Introduction
Cervical cancer (CC) is the fourth most common type
of malignancy in women worldwide, accounting for ~250,000
cancer-associated mortalities annually (1,2). The
majority of CC cases are as a result of human papillomavirus (HPV)
infections (3), which may explain why
~80% of new CC cases are in developing countries (4,5). An
increasing number of studies have revealed that HPV infection alone
is not sufficient to initiate the malignant changes that lead to
CC, and that other factors contribute to the carcinogenesis and
progression of CC (6,7). Therefore, screening the factors involved
in tumorigenesis may provide a new way to predict the progression
of CC early or to efficiently treat patients with CC.
MicroRNAs (miRNAs or miRs) are a class of small
noncoding RNAs that are 18–25 nucleotides in length, and which
function as key regulators of gene expression at the
post-transcriptional level (8). By
targeting the 3′-untranslated regions (3′-UTRs) of target mRNAs,
miRNA may lead to either translational repression or degradation of
mRNA (9,10). Several studies have demonstrated that
miRNAs are involved in regulating various biological processes,
including cell proliferation (11),
migration (10), invasion (10,12) and
drug resistance (13). miR-214, one
member of the miR-214 family, has been revealed to be aberrantly
expressed in several human cancer types, including breast cancer
(14), hepatocellular carcinoma
(15), lung cancer (13), esophageal squamous cell cancer
(16) and ovarian cancer (17). The dysregulation of miR-214 predicts a
poor prognosis in the aforementioned cancers (13–17).
Furthermore, the underlying molecular mechanism in these cancers
has been explored, and a number of target genes, including PTEN,
LHX6, GALNT7 and uncoupling protein 2, have been identified
(13,15–17).
However, the role of miR-214 in regulating human CC cells remains
to be explored.
Enhancer of zeste homolog 2 (EZH2) serves an
important role in regulating cell proliferation and the cell cycle
via regulating the methylation status of lysine 27 in histone H3
(H3K27) (18,19). A previous study demonstrated that
overexpression of EZH2 is associated with worse disease-free
survival rates and worse overall survival rates for patients with
breast cancer (20). EZH2 was
identified as a direct target of miR-214 in breast cancer (21). However, the association between EZH2
expression and miR-214 expression in human CC requires further
exploration.
In the present study, the expression and biological
function of miR-214 in human CC was evaluated. The expression of
miR-214 was identified to be downregulated in CC tissues compared
with the adjacent noncancerous tissues and EZH2 was identified as a
direct target of miR-214. EZH2 knockdown or miR-214 overexpression
could impair the cell proliferation of CC cell lines. Taken
together, these results indicate that EZH2 may function as an
oncogene and as a mediator of miR-214 in human CC.
Materials and methods
Clinical tissue samples
A total of 45 patients diagnosed as CC were enrolled
in the current study between August 2007 and October 2011, and none
of them had received any anti-cancer treatments. Fresh CC tissues
and corresponding adjacent noncancerous tissues were obtained from
each of the enrolled patients. All tissue samples were stored in
liquid nitrogen until further usage. The mean age of these patients
was 53.5±7.4 years, ranging between 45 and 69 years. The clinical
information of CC cases is presented in Table I. Written informed consent was
obtained from all enrolled patients. The current study was
performed according to the principles of the Declaration of
Helsinki. Ethics approval was granted by the Ethics Committee of
the Xuzhou Maternity and Child Health Care Hospital (Xuzhou,
China).
 | Table I.Association between miR-214 expression
and clinicopathological features. |
Table I.
Association between miR-214 expression
and clinicopathological features.
|
|
| miR-214 expression
level |
|
|---|
|
|
|
|
|
|---|
| Variables | n | High | Low | P-value |
|---|
| Age, years |
|
|
| 0.252 |
|
≥50 | 25 | 8 | 17 |
|
|
<50 | 20 | 6 | 14 |
|
|
Differentiation |
|
|
| 0.037 |
|
Well/Moderate | 17 | 5 | 12 |
|
|
Poor | 28 | 9 | 19 |
|
| Tumor stage |
|
|
| 0.012 |
|
I–II | 16 | 6 | 10 |
|
|
III | 29 | 8 | 21 |
|
| Lymph node
metastasis |
|
|
| 0.075 |
|
Negative | 18 | 5 | 13 |
|
|
Positive | 27 | 9 | 18 |
|
Cell culture
Human CC cell line HeLa and normal cervical cell
line Ect1/E6E7 were purchased from the American Type Culture
Collection (Manassas, VA, USA). These cells were cultured in RPMI
1640 medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% heat-inactivated fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin
and 100 µg/ml streptomycin, in a humidified atmosphere of 95% air
and 5% CO2 at a temperature of 37°C.
Transient transfection
The miR-214 mimic (5′-UGCCUGUCUACACUUGCUGUGC-3′),
miR-214 inhibitor (5′-GCACAGCAAGUGUAGACAGGCA-3′) and negative
control miRNA (5′-GUGUCUGUCCUUACGUGCUCCA-3′) were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The EZH2-targeting
small-interfering RNA (siRNA; 5′-AGUCUCAUGUACGCTGACUCUG-3′) and
negative control siRNA (5′-GUGUCUUCACGUUACCUAGAGC-3′) were also
purchased from Guangzhou RiboBio Co., Ltd. All cell transfections
were performed using Lipofectamine® 2000 reagent
(Invitrogen) according to the manufacturer's protocol and cultured
for 48 h prior to the following experiments. The final
concentration of miRNAs and siRNAs used for cell transfection was
100 nm.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cultured cells and
fresh-frozen tissues using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
To quantify the expression level of miR-214, a total of 5 µg RNA
was reverse transcribed into cDNA using M-MLV reverse transcriptase
(Promega Corporation, Madison, WI, USA). The temperature protocol
of first-strand cDNA synthesis was: 16°C for 30 min, 42°C for 30
min and 85°C for 5 min. The expression level of miR-214 was
normalized to human U6 snRNA. The following PCR primers were used:
U6: Forward, 5′-TGCGGGTGCTCGCTTCGGCAGC-3; reverse,
5′-CCAGTGCAGGGTCCGAGGT-3′ and miR-214: Forward,
5′-TGCGGACAGCAGGCACAGAC-3′; reverse, 5′-CCAGTGCAGGGTCCGAGGT-3′.
