Introduction
Worldwide, primary liver cancer is more common in
men than women, and is the second leading cause of
cancer-associated mortality among men in poorly developed countries
and the sixth in more developed countries (1). Although the diagnosis and the treatment
of hepatocellular carcinoma (HCC) have been improved, the most
efficient treatments for HCC are surgical removal of the tumor, and
chemo- or radiotherapy. However, HCC is not sensitive to
chemotherapy and easily develops resistance, which limits the
application of chemotherapy in the clinic (2–4). Targeted
therapy remains in an early stage of development, indeed, sorafenib
has only a modest effect on patient survival (5). The aforementioned treatments have been
demonstrated to be of limited efficacy and are not applicable to
all patients (6). Thus, a novel
antitumor agent with improved effectiveness may be a novel choice
for the therapeutic treatment of HCC.
Researchers have identified that epigenetic
alterations (in particular aberrant DNA methylation) were
associated with the occurrence of a number of types of cancer
(7). DNA methylation is an epigenetic
modification of DNA performed by DNA methyltransferase enzymes
(DNMTs), which catalyze the transfer of a methyl group from
S-adenosyl methionine to the cytosine target nucleotide producing
methylcytosine (5mC) (8). In the
development of mammalian, DNA methylation serves a key function,
which may affect gene repression, suppression of repetitive genomic
elements, X-chromosome inactivation and parental imprinting
(9,10). The hypomethylation of repetitive
elements results in genomic instability, while hypermethylation of
the promoter is associated with tumor suppressor genes (TSGs)
inactivation, affecting cell proliferation, apoptosis and DNA
repair. The majority of cancer cells exhibit aberrant DNA
hypermethylation localized in promoter regions, which are normally
unmethylated and encode tumor suppressors (11). Aberrant DNA methylation may cause
functional inactivation of these tumor suppressors and contribute
to tumorigenesis (12). However,
unlike other causes of gene inactivation, promoter methylation is a
reversible process. Inhibitors of DNMTs may reactivate
epigenetically silenced TSGs, decrease tumor cell growth and induce
cell apoptosis. Therefore, DNMT inhibitors may be used as potential
anticancer agents for cancer therapy (13–15).
Previously, certain DNMT activity inhibitors were
evaluated in preclinical and clinical studies, including
5-azacytidine (5-aza-C), decitabine (5-aza-2′-deoxycytidine,
5-aza-dC), 1-β-D-arabinofuranosyl-5-azacytosine, and
dihydro-5-azacytosine (16).
Decitabine has been approved by the Food and Drug Administration
for the treatment of myelodysplastic syndrome (17). All these drugs are nucleoside
inhibitors, able to incorporate into DNA and RNA, therefore they
have certain drawbacks that include instability and relatively high
toxicity (18,19). SGI-110 (previously designated S110), a
dinucleotide of 5-aza-2′-deoxycytidine and deoxyguanosine,
containing a 5-azaCdR moiety has been demonstrated to be effective
in inhibiting DNA methylation; however, its stability and
cytotoxicity levels are similar to decitabine (20).
SGI-1027, a non-nucleoside DNMT inhibitor, has been
reported as part of a novel class of relatively stable, highly
lipophilic quinoline-based (monoquaternary pyridinium analogue)
small-molecule inhibitors of DNMT1, DNMT3A and DNMT3B (21,22).
SGI-1027 may inhibit DNMT activity, induce the degradation of DNMT1
and reactivate TSGs; however, it is unable to bind to the RNA or
the minor groove of DNA. To the best of knowledge, the effects of
SGI-1027 on human HCC cells has not been previously researched,
therefore the objective of the present study was to explore the
effects of SGI-1027 on human HCC cells, understand the mechanisms
of action of SGI-1027 and provide useful information for a possible
application in HCC therapy.
Materials and methods
Preparation of SGI-1027
A 50 mmol/l stock solution of SGI-1027 (Selleck
Chemicals, Houston, TX, USA) was prepared in dimethylsulfoxide
(DMSO), which was used to prepare dosing solutions of 5, 10, 15,
20, 25, 30 and 35 µmol/l in culture medium, and 0.1% DMSO in the
culture medium used as a control. The stock solution was kept at
−20°C for long term storage.
Cell culture
The human HCC cell line-Huh7 was purchased from the
Cell bank of the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). Huh7 cells were maintained in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 100 U/ml
penicillin, 100 µg/ml streptomycin and 10% fetal bovine serum (FBS;
Life Technologies; Thermo Fisher Scientific, Inc.), and incubated
in a humidified atmosphere containing 5% CO2 at
37°C.
MTS assay
Cell viability was measured using a CellTiter 96
Aqueous One Solution cell proliferation assay kit (Promega
Corporation, Madison, WI, USA). Huh7 cells were seeded onto 96-well
plates at a concentration of 1×104 cells/well and
incubated in a humidified atmosphere containing 5% CO2
at 37°C for 24 h in DMEM supplemented with 10% FBS. The Huh7 cells
were subsequently treated with SGI-1027 (SGI-1027 was dissolved in
DMSO) at different concentrations (5, 10, 15, 20, 25, 30 and 35
µmol/l, with 0.1% DMSO as a control). Cell viability was measured
according to the manufacturer's protocol after 24 h. An ELISA
reader (BioTek Instruments, Inc., Winooski, VT, USA) was used to
measure the absorbance value at 490 nm. The viability ratio was
calculated according to the following formula: [(The absorbance of
experimental group-the absorbance of blank group)/(the absorbance
of untreated group-the absorbance of blank group)] ×100%.
