Introduction
The malignant tumor is usually with latent onset and
atypical symptoms, and many cases were found in the advanced stage
and could not undergo surgery. Therefore, radiotherapy and
chemotherapy become the primary forms of treatment for advanced
tumor and recurrent tumor (1). Since
radiotherapy and chemotherapy inhibit or kill tumor cells, they
have toxic action on the normal cells of the body. Therefore,
identification of effective, harmful antitumor drugs has become a
research hotspot. Barbadian, the national protected variety of
traditional Chinese medicine manufactured by Chinese Medicine
Factory Co., Ltd. (Xiamen, China) is mainly made up of natural
bezoar, snake gall, antelope's horn, pearl, pseudo-ginseng and
natural musk with the functions of eliminating dampness and heat,
activating blood, relieving internal heat or fever, excitation and
pain. Researchers have found that Barbadian exerts a good
anti-dullness effect (2) on the
adjuvant therapy of cancer.
In clinic, the combination of Barbadian and
chemotherapeutic drugs can relieve patients' pain and improve the
clinical symptoms, reduce the toxic and side effect of
chemoradiotherapy and prolong patients' lifetime through the
function of clearing heat and removing toxicity, but the mechanism
is undefined (3,4). In this study, the combination of
chemotherapeutic drug Tegafur and Barbadian was used to determine
the antitumor effect and initial exploration of antitumor
mechanism, which can provide reference and basis for further
research and clinical application.
Materials and methods
Materials and reagents
A total of 24 healthy female SPF Kunming mice
(weight, 18–25 g) were provided by the Laboratory Animal Center of
Shandong Luye Pharma Co., Ltd. (Yantai, China), the animal
certificate number of which was SCXK (Lu) 20090009. S-180 tumor
strains of mouse sarcoma cells were provided by the Shanghai Cell
Institute (Shanghai, China); Barbadian capsules (batch no. GYZZ
Z10940006) were provided by the Chinese Medicine Factory Co., Ltd.;
Tegafur capsules (batch no. GYZZ H20080802 were provided by
Shandong New Era Pharma; RPMI-1640 was provided by Gibco; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA); MTT was provided by
Sigma-Aldrich (St. Louis, MO, USA); FBS was provided by Sangon
Biotech Co., Ltd. (Shanghai, China); DMSO (AR) was provided by
Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China); mice
IL-1, IL-6, TNF-α ELISA kits were provided by Bio-Rad Laboratories,
Inc. (Hercules, CA, USA). The study was approved by the Ethics
Committee of Yantaishan Hospital (Yantai, China).
Instruments and equipments
The following instruments and equipment were used:
Super clean bench CW-CJ-2D [Purification Equipment (Suzhou) Co.,
Ltd., Suzhou, China]; CO2 constant temperature
incubation (Sanyo Electric Co., Ltd., Osaka, Japan); fully
automatic enzyme immunoassay analyzer EVOLIS (Bio-Rad Laboratories,
Inc.); fully automatic five classification hematology analyzer
LH750 (Beckman Coulter, Inc., Brea, CA, USA); fully automatic
biochemical analyzer DXC800 (Beckman Coulter, Inc.); electronic
scale YP10002 (Shanghai Youke Equipment & Instrument Co., Ltd.,
Shanghai, China); electric-heated thermostatic water bath HHS-21-6
(Changzhou Nuoji Instrument Co., Ltd.); biochemical incubator
DNP-9002BS-III (Shanghai Cimo Medical Devices Manufacturing Co.,
Ltd.).
Drug preparation
Preparation of Tegafur
The dose of Tegafur for human is 2 mg/kg, according
to the conversion formula of animal dose: DB = DA × RB/RA ×
(WA/WB)1/3 (DA and DB are the doses of per kg of AB two kinds of
animals, RA and RB are the shape coefficients of AB two kinds of
animals, WA and WB are the standard weights of animals) (5,6). The
Tegafur dose for the mice was 12.28 mg/kg, prepared with sterilized
saline and stored at 4°C in a refrigerator for standby
application.