The expression level of EZH2 was normalized to
GAPDH. The following PCR primers were used: EZH2: Forward,
5′-TTGTTGGCGGAAGCGTGTAAAATC-3′; reverse,
5′-TCCCTAGTCCCGCGCAATGAGC-3′ and GAPDH: Forward,
5′-TGAACGGGAAGCTCACTGG-3′; and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
RT-qPCR was performed using SYBR Premix Ex Taq™ kit
(Takara Biotechnology Co., Ltd., Dalian, China) on the 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The reaction condition was 95°C for 3 min, followed by 40
cycles of 95°C for 30 sec and 60°C for 30 sec. Relative expression
values from three independent experiments were calculated using the
2−∆∆Cq method (22).
Western blot analysis
Total protein was isolated from cultured cells and
tissues using RIPA lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) and was quantified using a BCA
protein quantification kit (Beyotime Institute of Biotechnology)
according to the manufacturer's protocol. The protein samples (50
µg) were separated using a 12% SDS-PAGE gel and then transferred to
a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was incubated with fat-free milk for 90 min at
room temperature, then incubated with primary antibodies against
EZH2 (cat. no. 4905) and GAPDH (cat. no. 2118; both 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) for 60 min at 4°C.
The membrane was then incubated with horseradish
peroxidase-conjugated secondary antibodies (cat. no. 7074; 1:500;
Cell Signaling Technology, Inc.) at room temperature for 60 min.
Bands were visualized using the BeyoECL Plus kit (Beyotime
Institute of Biotechnology). Densitometric analysis of the protein
bands was conducted using ImageJ 1.42 software (National Institutes
of Health, Bethesda, MD, USA). Analysis of each sample was repeated
three times.
Cell proliferation assay
To determine cell proliferation, an MTT assay was
performed. Cells were seeded into 96-well plates at a density of
2×103 cells/well in a volume of 100 µl RPMI 1640 medium
supplemented with 10% FBS, 100 IU/ml penicillin and 100 µg/ml
streptomycin, and incubated for 0, 24, 48 and 72 h. MTT solution
(10 µl) was added to each well at a final concentration of 0.5
mg/ml and the cells were cultured for another 4 h at 37°C. The
medium was removed and the precipitated formazan was dissolved in
100 µl DMSO. The absorbance of each well was measured at 570 nm
using the Thermo Multiskan Spectrum spectrophotometer (Thermo
Fisher Scientific, Inc.).
Target prediction and luciferase
reporter assay
Based on bioinformatics prediction algorithm
TargetScan 7.2 (http://www.targetscan.org/vert_72/), EZH2 was selected
as a candidate target of miR-214. The 3′-UTR of EZH2 that contains