Cell cycle assay
The cell cycle was measured using a Cell Cycle
Detection kit (KeyGen Biotech Co., Ltd., Nanjing, China). A total
of 2 ×105 Huh7 cells/well were seeded into a 6-well
plate and starved in serum-free medium on the second day of
culture. After 12 h starvation, the cells were treated with
different concentrations of SGI-1027 (10, 20 and 30 µmol/l, and
0.1%DMSO as control) for 24 h. The cells were trypsinized and
washed with cold PBS, then fixed overnight with 70% ethanol at 4°C.
The next day, the fixed cells were centrifuged at 1,200 × g at 4°C
for 5 min and washed once with cold PBS. Subsequently, the cells
were suspended in propidium iodide (PI)/RNase staining buffer at
room temperature for 30 min in the dark according to the
manufacturer's protocol. A FACScan flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) was used to analyze the DNA contents of
the cells. Each measurement collected at least 1×104
cells.
Apoptosis assay
Cell apoptosis was assessed using the Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit (KeyGen
Biotech Co., Ltd.). A total of 2×105 Huh7 cells/well
were seeded into a 6-well plate. After 24 h exposures to different
concentrations of SGI-1027 (10, 20 and 30 µmol/l, with 0.1% DMSO as
control), the cells were treated with 0.25% trypsin without EDTA.
According to the manufacturer's protocols, Annexin V-FITC and PI
were used for staining. The double-stained cells were measured
using a FACScan flow cytometer (BD Biosciences). At least
1×104 cells were measured.
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling
(TUNEL) assay
The TUNEL method was used to label apoptotic cells
using a TUNEL apoptosis detection kit (KeyGen Biotech Co., Ltd.)
according to the manufacturer's protocol. Following treatment with
SGI-1027 at a number of concentrations (10, 20 and 30 µmol/l, with
0.1% DMSO as control) for up to 24 h in a humidified atmosphere
containing 5% CO2 at 37°C in a 6-well plate, the cells
were washed twice with PBS and fixed in 1 ml of 4% paraformaldehyde
for 10 min at 4°C, and permeabilized with 0.1% Triton X100 at 25°C
for 5 min. The cells washed twice with PBS, stained by the TUNEL
mixture for 1 h at 37°C in darkness, and then stained by the
Streptavidin-Tetramethylrhodamine for 30 min at 37°C in darkness,
followed by staining with DAPI at 37°C for 10 min. The cells were
washed with PBS, then mounted and examined using fluorescence
microscopy (magnification, ×200) (Olympus IX71; Olympus
Corporation, Tokyo, Japan). Apoptotic cells were identified by the
condensation and fragmentation of their nucleus. The apoptotic
ratio was calculated using the following formula: Apoptotic cell
number/seeded cell number ×100%.
Western blot analysis
Huh7 cells were treated with SGI-1027 at different
concentrations (10, 20 and 30 µmol/l, with 0.1% DMSO as control)
for 24 h, and then washed twice with ice-cold PBS and lysed in
ice-cold protein lysis buffer containing 1% protease inhibitor
cocktail and phenylmethylsulfonyl fluoride. The lysates were
centrifuged at 10,000 × g for 10 min at 4°C. Protein concentrations
were determined using a bicinchoninic acid protein assay kit
(Beyotime Institute of Biotechnology, Shanghai, China). Total
proteins (25 µg/lane) were resolved using 12% SDS-PAGE, then
transferred to polyvinylidene difluoride (PVDF) membranes using a
wet transfer system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
at 70V at 4 °C. The PVDF membranes were blocked with Tris-buffered
saline plus Tween-20 (TBST; pH 8.0; Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) containing 5% nonfat milk at
room temperature for 1 h, and then incubated with primary
antibodies overnight at 4°C. Subsequent to washing with TBST at
room temperature (15 min/time, 3 times), membranes were incubated
with horseradish peroxidase-conjugated Immunoglobulin G (IgG) at
room temperature for 2 h. The probed proteins were detected using
an Enhanced Chemiluminescence kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. The
primary antibodies used in this study were rabbit anti-B cell
lymphoma (Bcl)-2 polyclonal antibody (1:1,000; cat. no. 12789-1-AP;
Proteintech Group, Inc. Chicago, IL, USA), mouse anti-Bcl
associated X-protein (BAX) monoclonal antibody (1:1,000; cat. no.
60267-1-Ig; Proteintech Group, Inc.) and mouse anti-β-actin
monoclonal antibody (1:1,000; cat. no. CW0096; CwBiotech, Beijing,
China). The secondary antibodies included goat anti-rabbit IgG
serum (1:20,000; cat, no. TA130015; OriGene Technologies, Inc.,
Beijing, China) and goat anti-mouse IgG serum (1:20,000; cat, no.