Preparation of Barbadian
The dose of Barbadian for human is 20 mg/kg. For
mice, the dose (5,6) of Barbadian was calculated according to
the above conversion method, 122.8 mg/kg, prepared with sterilized
saline and stored at 4°C in a refrigerator for standby
application.
Preparation of mixed liquor of Tegafur
and Barbadian
Sterilized saline was used to prepare the mixed
liquor of Tegafur and Barbadian with a final concentration of 24.56
and 245.6 mg/kg.
Preparation of mouse tumor models and
group administration
Preparation of mouse tumor models
S-180 myeloma cells of mice in the logarithmic phase
were selected and prepared in single-cell suspension with the
concentration of 1×107 cell/ml with normal saline under
aseptic condition, and injected into the armpit of the right limb
of the mice by subcutaneous injection, 0.2 ml/mouse (7–9).
Group administration and record of
observation index
The day of subcutaneous inoculation of tumor mass
was recorded as 0 day. On the 7th day after the inoculation of
tumor mass, the subcutaneous tumor could be touched by hand and the
diameter was 0.2–0.4 cm. The group experiment was started, and the
mice (n=6 per group) were randomly divided into 4 groups, i.e.,
combination therapy group, Tegafur group, Barbadian group and
normal saline control group. Corresponding test substances were
given to mice in each group by intragastric administration, 0.2
ml/mouse, once/day, for an interval for 2 days after continuous
administration for 5 days, recorded as 1 period, 3 periods were
subsequently performed. The appearance characteristics of mice were
recorded every day.
Determination of experimental index
Determination of antitumor rate
Mice were weighed 48 h after the final
administration, and then sacrificed. An autopsy was performed, the
tumor mass was separated and weighed to calculate the antitumor
rate. Inhibition ratio was calculated as: (average tumor weight of
control group - average tumor weight of drug group)/average tumor
weight of control group × 100% (10–12).
Determination of immune cells
Forty-eight hours after the final administration,
the eyeballs were removed to collect 0.2 ml anticoagulant, and the
five classification method was used to determine hemocyte. The five
classification method refers to the results of five common types of
white blood cells in peripheral blood fluid, namely, the percentage
and absolute value of eosinophils (EOS), basophils (BASO),
neutrophils (NEUT), monocytes (MONO) and lymphocytes (LYMPH),
analyzed by the hemocyte analyzer through physicochemical
techniques (13).
Determination of blood biochemistry
and the level of inflammatory mediators
Forty-eight hours after the final administration,
the eyeballs were removed to collect 0.8–1 ml blood, indoor
solidification for 30 min, and the supernatant was collected after
centrifugation (5,000 × g at 20°C for 5 min). The ELISA kit was
used to determine IL-1, IL-6, TNF-α and other indexes, and a fully
automatic biochemical analyzer DXC800 (Beckman Coulter, Inc., Brea,
CA, USA) was used to determine all the indexes (14–17) of
blood biochemistry.
Statistical analysis
Excel was used in recording the statistical
treatment. Data were presented as mean ± standard deviation (mean ±
SD). An F test was used to determine homogeneity of variance,
t-test was used to determine homoscedasticity or heteroscedasticity
according to homogeneity of variance. The difference was
statistically significant when P<0.05 (18–20).
