Introduction
Primary breast cancer is a common malignancy in
females and remains the leading cause of cancer-associated
mortalities among females globally despite advances in screening,
diagnosis, and treatment (1);
however, breast metastasis from an extramammary neoplasm is
uncommon, constituting only 0.5–2.0% of all mammary malignancies
(1,2).
The most common origins of breast metastasis are malignant
melanoma, lymphoma, lung cancer, ovarian carcinoma and soft tissue
sarcoma, followed by gastrointestinal and genitourinary tumor types
(3–6).
Gastric carcinoma is the third most common carcinoma
in Korean females, followed by breast and thyroid carcinoma in the
past decade (7). There were estimated
to have been ~1,000,000 new cases of gastric cancer in 2012, making
it the fifth most common malignancy and the third leading cause of
cancer mortality for females and males globally (8). Common sites of distant metastasis of
gastric cancer include the peritoneum, liver, lymph nodes, and
lungs. The breast is a rare site of metastasis in gastric cancer
(9). Metastatic tumors frequently
contain similar immunohistochemical characteristics to the primary
tumors, and it is important to determine whether the breast lesions
are primary or metastatic from gastric cancer, in order to
determine the surgical intervention required (10–17). Owing
to the low frequency of the disease, only sporadic cases or a small
series of cases of patients with breast metastases from gastric
cancer have been published so far (18). The majority of the previous studies
focused on clinical presentation and immunohistochemical
characteristics rather than specific treatment and prognostic
variables (2,19–22);
therefore, little is known about the biological behavior,
clinicopathological features, optimal treatment and prognosis of
this condition. Thus, clinical researchers may face numerous
challenges when conducting a prospective randomized case-control
clinical study to compare the treatment programs and outcomes in
this rare clinical entity. The previous study demonstrated that
treatment strategies, including intensive multi-agent chemotherapy,
surgery, radiation and targeted therapy, however, the treatment
strategies to achieve complete remission or partial remission
remain controversial (23). The
present study included 3 cases of gastric cancer with breast
metastasis from Renji Hospital (Shanghai, China) and 51 additional
cases from previous studies (9,10,12,13,23–66).
The primary origin, clinicopathological features, treatments and
survival data were systematically collected and analyzed in order
to evaluate whether these factors may serve potential roles as
prognostic and predictive biomarkers of patients with gastric
cancer and breast metastasis.
Materials and methods
Data collection
The inclusion criteria included: A pathological
diagnosis of gastric cancer with breast metastasis; and willing to
sign informed consent. The exclusion criteria included: No definite
pathology; and unwilling to sign informed consent. To obtain data
on studies detailing patients with gastric cancer with breast
metastasis, studies in databases from between January 1960 and
December 2016, including PubMed, MEDLINE, Embase, Google Scholar,
Wanfang Database, China Science, Technology Periodical Database and
China Journal Net, were assessed using the keywords ‘gastric or
stomach’, ‘tumor or cancer or carcinoma’, ‘breast or mammary’ and
‘metastasis’. All titles, abstracts and associated citations were
scanned and reviewed. Relevant references from which these data
were obtained have been included (9,10,12,13,23–66).
A total of three patients diagnosed with gastric cancer and breast
metastases in the Renji Hospital, from January 2003 to December
2017 were retrospectively reviewed. All patients were female with a
median age of 49.00±1.73 years old (age range, 48–51 years). Of
these patients, two of them were diagnosed with breast lesions 3
years following gastric cancer surgery, and one was concurrently
diagnosed with gastric cancer and breast metastasis. All of the
patients received chemotherapy, and one of them received surgery
for breast lesions. Ethical approval was obtained from Human
Clinical and Research Ethics Committees of Renji Hospital
Affiliated to Shanghai Jiaotong University and written informed
consent was obtained from the patients prior to the study. The
following data were collected: Epidemiological, symptomatological,
macroscopic presentation, pathological diagnosis, imaging
performance, time between primary gastric cancer diagnosis and
breast metastasis detection, treatment and prognosis.
Hematoxylin-eosin (H&E) and
immunohistochemical staining
Tissue samples derived from resected and core needle
biopsy specimens were fixed in 10% formalin at room temperature for
24 h, paraffin embedded and subjected to histological or
immunohistochemical analysis. Sections (4 µm) were heated at 58°C
for 2 h and then deparaffinized in xylene and hydrated with a
series of graded alcohols, including anhydrous ethanol for 5 min,
95% ethanol for 2 min, 90% ethanol for 2 min, 80% ethanol for 2 min
and 70% ethanol for 2 min. H&E staining was used for
histological analysis. Antigen recovery was performed by heating
and immersing the slides in citrate buffer (0.01 M, pH 6.0; cat.
no. P0020; Noble-Ryder Technology Co., Ltd., Beijing, China) in a
microwave oven (121°C) for 10 min twice. Endogenous peroxidase
activity was blocked using 3% hydrogen peroxide for 30 min at 20°C,
and the sections were incubated with anti-cytokeratin 7 (CK7; 1:50;
cat. no. OV-TL12/30; Dako; Agilent Technologies, Inc., Santa Clara,
CA, USA), CK20 (1:80; cat. no. M7019; Dako; Agilent Technologies,
Inc.), mucin 1 (1:50; cat. no. MRQ-17; AmyJet Scientific, Inc.,
Wuhan, China), Ki-67 (1:100; cat. no. MIB-1; Dako; Agilent
Technologies, Inc.), gross cystic disease fluid protein-15
(GCDFP-15; 1:50; cat. no. 23A3; Dako; Agilent Technologies, Inc.),
mammaglobin (1:100; cat. no. TA327698, OriGene Technologies, Inc.,
Beijing, China), villin (1:100; cat. no. 1D2C3) and
carcinoembryonic antigen (CEA; 1:100; cat. no. II-7; both Dako;
Agilent Technologies, Inc.), respectively, at 4°C overnight.
Subsequently, the sections were washed with PBS three times for 2
min and incubated with a biotinylated anti-mouse (cat. no. D0486)
/anti-rabbit secondary antibody (cat. no. D0487; 1:500; Dako;
Agilent Technologies, Inc.) at 37°C for 15 min. The signal was
detected with a 3,3′diaminobenzidine kit (Dako; Agilent
Technologies, Inc.). Finally, the sections were counterstained with
hematoxylin solution at room temperature for 5 min. The positive
immunostaining cells were counted and imaged under a light
microscope (Olympus BX43; Olympus Corporation, Tokyo, Japan) with a
magnification of ×100 and ×400. The negative control was conducted
by replacing the primary antibody with 0.1% bovine serum albumin
(cat. no. BAH62-0100; AmyJet Scientific, Inc.)/PBS.
