Introduction
Multiple myeloma (MM) is an aggressive hematologic
malignancy, which has short overall survival time (OS) despite
treatment with immunoregulatory drugs (IMiDs) and proteasome
inhibitors, including bortezomib (1).
It accounts for 1% of all malignant diseases and for slightly more
than 10% of all hematologic cancers (2). The mean age of affected individuals is
72 years for men (75% >70 years) and 71 years for women (79%
>70 years) (3). In addition to
genetic markers, there are further prognostic factors, including
lymphocyte counts and C-reactive protein (CRP) expression level,
which have been evaluated in recent years (4,5).
Immunophenotyping, including the analysis of CD19, CD45, CD56 and
CD117, is of great value to the prognosis and diagnosis of MM
according to recent reports (6,7). Pan et
al (8) observed that for patients
with MM, CD56+ was an independent prognostic factor for increased
OS. Ceran et al (9) suggested
that CD56- and CD117- groups were associated with advanced stages
of MM. However, these results are based on small sample sizes (50
cases and 34 cases) (8,9) and short follow-up period (~6 years)
(8) Importantly, bias originating
from different treatments was not accounted for, particularly in
patients that did not receive transplantations as a result of
financial status or by personal choice. Furthermore, at present, to
the best of our knowledge, HLA-DR has not been studied in terms of
patients with MM. The present study retrospectively analyzed
clinical parameters, including immunophenotypes and prognosis, of
80 patients newly diagnosed with MM that did not receive autologous
stem cell transplantation and allo-hematopoietic stem cell
transplantation and was based on uniform combination treatment of
bortezomib and thalidomide between January 2007 and December
2015.
Patients and methods
Patients
A total of 80 newly diagnosed patients (46 males and
34 females) with MM treated with a combination treatment including
thalidomide between January 2007 and December 2015 in Wuxi People's
Hospital (Wuxi, China) were analyzed retrospectively. Data were
thought to be related to MM when they were different from our
reference values. Our lab has its own reference values generated
from healthy patients who were examined in our hospital and were
considered as a control group Therefore, when patients with MM have
data outside these levels, this was taken indicate MM. All patients
had the full clinical information (age ≥65 years, sex, ISS staging
III, Complex Chromosome, Hypertension, Diabetes, treatment regimens
and survival data) including laboratory parameters (albumin <35
g/l, calcium >2.8 mmol/l, hemoglobin <100 g/l, lymphocyte
counts >1.3×109/l, lactate dehydrogenase >245 U/l,
serum creatinine concentrations >177 umol/l, C-reactive protein
(CRP) expression level >8 mg/l, Flow cytometry results, survival
data and immunoglobulin type of monoclonal protein) before any
therapy. Concurrence of autoimmune disease, human immunodeficiency
virus (HIV) and syphilis was excluded for all enrolled individuals.
Karyotypes detected were based on traditional reverse-banding
and/or G-banding techniques. Demographic and clinical
characteristics of the patients were collected by reviewing medical
charts and electronic records. Diagnostic criteria and risk
stratification of the disease were based on the International
Myeloma Working Group (IMWG) criteria and International Staging
System (ISS) (10,11).
Flow cytometry
Immunophenotype evaluation was performed using a
flow cytometer (FACS Canto; BD Biosciences, San Jose, CA, USA).
Analysis were performed, using the FACS DIVA 6.1.3 software (BD
Biosciences). All samples were anticoagulated with EDTA tube and
examined within 6 h. Approximately 100 ul of anticoagulated bone
marrow sample was labeled with pre-conjugated monoclonal antibodies
at 25°C for 20 min in the dark. Following incubation, red blood
cells were lysed and washed in PBS three times. These regents were
supplied by BD Biosciences. All erythrocyte-lysed bone marrow (BM)
samples obtained prior to treatments were stained using the
following 3-color surface combinations (They were not as a kit and
they were combined by our laboratory supplied by BD Biosciences),
with fluorescein (FITC), phycoerythrin (PE) and peridinin
chlorophyll protein complex (PerCP): CD2/CD19/CD45, CD3/CD56/CD45,
CD4/CD8/CD45, CD5/CD7/CD45, human leukocyte antigen-antigen
D-related (HLA-DR)/CD10/CD45, CD20/CD117/CD45, CD22/CD14/CD45,
CD3/CD13/CD45, CD20/CD33/CD45 and CD38/CD34/CD45. Positivity was
defined as ≥20% antibody expression and negativity as <20%
expression. IgG1 was stained with PE or with FITC as
isotope/negative control at 25°C for 15 min in the dark. Each
patient sample was divided into ten tubes to detect the various
immunophenotype combinations listed above. All positive results
from the same patient were recorded. MM are thought to be CD38+.
All samples were tested CD38+ (>20% expression). Samples
containing myeloid antigens, including CD13 and CD33 in ≥1 tube,
were recruited as myeloid antigens-positive. All agents were
provided by BD Biosciences. Cat. nos. IgG1, 349041; CD45, 652803;
HLA-DR, 347363; CD10, 340921; CD20, 652808; CD117, 340129; CD22,
347573; CD4/CD8, 340039; CD14, 557742; CD38, 340909; CD34, 652802;
CD13, 652820; CD33, 347787; CD2, 555327; CD19, 557791; CD3, 557052;
CD56, 556647; CD5, 561896; CD7, 555360. (Dilution with aqueous
buffered solution containing ≤0.09% sodium azide.)
Therapeutic regiments
All patients received thalidomide (100–125 mg/day)
daily as basic treatment. Bortezomib and dexamethasone were
additionally administered as induction and maintenance therapy.
Treatment included a 3-week cycle of 1.3 mg/m2
bortezomib on days 1, 4, 8 and 11, with 20 mg dexamethasone on days
1,2, 4,5, 8,9 and 11,12 (12,13). Bortezomib and dexamethasone were
administered at the same time. All patients received 1–3 cycles of
induction therapy followed by >2 cycles of maintenance therapy
according to the OS (Table I).
Hematopoietic stem cell transplantation was not performed as a
result of financial status or by personal choice; however, some
patients did not qualified for transplantation.
 | Table I.Total number of cycles that patients
with multiple myeloma and various immunophenotypes were
administered a combination treatment. |
Table I.
Total number of cycles that patients
with multiple myeloma and various immunophenotypes were
administered a combination treatment.
| Groups | Treatment cycles
[range (median)] | P-value |
|---|
| CD56+/CD117+ | 3–8 (5.5) | 0.873 |
| CD56+/CD117- or
CD56-/CD117+ | 1–8 (6.5) |
|
| CD56-/CD117- | 4–8 (6.5) |
|
| HLA-DR+ | 1–8 (4.5) | 0.753 |
| HLA-DR- | 2–8 (5.5) |
|
| CD56+/HLA-DR+ | 4–8 (6.5) | 0.456 |
| CD56+/HLA-DR- | 1–8 (5.5) |
|
| CD117+/HLA-DR+ | 2–4 (3) | 0.136 |
| CD117+/HLA-DR- | 4–8 (4) |
|
| CD13+ or
CD33+/HLA-DR+ | 2–4 (3) | 0.071 |
| CD13+ or
CD33+/HLA-DR- | 4–9 (5) |
|
| MPI score 0 | 4–10 (5) | 0.514 |
| MPI score 1 | 1–9 (4.5) |
|
| MPI score 2–3 | 1–8 (5) |
|
Efficacy and follow-up
Efficacy was evaluated using the response criteria
for MM provided by the IMWG (14). OS
was measured from the date of diagnosis to the date of death or the
last follow-up in September 2017. Progression free survival (PFS)
was calculated from the date of diagnosis to the first date of
confirmed progression or death by any cause.
Statistical analysis
Differences among groups were analyzed by
Kruskal-Wallis and Mann-Whitney U test for continuous parameters,
and by Chi-square tests for categorical data. OS and PFS were
analyzed using Kaplan-Meier tests. Differences between survival
curves were assessed for statistical significance using the
two-tailed log-rank test. Potential risk factors for OS and PFS
were evaluated in univariate and multivariate analyses using the
Cox proportional hazards regression model. Hazard ratios (HRs) were
estimated with 95% confidence intervals for the survival analysis.