Results
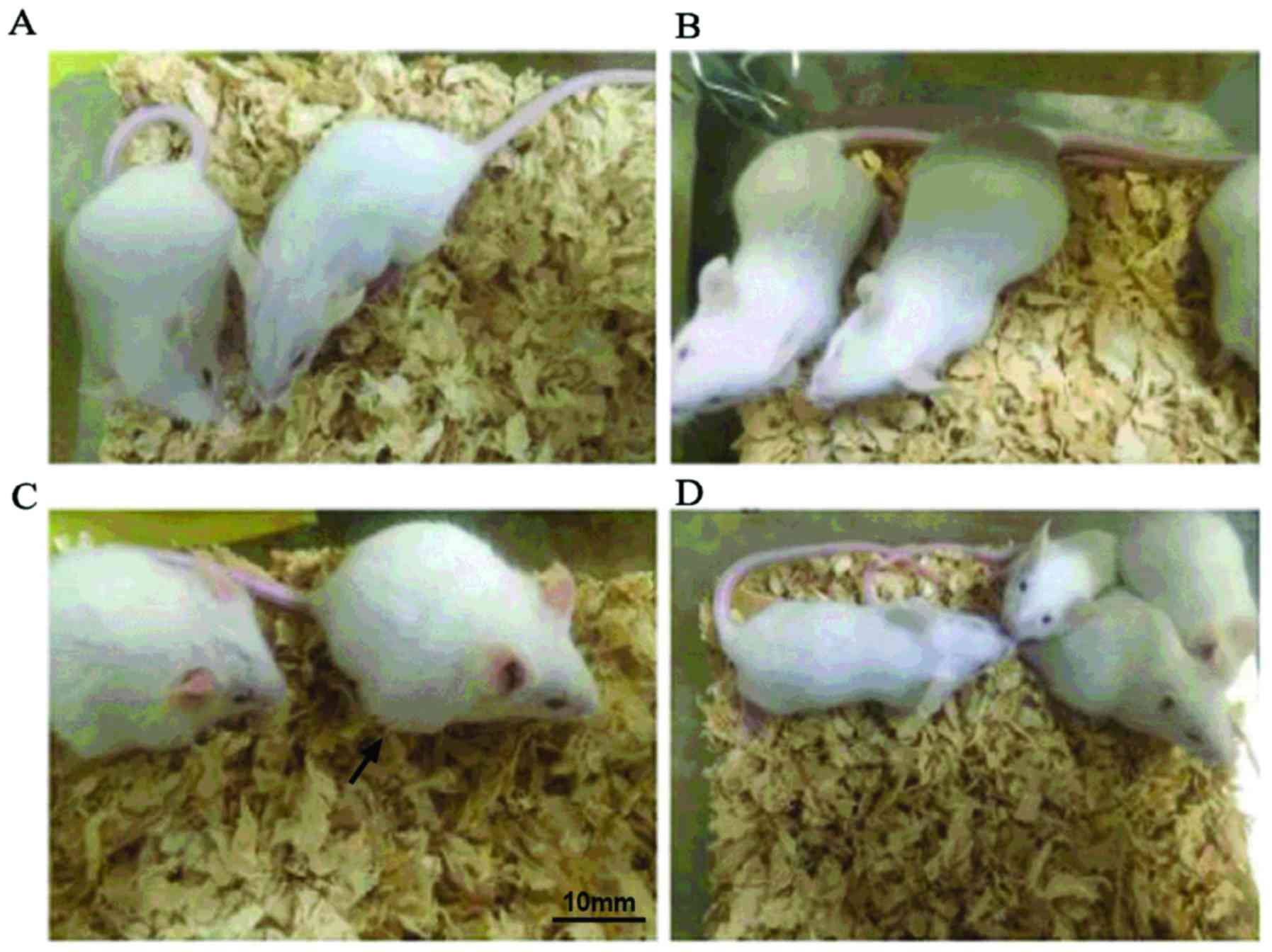
Effect of the combination of Tegafur
and Barbadian on the bodies of tumor-bearing mice
At the end of the experiment, the condition of mice
was good according to the visual inspection of combination therapy
group (Fig. 1A), Barbadian group
(Fig. 1B), normal saline group
(Fig. 1D); the fur of mice in Tegafur
group (Fig. 1C) were sparse, rough
and dispirited (Fig. 1).