Statistical analysis
All statistical analyses were performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA) and numeric parameters
presented as the mean ± standard deviation. Survival data were
defined as the time from breast metastasis until the date of
mortality or last follow-up, and median overall survival (OS) time
was estimated using the Kaplan-Meier method and the log-rank test
was used for comparison of outcomes. Multivariate analysis was
performed to confirm independent predictors by using a step-forward
logistic regression approach for the Cox proportional hazards
model, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinicopathological characteristics of
patients with primary gastric cancer
A total of 54 cases that were enrolled in the
present study, 51 of which were from previous studies (9,10,12,13,23–66).
The primary gastric cancer characteristics are summarized in
Table I. All but one of the patients
were female. A total of 30 cases were available with Borrmann
classification (67) data, including
Borrmann I in 3 cases (5.6%), II in 2 cases (3.7%), III in 17 cases
(31.5%) and IV in 8 cases (14.8%). For the primary gastric
carcinoma location, 33 cases were available for analysis. Of these
cases, 9 cases (16.7%) had lesions in the gastric corpus, 19
(35.2%) in the gastric antrum, 4 cases (7.4%) in the linitis
plastica and 1 case (1.9%) in the gastric fundus (Table I).
 | Table I.Clinicopathological information of
patients with primary gastric tumor types. |
Table I.
Clinicopathological information of
patients with primary gastric tumor types.
| Variables | Patients, n | % | Log rank-value | Univariate
P-value |
|---|
| Sex |
|
|
|
|
|
Female | 53 | 98.1 |
|
|
|
Male | 1 | 1.9 | 2.203 | 0.138 |
| Borrmann's
classification |
|
|
|
|
| I | 3 | 5.6 |
|
|
| II | 2 | 3.7 |
|
|
|
III | 17 | 31.5 |
|
|
| IV | 8 | 14.8 |
|
|
|
Unknown | 24 | 44.4 | 3.067 | 0.381 |
| Tumor position |
|
|
|
|
| Gastric
corpus | 9 | 16.7 |
|
|
| Gastric
antrum | 19 | 35.2 |
|
|
| Gastric
fundus | 1 | 1.9 |
|
|
| Linitis
plastica | 4 | 7.4 |
|
|
|
Unknown | 21 | 38.9 | 7.746 | 0.052 |
| Tumor size |
|
|
|
|
| T1 | 3 | 5.6 |
|
|
| T2 | 3 | 5.6 |
|
|
| T3 | 4 | 7.4 |
|
|
| T4 | 14 | 25.9 |
|
|
|
Unknown | 30 | 55.6 | 8.342 | 0.039 |
| Lymph node
involvement |
|
|
|
|
|
Positive | 19 | 35.2 |
|
|
|
Negative | 3 | 5.6 |
|
|
|
Unknown | 32 | 59.3 | 4.474 | 0.034 |
| Coexisting
metastasis in other organs |
|
|
|
|
|
Positive | 24 | 44.4 |
|
|
|
Negative | 20 | 37.0 |
|
|
|
Unknown | 10 | 18.5 | 0.090 | 0.764 |
| Initial stage |
|
|
|
|
| I | 2 | 3.7 |
|
|
| II | 4 | 7.4 |
|
|
|
III | 6 | 11.1 |
|
|
| IV | 24 | 44.4 |
|
|
|
Unknown | 18 | 33.3 | 4.231 | 0.238 |
| Histology |
|
|
|
|
| AC | 1 | 1.9 |
|
|
|
SIG | 37 | 68.5 |
|
|
|
PDA | 12 | 22.2 |
|
|
|
MAC | 3 | 5.6 |
|
|
|
Unknown | 1 | 1.9 | 0.605 | 0.895 |
Tumor-Node-Metastasis staging was performed
according to the 2003 American Joint Committee on Cancer staging
system (68). Information on patient
T staging were provided in the previous studies that were analyzed.
Not all data provided was complete, hence not all patients had
tumor staging data. Tumor size was available in only 24 cases; of
these, 14 cases (25.9%) were T4 gastric cancer types, 4 cases
(7.4%) were T3, 3 cases (5.6%) were T1 and 3 cases (5.6%) were T2.
A statistically significant difference was identified between the
different T groups (P=0.039). Of the 22 patients with lymph node
involvement data, 19 patients presented with lymph node
involvement, with a statistically significant difference between
the lymph node involvement groups (P=0.034). In the 44 cases with
data available on whether there were coexisting metastasis in other
organs, 24 cases (44.4%) were positive. Of the 24 patients with
coexisting metastasis in other organs, 4 had bone metastases and 14
had ovarian metastases. The remaining metastases were cutaneous (1
case), orbital metastases (1 case), liver (1 case) and other organs
(3 cases) (data not shown). The most common additional metastases
were ovarian (25.9%; data not shown). A total of 36 cases provided
information on the initial TNM stage as follows: 2 cases (3.7%) of
stage I disease; 4 cases (7.4%) of stage II; 6 cases (11.1%) of
stage III and 24 cases (44.4%) of stage IV. Staging information was
unavailable for 18 patients owing to unknown tumor size, lymph node
status or both (Table I).