TA130001; OriGene Technologies, Inc.). The images were analyzed
using the MicroChemi Bio-Imaging system (DNR Bio-Imaging Systems
Ltd., Neve Yamin, Israel) and quantified using Quantity One
software (version 4.5.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were performed three times. The data
were expressed as the mean ± standard deviation. GraphPad Prism
software (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA)
was used for data analysis. The differences were analyzed using a
one-way analysis of variance test followed by Dunnett's test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
SGI-1027 inhibits the viability of
Huh7 cells
In order to evaluate the effect of SGI-1027 on Huh7
cells, the cell viability was investigated using an MTS assay once
Huh7 cells were dose-dependently treated with SGI-1027 for 24 h.
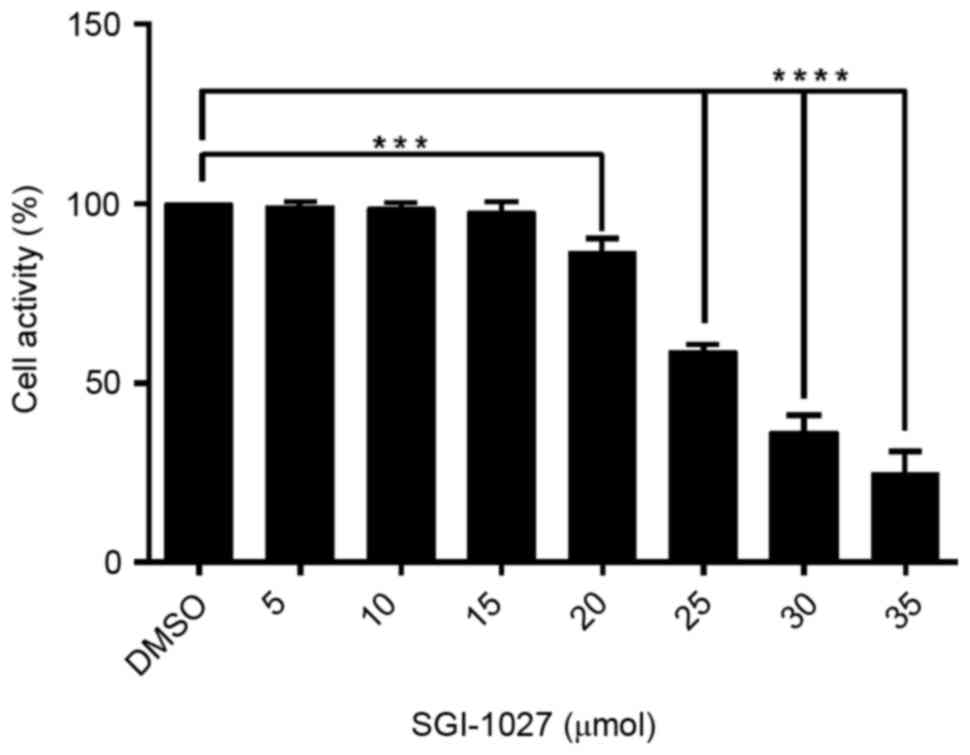
The results revealed that SGI-1027 significantly suppressed the
cell viability of Huh7 cells in a dose-dependent manner compared
with the DMSO control group, with an IC50 value of 27.30
µmol/l (Fig. 1).
SGI-1027 does not block the cell cycle
of Huh7 cells