the putative binding sites for miR-214 was amplified from human
genomic DNA and cloned into the 3′-UTR of Renilla luciferase
gene in the psiCHECK-2 receptor vector (Promega Corporation). The
putative miR-214 binding sites in EZH2 were then mutated and also
cloned into the psiCHECK-2 receptor vector. Following the cloning,
the cells were co-transfected with miR-214 mimic or negative
control miRNA and the wild-type or mutant 3′-UTR luciferase
constructs using Lipofectamine 2000 reagent. Cells were lysed 24 h
post-transfection to measure the luciferase activity using a
Dual-Luciferase Reporter Assay system (Promega Corporation),
according to the manufacturer's protocol. Renilla luciferase
activity was used to normalize the luciferase activity.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
The 75th percentile of the expression level of miR-214 was used to
classify the patients into a high (≥75th percentile) or low
(<75th percentile) miR-214 expression group. Data are presented
as the mean ± standard deviation (three repeats). χ2
test was used to evaluate associations between the expression of
miR-214 and clinicopathological characteristics. Survival curves
were generated using the Kaplan-Meier method and compared using the
log-rank test. Comparisons between two groups were conducted using
Student's t-test. Analysis of variance and Tukey's post-hoc test
were used when comparing the differences among multiple groups. Cox
univariate and multivariate regression analyses were used to
identify the independent predictor for the prognosis of patients
with CC. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-214 is downregulated in CC tissues
and a CC cell line
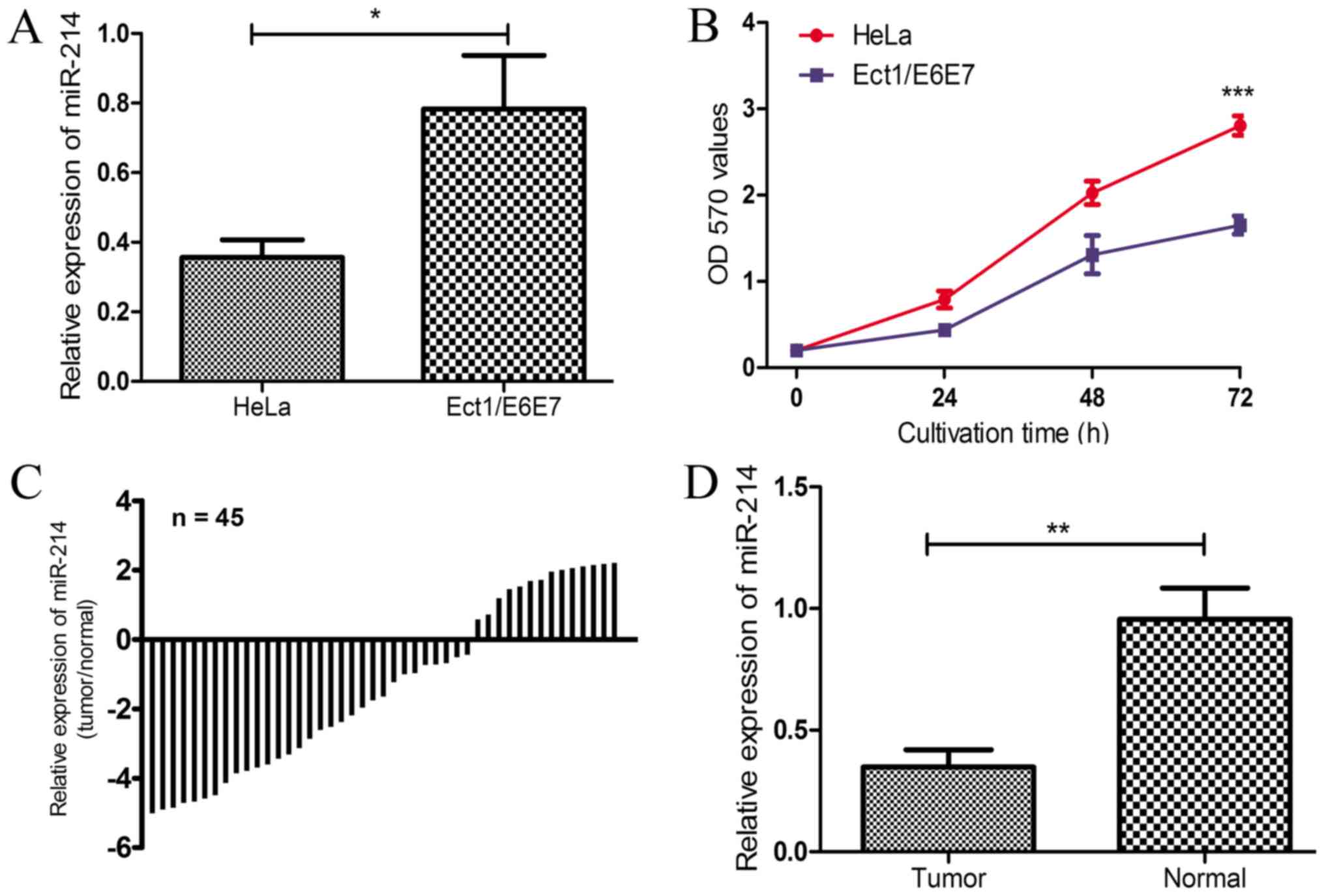
To explore whether miR-214 expression was altered in
CC, miR-214 expression levels in the CC and normal cervical cell
lines were examined. As revealed in Fig.
1A, the expression of miR-214 in the CC cell line, HeLa, was
significantly lower compared with that in the normal cervical cell
line, Ect1/E6E7 (P<0.05). The cell proliferation in HeLa cells
was significantly increased compared with the Ect1/E6E7 cells at 72
h (P<0.001; Fig. 1B). The
expression of miR-214 was also investigated in the 45 pairs of CC
tissues and corresponding adjacent non-cancerous tissues. Compared
with the paired normal tissues, miR-214 expression was decreased in
31 of 45 CC tissues (68.89%; Fig.
1C), which was demonstrated to be statistically significant
(P<0.01; Fig. 1D). The results
demonstrated that the expression of miR-214 was downregulated in CC
tissues and HeLa cells, which implies that miR-214 may serve an
important role in the progression of CC.