For pathological diagnoses, 37 cases (68.5%) were
identified with signet ring cell carcinoma (SIG), 12 cases (22.2%)
with poorly differentiated adenocarcinoma (PDA), 3 cases (5.6%)
with mucinous adenocarcinoma (MAC) and 1 case (1.9%) with
adenocarcinoma (AC) (Table I). The
immunohistochemistry images from one patient from Renji Hospital
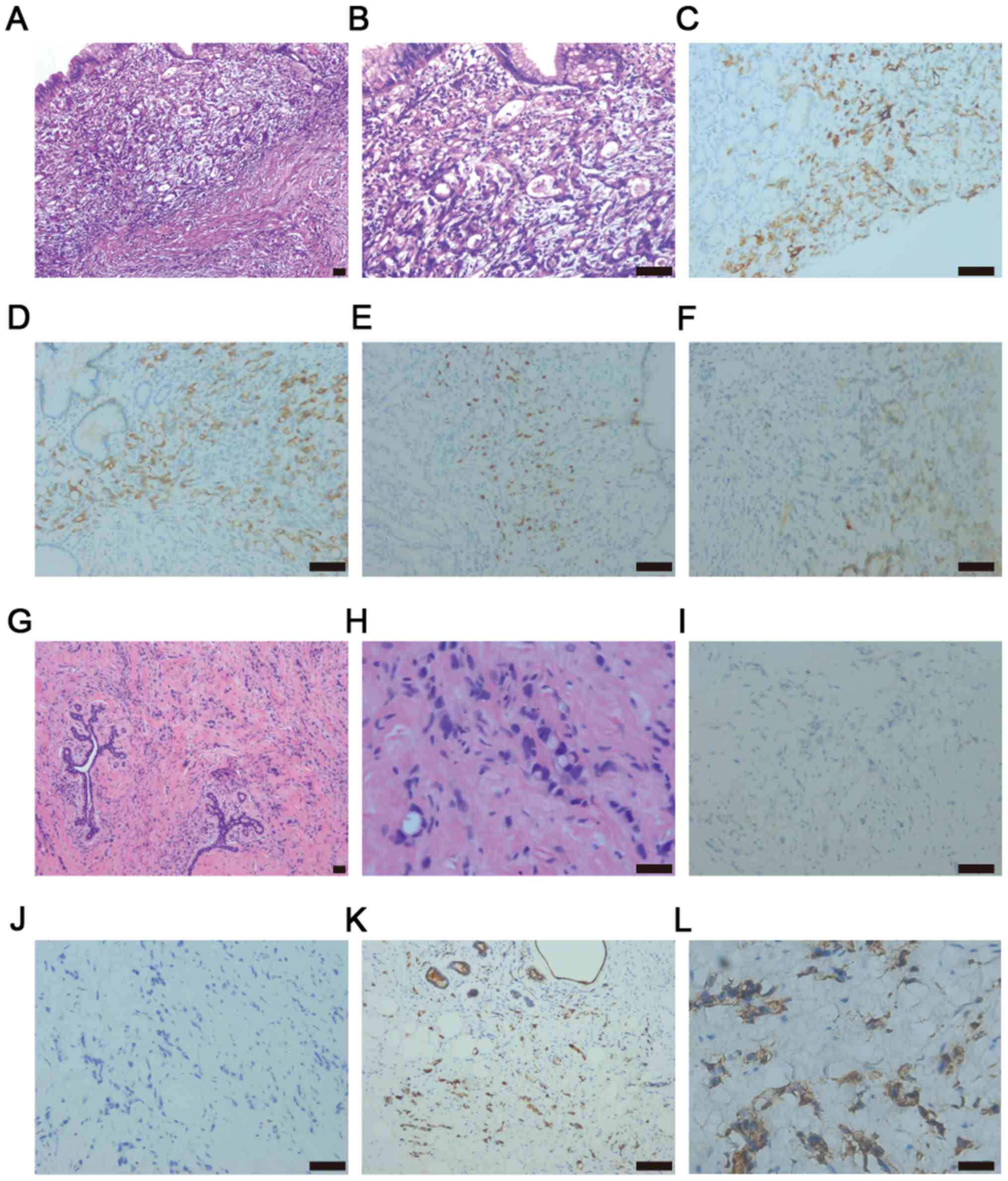
diagnosed with PDA and partial SIG are presented in Fig. 1A and B. This gastric cancer tumor
tissue was positive for CK7, CK20 and mucin 1, cell surface
associated-1 and was positive for Ki-67 (Fig. 1C-F).
Clinicopathological characteristics of
breast metastasis
Clinicopathological characteristics of the breast
metastases are presented in Table
II. These were the same aforementioned patients with gastric
cancer, but the clinical presentation data for breast lesions were
available only in 52 cases (96.3%). The median age of the patients
with breast metastasis diagnosis was 43 years (range, 22–72 years).
A significant difference was identified between the <45 and the
≥45 year old age groups (P=0.001). Upon physical examination,
palpable nodules and inflammatory changes in the breast were
identified in 40 cases (74.1%) and 12 cases (22.2%), respectively.
A total of 26 cases possessed lesions in the left breast (48.1%),
15 had lesions in the right breast (27.8%) and 13 were bilateral
(24.1%). Axillary lymph node involvement data were available in 44
cases (81.5%), 23 of which were positive for nodal involvement
(42.6%). Data from ultrasonic manifestation of breast metastasis
were available for 28 cases (51.9%), with nodules (19 cases; 35.2%)
being the most common manifestation (Table II). However, there was no significant
difference identified for any of these factors.
 | Table II.Clinicopathological characteristics
of patients with breast metastases. |
Table II.
Clinicopathological characteristics
of patients with breast metastases.
| Variables | Patients | % | Log rank-value | P-value |
|---|
| Sex |
|
|
|
|
|
Female | 53 | 98.1 |
|
|
|
Male | 1 | 1.9 | 2.203 | 0.138 |
| Age, years |
|
|
|
|
|
<45 | 29 | 53.7 |
|
|
|
≥45 | 25 | 46.3 | 10.867 | 0.001 |
|
Median | 43 |
|
|
|
| Clinical
presentation |
|
|
|
|
|
Nodule | 40 | 74.1 |
|
|
|
Inflammatory | 12 | 22.2 |
|
|
|
Unknown | 2 | 3.7 | 0.195 | 0.659 |
| Localization |
|
|
|
|
|
Bilateral | 13 | 24.1 |
|
|
|
Left | 26 | 48.1 |
|
|
|
Right | 15 | 27.8 | 4.367 | 0.113 |
| Axillary lymph node
involvement |
|
|
|
|
|
Positive | 23 | 42.6 |
|
|
|
Negative | 21 | 38.9 |
|
|
|
Unknown | 10 | 18.5 | 0.626 | 0.429 |
| Ultrasonography
manifestation |
|
|
|
|
|
Nodules | 19 | 35.2 |
|
|
| Skin
thickening | 4 | 7.4 |
|
|
|
Negative | 5 | 9.3 |
|
|
|
Unknown | 26 | 48.1 | 0.974 | 0.614 |
| Time between
occurrence of gastric cancer and breast metastasis |
|
|
|
|
|
Heterochronia | 30 | 55.6 |
|
|
|
Concomitant | 24 | 44.4 | 0.235 | 0.628 |
| Diagnostic
method |
|
|
|
|
| Needle
biopsy of breast | 32 | 59.3 |
|
|
| Surgery
of breast | 13 | 24.1 |
|
|
|
Unknown | 9 | 16.7 | 0.400 | 0.527 |
| Histology |
|
|
|
|
| AC | 6 | 11.1 |
|
|
|
SIG | 42 | 77.8 |
|
|
|
PDA | 4 | 7.4 |
|
|
|
MAC | 1 | 1.9 |
|
|
|
Unknown | 1 | 1.9 | 58.014 | <0.001 |
| Gastric
surgery |
|
|
|
|
|
Positive | 26 | 48.1 |
|
|
|
Negative | 19 | 35.2 |
|
|
|
Unknown | 9 | 16.7 | 0.003 | 0.959 |
| Chemotherapy |
|
|
|
|
|
Positive | 32 | 59.3 |
|
|
|
Negative | 6 | 11.1 |
|
|
|
Unknown | 16 | 29.6 | 1.117 | 0.290 |
The median interval between primary diagnosis and
metastatic presentation was 1.25 months (range, 0–72 months).