A prognostic index was designed using the variables that were the
most significant prognostic factors for the multivariate analysis.
The myeloma prognostic index (MPI) attributed 1 point for each
unfavorable factor. Risk categories were stratified into low
(score, 0), intermediate (score, 1) and high (score, 2–3). All data
were analyzed using SSPS (v21.0, IBM Corp., Armonk, NY, USA) and
P-values are two-sided. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics of patients
with MM
Baseline characteristics of the 80 patients with MM,
including age and ISS staging are presented in Table II. All patients were positive for
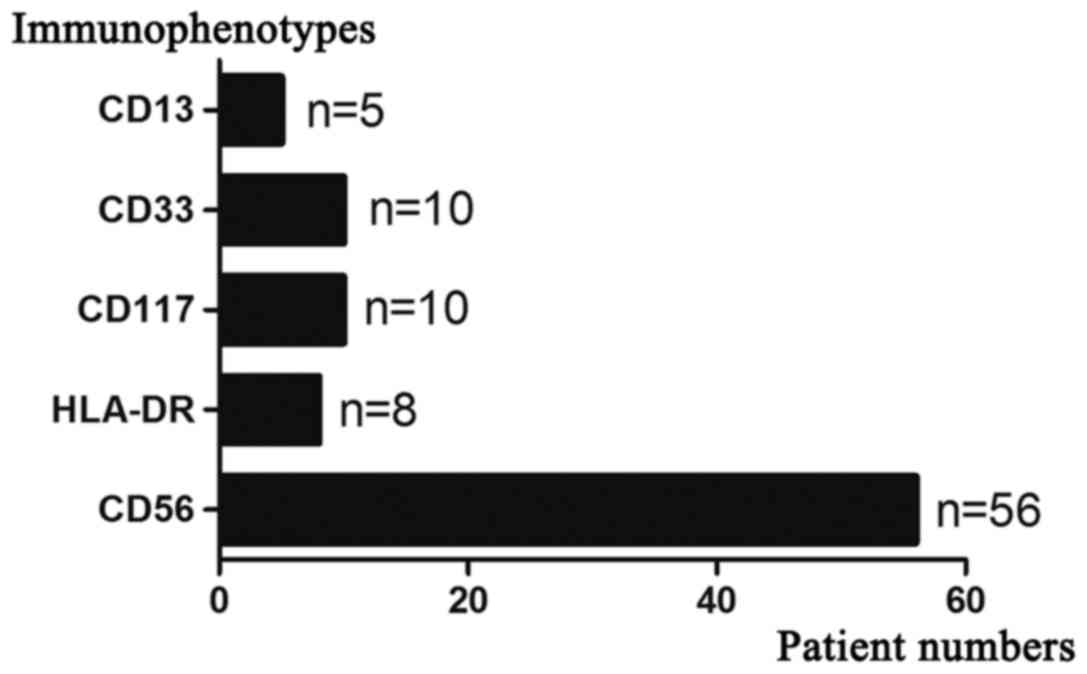
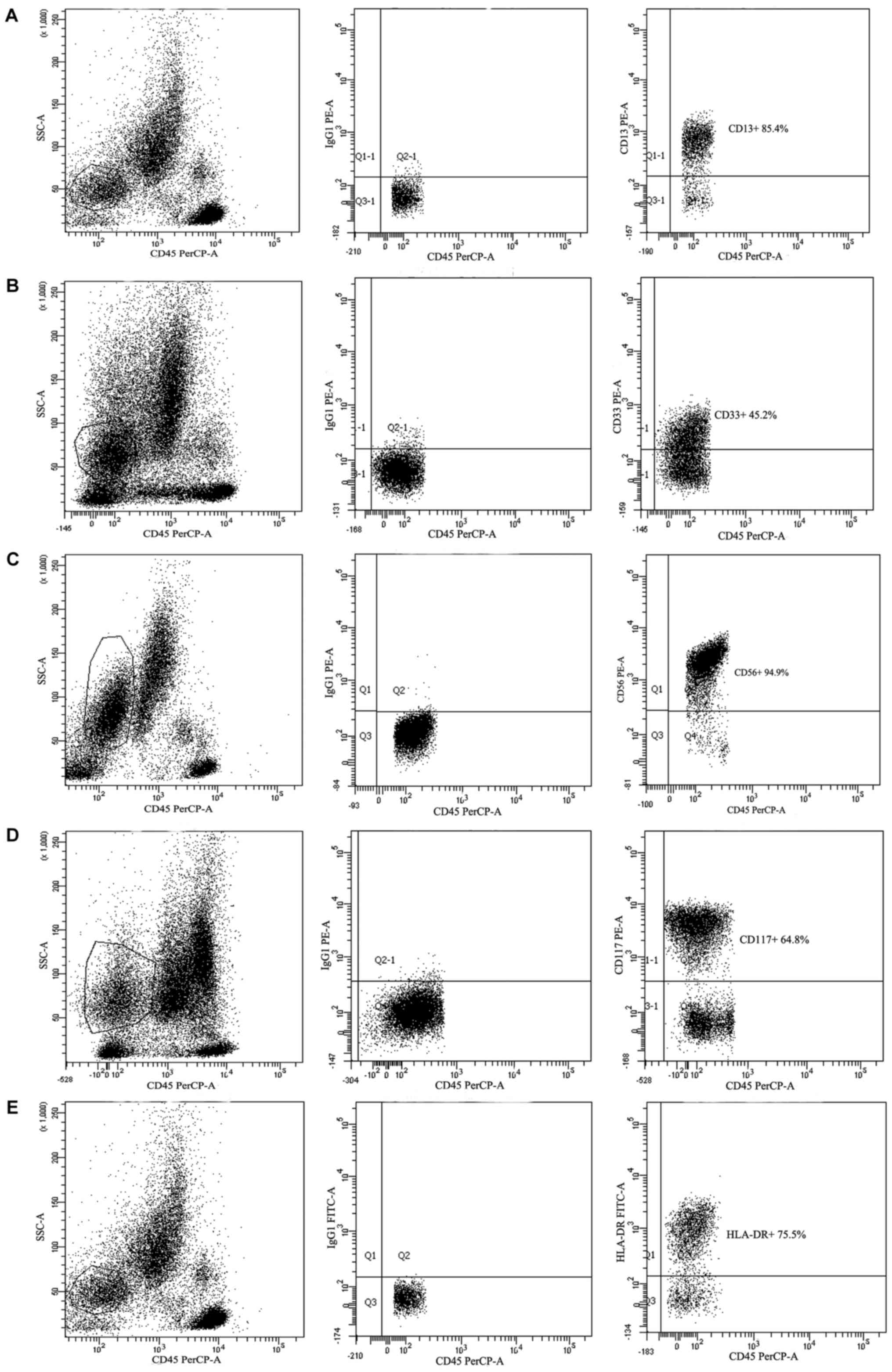
CD38 and further immunophenotypes are presented in Figs. 1 and 2.
All results were based on the flow cytometry measurements. Data
from different patients is presented in Fig. 2A-D and data in Fig. 2A and E were obtained from the same
patient. IgG1 was stained with PE (Fig.
2A-D) or with FITC (Fig. 2E) as
isotope/negative control.
 | Table II.Baseline characteristics for patients
newly diagnosed with multiple myeloma. |
Table II.
