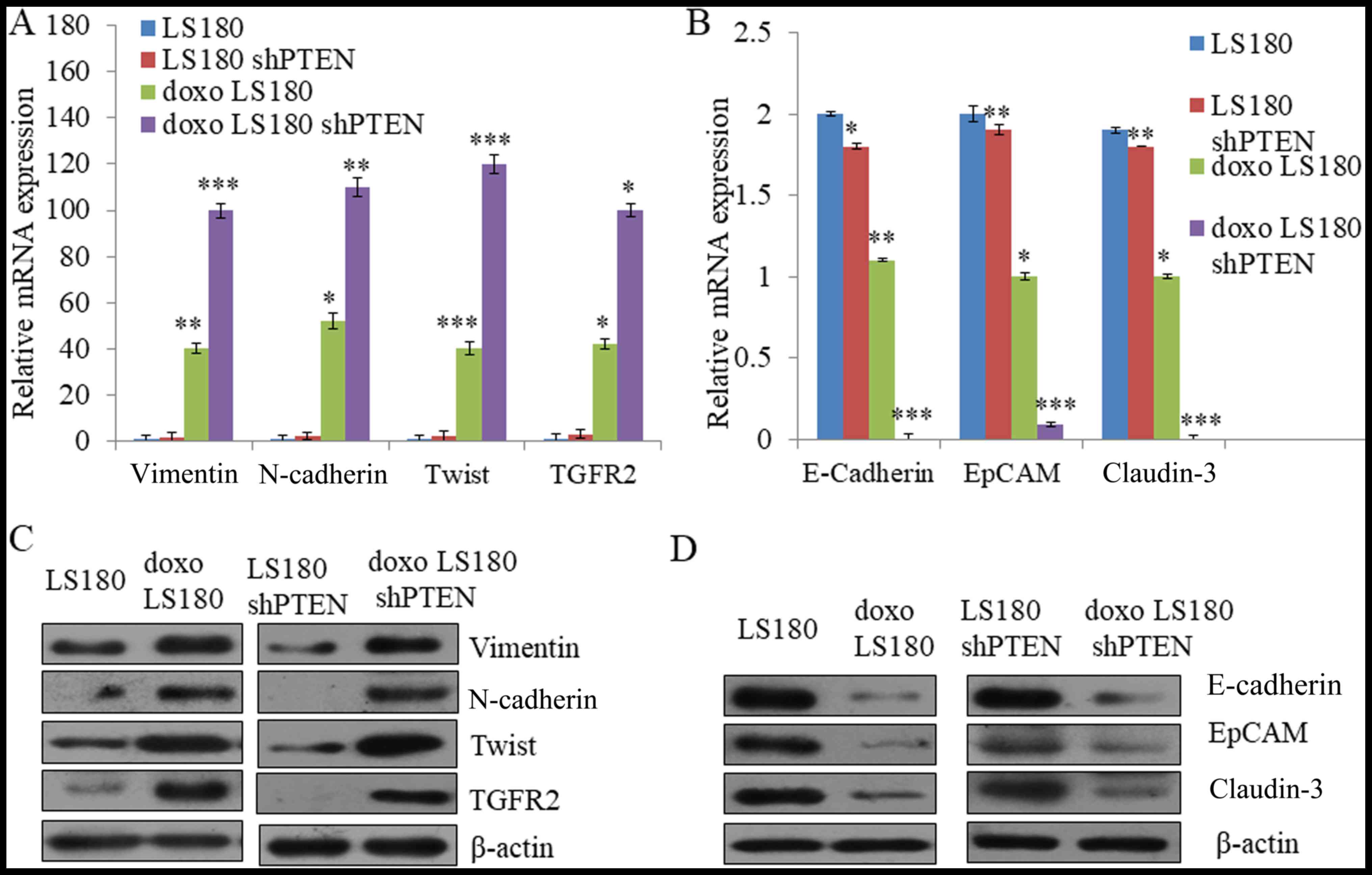 | Figure 4.Induction of EMT in LS180 by
doxorubicin resistance. (A) Quantification of mesenchymal markers
vimentin, N-cadherin, Twist and TGFR2 by RT-qPCR using GAPDH as the
normalizing marker. (B) Quantification of epithelial markers
E-cadherin, EpCAM and claudin-3 by RT-qPCR using GAPDH as
normalizing marker. Data presented here are means of three similar
experiments. ***P<0.001, **P<0.01, *P<0.05 vs. LS180. (C)
Assessment of EMT phenotype at protein level was analyzed by
western blotting in which the expression of epithelial markers was
decreased and the expression of mesenchymal markers in
doxorubicin-resistant cells was increased. (D) The expression of
E-cadherin, EpCAM and claudin-3 decreases in shPTEN
doxorubicin-resistant cells compared with parental cells shPTEN
LS180 cells. Data are presented the mean of three independent
experimental repeats. EMT, epithelial mesenchymal transition;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; sh, short hairpin; PTEN, phosphatase and tensin homolog;
doxo, doxorubicin-resistant; TGFR2; transforming growth factor β
receptor 2; EpCAM, epithelial cell adhesion molecule; E-cadherin,
epithelial cadherin; N-cadherin, neural cadherin. |
Discussion
Colon cancer is one of the leading cause of
cancer-associated mortalities worldwide, and drug resistance remain
a major challenge (29). However,
presently there are strategic approaches in pre-clinical trials,
including the combination of ATP-binding cassette (ABC)
transporters and Epidermal growth factor receptor (EGFR)
inhibitors, which are being administered in conjuction with
conventional anti-cancer drugs, which have proven effective against
drug resistance in colon cancer (30).
In the present study, a PTEN knockdown model of
LS180 cells was generated and LS180 and its PTEN knockdown model
were subjected to doxorubicin treatment for a period of 8–10 months
to generate a drug-resistant cell line against doxorubicin The
present study also demonstrated that the fraction of cancer-like
stem cells, which have been reported to be responsible for drug
resistance, was increased by the knockdown of PTEN (31). Furthermore, it was also observed that
the fraction of cancer stem cells was further increased in
