Introduction
Despite significant advances in the basic
understanding of tumor pathogenesis, improvement in surgical
techniques and new adjunct treatment, the median overall survival
rate of patients with glioblastoma (GB) has increased only by 3.3
months (from 11.3 to 14.6 months) until 2010 (1,2) with a
long-term survival rate above three years of only 3 to 5% (3). During the past decade, the consistent
implementation of surgical re-resection in recurrent GB has helped
to improve this devastating figure resulting in up to 25 months of
overall survival rate, and 11.9 months following the first
resection (4). The main predictive
factors of survival are tumor localization in eloquent areas of the
brain and the functional performance and tumor volume of the
patient.
In GB, a small cell population with stem cell-like
properties is resistant to chemotherapy. These cells exhibit unique
energy metabolic characteristics (5),
including low mitochondrial respiration and a preference for
hypoxia, to maintain their tumor-forming capacity (6). In vitro, hypoxia promotes the
survival of these clonogenic cancer stem cells (7) and inhibits their differentiation even if
the cultures are returned to normoxic conditions (8). Furthermore, approximately half of all
human GB respond to hypoxia by inducing c-Met, with subsequent
enhancement of tumor cell migration and invasion (9).
Treatment strategies utilizing reactive oxygen
(O2) species induce cell death by autophagy and
apoptosis in glioma cell cultures (10). Employing positron emission tomography
(PET) or advanced magnetic resonance imaging (MRI) techniques such
as MR spectroscopy enable the monitoring of local O2
levels (11). The intracellular in
vivo oxymetry in rats implanted with the aggressive glioma cell
line 9L demonstrates the preference for low tumor pO2
levels around 45 mmHg (12).
Treatment with bischloroethylnitrosourea (BCNU) resulted in an
immediate and significant increase in pO2 up to 140 mmHg
(12). These anti-tumorigenic
properties of oxygen are accompanied by its key role in regulating
the immunogenicity of primary human glioma cells (13). Furthermore, the application of
O2 plus ozone (O3) in two different human
neuroblastoma cell lines (SK-N-SH and SK-N-DZ) induced cell growth
inhibition at G2 phase and cell cycle perturbation in
both cell lines (14).
Ozone is a gas that is produced endogenously by
granulocytes. In the presence of an electron donor, reactive
O2 species are produced, particularly hydrogen peroxide
(15). Clinical O3
application has resulted in improved wound healing (16,17), and
the improvement of radiation-induced proctitis (18) and cystis (19), as well as the improvement of
osteonecrosis (20–22). The toxicity of inhaled O3
can be reduced by applying the gas for a calculated period and at
lower doses (23–25) although certain genetic variance in the
O3 response exist (26).
However, O3 acts antineoplastically in the presence of
carcinogens if administered by inhalation (27,28),
intra-vesicular (29) or
intra-peritoneal (30,31) instillation. Since O3
stimulates the release of immunoactive cytokines (32), these effects are more likely to be the
result of an immune-mediated reaction rather than being a direct
consequence of administration (33).
Studies have emphasized that O3 therapy
can be considered a serious adjuvant therapy in oncological
patients receiving radiochemotherapy (34). The present study reports data from the
off-label application of O2-O3 into the tumor
vicinity of GB following surgery in a limited series of patients.
In addition, the results of a systematic literature search on
O3 treatment following surgery for malignancies are
presented.
Materials and methods
Between January 2012 and December 2013, patients
diagnosed with GB at the Klinikum Amberg were offered an
intra-tumoral treatment with O2-O3 extending
the standard therapy as determined by the local tumor board.
Following extensive information about their options and the
possible side effects of the treatment, five patients provided
their informed consent, and the ethical committee of the Bavarian
National Medical Association approved the study, based on the
Helsinki Declaration of 1964, revised 2013 (EK-Nr. 2013-125).
Informed consent included publication of the case report and any
accompanying images. Part of the illustrative case was presented as
a poster on the Brain Tumor Meeting May 2013 in Berlin.
Treatment
Together with the re-resection in recurrent GB, a
Rickham reservoir was implanted with the tip ending in the middle
of the resection area. The exact catheter localization and the
extent of tumor resection were confirmed by an early (<72 h
post-operatively) contrast-enhanced MRI. Subsequently, five ml of
O2-O3 were applied monthly through the
reservoir at a concentration of 40 µg O3 per ml.
The standard adjuvant therapy following the initial
surgery for GB consisted of radiotherapy with a local boost and
chemotherapy according to Stupp et al (2); 75 mg/m2 body surface
temozolomide followed by cycles with 150 mg/m2 body
surface, five days per week for the irradiation period, followed by
a three week off/one week on schedule. Following progression or
recurrence and re-section, the patients were switched to PCV
chemotherapy (procarbacine 60 mg/m2 day 8 to 21, CCNU
100 mg/m2 day and vincristine 1.4 mg/m2 day
8+29).