Baseline characteristics for patients
newly diagnosed with multiple myeloma.
| Characteristic | Number or median
(range) |
|---|
| Age, years | 62 (24–80) |
| Sex |
|
|
Male | 46 |
|
Female | 34 |
| ISS stage |
|
|
I–II | 40 |
|
III | 40 |
| Hemoglobin,
g/l | 88 (45–148) |
| Lymphocyte count,
×109/l | 1.20
(0.16–3.95) |
| C-reactive protein,
mg/l | 5 (1–160) |
| Lactate
dehydrogenase, U/l | 128.50
(50–1,221) |
| Creatinine,
µmol/l | 97.90
(30.30–760.90) |
| Ca2+,
mmol/l | 2.20
(1.38–5.35) |
| Albumin, g/l | 28.20
(11.70–44.10) |
| β-2 microglobulin,
mg/l | 5.45
(1.50–81.88) |
| Isotope |
|
|
IgA | 24 |
|
IgG | 38 |
|
Non-secrete isotype | 2 |
| Light
chain only | 13 |
|
Others | 3 |
| Plasma cell in bone
marrow, % | 27.75
(10.50–95) |
| Chromosome |
|
| <3
abnormal | 66 |
| ≥3
abnormal | 14 |
| Hypertension | 30/50 |
|
Yes | 30 |
| No | 50 |
| Diabetes |
|
|
Yes | 17 |
| No | 63 |
Univariate and multivariate analysis
for OS and PFS
According to the univariate analysis, age ≥65 years,
ISS stage III, CRP levels >8 mg/l, lactate dehydrogenase
activity >245 U/l, ≥3 abnormal (complex) chromosomes and HLA-DR+
were associated with decreased OS, while CRP levels >8 mg/l,
complex chromosomes, diabetes, CD117+ and HLA-DR+ were associated
with decreased PFS (Table III). In
the multivariate analysis, HLA-DR+, ISS stage III and age ≥65 years
represented independent predictive factors for decreased OS and
HLA-DR+, CD117+ and diabetes were independently correlated with
decreased PFS based on the Cox proportional hazard model (Table IV).
 | Table III.Univariate analysis for overall
survival and progression-free survival in patients with multiple
myeloma. |
Table III.
Univariate analysis for overall
survival and progression-free survival in patients with multiple
myeloma.
| A, Overall
survival |
|---|
|
|---|
| Characteristic | B | Wald | HR (95% CI) | P-value |
|---|
| Age, ≥65 years | −0.617 | 3.984 | 1.853
(1.011–3.394) | 0.046a |
| Sex, male | −0.363 | 1.329 | 1.438
(0.776–2.665) | 0.249 |
| ISS stage, III | −0.725 | 5.417 | 2.066
(1.121–3.805) | 0.020a |
| Hemoglobin, <100
g/l | −0.548 | 2.267 | 1.729
(0.848–3.527) | 0.132 |
| Lymphocyte count,
>1.3×109/l | 0.519 | 2.646 | 1.681
(0.899–3.141) | 0.104 |
| C-reactive protein,
>8 mg/l | −0.645 | 4.071 | 0.525
(0.281–0.982) | 0.044a |
| Lactate
dehydrogenase, >245 U/l | −0.906 | 4.605 | 2.474
(1.082–5.657) | 0.032a |
| Creatinine, >177
µmol/l | −0.316 | 0.813 | 1.372
(0.690–2.726) | 0.367 |
| Ca2+,
>2.8 mmol/l | −0.289 | 0.483 | 1.335
(0.591–3.017) | 0.487 |
| Albumin, <35
g/l | −0.637 | 1.897 | 1.891
(0.764–4.683) | 0.168 |
| Chromosome, ≥3
abnormal | −0.900 | 7.190 | 2.459
(1.274–4.476) | 0.007a |
| Hypertension,
yes | −0.14 | 0.194 | 0.869
(0.466–1.622) | 0.660 |
| Diabetes, yes | −0.657 | 3.670 | 1.929
(0.985–3.779) | 0.055 |
| CD13, positive | −0.558 | 0.578 | 1.746
(0.415–7.349) | 0.447 |
| CD33, positive | −0.337 | 0.491 | 1.401
(0.546–3.597) | 0.483 |
| CD56, positive | −0.070 | 0.044 | 0.932
(0.486–1.789) | 0.833 |
| CD117,
positive | −0.886 | 3.373 | 2.426
(0.942–6.248) | 0.066 |
| HLA-DR,
positive | −1.143 | 8.18 | 3.135
(1.433–6.860) | 0.004a |
|
| B,
Progression-free survival |
|
|
Characteristic | B | Wald | HR (95%
CI) | P-value |
|
| Age, ≥65 years | −0.418 | 1.931 | 1.519
(0.842–2.738) | 0.165 |
| Sex, male | −0.408 | 1.705 | 1.503
(0.815–2.772) | 0.192 |
| ISS stage, III | −0.633 | 3.824 | 1.883
(0.999–3.552) | 0.051 |
| Hemoglobin, <100
g/l | −0.341 | 0.927 | 1.407
(0.702–2.817) | 0.336 |
| Lymphocyte count,
>1.3×109/l | 0.238 | 0.606 | 1.268
(0.697–2.308) | 0.436 |
| C-reactive protein,
>8 mg/l | −0.633 | 3.933 | 1.882
(1.007–3.517) | 0.047a |
| Lactate
dehydrogenase, >245 U/l | −0.696 | 2.798 | 2.006
(0.887–4.536) | 0.094 |
| Creatinine, >177
µmol/l | −0.250 | 0.498 | 1.284
(0.641–2.571) | 0.480 |
| Ca2+,
>2.8 mmol/l | −0.569 | 1.867 | 1.766
(0.781–3.995) | 0.172 |
| Albumin, <35
g/l | −0.505 | 1.306 | 1.657
(0.697–3.942) | 0.253 |
| Chromosome, ≥3
abnormal | −0.729 | 4.362 | 2.073
(1.046–4.107) | 0.037a |
| Hypertension,
yes | −0.079 | 0.060 | 0.924
(0.492–1.737) | 0.806 |
| Diabetes, yes | −0.735 | 4.553 | 2.086
(1.062–4.100) | 0.033a |
| CD13, positive | −0.162 | 0.049 | 1.175
(0.282–4.891) | 0.824 |
| CD33, positive | −0.850 | 3.156 | 2.340
(0.916–5.981) | 0.076 |
| CD56, positive | 0.118 | 0.125 | 1.125
(0.586–2.158) | 0.724 |
| CD117,
positive | −1.143 | 5.590 | 3.136
(1.216–8.087) | 0.018a |
| HLA-DR,
positive | −0.999 | 6.389 | 2.715
(1.251–5.892) | 0.011a |
 | Table IV.Multivariate analysis for overall
survival and progression-free survival in patients with multiple
myeloma. |
Table IV.
Multivariate analysis for overall
survival and progression-free survival in patients with multiple
myeloma.