To investigate the effect of SGI-1027 on the cell
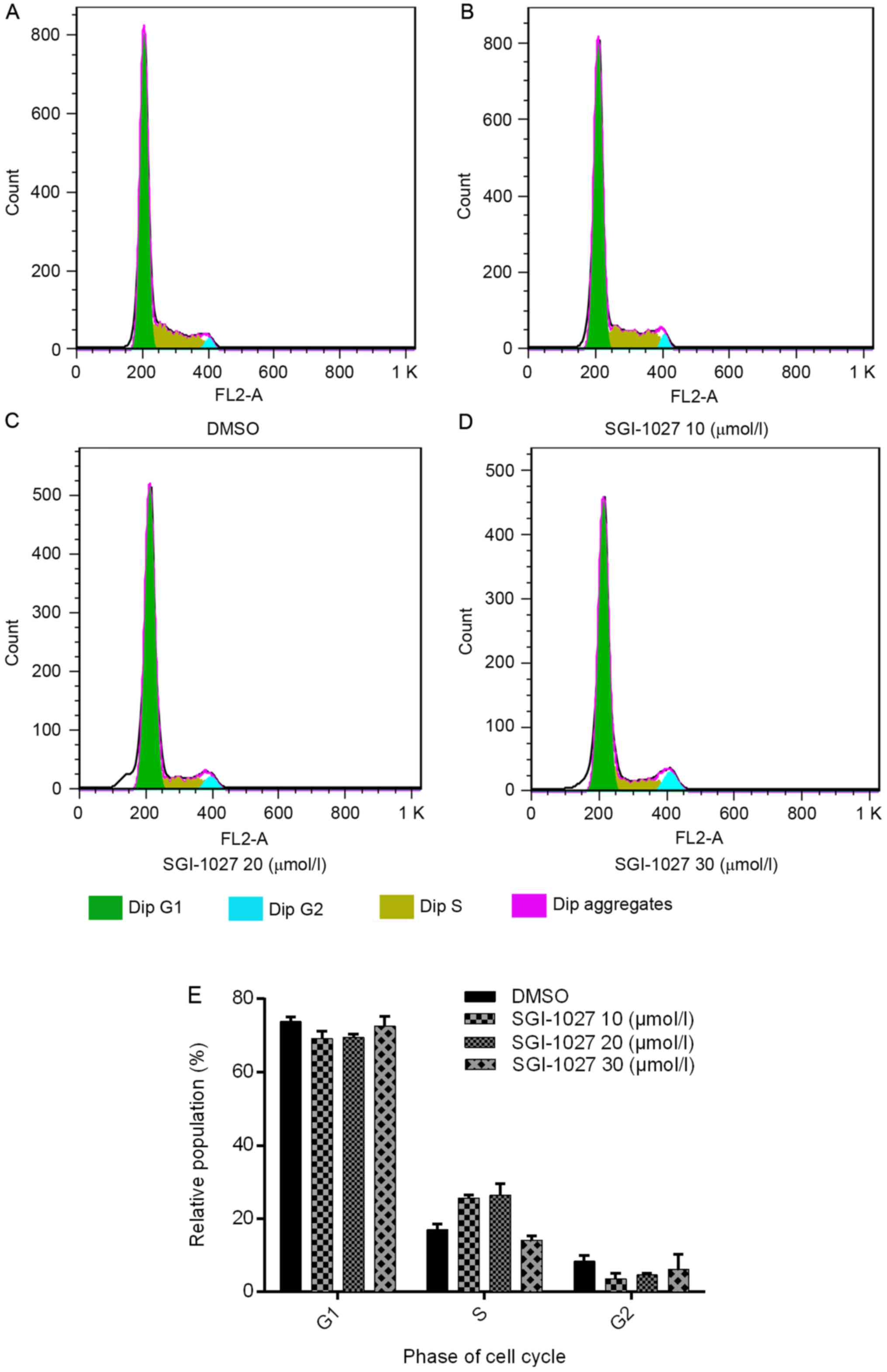
cycle, the cell cycle profiles of Huh7 cells were analyzed using
flow cytometry. However, no significant alterations of the cell
cycle phases were observed following treatment with distinct
concentrations of SGI-1027 for 24 h (P>0.05). The percentage of
cells accumulated in the G1 phase was 73.81±1.357, 69.17±2.021,
69.517±0.904 and 72.547±2.724% in the DMSO control, 10, 20 and 30
µmol/l SGI-1027 groups, respectively. The percentage of cells
accumulated in the S phase was 17.017±1.443, 25.623±0.869,
26.377±3.107 and 14.140±1.067% in the DMSO control, 10, 20 and 30
µmol/l groups, respectively. The percentage of cells accumulated in
the G2 phase was 8.377±1.542, 3.577±1.452, 4.633±0.503 and
6.11±4.116% in the DMSO control, 10, 20 and 30 µmol/l SGI-1027
groups, respectively (Fig. 2).
SGI-1027 induces cell apoptosis in
Huh7 cells
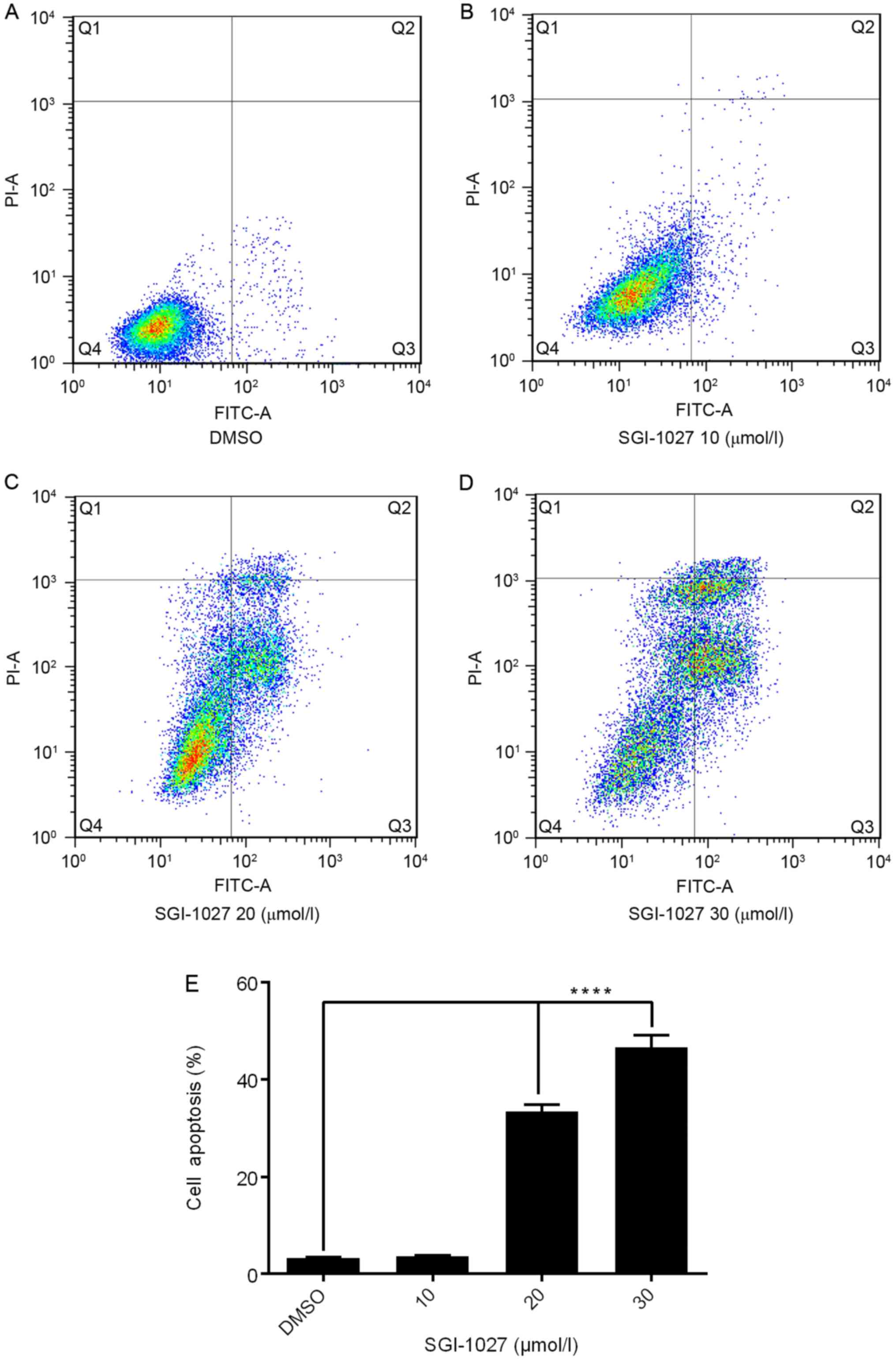
To confirm whether the SGI-1027 induced inhibition
of proliferation was due to a direct effect on apoptosis in Huh7
cells, flow cytometry analysis was performed using Annexin V-FITC
