Introduction
Mantle cell lymphoma (MCL) is a distinct entity of
B-cell non-Hodgkin lymphoma, accounting for 6–9% of malignant
lymphoma cases (1). MCL has a male
predominance and the majority of patients with MCL are >50 years
old (1). Due to the lack of specific
clinical manifestations, the majority of patients present with the
advanced stage at diagnosis. A diagnosis of MCL depends on the
pathological examination results. Biologically, MCL cells are
characterized by cluster of differentiation (CD)5+, CD20+, CD10-
and cyclin D1+. Although bone marrow, and the circulatory and the
gastrointestinal systems are often involved, primary bone MCL is
rare. To the best of our knowledge, only one case of primary spinal
MCL has been reported in the literature to date (2). The present study discussed a case of
bulky primary tibia MCL, for which complete remission was achieved
with R (rituximab)-CHOP (rituximab 600 mg on day 1,
cyclophosphamide 1.2 g on day 2, vindesine 4 mg on day 2,
epirubicin 96 mg on day 2, prednisone 50 mg every 12 h on days 2–6)
and R-DHAP (rituximab 600 mg on day 1, cisplatin 168 mg on day 2,
cytarabine 3.3 g/q12h on day 3, dexamethasone 40 mg on days
2–5).
Case report
A 50-year-old male presented with left tibia pain
with no apparent cause, at Fenghua District People's Hospital
(Ningbo, China) in September, 2014. An X-ray revealed that local
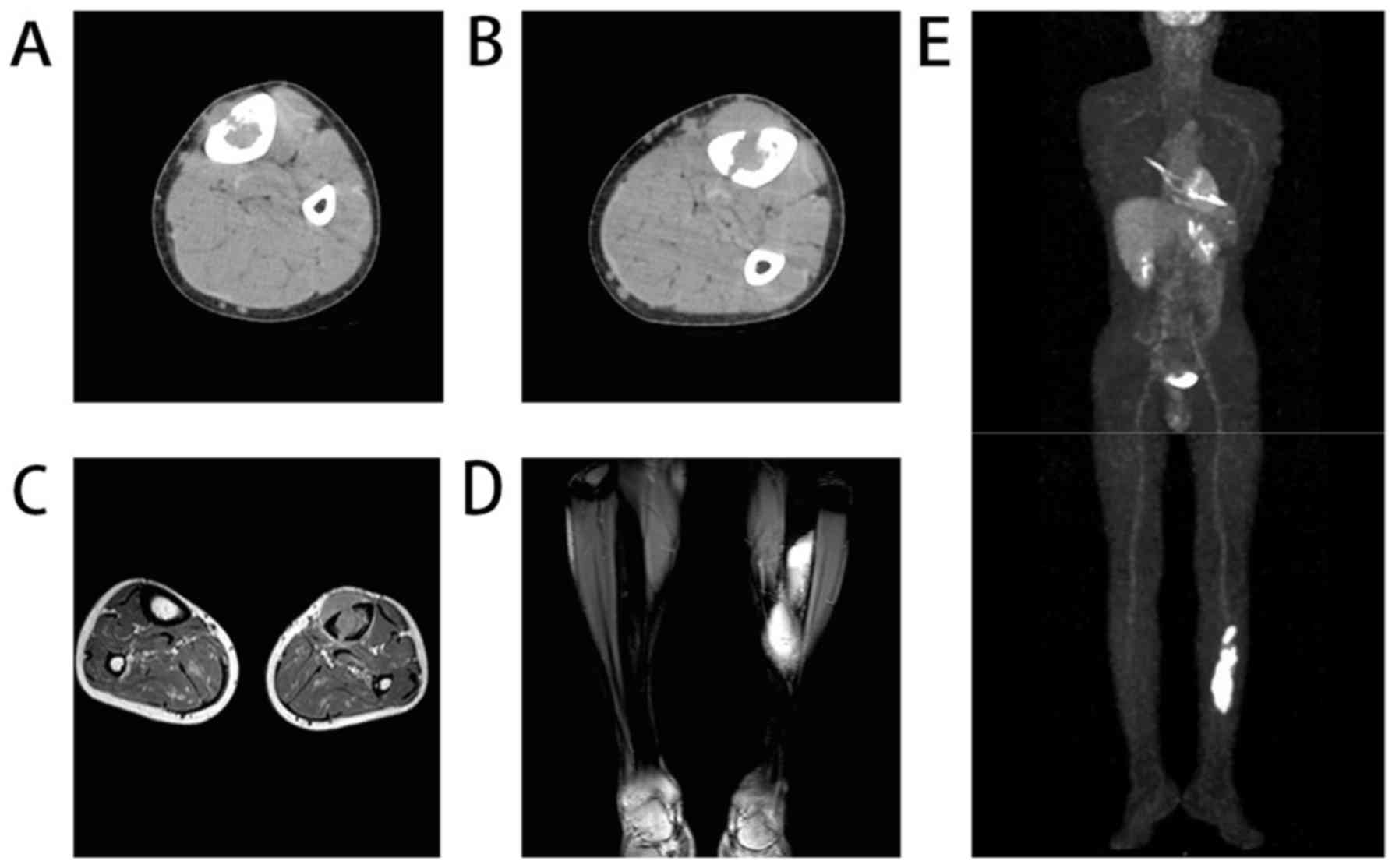
bone density was decreased. Computed tomography (CT) revealed local
cortical bone rupture with abnormal density in the medullary cavity
and surrounding soft tissue (Fig.