| A, Overall
survival |
|---|
|
|---|
| Characteristic | B | Wald | HR (95% CI) | P-value |
|---|
| Age, ≥65 years | −0.727 | 4.596 | 0.483
(0.249–0.940) | 0.032a |
| ISS stage, III | −0.755 | 5.139 | 0.470
(0.245–0.903) | 0.023a |
| C-reactive protein,
>8 mg/l | −0.525 | 2.309 | 0.529
(0.301–1.164) | 0.129 |
| Lactate
dehydrogenase, >245 U/l | −0.763 | 2.975 | 0.466
(0.196–1.110) | 0.085 |
| Chromosome, ≥3
abnormal | −0.689 | 3.700 | 0.052
(0.249–0.940) | 0.054 |
| HLA-DR,
positive | −1.102 | 6.528 | 0.332
(0.143–0.774) | 0.011a |
|
| B,
Progression-free survival |
|
|
Characteristic | B | Wald | HR (95%
CI) | P-value |
|
| C-reactive protein,
>8 mg/l | −0.627 | 3.643 | 0.534
(0.281–1.017) | 0.056 |
| Chromosome, ≥3
abnormal | −0.558 | 2.108 | 0.572
(0.269–1.216) | 0.146 |
| Diabetes, yes | −0.842 | 5.674 | 0.431
(0.216–0.861) | 0.017a |
| CD117,
positive | −1.082 | 4.639 | 0.339
(0.127–0.902) | 0.030a |
| HLA-DR,
positive | −0.902 | 4.238 | 0.404
(0.170–0.957) | 0.040a |
Comparison of the expression of
immunophenotypes based on 80 patients with MM
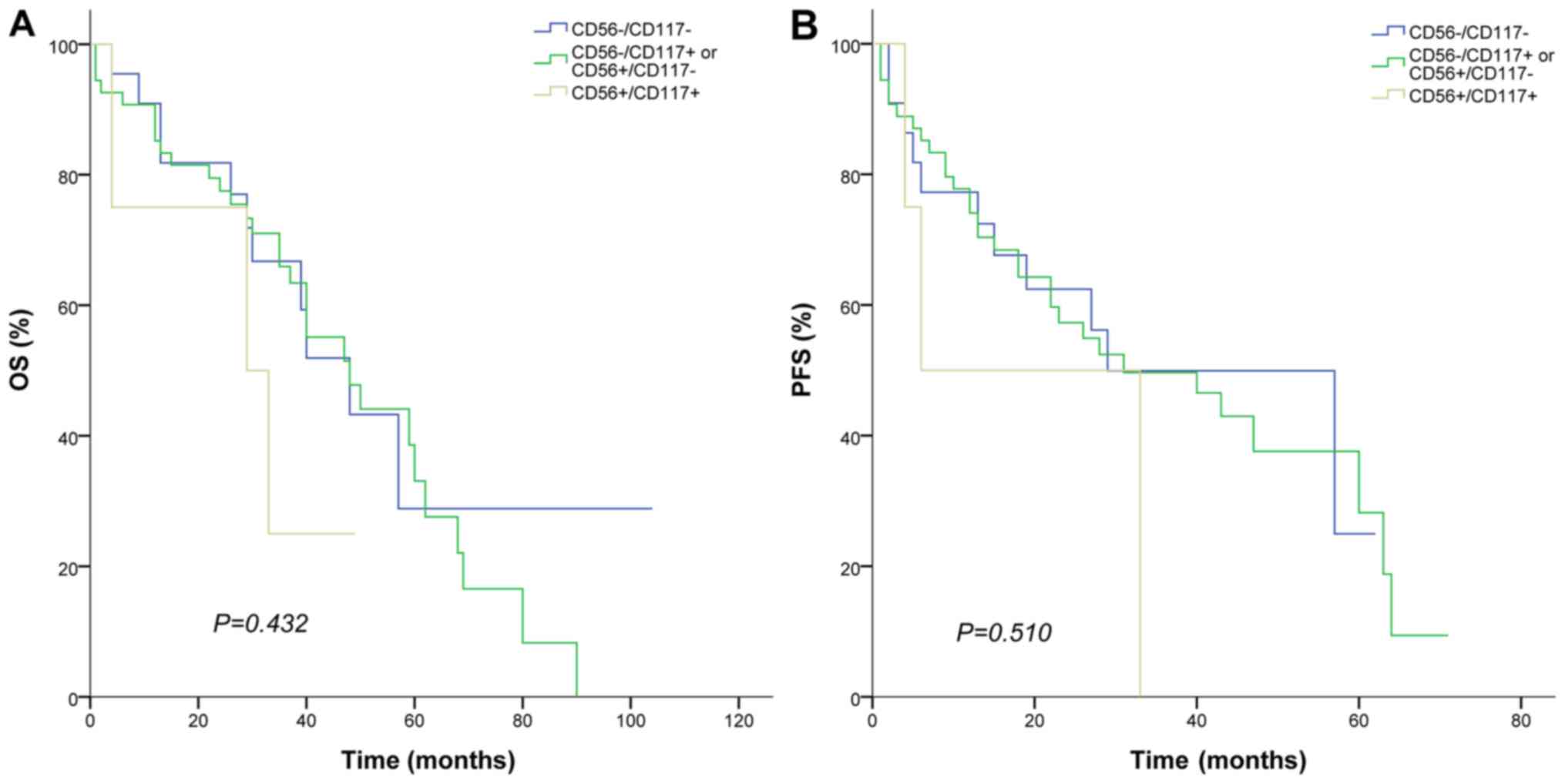
According to the expression of CD56 and CD117,
patients were divided into three groups: CD56+/CD117+, CD56-/CD117-
and CD56+/CD117- or CD56-/CD117+. No significant differences were
observed. (Table V). HLA-DR+ was
closely associated with complex chromosomes (Table VI) however there were no significant
differences among other factors (Table
VI). OS and PFS exhibited no significant differences among
these groups. (CD56+/CD117+, CD56-/CD117- and CD56+/CD117- or
CD56-/CD117+) (Fig. 3A and B).
 | Table V.Baseline characteristics for patients
newly diagnosed with multiple myeloma and varying CD56/CD117
immunophenotypes. |
Table V.
Baseline characteristics for patients
newly diagnosed with multiple myeloma and varying CD56/CD117
immunophenotypes.
|
| Number or median
(range) |
|
|---|
|
|
|
|
|---|
| Characteristic | CD56+/CD117+ | CD56+/CD117- or
CD56-/CD117+ | CD56-/CD117- | P-value |
|---|
| n | 4 | 54 | 22 |
|
| Age, years | 68.5 (43–75) | 61 (24–80) | 62 (47–75) | 0.62 |
| Sex, male | 3 | 28 | 15 | 0.35 |
| ISS stage III | 3 | 26 | 11 | 0.78 |
| Hemoglobin,
g/l | 85.50 (79–94) | 88 (45–131) | 83.50 (59–148) | 0.89 |
| Lymphocyte count,
×109/l | 2.34
(0.72–1.42) | 1.20
(0.16–3.45) | 1.24
(0.62–3.95) | 0.79 |
| C-reactive protein,
mg/l | 17 (2–49) | 5 (1–77) | 5 (1–160) | 0.59 |
| Lactate
dehydrogenase, U/l | 176 (109–315) | 127 (61–1,221) | 133 (50–365) | 0.23 |
| Creatinine,
µmol/l | 88.35
(55.20–117) | 97.50
(43.30–760.90) | 100.55
(30.30–372.70) | 0.65 |
| Ca2+,
mmol/l | 2.20
(1.99–2.31) | 2.20
(1.38–5.35) | 2.20
(1.93–2.95) | 0.78 |
| Albumin, g/l | 29.65
(17.10–34.10) | 27.70
(11.70–44.10) | 29.20
(20.10–42.70) | 0.81 |
| β-2 microglobulin,
mg/l | 7.30
(5.40–26.80) | 5.35
(1.50–81.88) | 5.45
(1.88–39.02) | 0.38 |
| Plasma cells in
bone marrow, % | 17.25
(10.50–75) | 27.75 (10–95) | 35.50 (12–86) | 0.77 |
| Chromosome, ≥3
abnormal | 1 | 10 | 3 | 0.78 |
| Hypertension,
yes | 1 | 20 | 9 | 0.93 |
| Diabetes, yes | 1 | 13 | 3 | 0.49 |
 | Table VI.Baseline characteristics for patients
newly diagnosed with multiple myeloma and varying HLA-DR
immunophenotypes. |
Table VI.
Baseline characteristics for patients
newly diagnosed with multiple myeloma and varying HLA-DR
immunophenotypes.