and PI double staining. It was identified that following
dose-dependent treatment with SGI-1027 for 24 h, in the control
group there were normal cells and rarely apoptotic cells, while in
SGI-1027 groups, the rates of apoptotic cells significantly
increased in a dose-dependent manner. The rates of apoptosis in
response to different concentrations of SGI-1027 were 3.242±0.204
(DMSO group), 3.672±0.123 (10 µmol/l), 33.49±1.317 (20 µmol/l) and
46.57±2.512% (30 µmol/l) (Fig.
3).
SGI-1027 induces morphological changes
in Huh7 cells
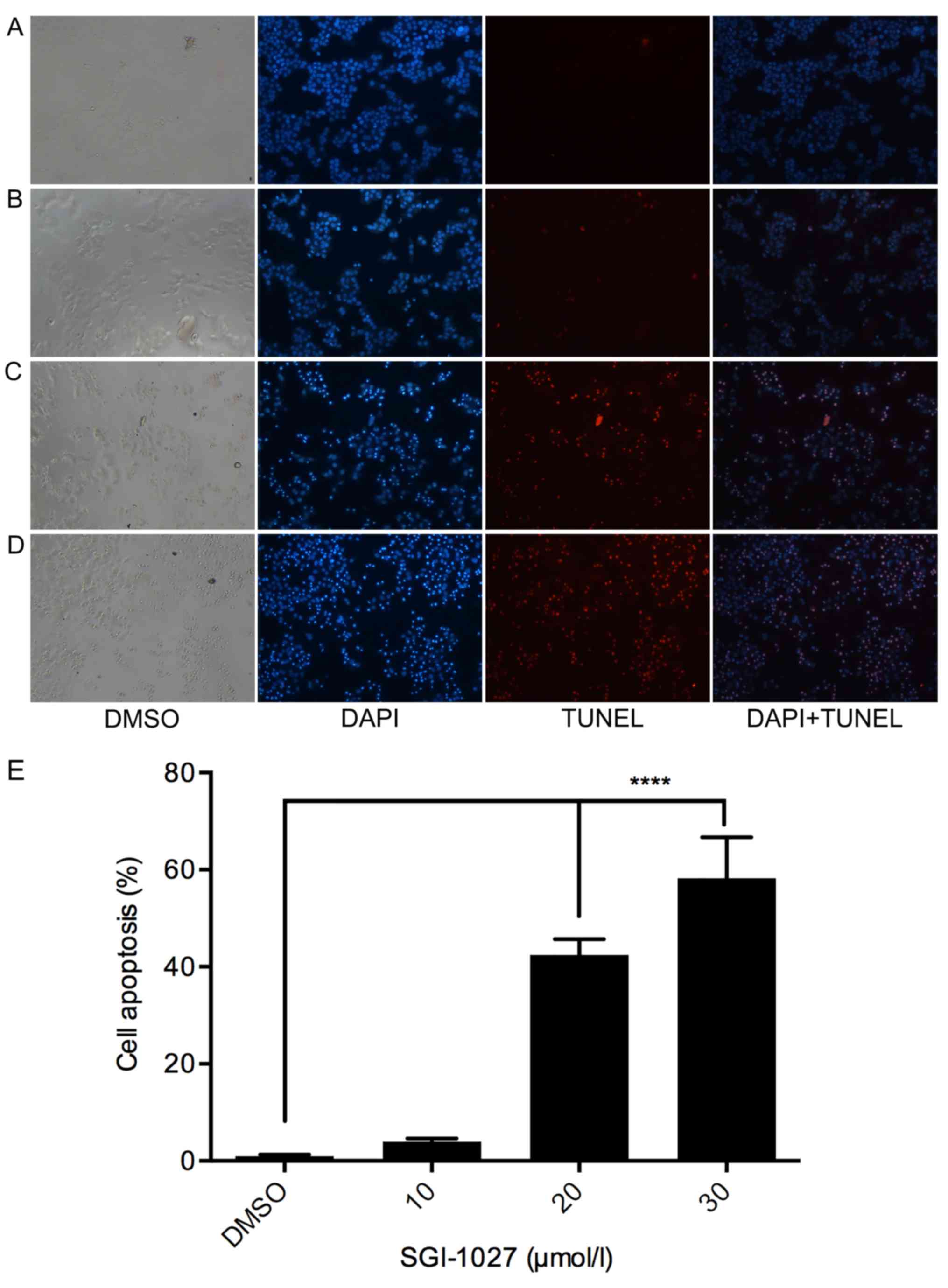
In the present study, the apoptotic effects of
SGI-1027 were ascertained in Huh7 cells by assessing morphological
changes. Subsequent to treatment with different doses of SGI-1027
for 24 h, Huh7 cells were stained with TUNEL. The results revealed
that in the chromatin of apoptotic cells the fluorescence dye
stains were observed more brightly compared with that of control
cells. The number of Huh7 cells adhering to the culture plates in
SGI-1027 treated groups was decreased compared with the control
group. The morphological changes of cell apoptosis included