1A). Infectious etiology was considered and cefaclor (250 mg,
every 8 h) was administered. After 1 month, the patient was
transferred to the Affiliated Hospital of Ningbo University due to
increased pain and presentation of a growing mass adjacent to the
left tibia. No B symptoms, including unexplained fever of >38°C,
drenching night sweats and loss of >10% body weight within 6
months, were reported except night sweats. There was no medical
history of any previous disease or pathology. On physical
examination, a 5×6-cm mass with a smooth surface was detected in
the right lower extremity.
Initial blood tests identified a lactate
dehydrogenase level of 646 U/l, β2-microglobulin level of 2388
ng/ml, C-reactive protein level of 4.7 mg/l, white cell count of
5,200 cells/µl, hemoglobin content of 15.0 g/l and a platelet count
of 169,000 platelets/µl. Screening tests for human immunodeficiency
virus, cytomegalovirus, EB virus, and hepatitis B and C were
negative.
CT (Fig. 1B) and
magnetic resonance imaging (MRI) (Fig. 1C
and D) scans demonstrated that the surrounding soft tissue mass
originated from the medullary cavity through the more severe region
of bone destruction. Positron emission tomography (PET)-CT
demonstrated increased glucose metabolism and standardized uptake
values in the left tibia and the left adrenal gland (Fig. 1E). No abnormal enlarged lymph nodes
were identified through ultrasonography or other examinations. An
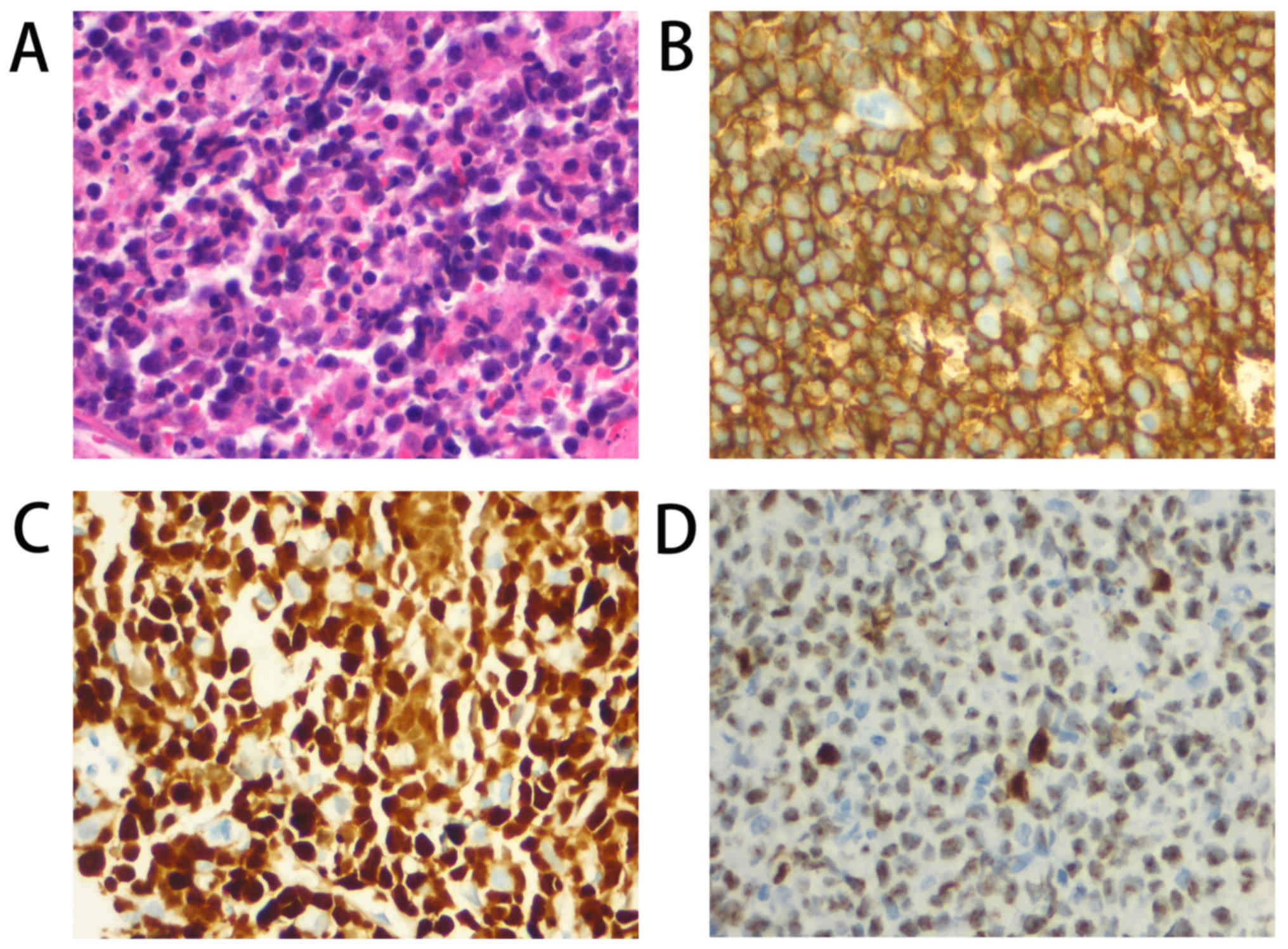
aspiration biopsy was performed on September 30, 2014. Histological
and immunohistochemical staining revealed typical characteristics
of MCL. Biopsy samples were fixed in formalin at 20°C for one night