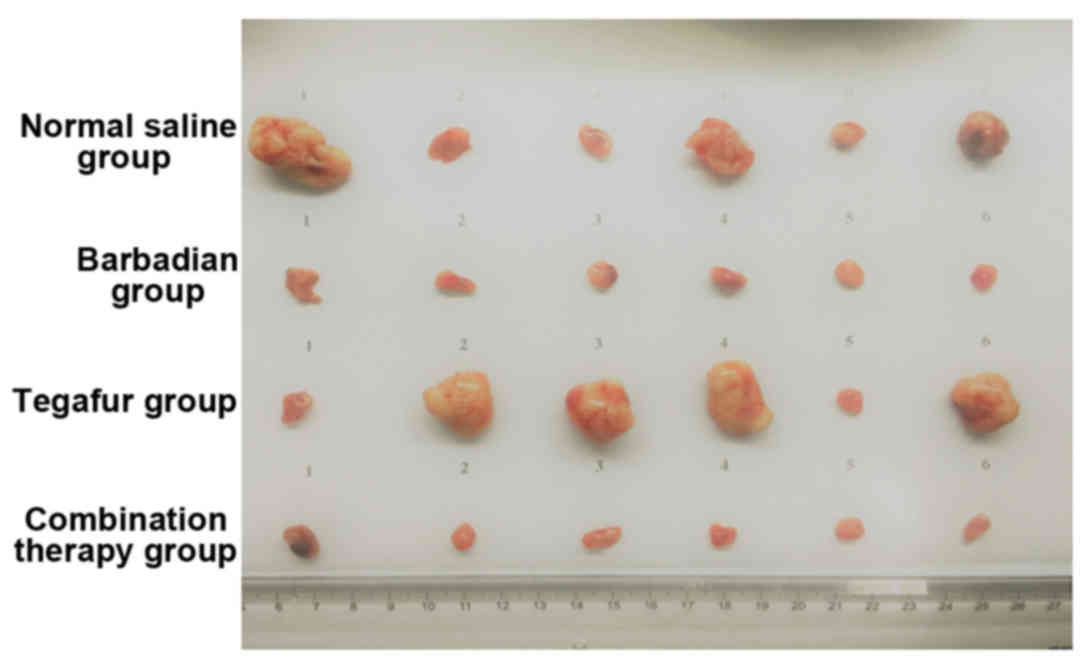
Effect of the combination of Tegafur
and Barbadian on the tumors of tumor-bearing mice
Tumor weight of the combination therapy and
Barbadian groups was significantly lower than that of the normal
saline group (P<0.05). Tumor weight of the Tegafur group was
higher than the normal saline group, but there was no significant
difference (P>0.05). The antitumor rate of the combination
therapy and Barbadian groups were 78.00 and 72.64%, respectively.
The antitumor rate of the Tegafur group was −89.00%, and the tumor
in this group was bigger than that in the normal saline control
group (Table I and Fig. 2).
 | Table I.The effect on the volume of mice tumor
by the Tegafur combined with Barbadian. |
Table I.
The effect on the volume of mice tumor
by the Tegafur combined with Barbadian.
| Group | No. of animals | Tumor volume
(mm3) |
|---|
| Normal saline
group | 6 | 230.02±31.25 |
| Combination therapy
group | 6 |
112.21±15.65a |
| Barbadian group | 6 |
130.56±13.22a |
| Tegafur group | 6 |
258.51±65.12a |
Effect of the combination of Tegafur
and Barbadian on immune cells of mice
According to the statistical analysis on immune
cells in the report of blood routine test, there was no significant
difference in the comparison of the total number of red blood cells
in the three drug groups and normal saline group: WBC in the
Barbadian group was significantly higher than that in the normal
saline group (P<0.01); LYMPH in the Barbadian group was
significantly higher than that in the normal saline group
(P<0.01), LYMPH in Tegafur group was significantly lower than
that in normal saline group (P<0.01); MONO in the three drug
groups was significantly higher than that in the normal saline
group (P<0.01); NEUT in the combination therapy (P<0.01) and
Barbadian (P<0.05) groups was significantly higher than that in
the normal saline group. No significant difference was found in EOS
in the three drug groups and normal saline group. BASO in the three
drug groups was significantly higher than that in normal saline
group (P<0.01); the other indexes were normal (Table II).
 | Table II.The effect of Tegafur combined with
Barbadian on the immune cells of mouse (mean ± SD, n=6). |
Table II.