|
| Number or median
(range) |
|
|---|
|
|
|
|
|---|
| Characteristic | HLA-DR- | HLA-DR+ | P-value |
|---|
| n | 72 | 8 |
|
| Age, years | 62.50 (56–75) | 61 (24–80) | 0.34 |
| Sex, male | 39 | 7 | 0.15 |
| ISS stage III | 38 | 2 | 0.26 |
| Hemoglobin,
g/l | 81.50 (64–112) | 88 (45–148) | 0.30 |
| Lymphocyte count,
×109/l | 1.19
(0.53–3.00) | 1.20
(0.16–3.95) | 0.89 |
| C-reactive protein,
mg/l | 10 (1–51) | 5 (1–160) | 0.44 |
| Lactate
dehydrogenase, U/l | 158 (69–1,221) | 127 (50–1,102) | 0.49 |
| Creatinine,
µmol/l | 88.95
(57.2–341.7) | 100.50
(30.30–760.90) | 0.59 |
| Ca2+,
mmol/l | 2.06
(1.93–3.25) | 2.20
(1.38–5.35) | 0.28 |
| Albumin, g/l | 28.90
(17.70–34.10) | 28.20
(11.70–44.10) | 0.46 |
| β-2 microglobulin,
mg/l | 4.10
(1.88–8.29) | 5.63
(1.50–81.88) | 0.13 |
| Plasma cells in
bone marrow, % | 38 (11.50–95) | 26.50
(10.50–92) | 0.46 |
| Chromosome, ≥3
abnormal | 9 | 5 | 0.002a |
| Hypertension,
yes | 28 | 2 | 0.70 |
| Diabetes, yes | 16 | 1 | 0.86 |
Analysis of OS and PFS in different
immunophenotypes
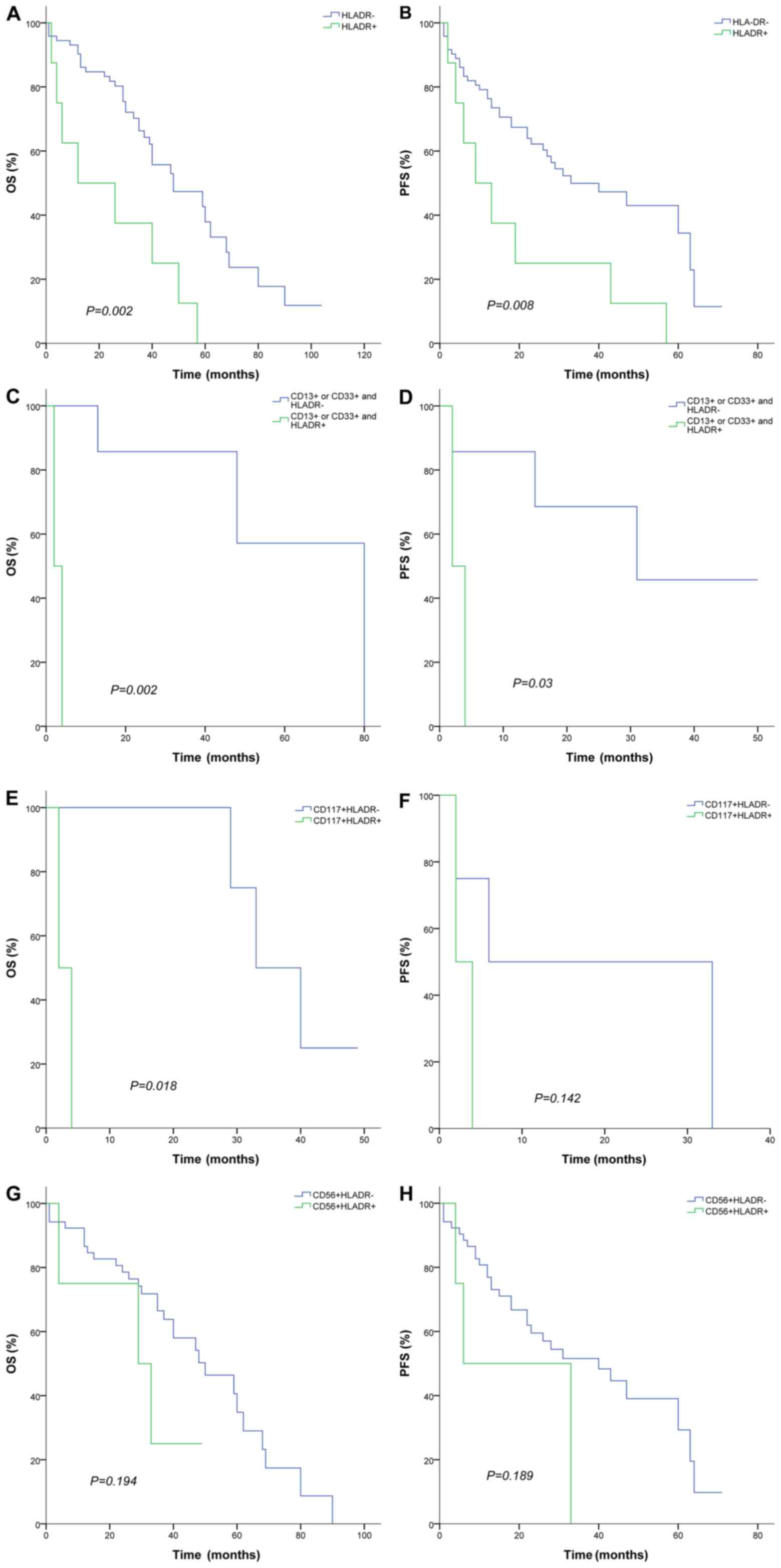
As presented in Fig.
4, the HLA-DR+ immunophenotype exhibited an adverse prognosis
compared with the HLA-DR- immunophenotype for OS (12 vs. 48 months;
P=0.002) and PFS (9 vs. 33 months; P=0.008; Fig. 4A and B). CD13+ or CD33+/HLA-DR+ groups
exhibited decreased OS (2 vs. 80 months; P=0.008) and PFS (2 vs. 31
months; P=0.03) compared with HLA-DR- immunophenotypes (Fig. 4C and D). A similar trend was observed
for CD117+/HLA-DR+ compared with CD117+/HLA-DR- immunophenotypes
for OS (2 vs. 33 months; P=0.018; Fig
4E). However, PFS did not exhibit a significant survival
difference between CD117+/HLA-DR+ and CD117+/HLA-DR- (2 vs. 6
months; P=0.142; Fig 4F). No
significant differences were observed for OS and PFS between
CD56+/HLA-DR+ and CD56+/HLA-DR- immunophenotypes (P=0.194 and
P=0.189; respectively; Fig 4G and
H).
Derivation of myeloma prognostic
index
The myeloma prognostic index (MPI) was derived from
the variables that were determined to be significant prognostic
factors for OS based on the multivariate analysis. HLA-DR+, age ≥65
years and ISS stage III exhibited an independent, unfavorable
significance for OS. Here, the MPI attributed 1 point for HLA-DR+,
old age (≥65 years) and ISS (stage III) and the final score was
obtained. Risk categories were stratified into low (score, 0),
intermediate (score, 1) and high (score, 2–3). The low,
intermediate and high MPI was suitable for risk stratification in
patients treated with bortezomib and thalidomide for OS (60, 47 and
33 months, respectively; P<0.001) and PFS (60, 28 and 12 months,
respectively; P=0.004; Fig. 5). Low
group and intermediate group had significant differences in OS (60
vs. 47 months; P=0.011) and PFS (60 vs. 28 months; P=0.038).