and decalcified with 20% ethylenediamine tetra-acetic acid for 5 h.
Then the samples were dehydrated at 20°C using 100, 100, 95 and 75%
graded ethanol series for 2 minutes each. Tumor tissue sections (3
µm thick) were sliced, deparaffinized in xylene and stained with
hematoxylin and eosin for 3 minutes at 20°C. The tissue sections
were visualized by a microscopy (TS100; magnification, ×400; Nikon
Corporation, Tokyo, Japan) and photographed by microscope camera
(DS-Ri2; Nikon Corporation, Tokyo, Japan). Immunohistochemical
staining was performed using the EnVision two-step staining method
(2). Mouse anti-CD5 (cat. no.
ZM-0280), anti-CD10 (cat. no. ZM-0283), anti-Ki-67 nuclear antigen
monoclonal antibody (cat. no. ZM-0165) and rabbit anti-CD 20 (cat.
no. ZA-0549), anti-Cyclin D1 (cat. no. ZA-0101) monoclonal
antibodies were purchased from ZSQB-BIO corporation (Beijing,
China). A pathological exam revealed diffuse, small-to-medium sized
cells (Fig. 2A). Immunohistochemical
staining performed as previously described (3) revealed that the cells were positive for
CD5, CD20 (Fig. 2B), CD99, B cell
CLL/lymphoma 6 (Bcl6), multiple myeloma oncogene, BCL2 apoptosis
regulator (Bcl2) and cyclin D1 (Fig.
2C), but not for terminal deoxynucleotidyl transferase or CD10.
Ki-67 was expressed in 80–90% of the cells (Fig. 2D). Cyclin D1 (CCND1)/immunoglobulin
heavy chain (IGH) kit (F01019-00) was purchased from Beijing GP
Medical Technologie, Ltd. (Beijing, China). Fluorescent in
situ hybridization (FISH) analysis (4) was positive for the rearrangement of
IGH/CCND1 and Bcl6 fragmentation. IGH/BCL2 fusion gene, C-MYC
breakage or P53 deletion were not detected. A bone marrow biopsy
revealed no bone marrow infiltration (data not shown).