The effect of Tegafur combined with
Barbadian on the immune cells of mouse (mean ± SD, n=6).
| Group | RBC
(×10−12/l) | WBC
(×10−9/l) | LYMPH
(×10−9/l) | MONO
(×10−9/l) | NEUT
(×10−9/l) | EOS
(×10−9/l) | BASO
(×10−9/l) |
|---|
| Normal saline
group | 7.65±0.70 | 3.48±0.77 | 2.39±0.11 | 0.62±0.03 | 0.25±0.03 | 0.04±0.013 | 0.085±0.008 |
| Combination therapy
group | 7.99±0.36 | 3.54±0.35 | 2.31±0.39 |
0.83±0.08b |
0.35±0.04b | 0.02±0.013 |
0.005±0.005b |
| Barbadian group | 7.75±0.33 |
4.76±0.45b |
3.26±0.29b |
0.89±0.06b |
0.31±0.02a | 0.02±0.017 |
0.040±0.013b |
| Tegafur group | 8.04±0.51 | 3.37±0.47 |
1.65±0.36b |
0.85±0.07b | 0.23±0.03 | 0.04±0.013 |
0.033±0.010b |
The effect of Tegafur combing with
Barbadian on inflammatory mediator of mouse
IL-1 in combination therapy group and Barbadian
group were significantly higher than that in normal saline group
(P<0.05). IL-6 in combination therapy group (P<0.01),
Barbadian group (P<0.05) and Tegafur group (P<0.05) were
significantly higher than that in the normal saline group. TNF-α in
the Tegafur group was significantly lower than that in normal
saline group (P<0.05) (Table
III).
 | Table III.The effect of Tegafur combined with
Barbadian on inflammatory mediator of mouse (mean ± SD, n=6). |
Table III.
The effect of Tegafur combined with
Barbadian on inflammatory mediator of mouse (mean ± SD, n=6).
| Group | IL-1 (pg/ml) | IL-6 (pg/ml) | TNF-α (pg/ml) |
|---|
| Normal saline
group | 3.47±0.80 | 3.00±0.64 | 44.00±9.65 |
| Combination therapy
group |
4.69±0.56a |
3.98±0.55b | 34.33±6.31 |
| Barbadian group |
4.82±1.60a |
3.58±0.35a | 38.17±8.91 |
| Tegafur group | 3.90±1.78 | 3.8±0.57a |
28.83±9.15a |
Effect of Tegafur combined with
Barbadian on blood biochemistry of mouse
According to the statistical analysis on 15 test
indexes (including total protein, albumin, globulin, ratio of
albumin to globulin, GPT, GOT, GPT/GOT, ALP, g-GGT, TBil, DBil,
total cholesterol, triglyceride, BUN, uric acid) of blood
biochemistry, there was a significant difference in three test
indexes for all the drug groups. The details are as follows: FBG in
combination therapy group (P<0.05) and Barbadian group
(P<0.01) were significantly higher than that in normal saline
group, FBG in Tegafur group was significantly lower than that in
normal saline group (P<0.01). Total cholesterol in the Tegafur
group was significantly higher than that in normal saline group
(P<0.05). BUN in the Tegafur group was significantly higher than
that in the normal saline group (P<0.05) (Table IV).
 | Table IV.The effect of Tegafur combined with
Barbadian on the blood biochemistry of mouse (mean ± SD, n=6). |
Table IV.