Intermediate group had longer OS than high group (47 vs. 33 months;
P=0.013) but their PFS did not show the same difference (28 vs. 12
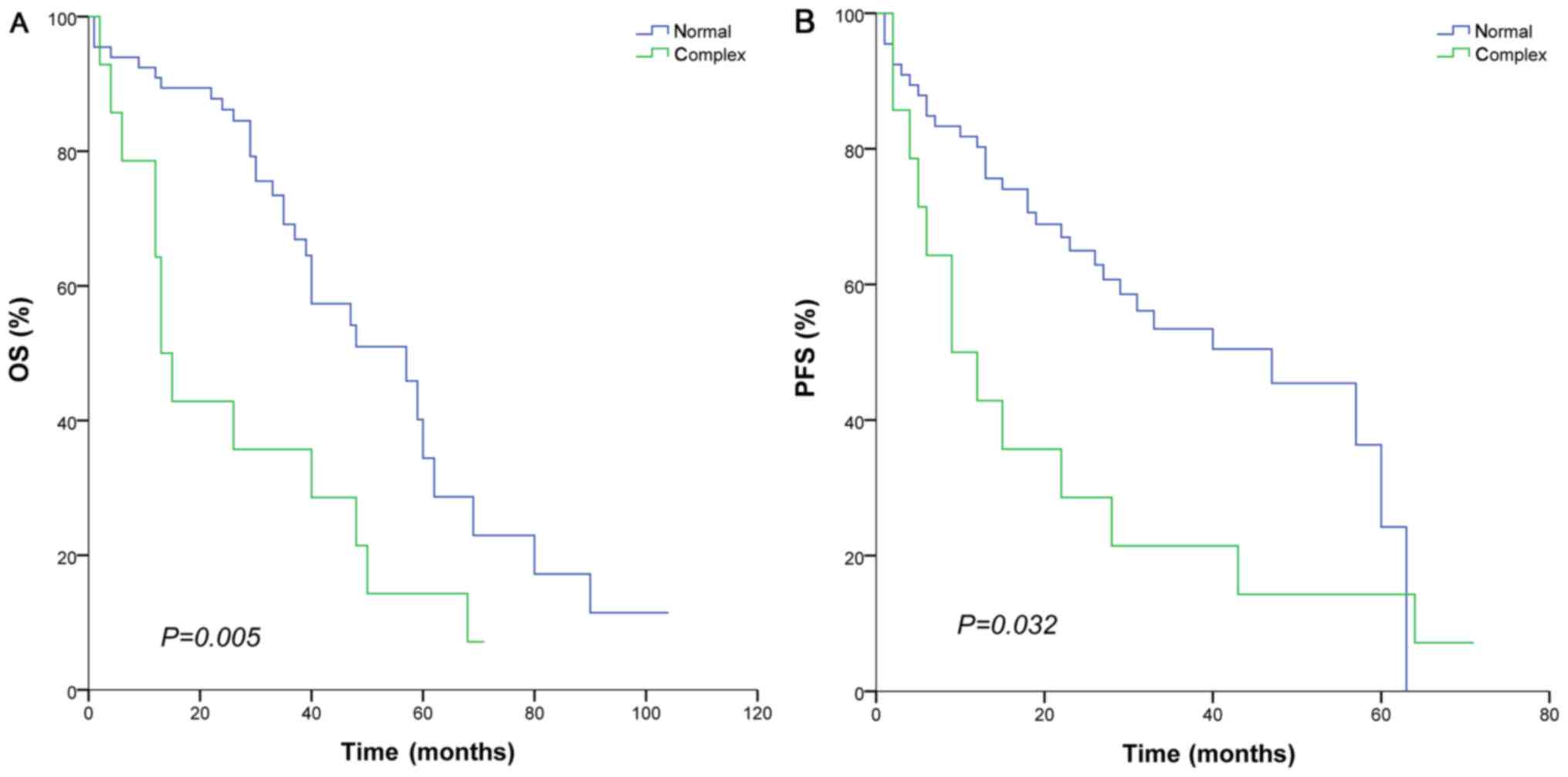
months; P=0.076). In addition, patients with complex chromosome had
a shorter OS (13 vs. 57 months; P=0.005) and PFS (9 vs. 47 months;
P=0.032) (Fig 6).
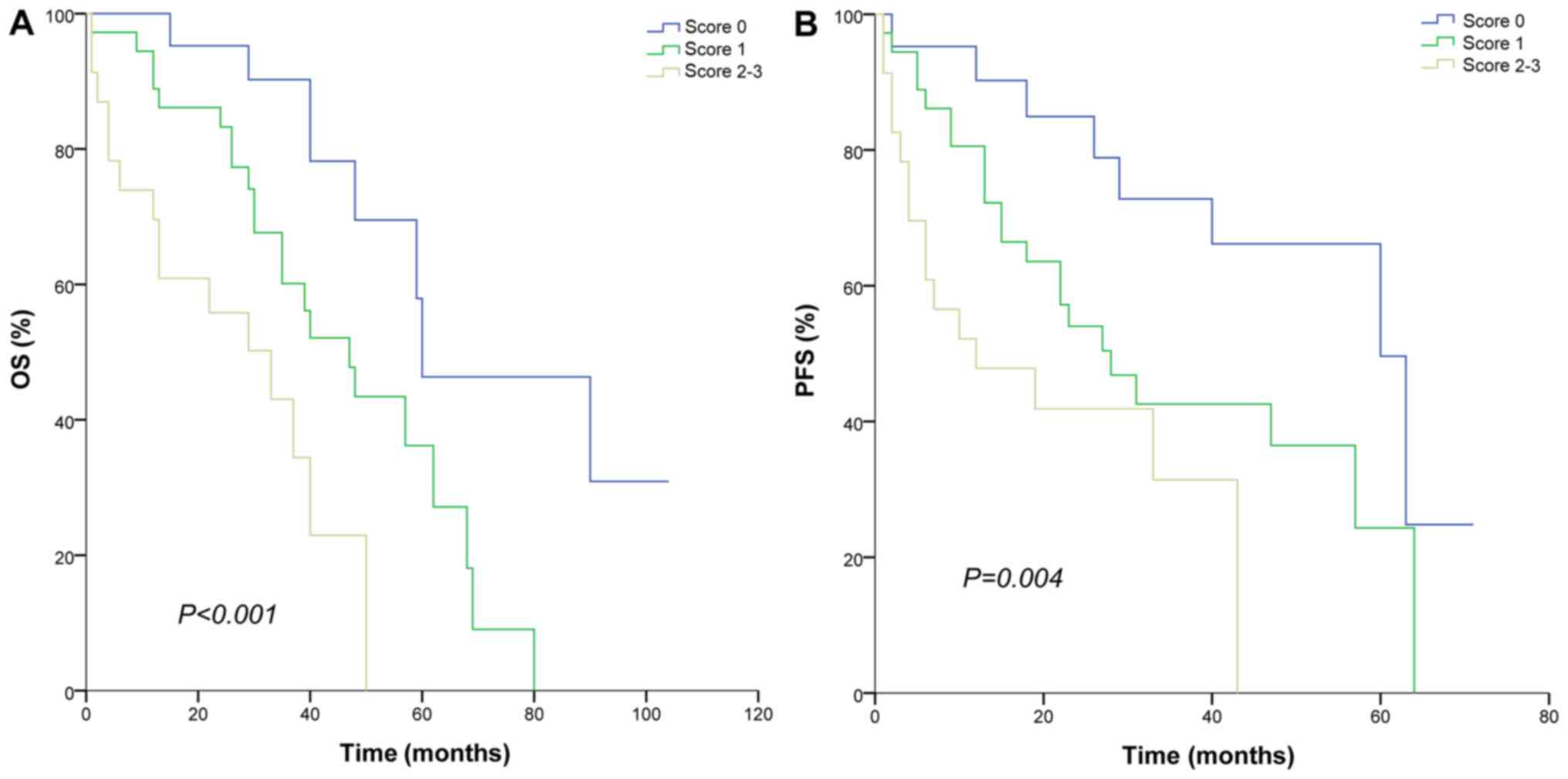 | Figure 5.Survival curve according to MPI
categories. (A) OS and (B) PSF for patients of different risk
categories. MPI attributed 1 point for HLA-DR+, age, ≥65 years and
international staging system stage III and risk categories were
stratified into low (score, 0), intermediate (score, 1) and high
(score, 2–3). MPI, myeloma prognostic index; OS, overall survival;
PFS, progression-free survival; HLA-DR, human leukocyte
antigen-antigen D-related. |
Discussion
As a currently incurable disease, research focusing
on MM is ongoing. Developments in the fields of biotechnology and
the identification of novel drugs have furthered the understanding
of the disease and aided the improvement of treatments (1). It has been demonstrated that MM in
different R-ISS stages consists of interphase fluorescence in
situ hybridization (FISH), β2-microglobulin, lactate
dehydrogenase and albumin, have different prognosis, R-ISS consists
of interphase fluorescence in situ hybridization (FISH),
β2-microglobulin, lactate dehydrogenase and albumin. Patients in
different stages had a different prognosis (15). Treatment with lenalidomide provides an
alternative choice for elderly patients and those unable to
tolerate chemotherapy (16,17). In addition, prognostic factors,
including peripheral lymphocytes and CRP, have been reported and
were demonstrated to have a varying prognostic significance in
traditional chemotherapy and novel drug protocols (4,5,18,19). This
highlights the importance of discovering and identifying prognostic
markers in MM for novel treatment approaches.
Immunophenotyping, as a critical cell indicator, is
a necessary step in the diagnosis of MM (6). It is known that MM plasma cells express
CD138 and CD38 at high levels, are positive for CD56, partially
positive for CD117 and do not express CD27. Normal bone marrow
plasma cells do not express CD138 and CD38. They are CD56 negative,
express CD19 weakly compared with MM plasma cells and express CD45
(6,7,20). In the
current study, all patients expressed CD38 and 70.00% (56 cases)
were CD56 and 13.75% (11 cases) were CD117 positive, which is in
accordance with previous reports (7,8).