Following a thorough examination, bulky stage
IE was diagnosed according to the Ann Arbor staging
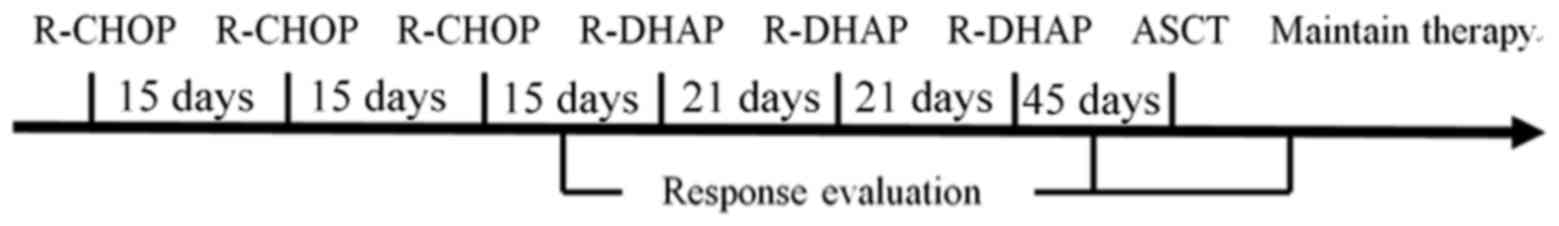
system (5). Sequential induction
therapy with three cycles of R-CHOP followed by three cycles of
R-DHAP followed by autologous stem cell transplantation (ASCT) was
adopted (Fig. 3). The first cycle of
R-CHOP began on October 17, 2014. The left lower limb mass was
reduced to 2×2 cm when the patient was re-assessed on October 31.
The second cycle of R-CHOP chemotherapy began on November 3. The
mass was almost gone by November 17. However, the third course of
chemotherapy, which was originally scheduled to begin on November
20, was cancelled due to severe lung infection occurred on November
18. Ceftazidime (800 mg, every 8 h) was administered for 3 days and
the patient still had high fever. Then biapenem (300 mg, every 12
h) and voriconazole (200 mg, every 12 h) was administered for 4
days. Pneumocystis carinii pneumonia was diagnosed on
November 25. Sulfamethoxazole (400 mg, every 8 h), Kosice (50 mg,
every 24 h), methylprednisolone (40 mg every 12 h) was
administered. Chest CT scans presented decreased shadow area on
November 28 and the dose of methylprednisolone was reduced (40 mg,
every day). The treatment for pneumonia ended on December 13.
During the treatment of the lung infection, the mass grew gradually
to 1×1 cm. The third course of R-CHOP began on December 20.
Considering the gradual enlargement of the mass and the long
interval between the second and third cycle of R-CHOP, another two
cycles of R-CHOP were administrated on January 7 and January 22,
2015. The first course of the following R-DHAP (rituximab 600 mg on
day 1, cisplatin 168 mg on day 2, cytarabine 3.3 g/q12h on day 3,
dexamethasone 40 mg on days 2–5) began on February 15. Complete
remission was achieved following another two cycles of R-DHAP,
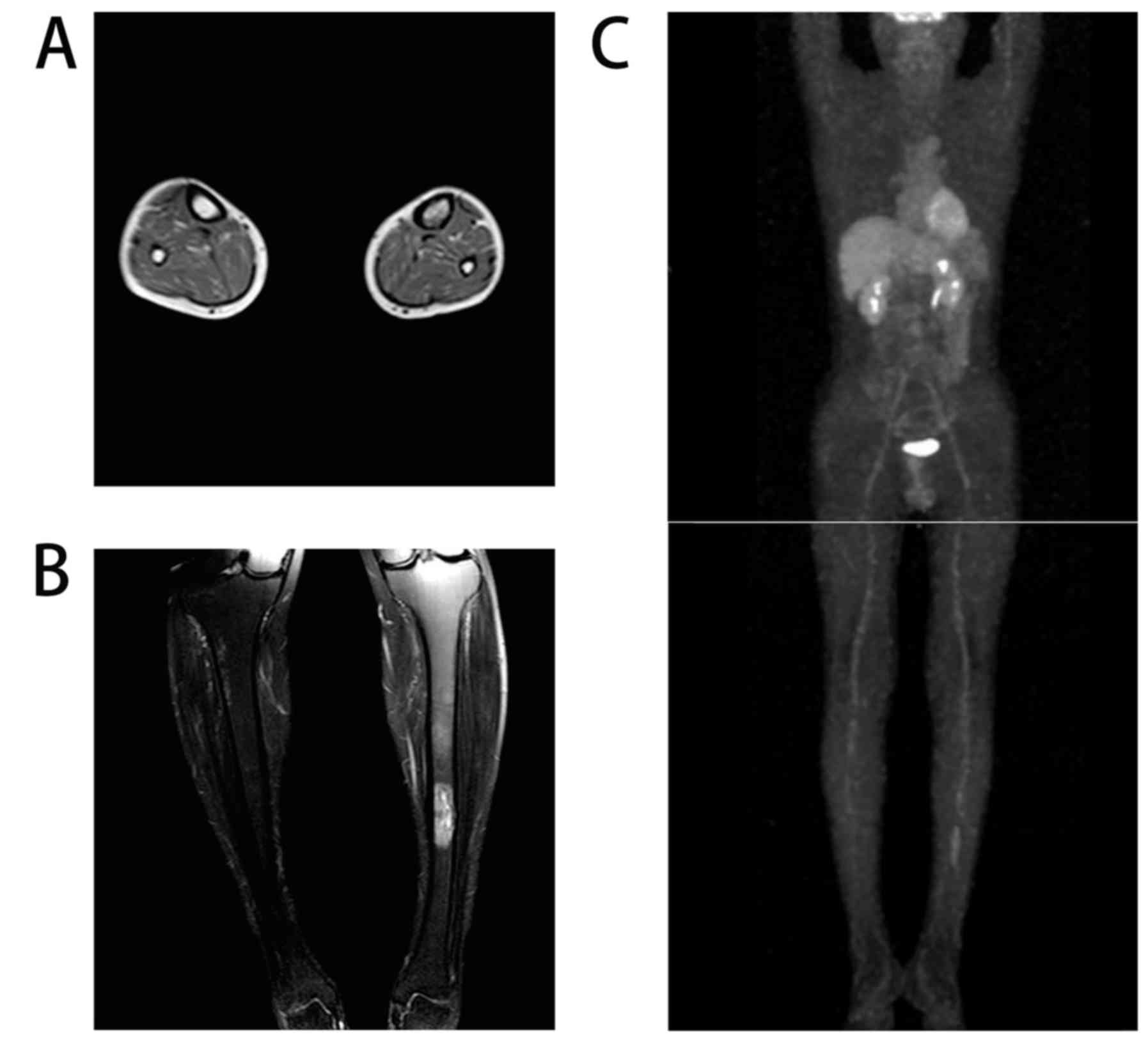
which respectively began on March 9 and 31, 2015. MRI revealed that
the range of abnormal signaling was notably reduced (Fig. 4A and B). PET-CT revealed a slight