The effect of Tegafur combined with
Barbadian on the blood biochemistry of mouse (mean ± SD, n=6).
| Group | FBG (mmol/l) | Total cholesterol
(mmol/l) | BUN (mmol/l) |
|---|
| Normal saline
group | 4.27±0.24 | 1.83±0.02 | 7.46±0.54 |
| Combination therapy
group |
5.43±0.93a | 1.98±0.73 | 8.02±0.75 |
| Barbadian group |
5.62±0.96b | 1.88±0.17 | 7.77±0.31 |
| Tegafur group |
2.60±0.35b |
2.15±0.28a |
9.68±0.98b |
Discussion
We know that chemotherapeutic drugs have a decisive
effect on antitumor treatment, but the severe adverse reactions,
such as myelosuppression, gastrointestinal reaction, liver and
kidney injury have restricted its effect and application to a
certain extent (21). Tegafur capsule
is a kind of new-style fluorouracil oral anticancer drug, which is
made up of FT, CDHP and OXO (22).
Its active constituent FT has good oral bioavailability, and can be
translated into fluorouracil in the body. CDHP can restrict the
decomposition of fluorouracil to make the stability of blood
concentration longer in the plasma and tumor tissues to strengthen
antineoplastic activity. OXO is distributed in the gastrointestinal
tract after oral administration, which can reduce the toxicity and
adverse reactions of fluorouracil in the gastrointestinal tract
(23). In this study, according to
the analysis on immune cells, there was no significant difference
of WBC, RBC, NEUT and EOS in the Tegafur and normal saline control
groups. MONO and BASO were increased compared to the normal saline
control group, and results were statistically significant. By
contrast, LYMPH was significantly reduced compared with the normal
saline control group. According to the analysis on cell factors,
there was no significant difference of IL-1 in the Tegafur and
normal saline groups, while IL-6 was significantly higher than that
in normal saline group. TNF-α was significantly lower than that in
the normal saline group. According to the analysis on blood
biochemistry, blood sugar in Tegafur group was significantly
reduced, whereas cholesterol and BUN were significantly increased.
The above results show that when Tegafur kills tumor cells, the
dead and injured tumor cells cause a series of immune responses
through the damage associate molecular pattern (DAMP). When the
immune response is started by DAMP and converted from innate
immunity into adaptive immune response, rapid division and
proliferation focused on T lymphocytes occurred, by this time, the
application of Tegafur restricted the DNA composition, when the
speed of Tegafur killing lymphocytes is faster than that of
oncocytes, the reduced T lymphocytes cannot restrict and kill
tumors, so that the result of the tumor growth is increased
(antitumor rate is −89%) and higher than normal saline group is
obtained.
In this study, Barbadian has good antitumor effect
without significant toxic and side effects. In this study, WBC,
TLC, MONO, NEUT and BASO in Barbadian group were higher than that
in the normal saline group. IL-1 and IL-6 were significantly
increased, while TNF-α had no significant difference compared with
the normal saline group. Blood sugar was significantly higher than
that in the normal saline control group, and there was a
significant difference of cholesterol and BUN compared with control
group. From the above analysis, it has been shown that the
Barbadian group has the significant function of increasing
immunity, compared with Tegafur group. The most significant
difference was that WBC and LYMPH were significantly increased,
while TNF-α had no significant decrease. Additionally, the main
drugs of Barbadian including bezoar, pseudo-ginseng, musk and pearl
have an immunomodulatory effect; bezoar 100 mg/kg can significantly
strengthen the phagocytic function (1) of mouse peritoneal macrophages (MPM),
pseudo-ginseng can strengthen NK cell viability and facilitate the
activity of macrophage, increasing the lethality (2) of tumors. The study by Hao et al
(24) has shown that sanchinoside 160
mg/kg can increase 92.0% of hemolytic plaque and significantly
increase the phagocytic rate and phagocytic index of MPM. Hao et
al have shown that pearl has good immunologic enhancement,
antitumor and radiation-proof effect (24). Those authors have shown that,
Barbadian has the effect of increasing the immunity of the
organism. This is in agreement with our result of the antitumor
rate of Barbadian group, which reached 72.68%.