Histological assessment
Tumor biopsies at primary resection and
recurrence(s) were evaluated by an experienced neuropathologist and
classified according to the criteria of the WHO 2007 classification
of tumors of the central nervous system. Prior to publication, the
histological results were reviewed and adapted to the current WHO
classification of 2016 (35). The
standard work-up for diagnostics comprised routine histological
stains, immunohistochemistry, including the p53 (BP53-12) and Ki-67
(Mib-1) antigens, and molecular diagnostics for
O-6-methylguanine-DNA-methyltransferase (MGMT) promoter
methylation and isocitrate dehydrogenase (IDH) 1/2
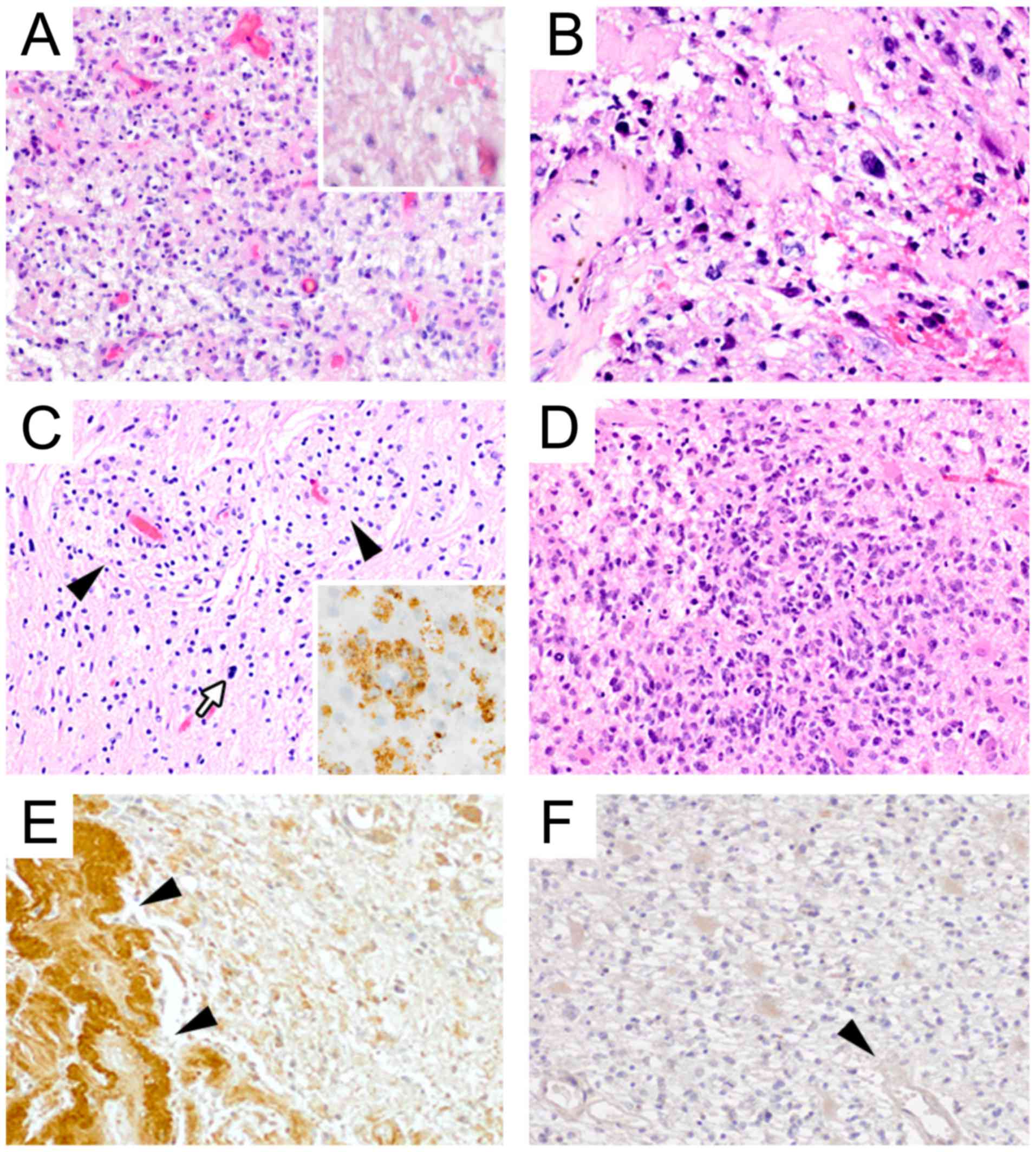
mutations. Additionally, immunohistochemistry for hypoxia-inducible
factor (HIF)-1α and HIF-2α was performed for investigative purposes
(Fig. 1). Immunohistochemistry on
formalin-fixed and paraffin-embedded tissue samples was performed
as described previously (36). In
brief, 5-mm sections were cut and slides were deparaffinized and,
following microwave antigen retrieval, stained using the EnVision+
Dual Link System-HRP (DAB+; K4065; Dako; Agilent Technologies GmbH,
Waldbronn, Germany) according to the manufacturer's protocol.
Anti-HIF-1α (NB100-123; Novus Biologicals, Ltd., Cambridge, UK) and
anti HIF-2α ha (NB100-122; Novus Biologicals, Ltd.) primary
antibodies were used at 1:50 in phosphate buffered saline
supplemented with 1% bovine serum albumin. Pre-treatment for
antigen retrieval was not applied.
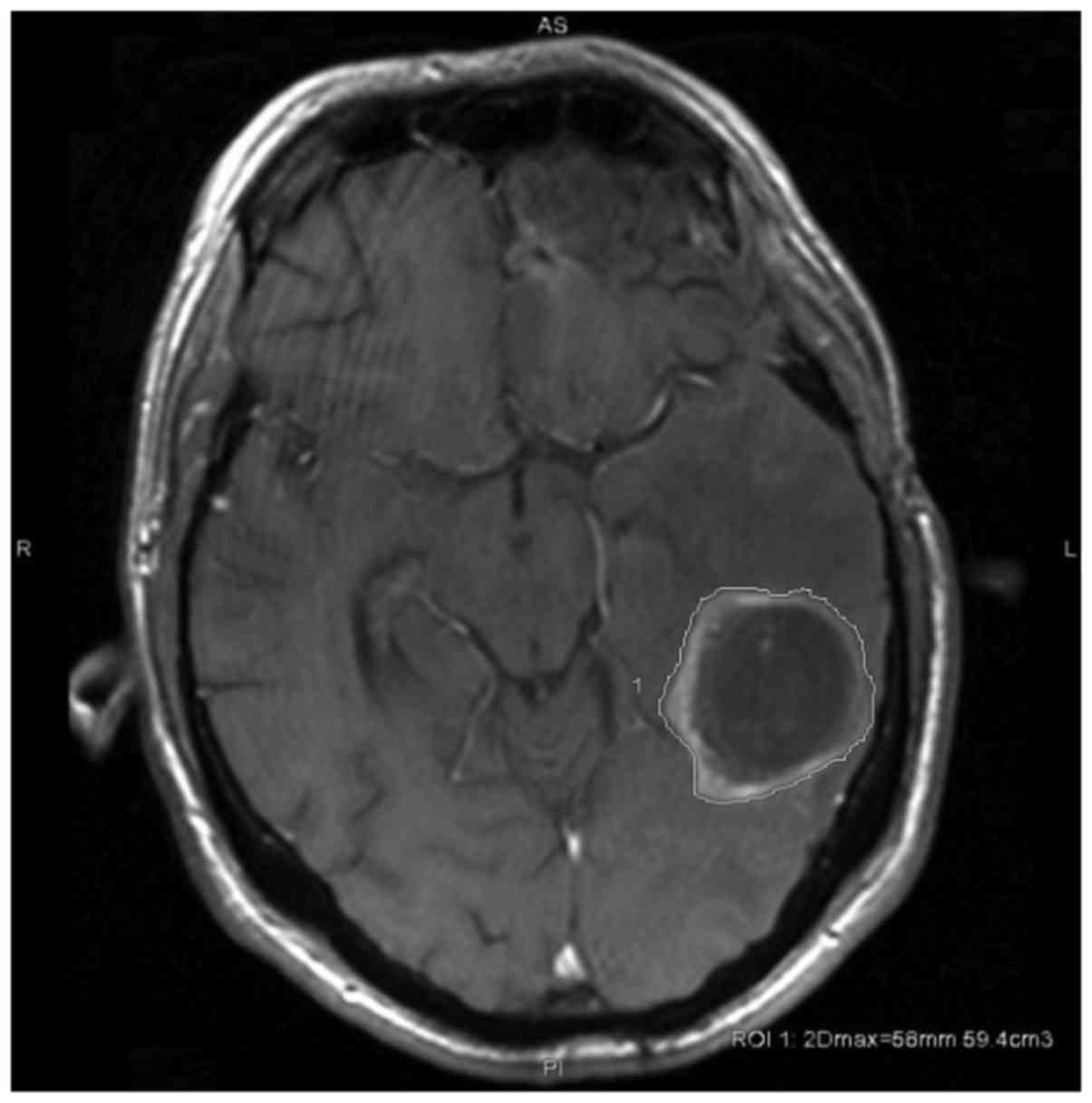
Magnetic resonance tumor volumetry. Volumetric
analysis was performed on a GE Advantage Workstation (GE Healthcare
Life Sciences, Little Chalfont, UK; version 4.7, Operation System
2.0 on a HELiOS 6.6.1 subsystem) by a radiologist. Tumor size and
volume were calculated by semi-automated contouring of tumor
borders on each T1 weighted-slice (post gadolinium) in
cm3 and maximum 2D diameter in mm, as outlined in
Fig. 2. Slice thickness was 1 and 5
mm, dependent on the subsequent protocols of MRI examination
sequences applied between January 2012 and July 2017. Enhancing
areas were considered as tumor with the exception of obvious
vessels, artifacts or postoperative resection defects.