Breast metastasis and gastric cancer were diagnosed simultaneously
in 24 cases (44.4%). Needle biopsy was performed for breast
metastasis diagnosis in 32 cases (59.3%) and surgery was performed
in 13 cases (24.1%). For breast histological diagnosis, 42 cases
(77.8%) were identified as SIG, 6 cases (11.1%) as AC, 4 cases
(7.4%) as PDA and 1 case (1.9%) as MAC, with a significant
difference identified between these groups (P<0.001). (Table II) Notably, 10 cases differed in
their pathology between the primary gastric cancer and breast
metastasis.
Estrogen receptor (ER) expression data were
available in 28 cases, progesterone receptor (PR) in 25 cases,
human epidermal growth factor receptor-2 (Her-2) in 15 cases and
GCDFP15 in 11 cases; all cases presented negative results. The
result of one patient is presented in Fig. 1G-H. Three years later, this patient
with metastatic breast cancer was diagnosed with primary gastric
cancer and was positive for CEA and villin, and negative for
mammaglobin (Fig. 1I-L).
Information on surgical treatment was available for
45 cases, of which 26 (48.1%) received gastric surgery, whilst the
other 19 (35.2%) did not. A total of 32 cases received chemotherapy
(59.3%), 6 cases did not, and 16 cases provided incomplete data
(Table II).
Survival
Data were available for 54 patients. The median
survival time was 8.6 months (range, 0–48 months). Univariate
analysis of the association among OS time, clinicopathological
factors, primary gastric tumor and breast metastasis
characteristics was performed, and gastric tumor size, gastric
lymph node involvement, age at breast metastasis diagnosis and
breast histology were all significantly associated with OS time
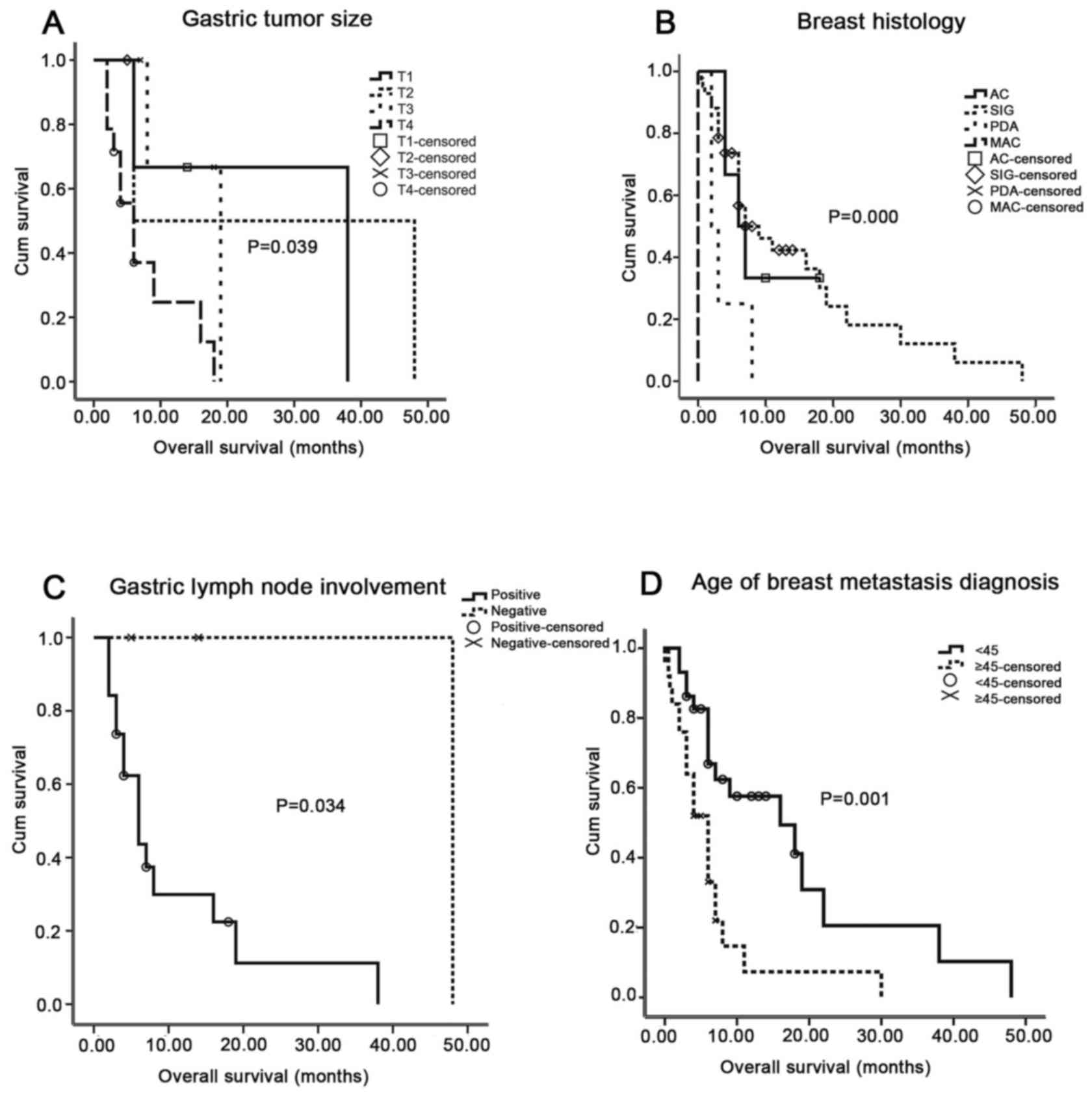
(P=0.039, 0.034, 0.001 and <0.001, respectively; Fig. 2). However, survival analysis revealed
that sex, Borrmann's classification, co-existing metastases in
other organs, initial stage, tumor position and primary gastric
cancer histology were not associated with OS time (P=0.138, 0.381,
0.764, 0.238, 0.052 and 0.895, respectively; Table I). At first, the association between
the number of breast metastases and the OS time was analyzed using
univariate analysis, and a significant association was identified
(P<0.05) (69). Clinical
presentation, localization of the breast metastasis, axillary lymph
node involvement, ultrasonography performance, diagnostic method
and time from occurrence of breast metastasis were also not
associated with OS time (P=0.659, 0.113, 0.429, 0.614, 0.527 and
0.628, respectively; Table II). In
addition, gastric surgery and chemotherapy were not associated with
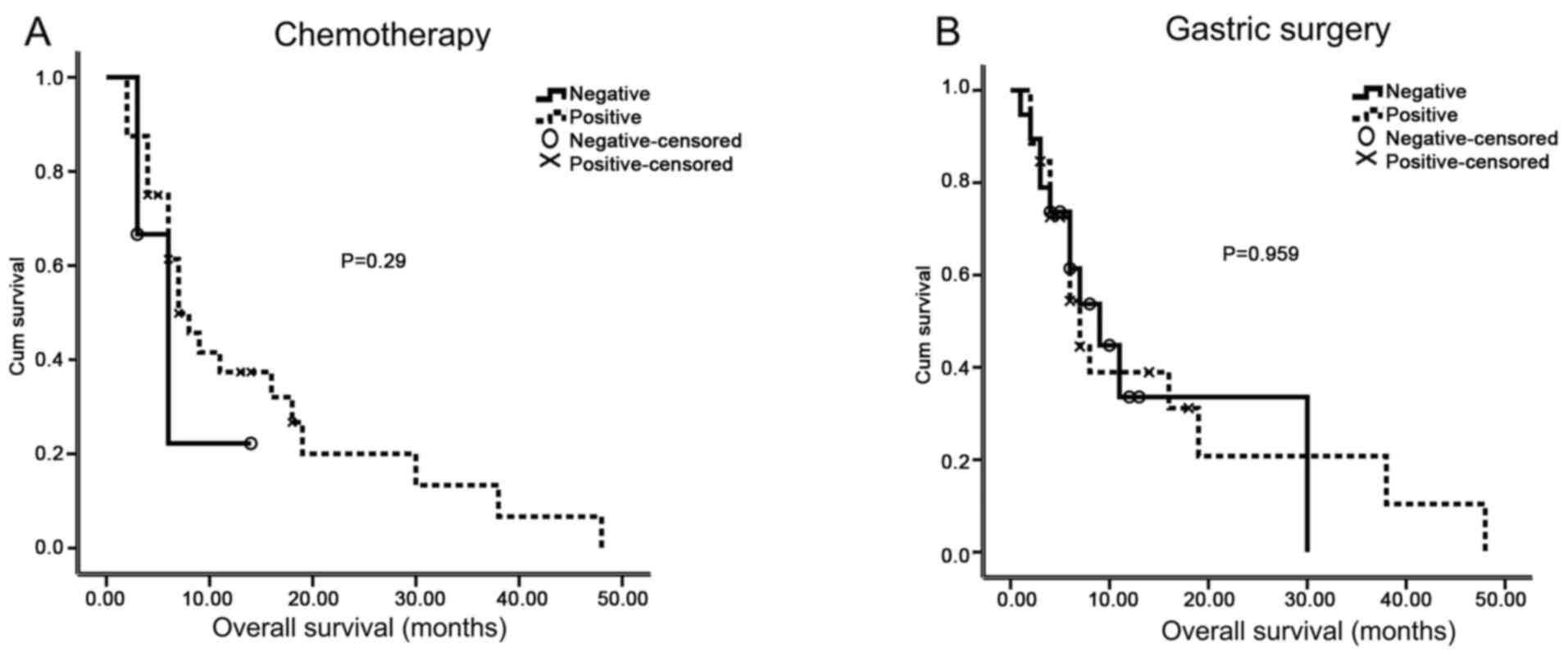
breast metastasis patient overall survival (P=0.959, 0.290,
respectively; Table II; Fig. 3). From further multivariate analysis,
the time to occurrence (P=0.017), age (P=0.009), histology
(P=0.045) and breast metastasis localization (P=0.043) were
significant independent OS time indicators (Table III).
 | Table III.Multivariate analysis of prognostic
factors for overall survival time in patients with breast
metastases. |
Table III.
Multivariate analysis of prognostic
factors for overall survival time in patients with breast
metastases.
| Variables | P-value | HR | 95% CI |