doxorubicin-resistant cells wherein it was identified that the
increased fraction of cancer stem cells was high in PTEN knockdown
model of LS180 as compared with doxorubicin-resistant LS180 cells.
This may be due to the fact that PTEN acts as a tumor suppressor by
negatively acting on the Akt signalling pathway, which is a pathway
that has been identified to be deregulated in the majority types of
cancer (31). PTEN mutations may
contribute to the failure of chemotherapy and therefore drug
resistance (32). The results of the
present study demonstrated that there was an increase in the levels
of IL6, IL2 and IL8. Knockdown of PTEN led to an increase in the
levels of IL6 which were further increased in the
doxorubicin-resistant cells. These results indicated that a
positive feedback loop is generated whereby IL6 increased
expression of the NF-κB pathway, which further enhances the
production of IL6, thus linking inflammation to the malignant
transformation of tumours. Doxorubicin resistance also induced
epithelial to mesenchymal transition in the colon cancer cell line
LS180 and its PTEN knockdown model as it was demonstrated by the
decrease in the expression of epithelial marker E-cadherin in
resistant cells as compared with parental cells. Additionally,
notable changes were shown in the expression of mesenchymal
markers, including vimentin and N-cadherin in the drug-resistant
cells compared with parental cells at the mRNA and protein level as
was examined by RT-qPCR and western blotting.
To conclude, investigating the underlying mechanisms
responsible for drug resistance is of great clinical significance.
The present study was able to demonstrate that doxorubicin
resistance may lead to the increased expression of IL6 signalling
pathway which was further activated by knockdown of PTEN, and this
effect may be reversed using an anti-IL6R antibody. Additionally,
the increase in IL6 levels due to doxorubicin resistance led to the
expansion of cancer stem cells and induced EMT.
Acknowledgements
Not applicable.
Funding
Financial assistance for the present study was
provided the National Natural Science Foundation of China (grant
no. 2015DB000475).
Availability of data and materials
In the present study the datasets generated and
analyzed are included in this published article.