Statistical analysis
Dichotomous or categorical parameters are presented
in frequencies and percentages. Metric variables are provided as
median and range. Statistical analyses were performed using SPSS
version 21 (IBM Corp., Armonk, NY, USA).
Systematic literature search
A systematic literature search was performed for
observational and clinical studies utilizing an O3
treatment for malignancies. Studies published between 01/01/1987
and 01/12/2017 were included, with no limitation regarding study
type and sample unit (observational or experimental, animal or
patient, in vitro or in vivo). The retrieval of
studies was performed in PubMed using the combined filter and
Medical Subject Headings (MeSH) term: [‘Ozone/surgery’ (Mesh) OR
‘Ozone/therapeutic use’ (Mesh) OR ‘Ozone/therapy’ (Mesh)) AND
‘Neoplasms’ (Mesh)] The records were screened based on title and
abstract independently. Finally, the remaining records were
evaluated by reading the full-text papers. All relevant
characteristics (study type, disease, route of O3
application, unit of analysis, sample size and main findings)
reported in the studies were extracted into an evidence table.
Results
The demographic and disease-related characteristics
are summarized in Table I. The
patient group comprised two women and three men with a median age
of 48 years at diagnosis (range 31 to 68 years), the Karnofsky
score (37) was 80% (range 50 to
90%).
 | Table I.Detailed patient characteristics. |
Table I.
Detailed patient characteristics.
|
| Patient no. |
|
|---|
|
|
|
|
|---|
| Characteristic | 1 | 2 | 3 | 4 | 5 | Median (range) |
|---|
| Sex | M | F | F | M | M |
|
| Age (years) | 59 | 68 | 48 | 31 | 45 | 48
(31–68) |
| Karnofsky index
(%) | 50 | 70 | 90 | 80 | 90 | 80
(50–90) |
| Initial
surgery | Biopsy | Biopsy | Complete tumor
resection and reservoir implantation | Partial tumor
resection | Subtotal tumor
resection |
|
| Surgery at
progression | Partial tumor
resection and reservoir implantation | Partial tumor
resection and reservoir implantation |
| Subtotal tumor
resection and reservoir implantation | Complete tumor
resection and reservoir implantation |
|
| Histology (WHO
2016) | Glioblastoma,
IDH-wildtype, WHO grade IV | Glioblastoma,
IDH-wildtype, WHO grade IV | Glioblastoma,
IDH-wildtype, WHO grade IV | (Secondary)
glioblastoma, IDH-mutant, WHO grade IV | Glioblastoma,
IDH-wildtype, WHO grade IV |
|
| MGMT
promoter methylation | 5% | 10% | 5% | 25% | Negative
(<3%) | 5%
(3–25) |
| IDH
mutation | WT | WT | WT | Mutant | WT |
| Ki67 proliferation
index | 20% | n.a. (Infiltrative
rim, no solid tumour core) | 40% | 30% | 30% | 30% (20–40) |
| p53 nuclear
accumulation | Some | 0 (negative) | 10% (heterog.) | 70–80% | 10% | 10% (0–75) |
| Radiotherapy | 50 Gy+10 Gy
boost | No | 50 Gy+10 Gy
boost | 50 Gy+10 Gy
boost | 46 Gy+14 Gy
boost |
|
| Chemotherapy | Temozolomide | Temozolomide | Temozolomide | Temozolomide | Temozolomide |
|
| Second-line
chemotherapy | PCV | PCV | None | None | PCV |
|
| Tumor volume at
diagnosis, cm3 | 10.5 | 13.4 | 44.1 | 136 | 29.4 | 29.4
(10.5–136) |
| Tumor volume at
progression before O2-O3, cm3 | 29.3 | 5.50 | n/a | 12.7 | Frontal, 8.63;
parietal, 11.6 | 16.5
(5.50–29.3) |
| Tumor volume at
start of O2-O3, cm3 | 13.3 | 0 | 3.03 | 0 | Frontal, 1.09;
parietal, 5.50 | 3.0 cm3
(0–13.3) |
| Tumor volume at
progression after O2-O3, cm3 | 25.5 | 14.4 | n/a | 27.5 | Frontal, 5.8;
parietal 65.2, | 26.5
(14.4–71.0) |
| Progression-free
survival, months | 38 | 12 | 53 | 29 | 10 | 6
(4–53) |
| Overall survival,
months | 40 | 46 | 53 | 31 | 16 | 40
(16–53) |
| Number of O2-O3
applications | 31 | 3 | 44 | 27 | 10 | 27
(3–44) |
| Procedure related
complications | Infection | – | – | – | Haemorrhage |
|
| Interval between
diagnosis and start of O2-O3, months | 6 | 9 | 0 | 4 | 4 | 4
(4–9) |
| Survival following
O2-O3, months | 33 | 37 | 50 | 27 | 11 | 34 (12–53) |
Primary treatment/initial surgery
In two patients the diagnosis was established
following a biopsy, in two patients following a partial resection.
In one patient the initial resection was complete and at her
request the O2-O3 therapy was started
following diagnosis.
In four out of five patients molecular
neuropathology was IDH wild-type GB; in the youngest patient
the GB exhibited an IDH mutation corresponding to
(secondary) GB, IDH-mutant. The MGMT promoter
methylation status ranged between <3 and 25%. The median Ki67
proliferation index was 30% (range 20 to 40%), and the median
nuclear score for p53 positivity was 20% (range 0 to 75%).
Of the five patients, four received a radiotherapy
with a median of 49 Gy (range, 46 to 50 Gy) accompanied by a local
boost of 11 Gy (range, 10 to 14 Gy). In all patients, the initial
chemotherapy was started with temozolomide according to Stupp et
al (2).