high-density shadow in the tibia with normal glucose metabolism
(Fig. 4C). Peripheral blood stem cell
collection began in April 13 and ASCT was conducted on May 12,
2015. For the past 2 years, rituximab at a dose of 600 mg was
administered every 3 months for maintenance therapy. To date, no
recurrence has been found by regular radiological follow-ups.
Discussion
Generally, primary bone lymphoma is considered to
consist of a single bone lesion with or without regional
lymphadenopathies (6). Although
skeletal involvement is not uncommon in other types of non-Hodgkin
lymphoma, the available literature on this in primary bone MCL is
limited (7). To the best of our
knowledge, only one case of primary spinal MCL has been previously
reported (2). In the present case
report, the imaging data provided evidence that MCL may have a
clear bone origin without involvement of lymph nodes.
Diagnosis of MCL should be based on pathological
examination of surgical specimens, preferably lymph nodes. The most
characteristic morphological feature is small- or medium-sized
lymphocytes with irregular nuclei. In addition,
immunohistochemistry for the detection of typical patterns of an
immunophenotype is mandatory. In the current case, the cells were
positive for cyclin D1, CD5 and CD20, but negative for CD10.
Additionally, FISH analysis detected IGH/CCND1 gene fusion and
consequently MCL was diagnosed.
MIPI, Ki-67 index and bulky mass are effective
markers in evaluating patient prognosis in MCL (8). In the current case, the patient was
classified to have a low-risk prognosis according to the MIPI.
Instead of local radiotherapy, systemic chemotherapy was adopted
due to the following: i) The large tumor burden and high rate of
proliferation, which was indicated by the rapidly enlarging leg
lump, increasing size of the tibia lesion and high Ki-67 index; and
ii) the possibility of increased glucose metabolism in the left
adrenal gland was caused by MCL metastasis could not be
excluded.