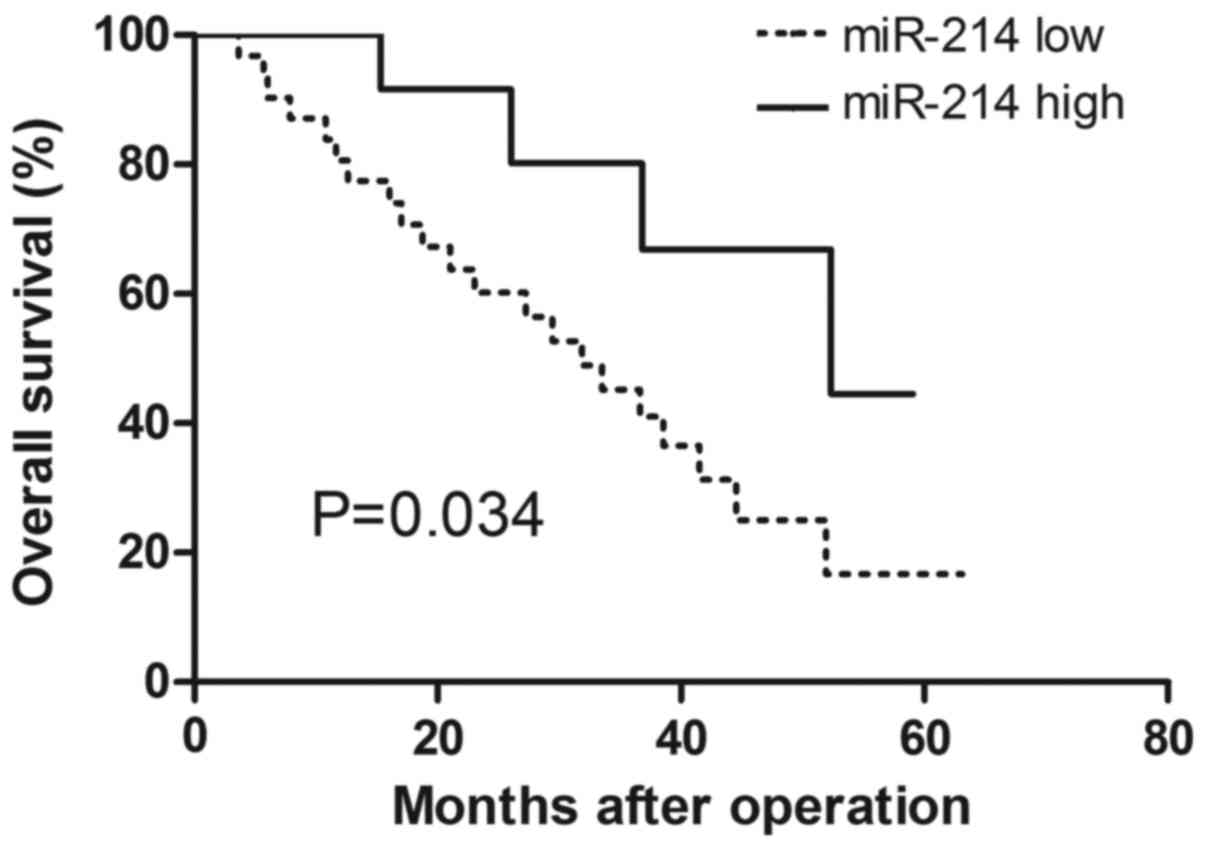
miR-214 expression is associated with
clinicopathological features of patients with CC
To investigate the association of miR-214 expression
and the clinical outcome of patients with CC, the 45 enrolled
patients were classified into two groups based on the expression
level of miR-214. The association between miR-214 expression and
clinicopathological features was analyzed (Table I). miR-214 expression was
significantly associated with tumor differentiation (P=0.037) and
tumor stage (P=0.012), while no association was identified between
miR-214 expression and age or lymph node metastasis. Patients with
high miR-214 expression exhibited significantly higher overall
survival probability compared with those with low miR-214
expression (P=0.034; Fig. 2)
according to the Kaplan-Meier analysis and log-rank test results.
These results indicated that miR-214 may contribute to CC
progression. Additionally, a Cox univariate regression analysis
revealed that low miR-214 expression (P=0.035), poor tumor
differentiation (P=0.044) and high tumor stage (P=0.033) were
associated with poorer survival rates of patients with CC (Table II). Similarly, a multivariate Cox
regression analysis revealed that low miR-214 expression (P=0.036),
poor tumor differentiation (P=0.042) and high tumor stage (P=0.020)
could be regarded as independent indicators for poor survival of
patients with CC (Table II).
 | Table II.Univariate and multivariate analyses
of overall survival rate. |
Table II.
Univariate and multivariate analyses
of overall survival rate.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| microRNA-214 | 2.401 | 1.065–5.413 | 0.035 | 2.392 | 1.060–5.397 | 0.036 |
| Age, years | 2.241 | 0.821–6.115 | 0.115 | – | – | – |
|