In addition to the exact diagnostic significance,
the prognostic value of immunophenotyping has been documented for
MM (21–24). Previously, Dahl et al (21) reported that the absence of CD56 may
contribute to extramedullar accumulation of tumor cells and
Bataille et al (22) reported
CD117+ patients exhibited an increased OS compared with negative
individuals. In addition, it was reported that the 3-year OS in
CD33+ patients was significantly decreased compared with CD33-
patients (23). Recently, Pan et
al (8) suggested that CD56 and
CD117 expression has a potential prognostic impact and it may be
associated with increased OS. Ceran et al (9) reported that CD56 and CD117 expression
levels are lower in advanced stages of MM. Qiu et al
(24) reported that OS and PFS in
CD56+ patients with MM are increased compared with CD56- patients
treated with lenalidomide or bortezomib-based therapies. It was
suggested that CD56 and CD117 negative expression are unfavorable
prognostic factors in patients with MM, based on data obtained from
small cohorts (34–50 participants), non-combination therapy and
uniformed protocols i.e., using novel agents and traditional
chemotherapies (8,9).
Opposing trends have also been reported. According
to data from Tang et al (25),
therapeutic efficacy of CD117+ patients was decreased compared with
CDl17- patients. Mateo et al (26) documented that patients with MM and
CD117+ treated with conventional therapy, including therapy
followed by autologous stem-cell transplantation, had decreased OS
and PFS compared with CD117- patients with MM. The prognostic
judgment based on immunophenotype indicators remains controversial.
According to the current study, no significant difference was
observed in OS and PFS between CD56+/CD117+ and CD56-/CD117-
patients and these markers did not appear to be associated with
poor prognosis. Differences in the described outcomes may be
attributed to the following: Previous reports (8,9,24) resulted from monotherapy, including
iMiDs, proteasome inhibitors or traditional chemotherapy, or
intermittent iMiDs administration and these results were based on
combination therapy, including thalidomide which were given
continuously in the presence or absence of chemotherapy; a majority
of patients tolerated small doses of thalidomide and peripheral
neuritis was accepted. A uniform protocol was also applied.
Elevation in CD117 and CD56 levels reported by Pan et al
(8) and Ceran et al (9) resulted from varying treatments,
including chemotherapies and novel agents. The influence of
different regimes and potential resulting bias was not accounted
for. Hundemer et al (27)
reported that a lack of CD56 expression on MM cells was not an
indicator for poor prognosis in patients with MM treated with a
high chemotherapy dose followed by autologous hematopoietic stem
cell transplantation. The present study suggested that CD117+
remained a negative factor in patients with MM treated with novel
agents. However, larger cohort studies are required to confirm
these conclusions.
According to univariate analysis, HLA-DR+ expression
was associated with decreased OS and PFS and similar results were
observed in combination with CD117+ or myeloid antigen (CD13+ or
CD33+) expression. In addition, HLA-DR+ expression was identified
as an independent factor for decreased OS and PFS. To the best of
the our knowledge, the current study is the first to report effects
of HLA-DR expression on OS and PFS in patients with MM.
The HLA gene, located on the short arm of chromosome
6, is a 4,000 kb gene complex composed of various tightly connected
gene clusters, which are known for maximum allele polymorphisms and
are closely associated with functions of the immune system in
humans (28). The HLA-DR antigen,
which is encoded by the HLA-II gene, most frequently appears on the
cytomembrane of macrophages and B lymphocytes and may aid the host
immune system to identify and attack tumor cell (28). Previously, research regarding HLA-DR
concentrated on diseases associated with the immune system
(29,30) and solid tumors. Diao et al
(31) indicated that overexpression
of HLA-DR is associated with decreased OS in patients with glioma,
while Sconocchia et al (32)
reported that HLA class II antigen served as a favorable prognostic
marker in patients with colorectal carcinoma. In hematological
malignances, certain reports have suggested that the HLA-DR status
determined by flow cytometry may be used to predict the prognosis
of patients with diffuse large B-cell lymphoma receiving R-CHOP
(rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone)
therapy (33,34). The current study demonstrated that
HLA-DR positive is an unfavorable factor for OS and PFS in patients
with MM. HLA-DR+ may be a valuable and simple prognostic marker in
the field of novel therapies. However, larger studies with
increased follow-up and additional researches are required in the
future to confirm the presented conclusions, particularly in
patients with transplantations. Co-expression with CD117 or myeloid
antigens (CD13 or CD33) did not alter the exhibited influence. This
may result from a close association with complex chromosomes.
Although t(4;14), t(14;16) and 17p- were previously reported as
unfavorable karyotypes in revised ISS for MM (R-ISS), further
unknown abnormalities may have been omitted as a result of
restrictions of the FISH probes (15). Certain complex karyotypes detected
were based on traditional reverse-banding and/or G-banding
techniques. According to the current study, complex chromosomes may
be a valuable prognostic factor, while complex karyotypes have
previously been described as an adverse prognostic factor (35). To the best of our knowledge the
association between complex chromosomes and immunophenotypes has
not been reported previously. However, these findings may be only
be applicable for a Chinese population.
As a first generation immunoregulator, thalidomide
is more widely used in China compared with lenalidomide, as a
result of its lower price and a majority of patients with MM
insisting on a combination therapy based on thalidomide and
bortezomib (36). Flow cytometry, as
an established technique, has been widely used in China (7). In association with simple ISS, R-ISS is
not common in basic hospitals, and age or MPI may provide a simple
diagnostic to predict prognosis in patients not undergoing
transplantation. The use of immunophenotypes as prognostic markers
is a simpler method than R-ISS. The current study suggested that
the expression of CD117 and HLA-DR may be prognostic markers for
novel therapies where combination therapy with thalidomide and
bortezomib is predominant, however, further associations for the
combination with transplantation should be studied.
There are several limitations associated with the
current study. Certain international prognostic factors, including
free light chain levels (FLC) were excluded as total light chain
levels not FLC were measured until 2012. Patient numbers in the
current study were increased compared with previous reports,
however, following subdivision of the cohort, individual groups
remained small (8,9). Future perspectives include the
recruitment of more patients and the analysis of more factors,
including FLC, to identify further markers of long-term prognosis
in patients with MM.
Acknowledgements
The authors would like to thank Mrs Ying Yin and Mrs
Zheng You for assistance with data collection.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JNY, JWZ and HFG provided the raw data. HW and XZ
analyzed and interpreted the patient data and drafted the
manuscript. CS designed the study and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by the
Institutional Review Board of Wuxi People's Hospital (Wuxi, China)
in accordance with the Declaration of Helsinki and informed consent
to participate in the study was obtained from participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cejalvo MJ and de la Rubia J: Which
therapies will move to the front line for multiple myeloma? Expert
Rev Hematol. 10:383–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson KC, Alsina M, Bensinger W,
Biermann JS, Cohen AD, Devine S, Djulbegovic B, Faber EA Jr,
Gasparetto C, Hernandez-Illizaliturri F, et al: Multiple myeloma,
version 1.2013. J Natl Compr Canc Netw. 11:11–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tadmor T: Do lymphocytes count in myeloma?
Are we absolutely sure? Leuk Lymphoma. 56:1193–1194. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim DS, Yu ES, Kang KW, Lee SR, Park Y,
Sung HJ, Choi CW and Kim BS: Myeloma prognostic index at diagnosis
might be a prognostic marker in patients newly diagnosed with
multiple myeloma. Korean J Intern Med. 32:711–721. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gertz MA and Buadi FK: Utility of
immunophenotyping of plasma cells in multiple myeloma. Leuk
Lymphoma. 28:1–2. 2015.
|
|
7
|
Li HQ and Zhai YP: Research progress on
multiple myeloma immunophenotyping and minimal residual disease
detected by flow cytometry. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
23:241–245. 2015.(In Chinese). PubMed/NCBI
|
|
8
|
Pan Y, Wang H, Tao Q, Zhang C, Yang D, Qin
H, Xiong S, Tao L, Wu F, Zhang J and Zhai Z: Absence of both CD56
and CD117 expression on malignant plasma cells is related with a
poor prognosis in patients with newly diagnosed multiple myeloma.