|---|
| Age at breast
metastasis diagnosis | 0.009 | 1.061 | 1.015–1.110 |
| Gastric
histology | 0.351 | 1.561 | 0.613–3.978 |
| Breast
histology | 0.045 | 3.662 | 1.029–13.036 |
| Clinical
presentation | 0.819 | 1.129 | 0.398–3.208 |
| Localization of
breast metastasis | 0.043 | 2.200 | 1.025–4.723 |
| Axillary lymph node
involvement | 0.617 | 0.779 | 0.293–2.071 |
| Time to occurrence
of the breast metastasis | 0.017 | 0.960 | 0.929–0.993 |
| Gastric
surgery | 0.051 | 3.283 | 0.996–10.823 |
Discussion
The estimated rate of occurrence of non-primary
breast malignancy reported in literature varies from 0.5–1.3% in
clinical observation to 1.7–6.6% in autopsy series (70,71). In
the present study, all but one of the patients were female, and
their median age was 43 years. In previous reports, the median age
at diagnosis of patients with gastric cancer with breast metastasis
was 46 years (9), and for patients
with primary breast cancer was 61 years in the USA and 56.99 years
in Japan (72,73). Breast metastases originating from
gastric carcinoma tend to occur at younger ages than primary breast
carcinomas (9). In the present study,
the age was defined as the age at the diagnosis of breast
metastasis. In univariate and multivariate analyses, patients aged
<45 years had longer survival times than those ≥45 years
(P=0.001 and P=0.009, respectively). Thus, an age at diagnosis of
breast metastasis of <45 years appears to be a positive
prognostic factor. Breast metastasis from gastric cancer is rare,
and the mechanism is not yet clear. The present study analyzed the
results of current clinical observations. In one previous study,
the majority of patients were at a higher stage (above AJCC stage
III) and Borrmann IV type (4). In the
present study, stage IV and Borrmann III types were the most
common. In univariate analysis, patients with T4 or those with
gastric lymph node involvement presented with the poorest
cumulative survival rates of all patients (P<0.05), as these
gastric cancer types are usually invasive. However, insufficient
data were available for gastric tumor size and lymph node
involvement; thus, these factors were not included in the
multivariate analysis.
Breast metastasis symptoms are unspecific in terms
of breast nodules, swelling, tenderness and pain compared with the
symptoms of primary breast cancer (10). In the present study, nodules (74.1%)
were a more common clinical symptom than inflammation (22.2%), and
axillary lymph node involvement was also common. Ultrasound results
including skin thickening and breast nodules, indistinguishable
from those of primary breast cancer, were unassociated with OS time
(P>0.05); thus, it suggests that it is difficult to diagnose
metastatic breast cancer by clinical presentation or diagnostic
imaging. Breast metastases were most common on the left side,
consistent with the results of a previous study (13). This laterality may indicate the
potential presence of another lymphatic pathway or preponderance to
the breast from other organs including the left supraclavicular
lymph node metastasis from gastric carcinoma. In multivariate
analysis, breast metastasis localization was a significant
prognostic factor of OS time (P=0.043).