Surgery for first
recurrence/progression: Further treatment
The patient who insisted on receiving the
O2-O3 treatment following the initial
diagnosis remains in remission. In the other four patients, the
first recurrence occurred in a median time of five months (range, 4
to 9 months). Together with the re-resection in recurrent GB, a
Rickham reservoir for O2-O3 application was
implanted. Five milliliter of O2-O3 were
applied monthly through the reservoir at a concentration of 40 µg
O3 per ml. The chemotherapy was changed in three out of
four patients to PCV; one patient refused further chemotherapy.
Overall survival and survival
following initiation of ozone treatment
The patients received a median of 27 (range, 3 to
44) O2-O3 applications. The overall median
survival rate was 40 months (range, 16 to 53 months). The median
survival rate following the first recurrence subsequent to the
initiation of the O2-O3 treatment was 34
months (range, 12 to 53 months). The progression-free survival rate
was positively associated with a more extensive surgical
re-resection (P=0.021), while the tumor volume was negatively
correlated (P=0.027).
In one patient, a local infection occurred resulting
in temporary removal of the reservoir. In another patient, a
hemorrhage in the tumor vicinity around the catheter four months
following the implantation necessitated the temporary removal of
the reservoir. Based on a total of 115 O2-O3
applications in five patients, the complication rate per
application was 1.7%.
Illustrative case
A 59-year-old male patient developed a seizure with
a persisting hemiparesis. MRI demonstrated a contrast-enhancing
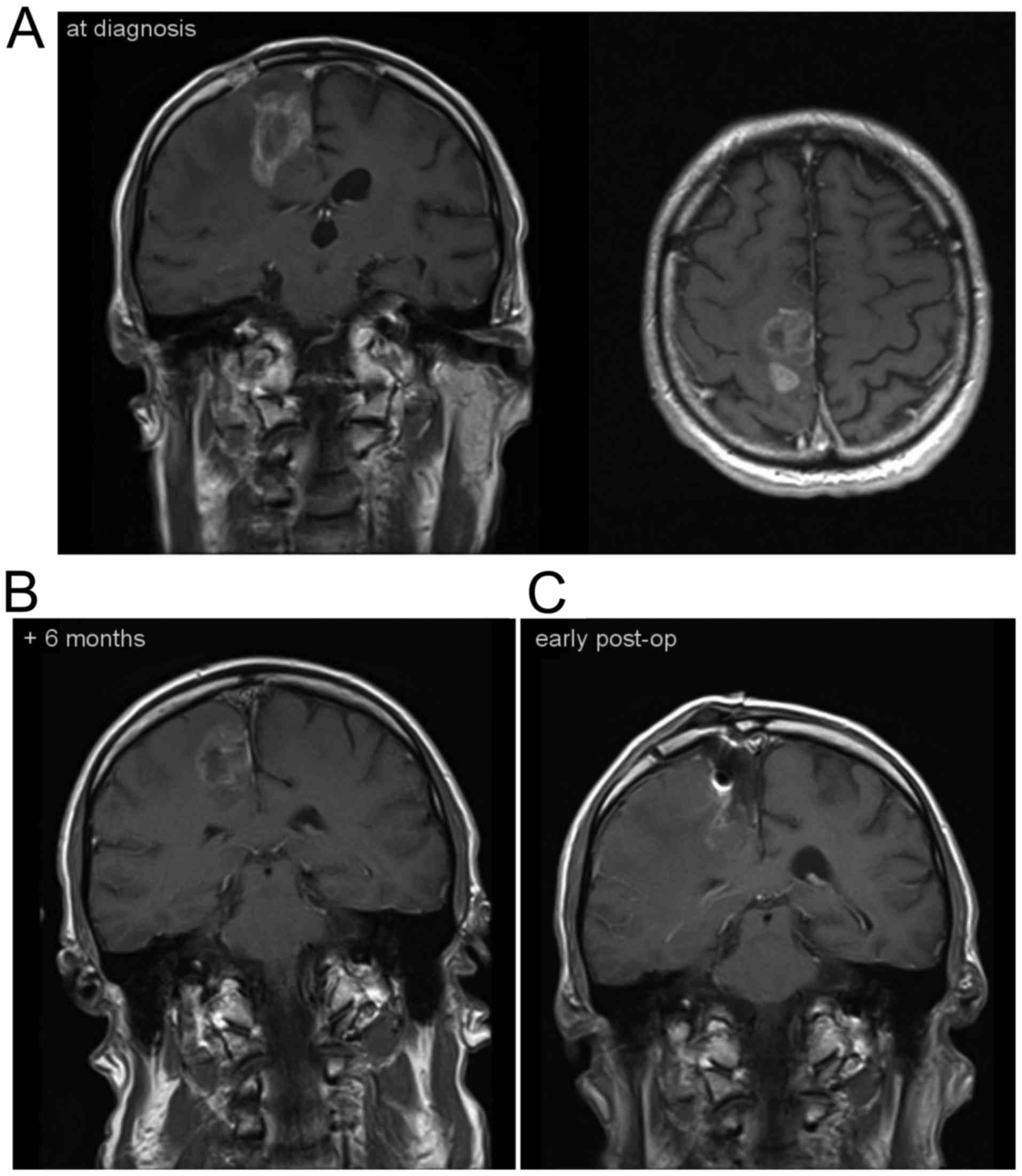
lesion in the motor cortex (10.5 cm3; Fig. 3A). The histological examination
confirmed a GB, IDH wild-type with a Ki-67 proliferation
rate of 20% and no relevant MGMT promoter methylation
(Fig. 1A). The patient was treated
with radiochemotherapy (50 Gy with local boost of 10 Gy),
temozolomide at 75 mg/m2 body surface for four weeks,
followed by one three weeks off and one cycle at 150
mg/m2. The symptoms worsened and a tumor progression was
evident at six months with a volume of 29.3 cm3
(Fig. 3B). At the time of tumor
resection, a catheter connected to a reservoir was placed in the
area of the former tumor cavity (Fig.
3C). Histology was performed and again demonstrated pleomorphic
astrocytic tumor cells and fibroid changes of tumor vessels as a
probable therapy-induced alteration (Fig.
1B). In addition, HIF-2α staining could be observed (Fig. 1E). O2-O3
treatment was employed monthly together with a second-line PCV
chemotherapy. Then, 13 months following surgery, the catheter was
removed due to a local infection accompanied by seizures. The
tissue demonstrated reactive and resorptive changes with an
accumulation of macrophages (Fig.
1C). Next, six weeks later, the catheter was re-implanted and
the intra-tumoral O2-O3 treatment was resumed
together with the PCV chemotherapy. The patient returned to work
and was in a stable neurological condition for almost 40 months.
However, paresis of the arm worsened and MRI demonstrated tumor
progression. A second re-section was performed, and presented with
the histology of a full-blown cell-rich tumor recurrence (Fig. 1D) but with lowered HIF-2α
immunostaining (Fig. 1F) compared
with the first recurrence and the situation prior to
O2-O3 treatment. Comparing the two time
points, no differences could be observed in HIF-1α staining. The
patient died 40 months after the initial diagnosis, and 33 months
following the initiation of O2-O3 treatment.
