Introduction
Lung cancer is a genetic disease that is mainly
caused by the activation of oncogenes (1), which can trigger abnormal cell
proliferation and apoptosis, as well as the development of drug
resistance in patients during treatment, leading to high mortality
(2). Treatment outcomes of
radiotherapy and chemotherapy are usually poor and adverse side
effects are obvious. Therefore, molecular targeted therapy has
attracted increased attention.
Janus protein tyrosine kinase (JAK) plays an
important role in cell signaling (3).
Activation of JAK can induce the phosphorylation of signal
transducer and activator of transcription (STAT) to form JAK-STAT
dimer, and the dimer will enter into the nucleus to induce
expression of target genes, and this pathway is called the JAK-STAT
signal pathway (4). STAT3 in the STAT
family is involved in cell proliferation, differentiation,
apoptosis, invasion and other cell activities (5). Activated STAT3 can activate some
anti-apoptotic genes to prolong the cell cycle (6). Therefore, abnormalities of JAK-STAT
pathway were often accompanied by the occurrence of tumors
(7). Studies have shown that STAT3 in
lung cancer is highly expressed (8).
Crizotinib is an ATP mimetic and can act on multiple targets in the
treatment of ALK or ROS1-positive lung cancer (9). The possible mechanism is related with
the inhibition of ALK or c-MET phosphorylation, which in turn leads
to the inhibition of cell proliferation (10). However, detailed mechanism is still
unclear. Therefore, in this study, crizotinib was used to treat
H2228 non-small cell lung adenocarcinoma cells and its effect was
observed on cell apoptosis and expression of JAK and STAT protein,
so as to explore the effects of crizotinib on lung cancer and the
role of JAK-STAT signaling pathways.
Materials and methods
Main materials
Crizotinib powder was from Selleckchem Biotech, Inc.
(Houston, TX, USA). 3–2,5-Diphenyl-2-H-tetrazolium bromide (MTT)
was purchased from Shanghai Solarbio Science & Technology Co.,
Ltd. (Shanghai, China). RPMI-1640 medium and PBS were from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). RIPA lysate was
purchased from Beyotime Institute of Biotechnology (Haimen, China).
Cell apoptosis kit was from Solarbio. Transwell chamber was from
Shanghai Yu Bo Biotechnology Co., Ltd. (Shanghai, China). Human
non-small cell lung H2228 cell line was from Shanghai Cell Bank of
Chinese Academy of Sciences (Shanghai, China). Gel imaging system
was from Thermo Fisher Scientific, Inc., (Waltham, MA, USA) and
microplate reader was provided by Bio-Rad Laboratories, Inc.,
(Hercules, CA, USA). The study was approved by the Ethics Committee
of Renji Hospital Shanghai Jiaotong University School of Medicine
(Shanghai, China). Patients who participated in this research,
signed the informed consent and had complete clinical data.
Cell culture
H2228 lung cancer cells containing the EML4-ALK
fusion gene were cultured in RPMI-1640 medium containing 10% fetal
bovine serum at 37°C under 5% CO2. The medium was
changed twice a week, and cells are harvested at logarithmic growth
phase for subsequent experiments.
MTT assay to detect cell inhibition
rate
Cells were collected at logarithmic growth phase and
inoculated into 96-well plates at a density of 1×107/l,
200 µl for each well. Cells were cultured at 37°C and 5%
CO2 for 2 days in incubator. After cell adhesion, 0
(control group), 20, 40, 80, 160 and 320 nmol/l (treatment groups)
crizotinib was added to treat the cells. After incubation for 3
days at 37°C and 5% CO2, liquid was discarded and 8 µl
MTT (5 g/l in PBS) was added to each well. After cell culture for
another 4 h, cell culture was terminated. After centrifugation at
1,800 × g for 5 min at 4°C, culture supernatant was discarded, and
150 µl DMSO was added to each well. The absorbance (A) of each well
was detected by microplate reader, and the cell inhibition rate and
IC50 value were calculated. Inhibition rate (%) = (1 - A
value of treatment group/A value of control group) × 100%.