blebbing, cell shrinkage, nuclear or chromatin condensation, DNA
fragmentation and apoptotic body formation. In contrast, the
morphology of normal cells was round and homogenous. In the present
results, the apoptotic morphological changes were observed in the
SGI-1027 treated groups, while few apoptotic cells were observed in
the control group. The percentage of apoptotic cells was
0.987±0.34, 3.883±0.667, 42.44±3.244 and 58.24±8.427% in the DMSO
control, 10, 20 and 30 µmol/l SGI-1027 groups, respectively
(Fig. 4).
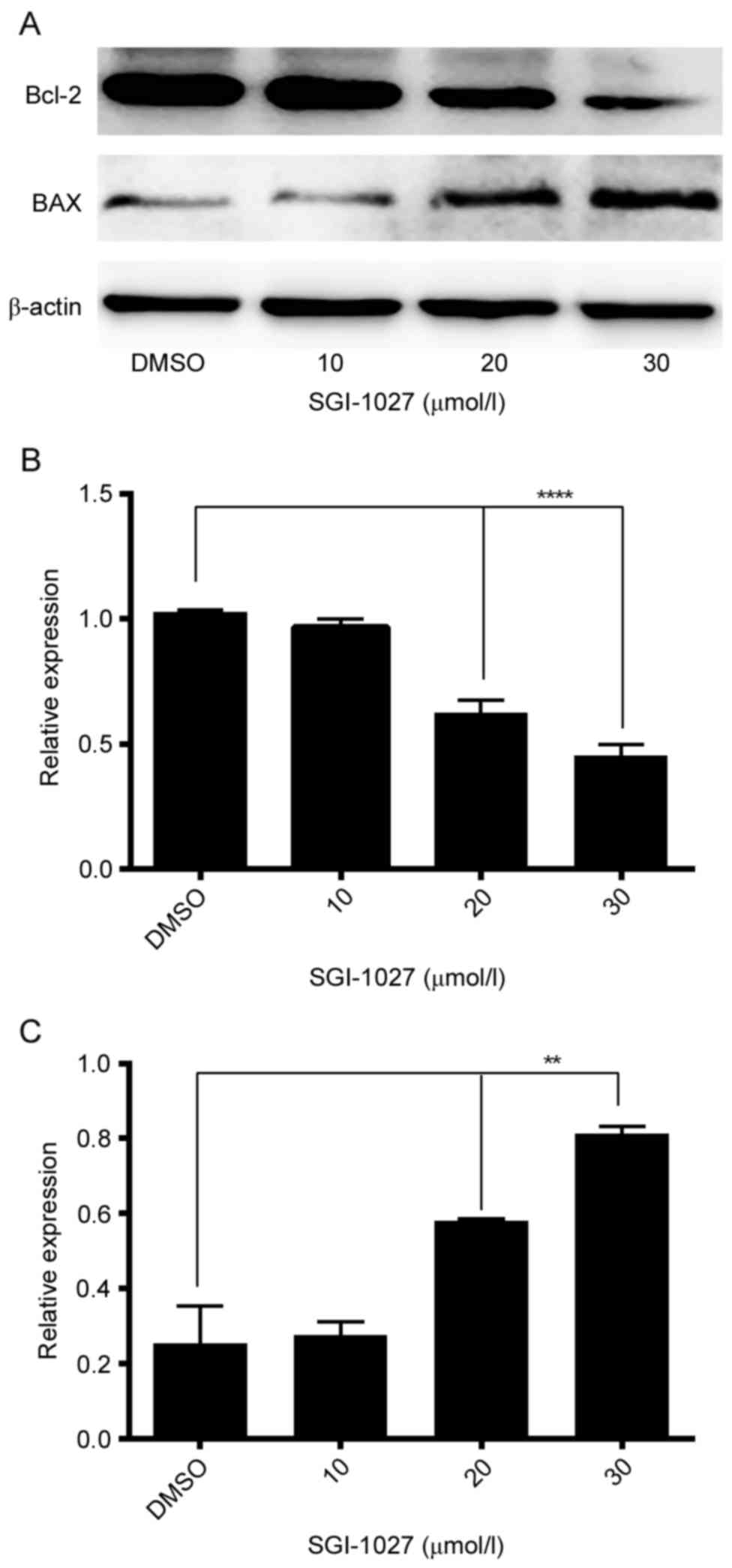
SGI-1027 affects the expression of
proteins associated with apoptosis
In order to investigate whether Bcl-2 and BAX
expression were involved in the apoptosis of Huh7 cells, the
expression levels of Bcl-2 and BAX were assessed using western
blotting. The results indicated that following treatment with
different concentrations of SGI-1027 (0.1% DMSO as control, 10, 20
and 30 µmol/l SGI-1027), Bcl-2 protein expression was significantly
downregulated, whereas BAX expression was significantly
upregulated, in a dose-dependent manner, in Huh7 cells (Fig. 5).