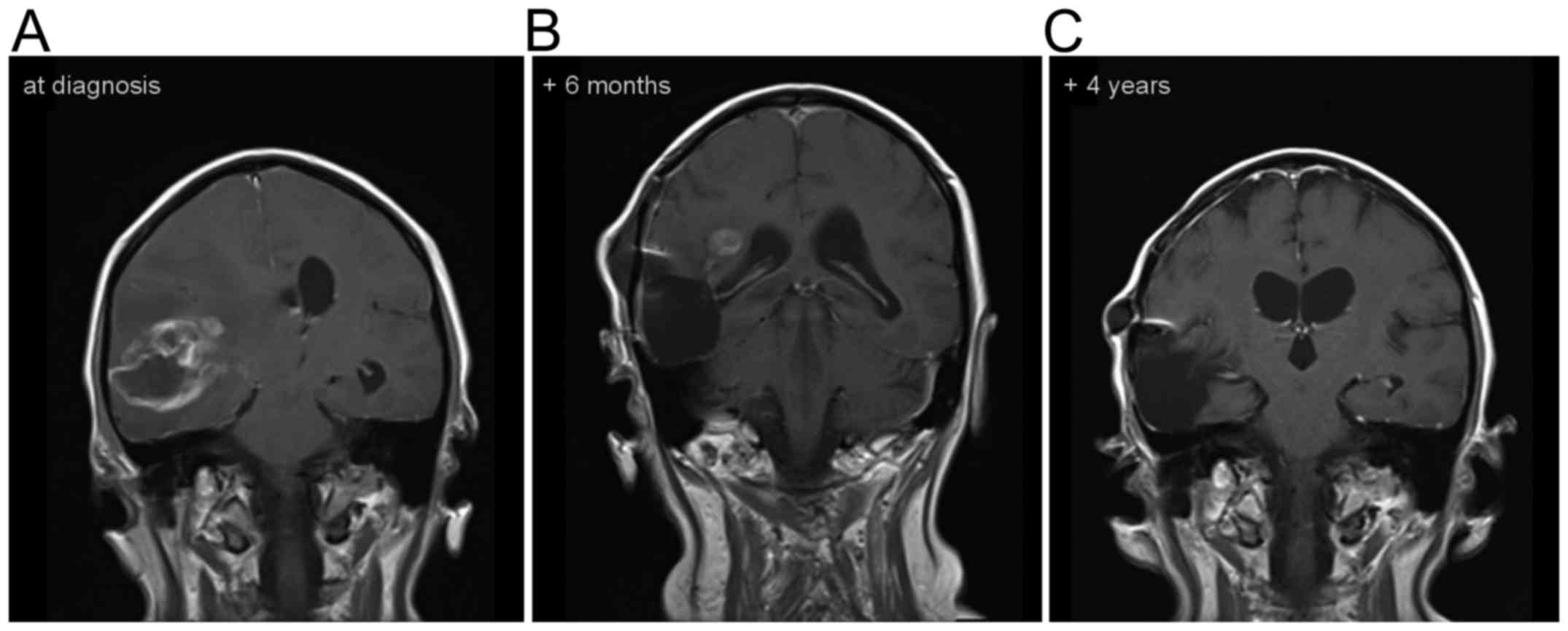
Another illustrative case is depicted in Fig. 4. This patient is still alive, more
than 4 years after the diagnosis GB.
Findings from the literature
search
The systematic literature search revealed 79
scientific studies. Following the screening of titles and
abstracts, 20 articles fulfilled the criteria for eligibility. In
three cases, no English full-text articles were available. Finally,
a total of seven clinical and ten basic research studies met the
eligibility criteria applied to the studies. Data extracted from
these studies are shown in Table
II.
 | Table II.Clinical and experimental studies
utilizing ozone. |
Table II.
Clinical and experimental studies
utilizing ozone.
| Author (year) | Study type | Disease | Route of
O3 application | Unit of
analysis | Sample size | Findings | (Refs.) |
|---|
| Batinjan et
al, 2014 | Case report | Radiation induced
osteonecrosis of the jaw | Alveolus
ozoning | Patients | N=1 | O3
improved wound healing | (16) |
| Brozoski et
al, 2014 | Case series | Biphoshonate
induced osteonecrosis of the jaw | Irrigation with
aqueous O3 4 mg/l | Patients | N=2 | O3
improved wound healing | (17) |
| Clavo et al,
2013 | Case series | Hemorrhagic
radiation-induced proctitis | Rectal insufflation
of O2-O3, O3 oil | Patients | N=17 |
O2-O3 reduced
endoscopic treatments (p< 0.063), reduced blood loss
(p<0.001), reduced median toxicity grades (p<0.001) | (18) |
| Clavo et al,
2005 | Case report | Radiation-induced
cystitis | Intra-vesicular
instillation of O3 at 20–25 g/ml | Patients | N=1 | O3
resulted in control of hematuria | (19) |
| Ripamonti et
al, 2011 | Prospective phase
I–II study | Osteonecrosis of
the jaw | O3 oil
suspension for 10 min for 10 local applications | Patients | N=10 | O3
induced spontaneous expulsion in 8 and new bone formation in 2
patients; in 2 patients no residual bone lesions were observed | (20) |
| Ripamonti et
al, 2012 | Case series | Osteonecrosis of
the jaw | O3
insufflation once daily for each pathological area | Patients | N=24 | O3
response rate was 75.0% (95% CI, 53.3–90.2%) in intention to treat
and 100% (95% CI, 81.5–100%) in per protocol analysis. No relapse
(follow-up mean 18 months, range 1–3 years) | (21) |
| Petrucci et
al, 2007 | Case series | Biphoshonate
induced osteonecrosis of the jaw | O3 once
daily, 7 days before to 7 days after surgery | Patients | N=12 | O3
resulted in 8 (75%) in resolution of osteonecrosis, in 4 patients
(25%) in improvement with persistence of lesio | (22) |
| Herbert et
al, 1996 | Basic research | Lung tumor
incidence | Inhalation
(O3 at 0.12, 0.5, 1.0 ppm, 6 h/day for lifetime) | Mice | N=100 total | O3
induced at the highest concentration the tumor incidence in female
mice | (23) |
| Ichinose et
al, 1992 | Basic research | Lung tumor
incidence | Inhalation
(O3 at 0.05 ppm for 13 months) | Rats | N=36 per group | O3 at
ambient level showed some tumor-enhancing action, although the
effect was small | (24) |
| Donaldson et al,
1991 | Basic research | Lung tumor
incidence | Inhalation (O3 at
0.2–0.8 ppm, 7 h/day for up to 4 days) | Rats | N=3, 13 groups | O3 at higher doses
decreased macrophages and increased neutrophils on day 1 and 2;
macrophages were larger | (25) |
| Hoogervorst et
al, 2003 | Basic research | Lung tumor
incidence | Inhalation
(O3 at 0.08 ppm for 13 weeks) | DNA
repair-deficient Xpa mice | N=20 per group | O3
induced cell proliferation and, dependent on the mouse strain, a
slight increase in tumor incidence | (26) |
| Witschi et
al, 1993 | Basic research | Lung tumor
incidence | Inhalation
(O3 at 0.8 ppm, 23 h/day for 24 weeks) | Hamsters | N=80 total | O3
increased the tumor incidence, but reduced the incidence following
treatment with the carcinogen N-nitrosodiethylamine | (27) |
| Last et al,
1987 | Basic research | Lung tumor
incidence | Inhalation
(O3 at 0.4 to 0.8 ppm, 7 h/day for 18 weeks) | Different mice
strains | N=31–37 per
group | O3 at
higher concentrations increased tumor incidence; following
treatment with the carcinogen urethane, O3 inhibited the
tumor development in A/J mice | (28) |
| Kiziltan et
al, 2015 | Basic research | Peritoneal
carcinomatosis | Intra-peritoneal
O3 at 20 or 40 µg/ml ± radiotherapy | Mice | N=60 total | O3 and
radiotherapy, either separately or concurrently, increased the
survival rates | (30) |
| Schulz et
al, 2008 | Basic research | Head and neck
squamous cell carcinoma | Intra-peritoneal
O2-O3 insufflation | Rabbits | N=59 total |
O2-O3 induced
significant tumor regression; effect reversed by immune suppression
(dexamethasone, cyclosporine A) | (31) |
| Rossmann et
al, 2014 | Basic research |
Papillomavirus-associated head and neck
cancer | Intra-peritoneal
O2-O3 insufflation | Rabbits | N=20 total |
O2-O3 resulted in
tumor eradication by immune ‘memory’ cells, and a significant
increase of peripheral white blood cells and CD3+ T cells | (32) |
All cohorts/samples were independent and no overlap