Flow cytometry
Cells were harvested at logarithmic growth phase,
and cell density was adjusted to 5×105/ml. Then 1 ml of
cells was added into each well of 6-well plates. After cell
adhesion, medium containing 300 nmol/l crizotinib was used to
replace the original medium. After cell culture for 1, 2 and 3
days, cells were collected and stained, followed by flow cytometry
(FACSCalibur; BD Biosciences, Detroit, MI, USA), to measure cell
apoptosis rate. This experiment was performed three times.
Transwell assay
Transwell chamber was placed in a 24-well culture
plate, and a small amount serum-free medium was added into the
upper chamber, followed by addition of Matrigel. The upper chamber
was added with 400 µl of cell suspension (5×104
cells/l), while the lower chamber was filled with 500 µl cell
culture medium containing 10% fetal bovine serum. Cells were
cultured under normal conditions, and 3 replicates were set for
each experiment. After cell culture 24 h, Transwell chambers were
collected and fixed with formaldehyde, followed by staining with
0.1% crystal violet. Ten visual fields were randomly selected under
a microscope to calculate the average number of cells that
penetrated the membrane.
Western blot analysis to detect the
expression of JAK and STAT proteins
RIPA solution was used to extract total protein from
cells according to the instructions. Protein concentration was
determined by BCA method. After blocking with 5% skimmed milk for 1
h, membranes were incubated with primary antibodies overnight at
4°C. After washing with TBST for 5 min ×3 times, membranes were
incubated with secondary antibodies at room temperature for 1 h.
Primary rabbit polyclonal JAK antibody (1:500; cat. no. ab47435),
mouse monoclonal STAT antibody (1:500; cat. no. AMAB90777), rabbit
polyclonal GAPDH antibody (1:500; cat. no. ab37168) and secondary
goat anti-rabbit (HRP) IgG antibody (1:2,000; cat. no. ab6721) were
all purchased from Abcam (Cambridge, MA, USA). Chemiluminescence
method was used to detect the signal, and signal was scanned by a
gel imager (Thermo Fisher Scientific, Inc.).
Statistical analysis
Statistical analysis was performed by using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Measurement data were
expressed as mean ± SD. Comparison between multiple groups was done
using One-way ANOVA test followed by post hoc test (Least
Significant Difference). t-test was used for comparison between two
groups, and repeated measurements variance analysis was used for
intragroup comparison. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of crizotinib on H2228 cell
proliferation
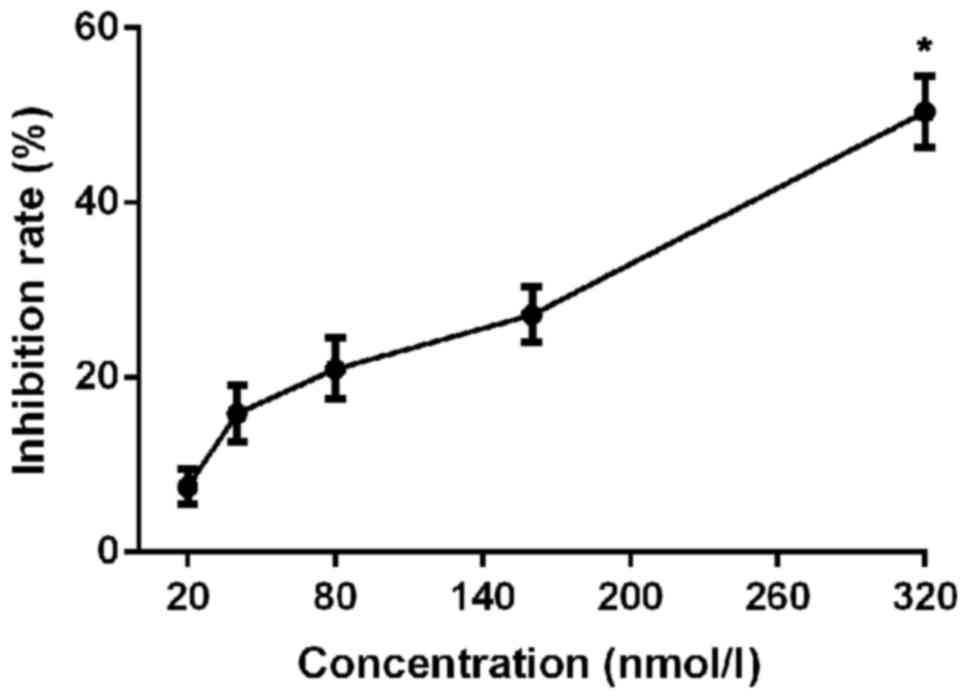
Cell proliferation was significantly inhibited after
crizotinib treatment for 3 days, and the inhibitory rate increased
with the increase of crizotinib concentration (p<0.01; Table I and Fig.