Differentiation | 2.342 | 1.022–5.366 | 0.044 | 2.556 | 1.034–6.314 | 0.042 |
| Tumor stage | 2.422 | 1.075–5.454 | 0.033 | 2.728 | 1.169–6.366 | 0.020 |
| Lymph node
metastasis | 2.438 | 0.945–6.292 | 0.066 | – | – | – |
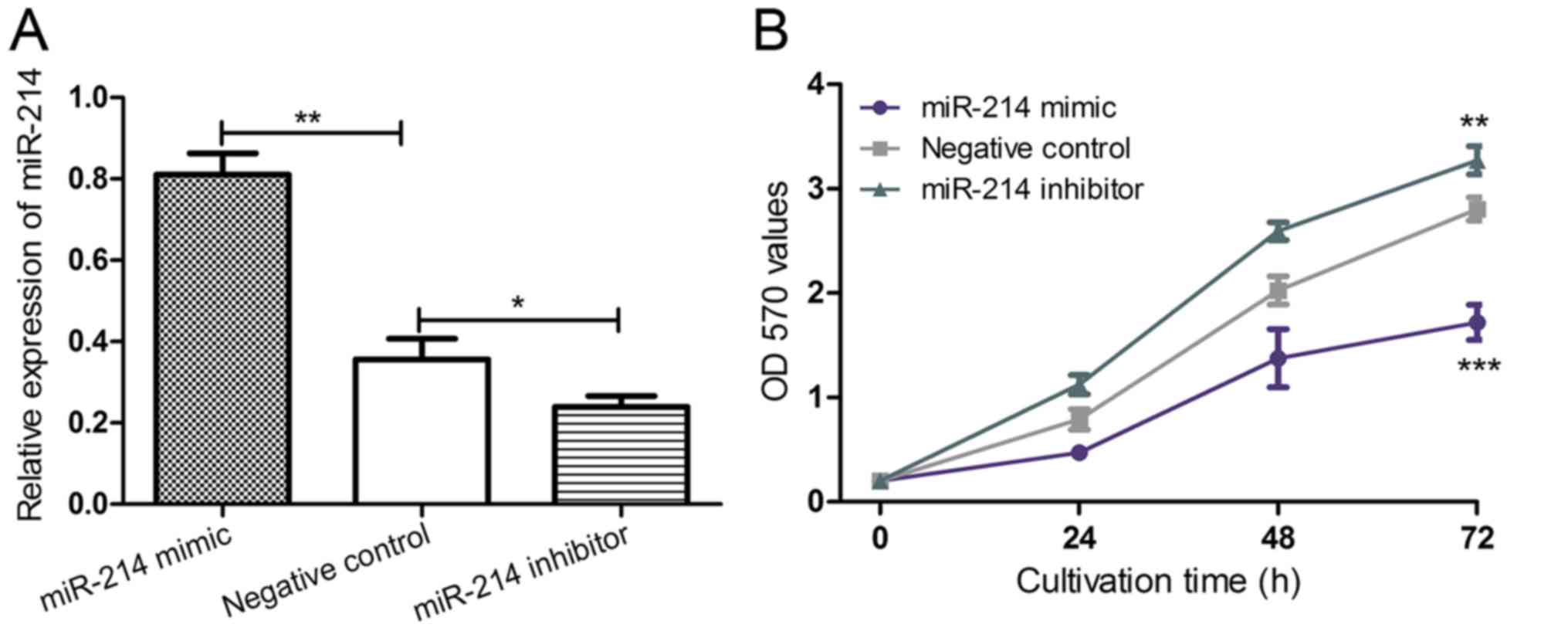
Upregulating miR-214 expression
inhibits the proliferation of CC cells
To further understand the biological function of
miR-214 expression in CC progression, HeLa cells were transfected
with an miR-214 mimic, an miR-214 inhibitor or negative control
miRNA to regulate the expression level of miR-214. Successful
transfection of the miRNAs was verified by RT-qPCR (Fig. 3A). The expression of miR-214 was
significantly downregulated using a miR-214 inhibitor compared with
the negative control (P<0.05; Fig.
3A), which significantly promoted HeLa cell proliferation
(P<0.01; Fig. 3B) compared with
the negative control. Conversely, the expression of miR-214 was
significantly upregulated using a miR-214 mimic (P<0.01;
Fig. 3A), which significantly
inhibited HeLa cell proliferation (P<0.001; Fig. 3B) compared with the negative control.
Collectively, the results indicated that miR-214 serves as a
proliferation inhibitor, which was consistent with the
aforementioned finding that miR-214 expression was reduced in a CC
cell line.
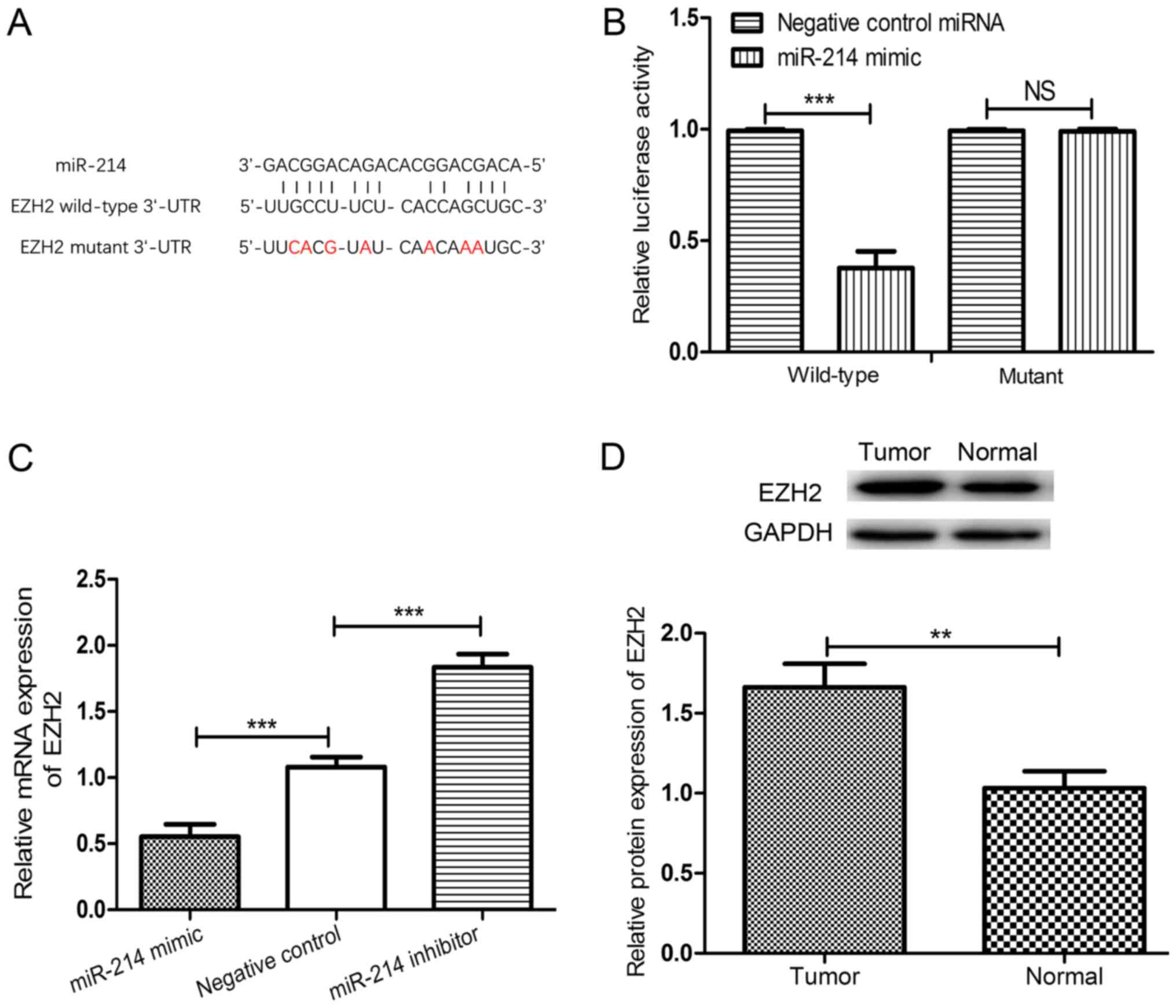
EZH2 is a direct target of
miR-214
Using the online TargetScan algorithm, the 3′-UTR of
EZH2 was identified to contain a putative target sequence for
miR-214 (Fig. 4A). To validate EZH2
as a target of miR-214 in CC, the luciferase activities of EZH2
were analyzed using a dual-luciferase reporter assay. As expected,
the miR-214 mimic significantly reduced the luciferase activity of
the wild-type 3′-UTR of EZH2 compared with the negative control
miRNA-transfected cells (P<0.001; Fig.