of included individuals could be identified. Overall, the sample
sizes of the clinical studies detected by the literature search
ranged from case reports to a prospective phase I–II study
including one to 24 patients. The basic research studies adopted
the guidelines for the care and use of animals and included 20 to
more than 100 animals.
Discussion
The rationale to perform an individual off-label
treatment in GB patients was three-fold: There was i) in
vitro and in vivo evidence for a preference for hypoxia
in GB, ii) in vitro and in vivo evidence for the
treatment efficacy of hyperoxia generating reactive O2
species in GB, and iii) in vivo evidence for the treatment
effect of O3 in stimulating the immune system in other
types of cancer (31,33).
Tumor cells, particularly those with stem cell-like
properties, exhibit a preference for hypoxia (6,12).
Therefore the intra-tumoral application of O2 was aimed
at establishing normoxia, thereby inhibiting proliferation and
migration (9) in addition to
promoting differentiation (5,8). Whether this target was accomplished, was
not verified. Five ml of O2-O3 were applied
intra-tumorally at a concentration of 40 µg ozone per ml once per
month. While direct intracellular oxymetry can be utilized in basic
research models, the measurement of tissue O2 levels in
the clinical setting is restricted to advanced and not readily
available techniques such as PET or MR spectroscopy (11).
HIFs are transcription factors that respond to
decreases in available O2 in the cellular environment,
or hypoxia. HIF-1 consists of an O2-sensitive α-subunit
and a constitutively expressed β-subunit, and belongs to a family
of transcription factors [PER/aryl hydrocarbon receptor nuclear
translocator (ARNT)/single minded (SIM)] (38–40).
HIF-1α facilitates, together with HIF-2α, O2 delivery
and cellular adaptation to hypoxia by stimulating multiple
biological processes, including erythropoesis, angiogenesis and
anaerobic glucose metabolism (41).
Histologically, following the intra-tumoral
O2-O3 application mainly reactive and
resorptive changes were identified (Fig.
1C). Notably, while relevant HIF-1α staining was not present
prior to or following the intra-tumoral O2-O3
application, the HIF-2α expression (particularly in tumor blood
vessels) was higher under the hypoxic conditions prior to, compared
with following, the treatment (Fig. 1E
and F).
Treatment strategies utilizing reactive
O2 species in glioma cell cultures have been
demonstrated to induce cell death by autophagy and apoptosis
(10) or at least cell growth
inhibition (14). Histologically, the
present illustrative case identified mainly reactive and resorptive
changes following the intra-tumoral O2-O3
application for a long time period. Only single scattered
pleomorphic astrocytic tumor cells could be detected (Fig. 1C).
O3, a gas that is produced endogenously
by granulocytes, induces the generation of reactive O2
species, stimulates the release of immunoactive cytokines (12) and regulates immunogenicity of human
glioma cells (13). The
intra-peritoneal insufflation of O2-O3
resulted in a tumoricidal immune response in experimental head and
neck squamous carcinoma (32) with
subsequent complete tumor remission (31). Oxidative stress converts the immune
response from a tumor permissive to a tumoricidal one, probably
through the stimulation of systemic T cells, resident macrophages
and dendritic cells (32).
Human natural killer cells as part of a systemic
immune response are most likely at least in part, responsible for
the absence of metastases in GB patients (42). The exposure of peripheral blood
mononuclear cells to a single dose of 1 µg/ml O3
increased the numbers of CD3-CD16+/56+ natural killer cells in
vitro (43). In human GB tissue
of long-term (>36 months) survivors, the number of CD 8, CD 20,
CD 25 and CD 95 positive lymphocytes was significantly increased
compared with short-term (<1 year) survivors. Therefore, the
O2-O3 application in GB may act as an
immunotherapy through the enhancement of human natural killer
cells. Histologically, in this context an accumulation of
macrophages was identified that immunohistochemically stained for
the CD68 (PGM1) antigen following the intra-tumoral
O2-O3 application in our illustrative case
(Fig. 1C).
The intra-peritoneal application of 1 ml
O3 at 20 or 40 µg/ml into the peritoneal cavity of mice
has been performed without side effects in a control group
confirming thereby the safety of the selected concentration
(30). Andreula et al
(44) safely injected four ml
O3 at 27 µg/ml into the lumbar disc and periganglionic
in 600 patients with clinical signs of lumbar disk nerve root
compression. The present study applied five ml O3 at 40
µg/ml into the tumor cavity and could not identify any direct cell
damage or necrosis around the cavity histologically. Nevertheless,
the safety of this application has to be confirmed by cell culture
of normal CNS tissue and glioma cells in addition to in vivo
studies.