To date, the initial regimen for younger patients
with MCL remains controversial. A phase II study from the Groupe
d'Etudes des Lymphomes de l'Adulte suggested that CHOP and DHAP
plus rituximab were safe and effective (9). According to the last European Society
for Medical Oncology guideline, a rituximab containing induction of
CHOP and cytarabine followed by ASCT and consolidation is
recommended (7). ASCT consolidation
is potentially curative and has become a standard approach for
patients with MCL (10). Despite the
high rate of complete response following systemic chemotherapy
followed by ASCT, patients do relapse. Rituximab maintenance
therapy following transplantation was demonstrated to prolong
event-free, progression-free and overall survival times in patients
with MCL (11).
Generally, MCL is an incurable disease; nonetheless,
early detection and treatment is essential to improve its
management. The present case report confirmed the possibility of
primary bone MCL with comprehensive and detailed clinical data.
Nevertheless, the level of evidence supporting definitive methods
of diagnosis and treatment of MCL remains low, and further studies
are required to confirm the appropriate methods of detection and
management, and to improve prognosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang Natural
Science Foundation (grant no. LY16H160005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SC collected the data and wrote the manuscript. MY
contributed to the conception of the study, revised the manuscript
and helped perform the analysis with constructive discussions.
Ethics approval and consent to
participate
This case report was approved by The Ethics
Committee of Ningbo University. Consent for publication was
obtained from the patient.
Patient consent for publication
Consent was obtained from all participants in the
present study.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang P, Lin J, Liu H, Shen H and Yang HL:
Primary bone mantle cell lymphomas with multiple vertebral
compression fractures: A case report. Oncol Lett. 13:1288–1292.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhao XF, Young KH, Frank D, Goradia A,
Glotzbecker MP, Pan W, Kersun LS, Leahey A, Dormans JP and Choi JK:
Pediatric primary bone lymphoma-diffuse large B-cell lymphoma:
Morphologic and immunohistochemical characteristics of 10 cases. Am
J Clin Pathol. 127:47–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li GP, Chen WZ, Huang HF, Chen JD, Lin XL
and Fu Q: Role of CyclinD1/IgH detection by FISH in differential
diagnostic significance between mantle cell lymphoma and chronic
lymphocytic leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
23:1314–1317. 2015.(In Chinese). PubMed/NCBI
|
|
5
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on Hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
6
|
Vitolo U, Seymour JF, Martelli M,
Illerhaus G, Illidge T, Zucca E, Campo E and Ladetto M; ESMO
Guidelines Committee, : Extranodal diffuse large B-cell lymphoma
(DLBCL) and primary mediastinal B-cell lymphoma: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 27:v91–v102. 2016.PubMed/NCBI
|
|
7
|
Dreyling M, Campo E, Hermine O,
Kluin-Nelemans HC, Le Gouill S, Rule S, Shpilberg O, Walewski J and
Ladetto M; ESMO Guidelines Working Group, : Newly diagnosed and
relapsed mantle cell lymphoma: ESMO clinical practice guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 25 Suppl
3:iii83–iii92. 2017. View Article : Google Scholar
|
|
8
|
Klener P, Fronkova E, Belada D, Forsterova
K, Pytlik R, Kalinova M, Simkovic M, Salek D, Mocikova H, Prochazka
V, et al: Alternating R-CHOP and R-cytarabine is a safe and
effective regimen for transplant-ineligible patients with a newly
diagnosed mantle cell lymphoma. Hematol Oncol. 36:110–115. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delarue R, Haioun C, Ribrag V, Brice P,
Delmer A, Tilly H, Salles G, Van Hoof A, Casasnovas O, Brousse N,
et al: CHOP and DHAP plus rituximab followed by autologous stem
cell transplantation in mantle cell lymphoma: A phase 2 study from
the Groupe d'Etude des Lymphomes de l'Adulte. Blood. 121:48–53.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tseng YD, Stevenson PA, Cassaday RD, Cowan
A, Till BG, Shadman M, Graf SA, Ermoian R, Smith SD, Holmberg LA,
et al: Total body irradiation is safe and similarly effective to
chemotherapy-only conditioning in autologous stem cell
transplantation for mantle cell lymphoma patients. Biol Blood
Marrow Transplant. 24:282–287. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Gouill S, Thieblemont C, Oberic L,
Moreau A, Bouabdallah K, Dartigeas C, Damaj G, Gastinne T, Ribrag
V, Feugier P, et al: Rituximab after autologous stem-cell
transplantation in mantle-cell lymphoma. N Eng J Med.
377:1250–1260. 2017. View Article : Google Scholar
|