Authors' contributions
The experiments was performed and analyzed by XYL
and XFL. Study design and manuscript preparation by HBW and JZ.
Ethics approval and consent to
participate
The present study has been approved by the Ethics
Committee of Xiangyang Central Hospital and written informed
consent was obtained from all participants.
Patient consent for publication
The present study participants provided consent for
the data and any associated images to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gandomani HS, Yousefi SM, Aghajani M,
Mohammadian-Hafshejani A, Tarazoj AA, Pouyesh V and Salehiniya H:
Colorectal cancer in the world: Incidence, mortality and risk
factors. Biomed Res Therapy. 4:102017. View Article : Google Scholar
|
|
2
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mishra J, Dromund J, Quazi SH, Karanki SS,
Shaw JJ, Chen B and Kumar N: Prospective of colon cancer treatments
and scope for combinatorial approach to enhanced cancer cell
apoptosis. Crit Rev Oncol Hematol. 86:232–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wáng YX, De Baere T, Idée JM and Ballet S:
Transcatheter embolization therapy in liver cancer: An update of
clinical evidences. Chin J Cancer Res. 27:96–121. 2015.PubMed/NCBI
|
|
5
|
Blakely T, Collinson L, Kvizhinadze G,
Nair N, Foster R, Dennett E and Sarfati D: Cancer care coordinators
in stage III colon cancer: A cost-utility analysis. BMC Health Serv
Res. 15:3062015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keating NL, Landrum MB, Lamont EB, Bozeman
SR, Krasnow SH, Shulman LN, Brown JR, Earle CC, Oh WK, Rabin M and
McNeil BJ: Quality of care for older patients with cancer in the
Veterans health administration versus the private sector: A cohort
study. Ann Intern Med. 154:727–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lam TJ, Meurs-Szojda MM, Gundlach L,
Belien JA, Meijer GA, Mulder CJ and Felt-Bersma RJ: There is no
increased risk for colorectal cancer and adenomas in patients with
diverticulitis: A retrospective longitudinal study. Colorectal Dis.
12:1122–1126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suraj R, Radhamani S, Meehan-Andrews T and
Bradley C: Role of a novel benzoxazine derivative in the
chemosensitization of colon cancer. Apoptosis. 22:988–1000. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadioglu O, Law BYK, Mok SWF, Xu SW,
Efferth T and Wong VKW: Mode of action analyses of neferine, a
bisbenzylisoquinoline alkaloid of lotus (Nelumbo nucifera) against
multidrug-resistant tumor cells. Front Pharmacol. 8:2382017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang XJ, Wang HY, Peng HG, Chen BF, Zhang
WY, Wu AH, Xu Q and Huang YZ: Codelivery of dihydroartemisinin and
doxorubicin in mannosylated liposomes for drug-resistant colon
cancer therapy. Acta Pharmacol Sin. 38:885–896. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu J, Zhang S, Wang R, Wu X, Zeng L and Fu
Z: Knockdown of PRDX2 sensitizes colon cancer cells to 5-FU by
suppressing the PI3K/AKT signaling pathway. Biosci Rep.
37:BSR201604472017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nedaeinia R, Avan A, Ahmadian M, Nia SN,
Ranjbar M, Sharifi M, Goli M, Piroozmand A, Nourmohammadi E, Manian
M, et al: Current status and perspectives regarding LNA-Anti-miR
oligonucleotides and microRNA miR-21 inhibitors as a potential
therapeutic option in treatment of colorectal cancer. J Cell
Biochem. 118:4129–4140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sambi M, Haq S, Samuel V, Qorri B, Haxho
F, Hill K, Harless W and Szewczuk MR: Alternative therapies for
metastatic breast cancer: Multimodal approach targeting tumor cell
heterogeneity. Breast Cancer (Dove Med Press). 9:85–93.