1). IC50 value was 311.26 nnol/l after
administration for 3 days. Crizotinib at a concentration of 300
nmol/l was used in follow-up experiments.
 | Table I.Inhibition rates of different
concentrations of crizotinib on H2228 cell proliferation (mean ±
SD). |
Table I.
Inhibition rates of different
concentrations of crizotinib on H2228 cell proliferation (mean ±
SD).
| Concentrations
(nmol/l) | Inhibition rate
(%) |
|---|
| 0 | 0 |
| 20 | 7.48±2.01 |
| 40 | 15.85±3.25 |
| 80 | 20.98±3.51 |
| 160 | 27.18±3.23 |
| 320 | 50.43±4.12 |
| F-value | 72.91 |
| P-value | <0.01 |
Effects of crizotinib on H2228 cell
apoptosis
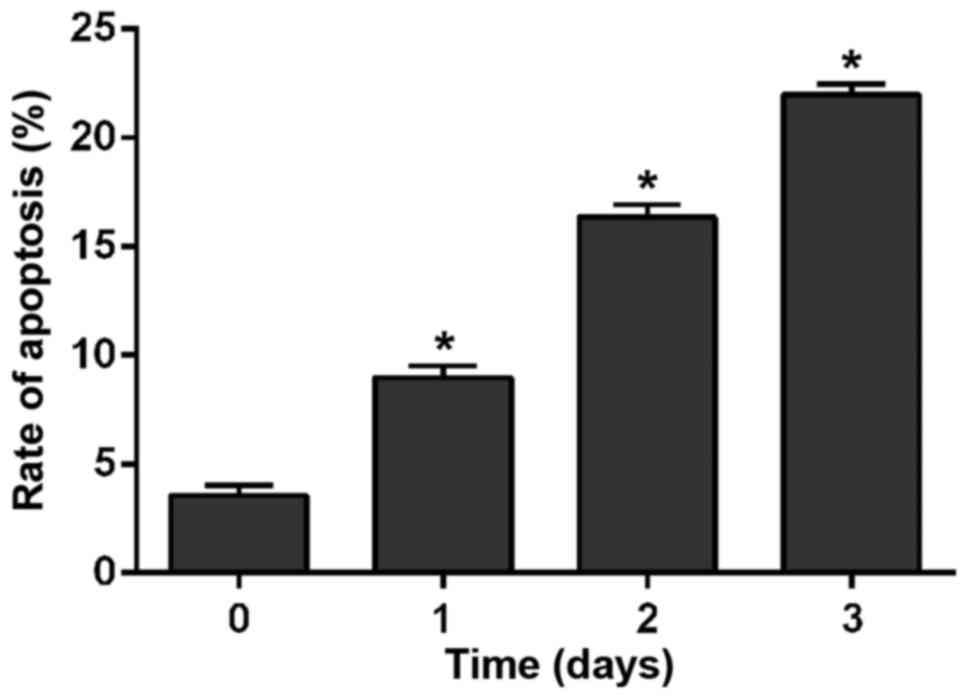
H2228 cells were cultured in medium containing 300
nmol/l crizotinib. After cell culture for 1, 2 and 3 days, cells
were detected by flow cytometry. As shown in Fig. 2, crizotinib obviously promoted cell
apoptosis (p<0.01). With the prolongation of time, apoptotic
rate of cells treated with crizotinib was significantly increased.
The apoptotic rate at the 1st day was obviously higher than that at
0 day (F=243.251, P<0.01); apoptotic rate at the 2nd day was
obviously higher than that at the 1st day (F=279.783, P<0.01)
and apoptotic rate at the 3rd day was obviously higher than that at
2nd day (F=167.524, P<0.01).