The median overall survival rate in our series
comprising of five patients was 40 months. Since in one patient the
O2-O3 treatment was started following
diagnosis, the median survival following recurrence was 30.5 months
(range, 12 to 37 months) in the remaining four patients. These data
outperform previously published data (3) and the data from a recent multicenter
trial including 505 patients from 20 institutions undergoing
re-resection in recurrent GB (4). In
that multicenter study, the median overall survival rate was 25
months and 11.9 months following the first resection. Furthermore,
one of the patients in the present study treated with local
O2-O3 in addition to the standard treatment
following initial surgery remains alive, 53 months following the
diagnosis of GB (Fig. 4A-C). The
patients in our series did not differ substantially with regard to
known prognostic factors: The median age at diagnosis was 48 years
(range, 31 to 68 years), the median Karnofsky score at presentation
80% (range, 50 to 90%), the median proliferation index 30% (range,
20 to 40%) and the median quantitative MGMT promoter
methylation was 5% (range, 3 to 25%).
However, the authors are aware that a study focusing
on long-term survivors following GB presented even better data
(45). That study retrospectively
identified 50 long-term GB survivors (>36 months). In this
selected cohort, the median progression-free survival rate was 25.4
months (range, 2.3 to 97.8 months) compared with six months (range,
4 to 52 months) in our series, and the overall survival rate of
long-term survivors was 55.9 months (range, 38.2 to 98.6 months)
compared with 40 months (range, 16 to 52 months) in our series.
The authors are aware of several limitations of the
present study. The exact dose and the timing of the application
were based on estimates and patient comfort. Optimization of both
should be performed either in a basic research setting utilizing
tissue oxymetry or mapping brain tissue O2 saturation in
the clinical setting with PET or MRI. Furthermore, this off-label
case series should be transferred into a clinical trial.
Taken together, the overall survival rate of our
patient series is longer than obtained in an unselected multicenter
study. Taking a closer look at a long-term GB survivor cohort
(44), both progression-free survival
and overall survival rate are slightly shorter. These results and
the existing evidence revealed by the systematic literature search
highlighted that O3 therapy could be considered a viable
adjuvant therapy in oncological patients receiving
radiochemotherapy (34). The case
series of the present study indicated the potential benefit and
efficacy of intra-tumoral O2-O3 application
following surgery for GB. Following this descriptive approach,
further observational and experimental research is warranted to
elucidate cellular and systemic effects, in addition to ensuring
safety by applying inference statistical analyses based on an
appropriate sample size.
Acknowledgements
Part of the illustrative case was presented as a
poster on the Brain Tumor Meeting May 2013 in Berlin (abstract no.
36).
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
RM designed the study. MJR performed the
histological examination and contributed to the drafting and
revision of the manuscript. FDS performed the literature search,
data analysis and interpretation and contributed to writing the
manuscript. MF analyzed and interpreted the radiological patient
data. AK revised the study design, structured the data aquisition,
analysed and interpreted the data and drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethical
committee of the Bavarian National Medical Association (approval
no. EK-Nr. 2013-125). All patients provided written informed
consent.
Patient consent for publication
Following extensive information about their options
and the possible side effects of the treatment, all patients
provided their informed consent, including the publication of the
case report and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Park JK, Hodges T, Arko L, Shen M, Iacono
Dello D, McNabb A, Bailey Olsen N, Kreisl TN, Iwamoto FM, Sul J, et
al: Scale to predict survival after surgery for recurrent
glioblastoma multiforme. J Clin Oncol. 28:3838–3843. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krex D, Klink B, Hartmann C, von Deimling
A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger
G, et al: Long-term survival with glioblastoma multiforme. Brain.
130:2596–2606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ringel F, Pape H, Sabel M, Krex D, Bock
HC, Misch M, Weyerbrock A, Westermaier T, Senft C, Schucht P, et
al: Clinical benefit from resection of recurrent glioblastomas:
Results of a multicenter study including 503 patients with
recurrent glioblastomas undergoing surgical resection. Neuro Oncol.
18:96–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kathagen A, Schulte A, Balcke G, Phillips
HS, Martens T, Matschke J, Günther HS, Soriano R, Modrusan Z,
Sandmann T, et al: Hypoxia and oxygenation induce a metabolic
switch between pentose phosphate pathway and glycolysis in glioma
stem-like cells. Acta Neuropathol. 126:763–780. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seidel S, Garvalov BK, Wirta V, von
Stechow L, Schänzer A, Meletis K, Wolter M, Sommerlad D, Henze AT,
Nistér M, et al: A hypoxic niche regulates glioblastoma stem cells
through hypoxia inducible factor 2 alpha. Brain. 133:983–995. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Y, Zhou Y, Shingu T, Feng L, Chen Z,
Ogasawara M, Keating MJ, Kondo S and Huang P: Metabolic alterations
in highly tumorigenic glioblastoma cells: Preference for hypoxia
and high dependency on glycolysis. J Biol Chem. 286:32843–32853.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bar EE, Lin A, Mahairaki V, Matsui W and
Eberhart CG: Hypoxia increases the expression of stem-cell markers
and promotes clonogenicity in glioblastoma neurospheres. Am J
Pathol. 177:1491–1502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eckerich C, Zapf S, Fillbrandt R, Loges S,
Westphal M and Lamszus K: Hypoxia can induce c-Met expression in
glioma cells and enhance SF/HGF-induced cell migration. Int J
Cancer. 121:276–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trejo-Solis C, Jimenez-Farfan D,
Rodriguez-Enriquez S, Fernandez-Valverde F, Cruz-Salgado A,
Ruiz-Azuara L and Sotelo J: Copper compound induces autophagy and
apoptosis of glioma cells by reactive oxygen species and JNK
activation. BMC Cancer. 12:1562012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mendichovszky I and Jackson A: Imaging
hypoxia in gliomas. Br J Radiol. 84:S145–S158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kadayakkara DK, Janjic JM, Pusateri LK,
Young WB and Ahrens ET: In vivo observation of intracellular
oximetry in perfluorocarbon-labeled glioma cells and
chemotherapeutic response in the CNS using fluorine-19 MRI. Magn
Reson Med. 64:1252–1259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Olin MR, Andersen BM, Litterman AJ, Grogan
PT, Sarver AL, Robertson PT, Liang X, Chen W, Parney IF, Hunt MA,
et al: Oxygen is a master regulator of the immunogenicity of
primary human glioma cells. Cancer Res. 71:6583–6589. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cannizzaro A, Falzacappa Verga CV,
Martinelli M, Misiti S, Brunetti E and Bucci B: O(2/3) exposure
inhibits cell progression affecting cyclin B1/cdk1 activity in
SK-N-SH while induces apoptosis in SK-N-DZ neuroblastoma cells. J
Cell Physiol. 213:115–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bocci VA: Scientific and medical aspects
of ozone therapy. State of the art. Arch Med Res. 37:425–435. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batinjan G, Zore Filipovic I, Vuletic M
and Rupic I: The use of ozone in the prevention of
osteoradionecrosis of the jaw. Saudi Med J. 35:1260–1263.