Leuk Res. 40:77–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ceran F, Falay M, Dagdas S and Ozet G: The
assessment of CD56 and CD117 expressions at the time of the
diagnosis in multiple myeloma patients. Turk J Haematol.
34:226–232. 2017.PubMed/NCBI
|
|
10
|
International Myeloma Working Group, .
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: A report of the
international myeloma working group. Br J Haematol. 121:749–757.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Blade J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cavo M, Rajkumar SV, Palumbo A, Moreau P,
Orlowski R, Blade J, Sezer O, Ludwig H, Dimopoulos MA, Attal M, et
al: International Myeloma Working Group consensus approach to the
treatment of multiple myeloma patients who are candidates for
autologous stem cell transplantation. Blood. 117:6063–6073. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palumbo A, Rajkumar SV, San Miguel JF,
Larocca A, Niesvizky R, Morgan G, Landgren O, Hajek R, Einsele H,
Anderson KC, et al: International Myeloma Working Group consensus
statement for the management, treatment, and supportive care of
patients with myeloma not eligible for standard autologous
stem-cell transplantation. J Clin Oncol. 32:587–600. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Durie BG, Harousseau JL, Miguel JS, Blade
J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J,
Sonneveld P, et al: International uniform response criteria for
multiple myeloma. Leukemia. 20:1467–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised international staging system
for multiple myeloma: A Report From International Myeloma Working
Group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benboubker L, Dimopoulos MA, Dispenzieri
A, Catalano J, Belch AR, Cavo M, Pinto A, Weisel K, Ludwig H,
Bahlis N, et al: Lenalidomide and dexamethasone in
transplant-ineligible patients with myeloma. N Engl J Med.
371:906–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gay F, Oliva S, Petrucci MT, Conticello C,
Catalano L, Corradini P, Siniscalchi A, Magarotto V, Pour L,
Carella A, et al: Chemotherapy plus lenalidomide versus autologous
transplantation, followed by lenalidomide plus prednisone versus
lenalidomide maintenance, in patients with multiple myeloma: A
randomised, multicentre, phase 3 trial. Lancet Oncol. 16:1617–1629.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jimenez-Zepeda VH, Reece DE, Trudel S,
Chen C, Franke N, Winter A, Tiedemann R and Kukreti V: Absolute
lymphocyte count as predictor of overall survival for patients with
multiple myeloma treated with single autologous stem cell
transplant. Leuk Lymphoma. 56:2668–2673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suriu C, Akria L, Azoulay D, Shaoul E,
Barhoum M and Braester A: Absolute lymphocyte count as a prognostic
marker in newly diagnosed multiple myeloma patients. Int J Lab
Hematol. 38:e56–59. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bataille R, Jego G, Robillard N,
Barille-Nion S, Harousseau JL, Moreau P, Amiot M and
Pellat-Deceunynck C: The phenotype of normal, reactive and
malignant plasma cells. Identification of ‘many and multiple
myelomas’ and of new targets for myeloma therapy. Haematologica.
91:1234–1240. 2006.PubMed/NCBI
|
|
21
|
Dahl IM, Rasmussen T, Kauric G and
Husebekk A: Differential expression of CD56 and CD44 in the
evolution of extramedullary myeloma. Br J Haematol. 116:273–277.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bataille R, Pellat-Deceunynck C, Robillard
N, Avet-Loiseau H, Harousseau JL and Moreau P: CD117 (c-kit) is
aberrantly expressed in a subset of MGUS and multiple myeloma with
unexpectedly good prognosis. Leuk Res. 32:379–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sahara N, Ohnishi K, Ono T, Sugimoto Y,
Kobayashi M, Takeshita K, Shigeno K, Nakamura S, Naito K, Tamashima
S, et al: Clinicopathological and prognostic characteristics of
CD33-positive multiple myeloma. Eur J Haematol. 77:14–18. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu Q, Zhu P, Wang MJ, Lu XZ, Dong YJ, Sun
YH, Wang LH, Zhang Y, Bu DF, Wang WS, et al: Expression of CD56 and
CD19 in patients with newly diagnosed multiple myeloma and their
relationship with karyotypes and prognosis. Zhongguo Shi Yan Xue Ye
Xue Za Zhi. 24:1071–1078. 2016.(In Chinese). PubMed/NCBI
|
|
25
|
Tang HL, Shu MM, Dong BX, Gu HT, Liang R,
Bai QX, Yang L, Zhang T, Gao GX and Chen XQ: Influence of CD117
expression on response of multiple myeloma patients to
chemotherapy. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 23:1346–1351.
2015.(In Chinese). PubMed/NCBI
|
|
26
|
Mateo G, Montalban MA, Vidriales MB,
Lahuerta JJ, Mateos MV, Gutierrez N, Rosinol L, Montejano L, Blade
J, Martinez R, et al: Prognostic value of immunophenotyping in
multiple myeloma: A study by the PETHEMA/GEM cooperative study
groups on patients uniformly treated with high-dose therapy. J Clin
Oncol. 26:2737–2744. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hundemer M, Klein U, Hose D, Raab MS,
Cremer FW, Jauch A, Benner A, Heiss C, Moos M, Ho AD and
Goldschmidt H: Lack of CD56 expression on myeloma cells is not a
marker for poor prognosis in patients treated by high-dose
chemotherapy and is associated with translocation t(11;14). Bone
Marrow Transplant. 40:1033–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Complete sequence and gene map of a human
major histocompatibility complex. The MHC sequencing consortium.
Nature. 401:921–923. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hasan ZN, Zalzala HH, Mohammedsalih HR,
Mahdi BM, Abid LA, Shakir ZN and Fadhel MJ: Association between
human leukocyteantigen-DR and demylinating Guillain-Barre syndrome.
Neurosciences. 19:301–305. 2014.PubMed/NCBI
|
|
30
|
Nada AM and Hammouda M: Immunoregulatory T
cells, LFA-3 and HLA-DR in autoimmune thyroid diseases. Indian J
Endocrinol Metab. 18:574–581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Diao J, Xia T, Zhao H, Liu J, Li B and
Zhang Z: Overexpression of HLA-DR is associated with prognosis of
glioma patients. Int J Clin Exp Pathol. 8:5485–5490.
2015.PubMed/NCBI
|
|
32
|
Sconocchia G, Eppenberger-Castori S,
Zlobec I, Karamitopoulou E, Arriga R, Coppola A, Caratelli S,
Spagnoli GC, Lauro D, Lugli A, et al: HLA class II antigen
expression in colorectal carcinoma tumors as a favorable prognostic
marker. Neoplasia. 16:31–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rimsza LM, Leblanc ML, Unger JM, Miller
TP, Grogan TM, Persky DO, Martel RR, Sabalos CM, Seligmann B,
Braziel RM, et al: Gene expression predicts overall survival in
paraffin-embedded tissues of diffuse large B-cell lymphoma treated
with R-CHOP. Blood. 112:3425–3433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamamoto W, Nakamura N, Tomita N, Takeuchi
K, Ishii Y, Takahashi H, Watanabe R, Takasaki H, Motomura S,
Kobayashi S, et al: Human leukocyte antigen-DR expression on flow
cytometry and tumor-associated macrophages in diffuse large B-cell
lymphoma treated by rituximab, cyclophosphamide, doxorubicin,
vincristine and prednisone therapy: Retrospective cohort study.
Leuk Lymphoma. 55:2721–2727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nemec P, Zemanova Z, Kuglik P, Michalova
K, Tajtlova J, Kaisarova P, Oltova A, Filkova H, Holzerova M,
Balcarkova J, et al: Complex karyotype and translocation t(4;14)
define patients with high-risk newly diagnosed multiple myeloma:
Results of CMG2002 trial. Leuk Lymphoma. 53:920–927. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen SY: Clinical effect of bortezomib
combined with dexamethasone and thalidomide on treatment of
multiple myeloma. J Clin Med Practice. 18:1–126. 2014.
|