Effects of crizotinib on H2228 cell
migration
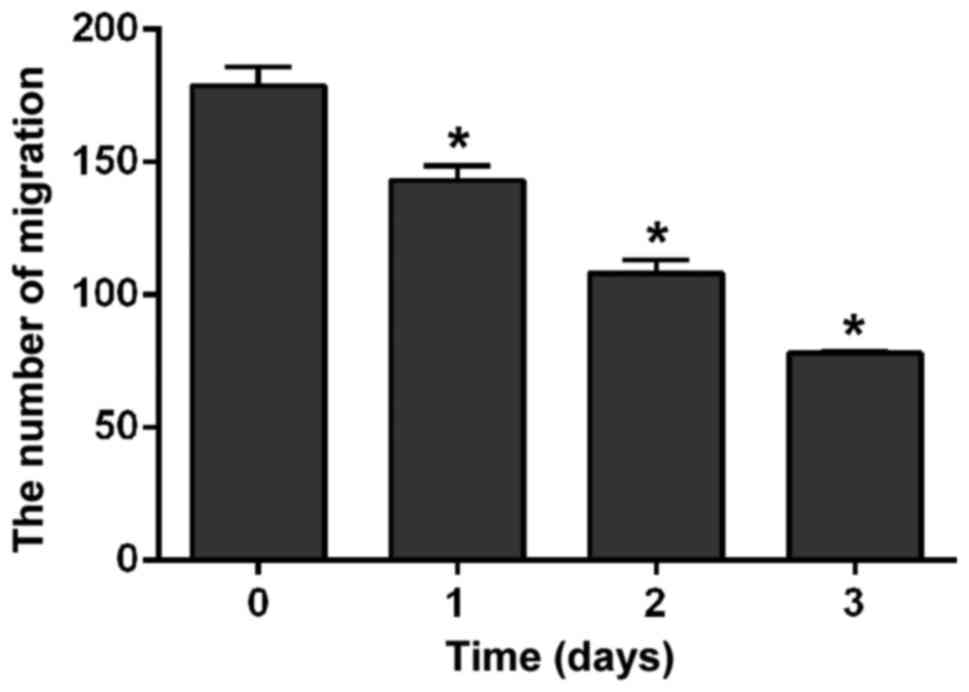
H2228 cells were cultured in medium containing 300
nmol/l crizotinib. Results of Transwell assay showed that the
number of cells that infiltrated the membrane was significantly
reduced with the prolonged treatment (p<0.01). The number of
cells at the 1st day was obviously lower than that at 0 day
(F=48.216, p=0.002); the number of cells at the 2nd day was
obviously lower than that at the 1st day (F=68.398, p=0.001) and
the number of cells at the 3rd day was obviously lower than that at