Biopsy and surgery on the breast masses were
generally performed for diagnosis. Needle biopsies of the breast,
including core needle biopsy or fine needle aspiration, may
differentiate between primary and metastatic breast tumor types
(74,75). In the present study, a needle biopsy
was used for 59.3% of the patients, providing a quick and accurate
diagnosis, thus avoiding an unnecessary mastectomy.
Immunohistochemistry was the primary method for identifying the
tumor origin (32,34). Of all the patients in a previous
study, ~77.8% presented with breast SIG, and 68.5% of patients
presented with gastric SIG, which accounted for ~10% of the gastric
cases (11). In previous studies, SIG
of the stomach was often observed in younger women (aged <40
years), and SIG of the primary breast was rare (43,44,48,68).
Differing histology of breast metastases was associated with OS
time in univariate (P<0.001) and multivariate analyses (P=0.045)
in the present study. In one reported case, immunohistochemical
staining for breast metastasis from gastric cancer was negative for
ER, PR, erb-B2 receptor tyrosine kinase 2 and GCDFP15 but positive
for CEA, CK7 and CK20 (24). This
phenomenon was also observed in the present study. It was
identified that that breast tumors were metastatic from gastric
carcinoma using immunohistochemistry. However, chemokines or
chemokine receptors associated with breast metastases in gastric
carcinoma were not identified in the present study; this should be
investigated further.
In the metastatic process, mammary involvement may
either be the first step or there may be a polymetastatic context
(63). In the present study, 44.4% of
patients suffered from concurrent metastasis at the time of breast
metastasis, and 44.4% of patients presented with gastric cancer and
synchronous breast metastases. The median interval between
diagnosis of the primary disease and identification of the
metastatic lesion was only 1.25 months. This result indicated that
gastric carcinoma with breast metastasis progresses rapidly and
that the potential of metastasis from gastric carcinoma is high,
even in patients with no history of gastric cancer. The time to
occurrence of breast metastasis was a significant independent OS
time indicator in the multivariate analysis (P=0.017). In the
present study, the overall prognosis of patients with breast
metastasis from gastric carcinoma was generally poor; the median
survival time was only 8.6 months. For clinical treatment, careful
attention is required, particularly when a breast lesion is the
first manifestation of an unknown primary malignancy. Unexpectedly,
surgical intervention and chemotherapy were not associated with the
survival of patients with breast metastases in the present study.
However, previous advances in the development of anticancer agents,
including trastuzumab and apatinib, have improved the prognosis of
patients with unresectable advanced or recurrent gastric cancer
(1,24). Although there are no current clinical
case reports to confirm this, to the best of our knowledge, these
novel drugs may be effective in treating this rare disease.
A number of limitations should be noted regarding
the retrospective design and long time span of the present study.
Furthermore, with a few exceptions, certain information concerning
the primary tumor and prognosis was unavailable, despite this being
a large study concerning this disease. Nevertheless, the present
study may shed light on the different factors that contribute to
the improved survival rates of patients with this disease and
provide impetus for future research on gastric cancer with breast
metastasis.
Acknowledgements
The authors would like to thank the following
investigators for supporting this article by providing information
on their studies: Dr Fengchun Zhang, Department of Oncology, Suzhou
Kowloon Hospital, Shanghai Jiaotong University School of Medicine
(Suzhou, China); Dr Qiang Liu, Department of Pathology, Renji
Hospital, School of Medicine, Shanghai Jiaotong University.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301858) and the
Suzhou Science and Technology Project (grant nos. SYS201404 and
SYS201508).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
All authors read and approved the final manuscript.
YX, YM and WL participated in selecting cases and the writing of
the manuscript. HW designed and supervised the project. YX and JL
collected the clinical details of the patients and carried out
statistical analysis.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Renji Hospital Affiliated to Shanghai Jiaotong
University and written informed consent was obtained from all
patients.
Patient consent for publication
All patients signed written informed consent for the
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee SK, Kim WW, Kim SH, Hur SM, Kim S,
Choi JH, Cho EY, Han SY, Hahn BK, Choe JH, et al: Characteristics
of metastasis in the breast from extramammary malignancies. J Surg
Oncol. 101:137–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Domanski HA and Mas-Morillas A: Breast
metastases from pancreatic and ovarian carcinoma. Diagn Cytopathol.
21:154–155. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta S, Gupta MK, Gupta R and Mishra RS:
Breast metastasis of cervical carcinoma diagnosed by fine needle
aspiration cytology. A case report. Acta Cytol. 42:959–962. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heggarty P, McCusker G and Clements WD:
Bilateral breast metastases from a renal carcinoma. Int J Clin
Pract. 52:443–444. 1998.PubMed/NCBI
|
|
6
|
Kayikçioğlu F, Boran N, Ayhan A and Güler
N: Inflammatory breast metastases of ovarian cancer: A case report.
Gynecol Oncol. 83:613–616. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adamovich TL and Simmons RM: Ductal
carcinoma in situ with microinvasion. Am J Surg. 186:112–116. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iesato A, Oba T, Ono M, Hanamura T,
Watanabe T, Ito T, Kanai T, Maeno K, Ishizaka K, Kitabatake H, et
al: Breast metastases of gastric signet-ring cell carcinoma: A
report of two cases and review of the literature. Onco Targets
Therapy. 8:91–97. 2015.
|
|
10
|
Kwak JY, Kim EK and Oh KK: Radiologic
findings of metastatic signet ring cell carcinoma to the breast
from stomach. Yonsei Med J. 41:669–672. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Otsuji E, Yamaguchi T, Sawai K and
Takahashi T: Characterization of signet ring cell carcinoma of the
stomach. J Surg Oncol. 67:216–220. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hasegawa S, Yoshikawa T, Yoshida T,
Osaragi T, Cho H, Tsuburaya A, Kobayashi O and Sairenji M: A case
of breast metastasis of gastric cancer. Gan to Kagaku Ryoho.
34:1115–1118. 2007.(In Japanese). PubMed/NCBI
|
|
13
|
Soler Parrell C, Palacios Marqués A, Saco
López L, De Las Heras Bermejo R and Martínez Pertusa S: Breast
metastatic localization of signet-ring cell gastric carcinoma. ISRN
Obstet Gynecol. 2011:4261502011.PubMed/NCBI
|
|
14
|
Christophorou MA, Ringshausen I, Finch AJ,
Swigart LB and Evan GI: The pathological response to DNA damage
does not contribute to p53-mediated tumour suppression. Nature.
443:214–217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsuoka S, Ballif BA, Smogorzewska A,
McDonald ER III, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini
N, Lerenthal Y, et al: ATM and ATR substrate analysis reveals
extensive protein networks responsive to DNA damage. Science.