2014.PubMed/NCBI
|
|
17
|
Brozoski MA, Lemos CA, Da Graca
Naclério-Homem M and Deboni MC: Adjuvant aqueous ozone in the
treatment of bisphosphonate induced necrosis of the jaws: report of
two cases and long-term follow-up. Minerva Stomatol. 63:35–41.
2014.PubMed/NCBI
|
|
18
|
Clavo B, Ceballos D, Gutierrez D, Rovira
G, Suarez G, Lopez L, Pinar B, Cabezon A, Morales V, Oliva E, et
al: Long-term control of refractory hemorrhagic radiation proctitis
with ozone therapy. J Pain Symptom Manage. 46:106–112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clavo B, Gutiérrez D, Martín D, Suárez G,
Hernández MA and Robaina F: Intravesical ozone therapy for
progressive radiation-induced hematuria. J Altern Complement Med.
11:539–541. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ripamonti CI, Cislaghi E, Mariani L and
Maniezzo M: Efficacy and safety of medical ozone (O(3)) delivered
in oil suspension applications for the treatment of osteonecrosis
of the jaw in patients with bone metastases treated with
bisphosphonates: Preliminary results of a phase I–II study. Oral
Oncol. 47:185–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ripamonti CI, Maniezzo M, Boldini S, Pessi
MA, Mariani L and Cislaghi E: Efficacy and tolerability of medical
ozone gas insufflations in patients with osteonecrosis of the jaw
treated with bisphosphonates-Preliminary data: Medical ozone gas
insufflation in treating ONJ lesions. J Bone Oncol. 1:81–87. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petrucci MT, Gallucci C, Agrillo A,
Mustazza MC and Foà R: Role of ozone therapy in the treatment of
osteonecrosis of the jaws in multiple myeloma patients.
Haematologica. 92:1289–1290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herbert RA, Hailey JR, Grumbein S, Chou
BJ, Sills RC, Haseman JK, Goehl T, Miller RA, Roycroft JH and
Boorman GA: Two-year and lifetime toxicity and carcinogenicity
studies of ozone in B6C3F1 mice. Toxicol Pathol. 24:539–548. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ichinose T and Sagai M: Combined exposure
to NO2, O3 and H2SO4-aerosol and lung tumor formation in rats.
Toxicology. 74:173–184. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Donaldson K, Brown GM, Brown DM, Slight J,
Maclaren WM and Davis JM: Leukocyte-mediated epithelial injury in
ozone-exposed rat lung. Res Rep Health Eff Inst. 1–27.
1991.PubMed/NCBI
|
|
26
|
Hoogervorst EM, de Vries A, Beems RB, van
Oostrom CT, Wester PW, Vos JG, Bruins W, Roodbergen M, Cassee FR,
Vijg J, et al: Combined oral benzo[a]pyrene and inhalatory ozone
exposure have no effect on lung tumor development in DNA
repair-deficient Xpa mice. Carcinogenesis. 24:613–619. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Witschi H, Wilson DW and Plopper CG:
Modulation of N-nitrosodiethylamine-induced hamster lung tumors by
ozone. Toxicology. 77:193–202. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Last JA, Warren DL, Pecquet-Goad E and
Witschi H: Modification by ozone of lung tumor development in mice.
J Natl Cancer Inst. 78:149–154. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teke K, Ozkan TA, Cebeci OO, Yilmaz H,
Keles ME, Ozkan L, Dillioglugil MO, Yildiz DK and Dillioglugil O:
Preventive effect of intravesical ozone supplementation on
n-methyl-n-nitrosourea-induced non-muscle invasive bladder cancer
in male rats. Exp Anim. 66:191–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiziltan HŞ, Bayir AG, Yucesan G, Eris AH,
İdin K, Karatoprak C, Aydin T, Akcakaya A and Mayadagli A: Medical
ozone and radiotherapy in a peritoneal, Erlich-ascites, tumor-cell
model. Altern Ther Health Med. 21:24–29. 2015.PubMed/NCBI
|
|
31
|
Schulz S, Haussler U, Mandic R, Heverhagen
JT, Neubauer A, Dünne AA, Werner JA, Weihe E and Bette M: Treatment
with ozone/oxygen-pneumoperitoneum results in complete remission of
rabbit squamous cell carcinomas. Int J Cancer. 122:2360–2367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rossmann A, Mandic R, Heinis J, Höffken H,
Küssner O, Kinscherf R, Weihe E and Bette M: Intraperitoneal
oxidative stress in rabbits with papillomavirus-associated head and
neck cancer induces tumoricidal immune response that is adoptively
transferable. Clin Cancer Res. 20:4289–4301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bocci V: Does ozone really ‘cure’ cancer?
Int J Cancer. 123:12222008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luongo M, Brigida AL, Mascolo L and
Gaudino G: Possible therapeutic effects of ozone mixture on hypoxia
in tumor development. Anticancer Res. 37:425–435. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Delic S, Lottmann N, Jetschke K,
Reifenberger G and Riemenschneider MJ: Identification and
functional validation of CDH11, PCSK6 and SH3GL3 as novel glioma
invasion-associated candidate genes. Neuropathol Appl Neurobiol.
38:201–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Karnofsky DA: The bases for cancer
chemotherapy. Stanford Med Bull. 6:257–269. 1948.PubMed/NCBI
|
|
38
|
Kewley RJ, Whitelaw ML and Chapman-Smith
A: The mammalian basic helix-loop-helix/PAS family of
transcriptional regulators. Int J Biochem Cell Biol. 36:189–204.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Semenza GL: Regulation of mammalian O2
homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol.
15:551–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wenger RH, Stiehl DP and Camenisch G:
Integration of oxygen signaling at the consensus HRE. Sci STKE.
2005:re122005.PubMed/NCBI
|
|
41
|
Semenza GL: HIF-1 and mechanisms of
hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee SJ, Song L, Yang MC, Mao CP, Yang B,
Yang A, Jeang J, Peng S, Wu TC and Hung CF: Local administration of
granulocyte macrophage colony-stimulating factor induces local
accumulation of dendritic cells and antigen-specific CD8+ T cells
and enhances dendritic cell cross-presentation. Vaccine.
33:1549–1555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kucuksezer UC, Zekiroglu E, Kasapoglu P,
Adin-Cinar S, Aktas-Cetin E and Deniz G: A stimulatory role of
ozone exposure on human natural killer cells. Immunol Invest.
43:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Andreula CF, Simonetti L, De Santis F,
Agati R, Ricci R and Leonardi M: Minimally invasive oxygen-ozone
therapy for lumbar disk herniation. AJNR Am J Neuroradiol.
24:996–1000. 2003.PubMed/NCBI
|
|
45
|
Adeberg S, Bostel T, König L, Welzel T,
Debus J and Combs SE: A comparison of long-term survivors and
short-term survivors with glioblastoma, subventricular zone
involvement: A predictive factor for survival? Radiat Oncol.
9:952014. View Article : Google Scholar : PubMed/NCBI
|