2017.PubMed/NCBI
|
|
14
|
Schoumacher M and Burbridge M: Key roles
of AXL and MER receptor tyrosine kinases in resistance to multiple
anticancer therapies. Curr Oncol Rep. 19:192017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng D, Wu W, Dong N, Jiang X, Xu J, Zhan
X, Zhang Z and Hu Z: Mxd1 mediates hypoxia-induced cisplatin
resistance in osteosarcoma cells by repression of the PTEN tumor
suppressor gene. Mol Carcinog. 56:2234–2244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
El Ouar I, Braicu C, Naimi D, Irimie A and
Berindan-Neagoe I: Effect of Helix aspersa extract on TNFα, NF-κB
and some tumor suppressor genes in breast cancer cell line Hs578T.
Pharmacogn Mag. 13:281–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mantamadiotis T: Towards targeting
PI3K-dependent regulation of gene expression in brain cancer.
Cancers (Basel). 9:E602017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ban HS, Kim BK, Kim HM, Harmalkar D, Nam
M, Park SK, Lee K, Park JT, Kim I, et al: The novel
hypoxia-inducible factor-1α inhibitor IDF-11774 regulates cancer
metabolism, thereby suppressing tumor growth. Cell Death Dis.
8:e28432017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tesio M, Trinquand A, Ballerini P,
Hypolite G, Lhermitte L, Petit A, Ifrah N, Baruchel A, Dombret H,
Macintyre E and Asnafi V: Age-related clinical and biological
features of PTEN abnormalities in T-cell acute lymphoblastic
leukaemia. Leukemia. 31:2594–2600. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Dong X, Wang Z, Liu W, Deng N, Ding
Y, Tang L, Hla T, Zeng R, Li L and Wu D: Regulation of PTEN by Rho
small GTPases. Nat Cell Biol. 7:399–404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Solari F, Bourbon-Piffaut A, Masse I,
Payrastre B, Chan AM and Billaud M: The human tumour suppressor
PTEN regulates longevity and dauer formation in Caenorhabditis
elegans. Oncogene. 24:20–27. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bar N and Dikstein R: miR-22 forms a
regulatory loop in PTEN/AKT pathway and modulates signaling
kinetics. PLoS One. 5:e108592010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chalhoub N and Baker SJ: PTEN and the
PI3-Kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keniry M and Parsons R: The role of PTEN
signaling perturbations in cancer and in targeted therapy.
Oncogene. 27:5477–5485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korkaya H, Kim GI, Davis A, Malik F, Henry
NL, Ithimakin S, Quraishi AA, Tawakkol N, D'Angelo R, Paulson AK,
et al: Activation of an IL-6 inflammatory loop mediates trastuzumab
resistance in HER2+ breast cancers by expanding the cancer stem
cell population. Mol Cell. 47:570–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zarogoulidis P, Yarmus L, Darwiche K,
Walter R, Huang H, Li Z, Zaric B, Tsakiridis K and Zarogoulidis K:
Interleukin-6 cytokine: A multifunctional glycoprotein for cancer.
Immunome Res. 9:165352013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Badawy A, Ghoneim MA, Gabr MM, Salah
RA, Mohamed IK, Amer M and El-Badri N: Cancer cell-soluble factors
reprogram mesenchymal stromal cells to slow cycling, chemoresistant
cells with a more stem-like state. Stem Cell Res Ther. 8:2542017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu T, Li Z, Gao CY and Cho CH: Mechanisms
of drug resistance in colon cancer and its therapeutic strategies.
World J Gastroenterol. 22:6876–6889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hammond WA, Swaika A and Mody K:
Pharmacologic resistance in colorectal cancer: A review. Ther Adv
Med Oncol. 8:57–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pu H, Zheng Q, Li H, Wu M, An J, Gui X, Li
T and Lu D: CUDR promotes liver cancer stem cell growth through
upregulating TERT and C-Myc. Oncotarget. 6:40775–40798. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Phadngam S, Castiglioni A, Ferraresi A,
Morani F, Follo C and Isidoro C: PTEN dephosphorylates AKT to
prevent the expression of GLUT1 on plasmamembrane and to limit
glucose consumption in cancer cells. Oncotarget. 7:84999–85020.
2016. View Article : Google Scholar : PubMed/NCBI
|