316:1160–1166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan N, Koritzinsky M, Zhao H, Bindra R,
Glazer PM, Powell S, Belmaaza A, Wouters B and Bristow RG: Chronic
hypoxia decreases synthesis of homologous recombination proteins to
offset chemoresistance and radioresistance. Cancer Res. 68:605–614.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobhian B, Shao G, Lilli DR, Culhane AC,
Moreau LA, Xia B, Livingston DM and Greenberg RA: RAP80 targets
BRCA1 to specific ubiquitin structures at DNA damage sites.
Science. 316:1198–1202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akcay MN: Metastatic disease in the
breast. Breast. 11:526–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quinn JE, Kennedy RD, Mullan PB, Gilmore
PM, Carty M, Johnston PG and Harkin DP: BRCA1 functions as a
differential modulator of chemotherapy-induced apoptosis. Cancer
Res. 63:6221–6228. 2003.PubMed/NCBI
|
|
20
|
Wei J, Costa C, Ding Y, Zou Z, Yu L,
Sanchez JJ, Qian X, Chen H, Gimenez-Capitan A, Meng F, et al: mRNA
expression of BRCA1, PIAS1, and PIAS4 and survival after
second-line docetaxel in advanced gastric cancer. J Natl Cancer
Inst. 103:1552–1556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yamaguchi H, Kitayama J, Ishigami H, Emoto
S, Yamashita H and Watanabe T: A phase 2 trial of intravenous and
intraperitoneal paclitaxel combined with S-1 for treatment of
gastric cancer with macroscopic peritoneal metastasis. Cancer.
119:3354–3358. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng YF, Imano M, Itoh T, Satoh T, Chiba
Y, Imamoto H, Tsubaki M, Nishida S, Yasuda T and Furukawa H: A
phase II trial of perioperative chemotherapy involving a single
intraperitoneal administration of paclitaxel followed by sequential
S-1 plus intravenous paclitaxel for serosa-positive gastric cancer.
J Surg Oncol. 111:1041–1046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu CP and Tang SH: Gastric cancer with
breast metastasis in young patient: A case report. Guangdong Med J.
27602013.(In Chinese).
|
|
24
|
He CL, Chen P, Xia BL, Xiao Q and Cai FL:
Breast metastasis of gastric signet-ring cell carcinoma: A case
report and literature review. World J Surg Oncol. 13:1202015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Briest S, Horn LC, Haupt R, Schneider JP,
Schneider U and Höckel M: Metastasizing signet ring cell carcinoma
of the stomach-mimicking bilateral inflammatory breast cancer.
Gynecol Oncol. 74:491–494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luk YS, Ka SY, Lo SS, Chu CY and Ma MW: An
unusual case of gastric cancer presenting with breast metastasis
with pleomorphic microcalcifications. J Breast Cancer. 15:356–358.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Madan AK, Ternovits C, Huber SA, Pei LA
and Jaffe BM: Gastrointestinal metastasis to the breast. Surgery.
132:889–893. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sato T, Muto I, Fushiki M, Hasegawa M,
Hasegawa M, Sakai T and Sekiya M: Metastatic breast cancer from
gastric and ovarian cancer, mimicking inflammatory breast cancer:
Report of two cases. Breast Cancer. 15:315–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin R and Ma XG: A case report of
metastatic signet ring cell carcinoma to the breast from stomach.
Acta Acad Med Militaris Tertiae. 2170. 2005.(In Chinese).
|
|
30
|
Ren XQ: Gastric cancer with bilateral
orbital, double breasts and multiple bone metastasis: A case
report. Chin Commun Doctors. 13:2962011.
|
|
31
|
Lin RY, Yang YM and Chen HY: 1 case of
bilateral breast and axillary lymph node metastasis after gastric
cancer surgery. Canc Res Prev Treat. 4:2181990.(In Chinese).
|
|
32
|
Weng HY, Zhang B, Lin YL and Hong M:
Bilateral breast metastasis of gastric cancer: A case report.
Hainan Med J. 22:1322011.
|
|
33
|
Qi H, Yu XT and Kong FH: Gastric
adenocarcinoma with mammary glands metastasis: A case report. J
China Tradit Chin Med Inform. 04:398–399. 2012.
|
|
34
|
Tang F, Jiang GY and Wang NX: Gastric
cancer with breast metastasis: A case report and literature review.
Chin J Breast Dis (electronic version). 6:707–712. 2012.(In
Chinese).
|
|
35
|
Hong YZ and Gao XY: Gastric cancer with
breast metastasis: A case report. Chin J Gen Surg. 22:1272013.
|
|
36
|
Wang ZC, Wu B and Li J: Metastatic gastric
cancer to the breast: A case report. Chin J Gen Surg.
20:9392011.
|
|
37
|
Wang M, Zhao X and Wu WX: Gastric
carcinoma with breast metastasis: A case report. Chin J Clin Oncol.
52007.
|
|
38
|
Ye CM, Xue MX, Chen B, Huang ZM and Zeng
FH: A gastric cancer patient with breast metastasis. Chin J Gen
Surg. 5692007.
|
|
39
|
Zhu JD and Fang JX: Breast metastasis from
gastric cancer: A case report. Chin J Prac Surg. 1131994.
|
|
40
|
Li DF, Liang XL, Qi QH and Jiang QM:
Gastric cancer with breast recurrence: A case report. J Qilu Oncol.
3742001.
|
|
41
|
Wei XH: Gastric cancer with breast
metastasis: A rare case report. Inner Mongolia Med J. 4362004.
|
|
42
|
Yang LF: A case of breast metastasis after
4 years of gastric cancer surgery. J Clin Surg. 172011.(In
Chinese).
|
|
43
|
Yang J, Li A and Zhao XH: A case report of
poorly differentiated adenocarcinoma of stomach with male breast
metastasis. J Rare Uncommon Dis. 50–51. 2001.
|
|
44
|
Shi L, Zhao W, Chen P, Wang H, Fang X and
He CL: Metastatic tumor to breast from primary gastric carcinoma: A
case report. Chin J Gen Surg. 3572016.
|
|
45
|
Zheng YJ, Deng YC, Hua C, Liu YB and Li
ZD: A case of breast metastasis after gastric cancer surgery. Chin
J Pract Surg. 512005.
|
|
46
|
Tang Y, Chen W and Wang Y:
Ultrasonographic manifestation of breast cancer metastasis of
gastric cancer: A case report. Chin J Ultrasound Med. 3432009.(In
Chinese).
|
|
47
|
Schmutzer KJ, Zaki AE and Regan JF:
Gastric carcinoma in a 22-year-old Negro woman with metastases to
ovaries and breast. A case report. J Natl Med Assoc. 65:426–428
passim. 1973.PubMed/NCBI
|
|
48
|
Boutis AL, Andreadis C, Patakiouta F and
Mouratidou D: Gastric signet-ring adenocarcinoma presenting with
breast metastasis. World J Gastroenterol. 12:2958–2961. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sandison AT: Metastatic tumours in the
breast. Br J Surg. 47:54–58. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nance FC, MacVaugh H III and Fitts WT Jr:
Metastatic tumor to the breast simulating bilateral primary
inflammatory carcinoma. Am J Surg. 112:932–935. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Togo S and Kasumi F: A case of gastric
cancer metastatic to the breast. Gan no rinsho. 386–393. 1980.(In
Japanese).
|
|
52
|
Nielsen M, Andersen JA, Henriksen FW,
Kristensen PB, Lorentzen M, Ravn V, Schiødt T, Thorborg JV and
Ornvold K: Metastases to the breast from extramammary carcinomas.