Discussion
Epigenetic changes frequently occur in certain types
of cancer via inactivation of TSGs, in particular, hypermethylation
of the promoter region of genes is the most important epigenetic
mechanism for silencing TSGs and causing tumorigenesis (7,15,23,24).
Unlike other gene expression mutations, methylation does not alter
the DNA base sequence making DNA methylation reversible. An
important component of the mammalian methylation machinery is
DNMTs; therefore, DNMT inhibitors are considered as promising
therapeutic targets (16).
Although DNA hypomethylating agents 5-aza-C and
5-aza-dC, and their analogues have been used in cancer therapy,
their instability in solutions and toxicity have limited their
clinical application, SGI-1027 is a quinolone-based small molecule
non-nucleoside inhibitor, which is able to inhibit DNMTs and
reactivate TSGs (21). Furthermore,
it does not bind to the DNA or RNA, is relatively stable and
exhibits lower toxicity compared with the nucleoside class DNMT
inhibitors (21). To the best of our
knowledge, four studies have investigated the inhibitory effect of
SGI-1027 on DNMTs; however, the ability of SGI-1027 to inhibit
proliferation and induce apoptosis in tumor cells remains scarcely
studied (21,22,25,26). One
of the aforementioned studies, using citofluorimetric analysis,
identified that SGI-1027 may induce apoptosis in human U937 cells;
however, the study did not clarify the underlying molecular
mechanism of apoptosis caused by SGI-1027 (25). There appears to be no research
regarding the effects of SGI-1027 on human HCC cell lines.
In the present study, the MTS assay results revealed
that SGI-1027 significantly inhibited the viability of Huh7 cells
in a dose-dependent manner. Usually, the inhibition of cell
viability involves blocking of cell cycle progression and
apoptosis. However, in the present data, no significant alterations
to the cell cycle phases were observed following SGI-1027
treatment. Although, the data demonstrated that SGI-1027 was
associated with apoptosis in Huh7 cells. Flow cytometric analysis
revealed that with increasing SGI-1027 concentrations, the early
and late apoptotic cells proportions also increased. Furthermore,
SGI-1027-treated Huh7 cells demonstrated significant
apoptosis-associated morphological alterations.
The ability of cancer cells to inhibit apoptosis is
essential for carcinogenesis. Programmed cell death has been
identified as an effective mechanism to prevent cancer, and cell
apoptosis is a core process of programmed cell death (27). The results of the present study
suggested that Huh7 cells treated with SGI-1027 followed the
typical apoptotic pathway. Bcl-2 family proteins have been
suggested to be the primary regulators of apoptosis in the
mitochondrial-mediated pathway, and BAX induced mitochondrial outer
membrane permeabilization is considered as an important control
switch of apoptosis (28). Bcl-2 is
an anti-apoptotic protein, while BAX is a critical pro-apoptotic
protein, which may increase the permeability of mitochondrial
membranes and accelerate cell apoptosis (29,30). In
this research, it was demonstrated that SGI-1027 significantly
downregulated Bcl-2 expression and upregulated BAX expression,
suggesting that the apoptosis of Huh7 cells resulting from SGI-1027
occurred via the mitochondrial-mediated pathway.