the 2nd day (F=53.912, p=0.02) (Fig.
3).
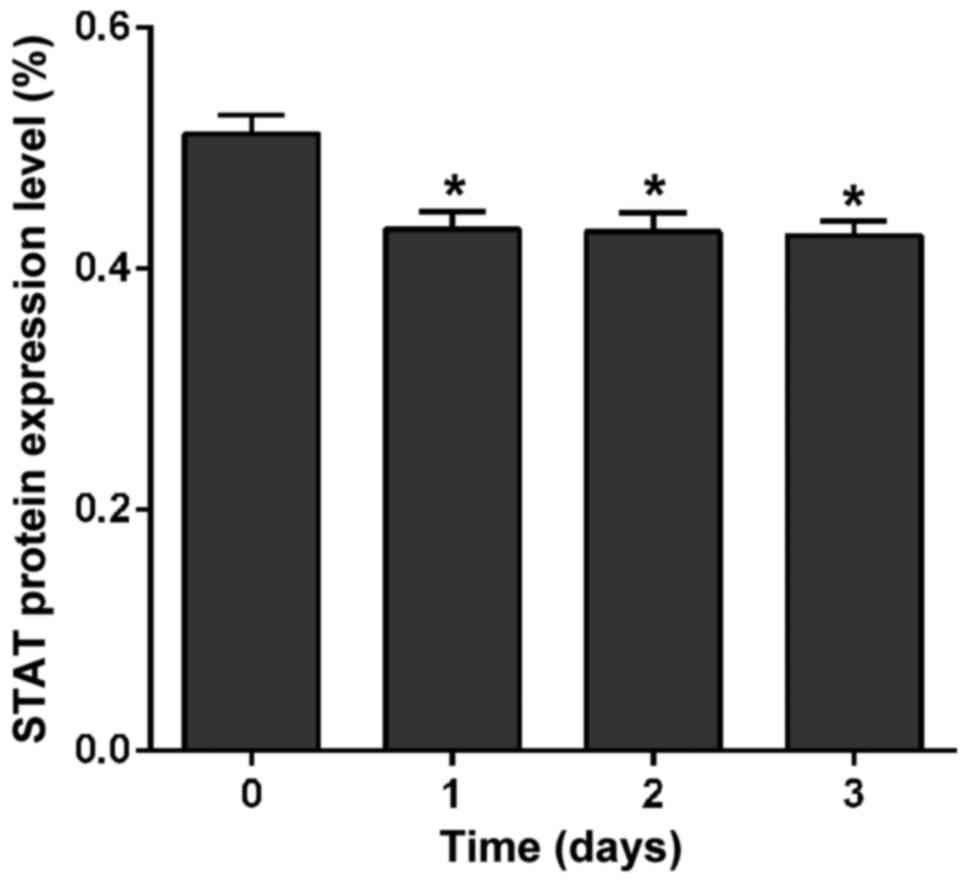
Effects of crizotinib on expression of
JAK and STAT proteins
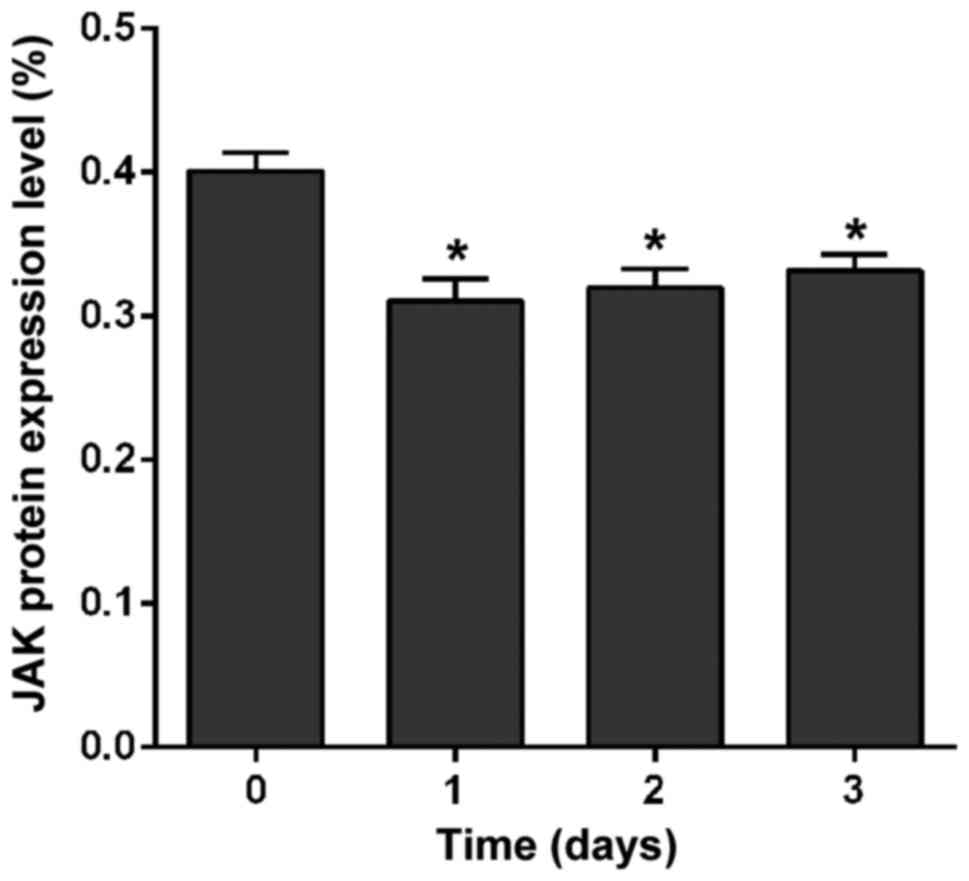
Effect of crizotinib on the expression of JAK and
STAT was detected by western blot analysis. Results showed that
crizotinib significantly downregulated the expression of JAK
protein after treatment for 1 day (p<0.01), but there was no
significant difference at the 1st, 2nd, or 3rd day in the level of
JAK protein (p=0.47) (Fig. 4).