Acta Pathol Microbiol Scand A. 89:251–256. 1981.PubMed/NCBI
|
|
53
|
Kasuga Y, Senga O, Hanamura N, Iida F,
Oota H, Katsuyama T and Tsuchiya S: A case of bilateral metastatic
breast carcinoma from gastric carcinoma. Gan No Rinsho Jpn J Cancer
Clin. 32:407–412. 1986.(In Japanese).
|
|
54
|
Tachibana S, Asano M, Miya K, Hino T,
Furuichi N and Misawa K: A case report of gastric cancer with
breast metastasis. J Clin Surg. 41:1719–1722. 1986.
|
|
55
|
Alexander HR, Turnbull AD and Rosen PP:
Isolated breast metastases from gastrointestinal carcinomas: Report
of two cases. J Surg Oncol. 42:264–266. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hamby LS, McGrath PC, Cibull ML and
Schwartz RW: Gastric carcinoma metastatic to the breast. J Surg
Oncol. 48:117–121. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mishina Y, Ohtsuka K, Yamamura H, Kanai T,
Kitagawa Y, Kawai M and Koyama Y: A case or metastasis of gastric
cancer to the mammary gland. Nihon Rinsho Geka Gakkai Zasshi (J Jpn
Surg Assoc). 54:112–117. 1993.
|
|
58
|
Cavazzini G, Colpani F, Cantore M, Aitini
E, Rabbi C, Taffurelli M, Pari F, Bellomi A, Bertuzzi A and
Smerierl F: Breast metastasis from gastric signet ring cell
carcinoma, mimicking inflammatory carcinoma. A case report. Tumori.
79:450–453. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kudo H, Sakatani T, Shibata S, Ishiguro M,
Nishidoi H and Murakami S: A case of gastric cancer metastasized to
the breasts. J Jpn Surg Assoc. 60:1486–1489. 1999.(In Japanese).
View Article : Google Scholar
|
|
60
|
Di Cosimo S, Ferretti G, Fazio N, Mandalà
M, Curigliano G, Bosari S, Intra M and Latronico A: Goldhirsch A:
Breast and ovarian metastatic localization of signet-ring cell
gastric carcinoma. Ann Oncol. 14:803–804. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Qureshi SS, Shrikhande SV, Tanuja S and
Shukla PJ: Breast metastases of gastric signet ring cell carcinoma:
A differential diagnosis with primary breast signet ring cell
carcinoma. J Postgrad Med. 51:125–127. 2005.PubMed/NCBI
|
|
62
|
Isobe T, Yokoyama G, Fujii T, Kawamura D,
Nakagawa S and Shirouzu K: A case of breast metastasis of gastric
cancer progressed rapidly. Nihon Rinsho Geka Gakkai Zasshi (J Jpn
Surg Assoc). 67:2800–2804. 2006. View Article : Google Scholar
|
|
63
|
Makni Krichen S, Abbes K, Khanfir A,
Frikha M and Boudawara Sellami T: Metastatic signet ring cell
carcinoma to the breast from stomach. Cancer Radiotherapie: J De La
Soc Francaise De Radiother Oncol. 11:276–279. 2007.(In French).
|
|
64
|
Gugić D, Josipa F, Šambić P, Mrčela M and
Romić S: Breast metastases from gastric carcinoma-a case report.
Libri Oncologici. 35:59–62. 2007.
|
|
65
|
Timuçin Ç, Abdullah A, Semir P and
Abdurrahman I: Gastric ring cell carcinoma metastasis to the
breast: Two case reports. Turk J Cancer. 39:62–65. 2009.
|
|
66
|
Avgerinou G, Flessas I, Hatziolou E,
Zografos G, Nitsios I, Zagouri F, Katsambas A and Kanitakis J:
Cutaneous metastasis of signet-ring gastric adenocarcinoma to the
breast with unusual clinicopathological features. Anticancer Res.
31:2373–2378. 2011.PubMed/NCBI
|
|
67
|
Kajitani T: The general rules for the
gastric cancer study in surgery and pathology. Part I. Clinical
classification. Jpn J Surg. 11:127–139. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Marano L, Boccardi V, Braccio B, Esposito
G, Grassia M, Petrillo M, Pezzella M, Porfidia R, Reda G, Romano A,
et al: Comparison of the 6 and 7th editions of the AJCC/UICC TNM
staging system for gastric cancer focusing on the ‘N’
parameter-related survival: The monoinstitutional NodUs Italian
study. World J Surg Oncol. 13:2152015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Diab SG, Elledge RM and Clark GM: Tumor
characteristics and clinical outcome of elderly women with breast
cancer. J Natl Cancer Inst. 92:550–556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bohman LG, Bassett LW, Gold RH and Voet R:
Breast metastases from extramammary malignancies. Radiology.
144:309–312. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hajdu SI and Urban JA: Cancers metastatic
to the breast. Cancer. 29:1691–1696. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Verdial FC, Etzioni R, Duggan C and
Anderson BO: Demographic changes in breast cancer incidence, stage
at diagnosis and age associated with population-based mammographic
screening. J Surg Oncol. 115:517–522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kinoshita T, Fukui N, Anan K, Iwamoto T,
Niikura N, Kawai M, Hayashi N, Tsugawa K, Aogi K, Ishida T, et al:
Comprehensive prognostic report of the Japanese Breast Cancer
Society Registry in 2004. Breast Cancer. 23:39–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kobayashi G and Cobb C: A case of
amelanotic spindle-cell melanoma presenting as metastases to breast
and axillary lymph node: Diagnosis by FNA cytology. Diagn
Cytopathol. 22:246–249. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Deshpande AH, Munshi MM, Lele VR and
Bobhate SK: Aspiration cytology of extramammary tumors metastatic
to the breast. Diagn Cytopathol. 21:319–323. 1999. View Article : Google Scholar : PubMed/NCBI
|