In conclusion, the data gathered reveal that
SGI-1027 inhibits Huh7 cell proliferation and induces apoptosis
in vitro. We hypothesize that SGI-1027 may be able to
reverse the aberrant DNA methylation of certain pro-apoptosis genes
and trigger their re-expression. It is suggested that SGI-1027 may
be a potential compound for the treatment of HCC, providing a novel
approach for HCC treatment. However, further research is required
to elucidate the precise molecular mechanisms underlying the
effects of SGI-1027 on apoptosis induction in HCC, and assess the
application of the present results to the treatment of human
HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the science and
technology project of Shenyang (grant no. F13-212-9-00).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JZ conceived the study. NS designed the study. NS,
CZ, BZ and AJ performed the experiment. NS and CZ analyzed the
data. NS wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
DNMTs
|
DNA methyltransferase enzymes
|
|
TSGs
|
tumor suppressor genes
|
|
5-aza-C
|
5-azacytidine
|
|
5-aza-dC
|
5-aza-2-deoxycytidine
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeo W, Mok TS, Zee B, Leung TW, Lai PB,
Lau WY, Koh J, Mo FK, Yu SC, Chan AT, et al: A randomized phase III
study of doxorubicin versus cisplatin/interferon
alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy
for unresectable hepatocellular carcinoma. J Natl Cancer Inst.
97:1532–1538. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gish RG, Porta C, Lazar L, Ruff P, Feld R,
Croitoru A, Feun L, Jeziorski K, Leighton J, Gallo J and Kennealey
GT: Phase III randomized controlled trial comparing the survival of
patients with unresectable hepatocellular carcinoma treated with
nolatrexed or doxorubicin. J Clin Oncol. 25:3069–3075. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu
P, Chen KK, Lopez JP, Poon RT and Fan ST: Octamer 4 (Oct4) mediates
chemotherapeutic drug resistance in liver cancer cells through a
potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology.
52:528–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chua CW and Choo SP: Targeted therapy in
hepatocellular carcinoma. Int J Hepatol. 2011:3482972011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dai ZJ, Tang W, Lu WF, Gao J, Kang HF, Ma
XB, Min WL, Wang XJ and Wu WY: Antiproliferative and apoptotic
effects of β-elemene on human hepatoma HepG2 cells. Cancer Cell
Int. 13:272013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esteller M: Epigenetic gene silencing in
cancer: The DNA hypermethylome. Hum Mol Genet 16 Spec No.
1:R50–R59. 2007. View Article : Google Scholar
|
|
8
|
Holliday R and Pugh JE: DNA modification
mechanisms and gene activity during development. Science.
187:226–232. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng S, Jacobsen SE and Reik W: Epigenetic
reprogramming in plant and animal development. Science.
330:622–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonasio R, Tu S and Reinberg D: Molecular
signals of epigenetic states. Science. 330:612–616. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feinberg AP and Tycko B: The history of
cancer epigenetics. Nat Rev Cancer. 4:143–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taby R and Issa JP: Cancer epigenetics. CA
Cancer J Clin. 60:376–392. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kondo Y: Epigenetic cross-talk between DNA
methylation and histone modifications in human cancers. Yonsei Med
J. 50:455–463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghoshal K and Bai S: DNA
methyltransferases as targets for cancer therapy. Drugs Today
(Barc). 43:395–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oki Y, Aoki E and Issa JP:
Decitabine-bedside to bench. Crit Rev Oncol Hematol. 61:140–152.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gorbunova V, Seluanov A, Mittelman D and
Wilson JH: Genome-wide demethylation destabilizes CTG.CAG
trinucleotide repeats in mammalian cells. Hum Mol Genet.
13:2979–2989. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mund C, Hackanson B, Stresemann C, Lübbert
M and Lyko F: Characterization of DNA demethylation effects induced
by 5-Aza-2′-deoxycytidine in patients with myelodysplastic
syndrome. Cancer Res. 65:7086–7090. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoo CB, Jeong S, Egger G, Liang G,
Phiasivongsa P, Tang C, Redkar S and Jones PA: Delivery of
5-aza-2′-deoxycytidine to cells using oligodeoxynucleotides. Cancer
Res. 67:6400–6408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Datta J, Ghoshal K, Denny WA, Gamage SA,
Brooke DG, Phiasivongsa P, Redkar S and Jacob ST: A new class of
quinoline-based DNA hypomethylating agents reactivates tumor
suppressor genes by blocking DNA methyltransferase 1 activity and
inducing its degradation. Cancer Res. 69:4277–4285. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gros C, Fleury L, Nahoum V, Faux C,
Valente S, Labella D, Cantagrel F, Rilova E, Bouhlel MA,
David-Cordonnier MH, et al: New insights on the mechanism of
quinoline-based DNA Methyltransferase inhibitors. J Biol Chem.
290:6293–6302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Palii SS and Robertson KD: Epigenetic
control of tumor suppression. Crit Rev Eukaryot Gene Expr.
17:295–316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kulis M and Esteller M: DNA methylation
and cancer. Adv Genet. 70:27–56. 2010.PubMed/NCBI
|
|
25
|
Garcia-Dominguez P, Dell'aversana C,
Alvarez R, Altucci L and de Lera AR: Synthetic approaches to DNMT
inhibitor SGI-1027 and effects on the U937 leukemia cell line.
Bioorg Med Chem Lett. 23:1631–1635. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ronen M, Avrahami D and Gerber D: A
sensitive microfluidic platform for a high throughput DNA
methylation assay. Lab Chip. 14:2354–2362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x(L): Keep your friends close but your enemies
closer. Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siddiqui WA, Ahad A and Ahsan H: The
mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update.
Arch Toxicol. 89:289–317. 2015. View Article : Google Scholar : PubMed/NCBI
|