4B). However, no significant differences were identified
between cells transfected with negative control miRNAs and the
miR-214 mimic when co-transfected with the mutated 3′-UTR of EZH2.
The expression of EZH2 in HeLa cells transfected with an miR-214
mimic, an miR-214 inhibitor or negative control miRNA was measured.
Upregulating miR-214 expression significantly downregulated the
expression of EZH2, whereas downregulating miR-214 expression
significantly upregulated the expression of EZH2 in HeLa cells
compared with HeLa cells transfected with the negative control
miRNA (both P<0.001; Fig. 4C).
Additionally, the expression of EZH2 in CC tissues and adjacent
noncancerous tissues was examined and, as expected, the expression
of EZH2 in CC tissues was significantly higher compared with that
of the adjacent non-cancerous tissues (P<0.01; Fig. 4D).
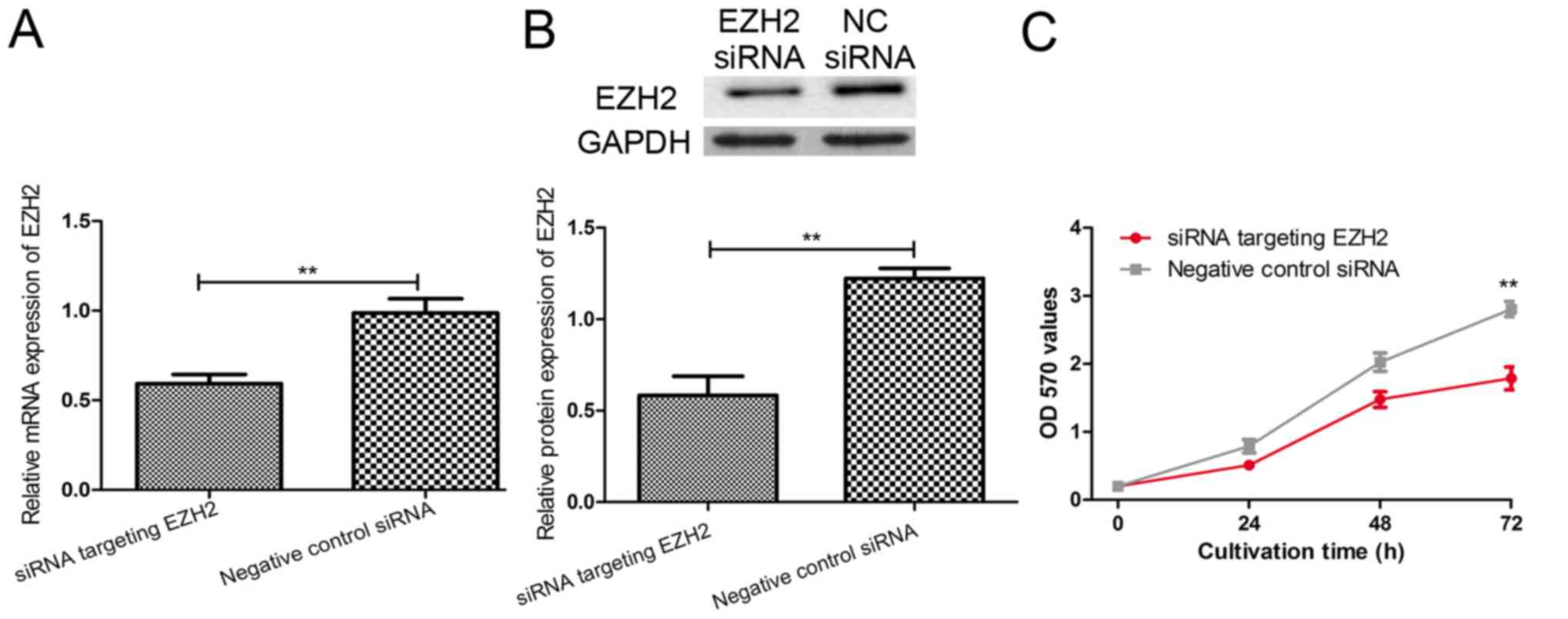
Inhibiting EZH2 expression reduces the
proliferation of CC cells
To explore the effect of EZH2 on the proliferation
capacity of HeLa cells induced by a miR-214 mimic, an EZH2-specific
siRNA was introduced into HeLa cells. As revealed in Fig. 5A and B, EZH2-specific siRNA
significantly downregulated the expression of EZH2 at the mRNA and
protein levels (both P<0.01). Furthermore, the proliferation of
HeLa cells was significantly inhibited by EZH2-specific siRNA at 72
h (P<0.01; Fig. 5C). Taken
together, these results demonstrated that miR-214 acts as a cell
proliferation inhibitor partly through regulating the expression of
EZH2.