Crizotinib also significantly downregulated the expression of STAT
protein after treatment for 1 day (p<0.01), but no significant
difference was found either at the 1st, 2nd, or 3rd day in the
level of STAT protein (p=0.91) (Fig.
5).
Discussion
Apoptosis is an essential process in normal life,
and abnormal apoptosis may trigger the occurrence of tumors
(11). Inducing tumor cell apoptosis
can effectively treat tumors (12).
This study found that crizotinib can promote apoptosis and inhibit
cell proliferation of cells of non-small cell lung adenocarcinoma
cell line H2228, which is consistent with the findings reported by
Anh et al (13), indicating
the application value of crizotinib in the treatment of lung
adenocarcinoma. Cell migration is cause of tumor metastasis and
deaths of patients (14). In this
study, results of Transwell assay showed that crizotinib can
inhibit the migration of cancer cells in lung adenocarcinoma, which
may be helpful for the treatment of lung cancer.
JAK-STAT signaling pathway is involved in cell
growth, differentiation, immune and other functions, and abnormal
JAK-STAT signaling can easily lead to the occurrence of tumors
(15). Abnormal activation of JAK2
causes aberrant phosphorylation of STAT5, resulting in
myeloproliferative tumors (16).
Abnormal JAK2-STAT3 signaling and leukemia (17), and lymphoma (18) are closely related. Abnormal activation
of STAT3 is also prevalent in lung cancer cells, and sustained high
expression of STAT3 appears in early stage of non-small cell lung
adenocarcinoma (19), and sustained
activation of JAK-STAT3 signaling pathway can enhance tumor cell
invasion and migration (20). In this
study, results of western blot analysis showed that crizotinib
significantly inhibited the expression of JAK and STAT proteins,
indicating that crizotinib can promote apoptosis and inhibit the
proliferation and migration of lung cancer cells. It can be seen
that crizotinib might promote apoptosis and inhibit the
proliferation and migration of lung cancer cells by inhibiting the
expression level of JAK and STAT and inhibiting the activation of
JAK-STAT signaling pathway, thus achieving the therapeutic effect
of the treatment of lung cancer. This conclusion is consistent with
the above theory. In addition, this study found that although
crizotinib inhibited the expression of JAK and STAT, no increased
inhibitory effects were observed after prolonged crizotinib
treatment, indicating that long-term administration may not improve
the condition further, possibly due to the development of drug
resistance, which is a common problem in the treatment of lung
cancer (21). This study only
provides a theoretical basis for crizotinib in the treatment of
lung cancer by regulating the JAK-STAT pathway. The resistance of
crizotinib to lung cancer still needs to be further studied.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL wrote the manuscript and helped with cell
culture. SW and HC performed MTT assay. YH and GQ contributed to
flow cytometry. LL and YL were responsible for Transwell assay and
western blot analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Renji Hospital Shanghai Jiaotong University School of Medicine
(Shanghai, China). Patients who participated in this research,
signed the informed consent and had complete clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Watza D, Cote ML and Schwartz AG: Lung
Cancer: geneticseLS. John Wiley & Sons, Ltd.; Chichester: 2017,
View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wakeford R and Tawn EJ: Paternal
irradiation and leukemia in offspring. Radiat Res. 154:222–223.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pappa E, Nikitakis N, Vlachodimitropoulos
D, Avgoustidis D, Oktseloglou V and Papadogeorgakis N:
Phosphorylated signal transducer and activator of transcription-1
immunohistochemical expression is associated with improved survival
in patients with oral squamous cell carcinoma. J Oral Maxillofac
Surg. 72:211–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leeman RJ, Lui VW and Grandis JR: STAT3 as
a therapeutic target in head and neck cancer. Expert Opin Biol
Ther. 6:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Proietti C, Salatino M, Rosemblit C,
Carnevale R, Pecci A, Kornblihtt AR, Molinolo AA, Frahm I, Charreau
EH, Schillaci R, et al: Progestins induce transcriptional
activation of signal transducer and activator of transcription 3
(Stat3) via a Jak- and Src-dependent mechanism in breast cancer
cells. Mol Cell Biol. 25:4826–4840. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gonçalves J and Silva AP: Methamphetamine
and the JAK/STAT pathwayNeuropathology of Drug Addictions and
Substance Misuse. Preedy VR: 2. 1st edition. Academic Press;
Cambridge, MA: pp. 147–154. 2016, View Article : Google Scholar
|
|
8
|
Schütz A, Röser K, Klitzsch J, Lieder F,
Aberger F, Gruber W, Mueller KM, Pupyshev A, Moriggl R and
Friedrich K: Lung adenocarcinomas and lung cancer cell lines show
association of MMP-1 expression with STAT3 activation. Trans Oncol.