The combination of Tegafur and Barbadian has shown
the best antitumor rate at 78% in this study. In addition, MONO,
NEU, and BASO in combination therapy group were higher than the
normal saline control group, and no significant difference is found
in other cells compared with the normal saline control group. IL-1
and IL-6 of combination therapy group were significantly higher,
while TNF-α had no significant difference compared with the normal
saline group. Blood sugar in the combination therapy group was
significantly higher than that in the normal saline control group,
no difference is found in cholesterol and BUN compared with control
group. The above analysis revealed that, compared with Tegafur
group, combination therapy group has a milder inflammatory
response, the most significant difference being for LYMPH, whereas
TNF-α was not lower than the normal saline group and without a
significant difference, with the antitumor rate reaching 78%. The
possible reason may be the effect of protecting liver and
gallbladder of Barbadian has accelerated the metabolism of Tegafur
and reduced the toxic effect, improved Tegafur-induced decrease of
blood glucose, leading to cholesterol and BUN recover. The key
point is LYMPH and TNF-α both returned to normal as indicated by
the body hair of mice in the combination group which was glossy and
smooth, the mental status of mice was good, and a significant
difference was identified compared with the toxic mice in the
Tegafur group. Tegafur combined with Barbadian complement each
other, they can relieve the toxic and side effects of
chemotherapeutic drugs thereby inhibiting the tumor and reaching
the desired effect.
In conclusion, this study has shown that the
combination of Tegafur and Barbadian has a significant effect of
inhibiting mice S-180 sarcoma. The single use of chemotherapeutic
drug Tegafur has no significant inhibitory effect on mice S-180
sarcoma, whereas the single use of Barbadian has good antitumor
effect and can resist the significant decrease of lymphocytes
caused by chemotherapeutic drug Tegafur. Barbadian therefore, not
only exerts a good antitumor effect but can also protect the immune
system of the organism, which has a good development prospect.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Science and
Technology Development Project of Yantai, China (project no.
2013YD008).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
EZ contributed to the study design, data acquisition
and analysis and drafted the manuscript; AF was involved in data
acquisition and revision of the manuscript; WC and XW assisted in
the performance of the statistical analysis with constructive
discussions. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Yantaishan Hospital (Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu L, Zheng Y, Zhang Z, Ding C and Lu Z:
Clinical observation on oral administration of single drug Tegafur
in the treatment of old advanced gastric cancer. Pract J Cancer.
26:294–296. 2011.(In Chinese).
|
|
2
|
Geng D, Zhang L and Sui M: Study on
Barbadian capsules assist advanced tumor chemotherapy. Shanxi Med
J. 6:562–564. 2010.(In Chinese).
|
|
3
|
Xu J: Study on the anti-tumor effect of
Barbadian (Huatai TCM). J Shantou Univ Med Coll. 1:25–28. 1994.(In
Chinese).
|
|
4
|
Zhu X and Zhang X: Clinical observation on
the prevention and treatment of toxic and side effects of tumor
chemotherapy of Barbadian. China's Naturopathy. 5:45–46. 2006.
|
|
5
|
Zhu K: The inhibition effect of S-1
combined with arctigenin injection on H22 tumor-bearing mice tumor.
J Shandong Med Coll. 35:333–335. 2013.(In Chinese).
|
|
6
|
Qiang G, Na L and Qian Z: Research
progress of apoptosis gene in the malignant tumor. J Shanxi Coll
Tradit Chin Med. 1:61–62. 2006.
|
|
7
|
Shuo Z: Experimental study on apoptosis of
mice with Xiao Chaihu decoction and 5-fluorouracil-induced Hca-f
liver cancer. Liaoning Univ Tradit Chin Med. 2009.
|
|
8
|
Feng X: Inflammation regulation and sex
difference of macrophage phagocytize apoptotic cells of mice.