Discussion
Dysregulation of miRNAs has been revealed in
numerous human cancers and thus increasing research efforts have
been made in this field (9–17,23). It
has previously been demonstrated that miRNAs may function as a
novel class of tumorigenic and tumor-suppressing factors (24). miRNAs are involved in the tumor
initiation and progression processes with two mechanisms: Certain
miRNAs are directly involved in cancer development by controlling
cell differentiation and apoptosis, and other miRNAs are involved
in cancer by targeting cancer oncogenes and/or tumor suppressors
(24). Understanding the molecular
mechanisms of miRNAs in cancers may provide new insights into the
molecular basis of cancers, and new biomarkers for cancer diagnosis
and cancer therapy.
The differential expression of miR-214 in human
cancers has been reported previously (13,15–17). In
the current study, a decrease in the expression of miR-214 was
identified in human CC tissues and the CC cell line, HeLa, which is
inconsistent with a previous study (25). In addition, the clinical significance
of miR-214 expression in CC was studied. The current study
demonstrated that low expression of miR-214 was associated with
poor tumor differentiation and high tumor stage, and that patients
with low miR-214 expression exhibited a worse 5-year overall
survival rate. The multivariate analysis demonstrated that the
miR-214 expression, tumor differentiation and tumor stage were
independent predictors for the prognosis of patients with CC, which
highlighted the importance of miR-214 expression in CC. Zhao et
al (26) reported that the
aberrant expression of miR-214 is associated with the growth of a
lung cancer cell line. Therefore, the effect of miR-214 expression
on HeLa cell proliferation was also investigated. In the current
study, the transfection of an miR-214 mimic into HeLa cells
resulted in the upregulation of miR-214, which led to decreased
cell proliferation. The transfection of an miR-214 inhibitor into
HeLa cells resulted in decreased miR-214 expression, but increased
cell proliferation. This finding mirrors the role of miR-214 as a
tumor suppressor gene. The deregulation of miR-214 in a CC cell
line compared with a normal cervical cell line may account for the
aberrant growth behavior of the CC cell line.
Several targets of miR-214, including ARL2, FOXM1
and HMGA1 have been identified in recent years (27–29). To
explore the underlying mechanisms of the effects of miR-214 in CC,
its potential target genes were explored using bioinformatics
analysis and several genes were predicted as target genes of
miR-214. The EZH2 gene was selected as a potential target to
investigate in the current study as it was widely reported to be
ectopically expressed in human cancers and associated with poor
prognosis (20,21,30). More
importantly, EZH2 may be regulated by miR-214 in skeletal muscle
cells (31), erythroid cells
(32), cardiac myofibroblasts
(33) and in the process of cardiac
hypertrophy (34). Recently, Xu et
al (35) demonstrated that EZH2
may also be regulated by miR-214 in glioma cells. Therefore, a
dual-luciferase reporter assay was used in the current study to
confirm that EZH2 is a direct target of miR-214 in CC. Notably,
EZH2 expression may be regulated by miR-214, as the upregulation of
miR-214 by a miR-214 mimic decreased EZH2 expression and the
downregulation of miR-214 by a miR-214 inhibitor increased EZH2
expression in CC. Additionally, the role of EZH2 on HeLa cell
proliferation was examined and, and as expected, downregulating the
expression of EZH2 decreased cell proliferation, which is in
accordance with a previous study (30). Taken together, these results indicate
that EZH2 is a target gene of miR-214, which could potentially help
to unravel the mechanism of miR-214 in the regulation of CC
progression.
In conclusion, miR-214 was expressed at a low level
in CC tissues when compared with adjacent non-cancerous tissues,
and the overexpression of miR-214 inhibited cell proliferation. A
novel target gene of miR-214, EZH2, was revealed to be upregulated
in HeLa cells, a CC cell line. These findings indicated that
inhibition of miR-214 in CC may contribute to the malignant
phenotype by maintaining a high level of EZH2. Thus, the
identification of miR-214 and its target gene, EZH2, in CC may aid
in improving the prognosis for CC patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY and YL contributed equally to the study. YY, YL
and HS conceived and designed the study. YY, YL, GL, LL, PG and HS
performed the experiments. YY, YL and HS wrote the paper. YY, YL
and HS reviewed and edited the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
Ethics approval was granted by the Ethics Committee
of the Xuzhou Maternity and Child Health Care Hospital (Xuzhou,
China). Written informed consent was obtained from all
participants.
Patient consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN and Gaffney DK: Cervical cancer: A global health
crisis. Cancer. 123:2404–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kent A: HPV vaccination and testing. Rev
Obstet Gynecol. 3:33–34. 2010.PubMed/NCBI
|
|
6
|
Hildesheim A and Wang SS: Host and viral
genetics and risk of cervical cancer: A review. Virus Res.
89:229–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin CM, Astbury K and O'Leary JJ:
Molecular profiling of cervical neoplasia. Expert Rev Mol Diagn.
6:217–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:pp. 15524–15529. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Hong W, Zhou C, Jiang Z, Wang G,
Wei G and Li X: miR-539 inhibits FSCN1 expression and suppresses
hepatocellular carcinoma migration and invasion. Oncol Rep.
37:2593–2602. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Z, Wang M, He Q, Li Z, Zhao Y, Wang
W, Ma J, Li Y and Chang G: MicroRNA-98 rescues proliferation and
alleviates ox-LDL-induced apoptosis in HUVECs by targeting LOX-1.