8:97–105. 2015. View Article : Google Scholar
|
|
9
|
de la Bellacasa Puig R, Karachaliou N,
Estrada-Tejedor R, Teixidó J, Costa C and Borrell JI: ALK and ROS1
as a joint target for the treatment of lung cancer: A review.
Transl Lung Cancer Res. 2:72–86. 2013.PubMed/NCBI
|
|
10
|
Xu W, Kim JW, Jung WJ, Koh Y and Yoon SS:
Crizotinib in combination with everolimus synergistically inhibits
proliferation of ALK-positive anaplastic large cell lymphoma.
Cancer Res Treat. Jun 19–2017.(Epub ahead of print). https://doi.org/10.4143/crt.2016.357
|
|
11
|
Adlakha YK and Saini N: MicroRNA: A
connecting road between apoptosis and cholesterol metabolism.
Tumour Biol. 37:8529–8554. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han Y, Park S, Kinyua AW, Andera L, Kim KW
and Kim I: Emetine enhances the tumor necrosis factor-related
apoptosis-inducing ligand-induced apoptosis of pancreatic cancer
cells by downregulation of myeloid cell leukemia sequence-1
protein. Oncol Rep. 31:456–462. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahn HK, Jeon K, Yoo H, Han B, Lee SJ, Park
H, Lee MJ, Ha SY, Han JH, Sun JM, et al: Successful treatment with
crizotinib in mechanically ventilated patients with ALK positive
non-small-cell lung cancer. J Thorac Oncol. 8:250–253. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Roth W: Cell death in malignant tumors.
Relevance of cell death regulation for metastasis. Pathologe. 36
Suppl 2:181–184. 2015.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song X, Zhang Z, Wang S, Li H, Zuo H, Xu
X, Weng S, He J and Li C: A Janus Kinase in the JAK/STAT signaling
pathway from Litopenaeus vannamei is involved in antiviral immune
response. Fish Shellfish Immunol. 44:662–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee TS, Kantarjian H, Ma W, Yeh CH, Giles
F and Albitar M: Effects of clinically relevant MPL mutations in
the transmembrane domain revealed at the atomic level through
computational modeling. PLoS One. 6:e233962011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rozovski U, Wu JY, Harris DM, Liu Z, Li P,
Hazan-Halevy I, Ferrajoli A, Burger JA, O'Brien S, Jain N, et al:
Stimulation of the B-cell receptor activates the JAK2/STAT3
signaling pathway in chronic lymphocytic leukemia cells. Blood.
123:3797–3802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang J, Liu Y, Tang Y, Li L, Zeng R, Zeng
S and Zhong M: ALDH1A1 induces resistance to CHOP in diffuse large
B-cell lymphoma through activation of the JAK2/STAT3 pathway. Onco
Targets Ther. 9:5349–5360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang R, Wang X, Jin Z and Li K:
Association of nuclear PIM1 expression with lymph node metastasis
and poor prognosis in patients with lung adenocarcinoma and
squamous cell carcinoma. J Cancer. 7:324–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu Q, Shen D, He L, Wang H, Liu C and
Zhang W: Prognostic significance of SOCS3 and its biological
function in colorectal cancer. Gene. 627:114–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue F, Zhu L, Meng QW, Wang L, Chen XS,
Zhao YB, Xing Y, Wang XY and Cai L: FAT10 is associated with the
malignancy and drug resistance of non-small-cell lung cancer. Onco
Targets Ther. 9:4397–4409. 2016. View Article : Google Scholar : PubMed/NCBI
|