Peking Union Medical College. 2011.
|
|
9
|
Zhao D, Xie X, Li M and Wang S:
Experimental study on the anticancer effect of Curcumine on mice of
S180 in vivo. J Xi'an Jiaotong University (Medical Sciences).
1(70–73): 822007.(In Chinese).
|
|
10
|
Du R, Wang XJ, Xu Q, Ruan X, Liu XS, Qu
YQ, Yang LH and Xiao YY: In vivo and in vitro anti-tumor effect of
ge-enriched barley seedlings. Food Science. 23:371–374. 2010.(In
Chinese).
|
|
11
|
Zhang Z, Zhao L, Zhang H and Bo L:
Influence on the anti-tumor effect before and after the preparation
of the Traditional Chinese Medicine rhizoma typhonii in mice.
Zhonghua Zhongyiyao Zazhi. 7:1009–1011. 2010.
|
|
12
|
Liu Q and Meng Q: Discussion on in vivo
anti-tumor effect and mechanism of Sargassum hemiphyllum
polysaccharides. J First Mil Med Univ. 4:434–436. 2004.
|
|
13
|
Davis BH and Bigelow NC: Performance
evaluation of a hematology blood counter with five-part leukocyte
differential capability. Am Clin Lab. 18:8–9. 1999.PubMed/NCBI
|
|
14
|
Lin Y, Xiao J, He C, Tu H and Wei Y:
Expression of IL-1β, IL-6, leptin and the receptors in gastric
cancer tissue. J Xi'an Jiaotong Univ (Med Sci).
|
|
15
|
Tan D, Xu X, Li T, Yi W and Han Z: Study
on cell morphology of drought stress-induced programmed cell death
of Malus sieversii. Huabei Nongxuebao. 1:50–55. 2007.
|
|
16
|
Run L, Jing L and Shen X: Experimental
study on anti-tumor effect and attenuation of Barbadian. Conference
Proceedings of International Biological Medicine and Biotechnology
Forum (Hong Kong). 37:40–41. 2006.
|
|
17
|
Raison CL, Capuron L and Miller AH:
Cytokines sing the blues: Inflammation and the pathogenesis of
depression. Trends Immunol. 27:24–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gatti Lobo L, Tunes Zambaldi M, de Lábio
RW, Silva LC, de Arruda Cardoso Smith M and Payão Marques SL:
Interleukin-6 polymorphism and Helicobacter pylori infection in
Brazilian adult patients with chronic gastritis. Clin Exp Med.
5:112–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strieter RM, Chensue SW, Basha MA,
Standiford TJ, Lynch JP, Baggiolini M and Kunkel SL: Human alveolar
macrophage gene expression of interleukin-8 by tumor necrosis
factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J
Respir Cell Mol Biol. 2:321–326. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
North RJ, Dunn PL and Havell EA: A role
for tumor necrosis factor in poly(I:C)-induced hemorrhagic necrosis
and T-cell-dependent regression of a murine sarcoma. J Interferon
Res. 11:333–340. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coburn JM and Kaplan DL: Engineering
biomaterial-drug conjugates for local and sustained
chemotherapeutic delivery. Bioconjug Chem. 26:1212–1223. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shirasaka T and Taguchi T: Timeline from
discovery of 5-FU to development of an oral anticancer agent S-1
and its drug concept. Gan To Kagaku Ryoho. 33 Suppl 1:4–18.
2006.(In Japanese). PubMed/NCBI
|
|
23
|
Wan Y, Hui H, Wang X, Wu J and Sun S:
Comparison of the efficacy and safety of capecitabine or tegafur,
gimeracil and oteracil potassium capsules combined with oxaliplatin
chemotherapy regimens in the treatment of advanced gastric cancer.
Zhonghua Zhong Liu Za Zhi. 38:28–34. 2016.(In Chinese). PubMed/NCBI
|
|
24
|
Hao Z and Xu Z: Effect of sanchinoside on
immune function in mice. Chinese Traditional Patent Medicine.
8:31–32. 1986.(In Chinese).
|