Exp Ther Med. 13:1702–1710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu Y, Wu G, Yan W, Zhan H and Sun P:
miR-146b-5p regulates cell growth, invasion, and metabolism by
targeting PDHB in colorectal cancer. Am J Cancer Res. 7:1136–1150.
2017.PubMed/NCBI
|
|
13
|
Liao J, Lin J, Lin D, Zou C, Kurata J, Lin
R, He Z and Su Y: Down-regulation of miR-214 reverses erlotinib
resistance in non-small-cell lung cancer through up-regulating LHX6
expression. Sci Rep. 7:7812017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Tian Y, Li F, Zhao Z, Jiang X, Zhao
C, Han X and Zhang L: Tumor-suppressing roles of miR-214 and
miR-218 in breast cancer. Oncol Rep. 35:3178–3184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu G, Wang J, Xu K and Dong J: Dynamic
regulation of uncoupling protein 2 expression by microRNA-214 in
hepatocellular carcinoma. Biosci Rep. 36(pii): e003352016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Q, Xu L, Li C, Yuan Y, Huang S and Chen
H: MiR-214 inhibits invasion and migration via downregulating
GALNT7 in esophageal squamous cell cancer. Tumor Biol.
37:14605–14614. 2016. View Article : Google Scholar
|
|
17
|
Zhang Q and Zhang S: MiR-214 promotes
radioresistance in human ovarian cancer cells by targeting PETN.
Biosci Rep. 27:BSR201703272017. View Article : Google Scholar
|
|
18
|
Margueron R and Reinberg D: The Polycomb
complex PRC2 and its mark in life. Nature. 469:343–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Di Croce L and Helin K: Transcriptional
regulation by Polycomb group proteins. Nat Struct Mol Biol.
20:1147–1155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kleer CG, Cao Q, Varambally S, Shen R, Ota
I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:pp. 11606–11611. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Derfoul A, Juan AH, Difilippantonio MJ,
Palanisamy N, Ried T and Sartorelli V: Decreased microRNA-214
levels in breast cancer cells coincides with increased cell
proliferation, invasion and accumulation of the Polycomb Ezh2
methyltransferase. Carcinogenesis. 32:1607–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2011.
View Article : Google Scholar
|
|
23
|
Yang LH, Yin SY, He RQ, Mo WJ, Pang YY, Wu
YZ, Peng ZG and Gan TQ: Prospective target genes and pathways of
miR-30a-5p in colorectal cancer: An investigation using TCGA and
bioinformatics analysis. Int J Clin Exp Med. 10:4373–4385.
2017.
|
|
24
|
Zhang B, Pan X, Cobb GP and Anderson TA:
MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2017. View Article : Google Scholar
|
|
25
|
Yang Z, Chen S, Luan X, Li Y, Liu M, Li X,
Liu T and Tang H: MicroRNA-214 is aberrantly expressed in cervical
cancers and inhibits the growth of HeLa cells. IUBMB Life.
61:1075–1082. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao X, Lu C, Chu W, Zhang Y, Zhang B,
Zeng Q, Wang R, Li Z, Lv B and Liu J: microRNA-214 governs lung
cancer growth and metastasis by targeting carboxypeptidase-D. DNA
Cell Biol. 35:715–721. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng R, Men J, Ma R, Wang Q, Wang Y, Sun Y
and Ren J: miR-214 down-regulates ARL2 and suppresses growth and
invasion of cervical cancer cells. Biochem Biophys Res Commun.
484:623–630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang JM, Ju BH, Pan CJ, Gu Y, Li MQ, Sun
L, Xu YY and Yin LR: MiR-214 inhibits cell migration, invasion and
promotes the drug sensitivity in human cervical cancer by targeting
FOXM1. Am J Transl Res. 9:3541–3557. 2017.PubMed/NCBI
|
|
29
|
Chandrasekaran KS, Sathyanarayanan A and
Karunagaran D: MicroRNA-214 suppresses growth, migration and
invasion through a novel target, high mobility group AT-hook 1, in
human cervical and colorectal cancer cells. Br J Cancer.
115:741–751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Y, Liu T, Bao X, He M, Li L and Yang
X: Increased EZH2 expression is associated with proliferation and
progression of cervical cancer and indicates a poor prognosis. Int
J Gynecol Pathol. 33:218–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Juan AH, Kumar RM, Marx JG, Young RA and
Sartorelli V: Mir-214-dependent regulation of the polycomb protein
Ezh2 in skeletal muscle and embryonic stem cells. Mol Cell.
36:51–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao M, Liu Y, Chen Y, Yin C, Chen JJ and
Liu S: miR-214 protects erythroid cells against oxidative stress by
targeting ATF4 and EZH2. Free Radic Biol Med. 92:39–49. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu WS, Tang CM, Xiao Z, Zhu JN, Lin QX,
Fu YH, Hu ZQ, Zhang Z, Yang M, Zheng XL, et al: Targeting EZH1 and
EZH2 contributes to the suppression of fibrosis-associated genes by
miR-214-3p in cardiac myofibroblasts. Oncotarget. 7:78331–78342.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang T, Zhang GF, Chen XF, Gu HH, Fu SZ,
Xu HF, Feng Q and Ni YM: MicroRNA-214 provokes cardiac hypertrophy
via repression of EZH2. Biochem Biophys Res Commun. 436:578–584.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu C, He T, Li Z, Liu H and Ding B:
Regulation of HOXA11-AS/miR-214-3p/EZH2 axis on the growth,
migration and invasion of glioma cells. Biomed Pharmacother.
95:1504–1513. 2017. View Article : Google Scholar : PubMed/NCBI
|