Introduction
Breast cancer is a group of heterogeneous diseases
with different clinical and histological forms (1). The molecular and clinical heterogeneity
of breast cancer renders it necessary to identify biomarkers of
clinical outcomes so that patients can be treated with the most
appropriate chemotherapeutic protocols (2). Therefore, identification of biomarkers
that will predict breast cancer to chemotherapeutic drugs is
important for the future development of individualized treatment
for patients with breast cancer.
Poly(ADP-ribose) polymerases (PARPs) constitute a
family of enzymes that catalyze poly(ADP-ribosylation) of
DNA-binding proteins and perform a key role in the regulation of
transcription, genome stability, energy metabolism, tumorigenesis
and cellular responses to DNA damage (3,4). The PARP
superfamily is composed of 17 members, of which PARP-1, PARP-2 and
PARP-3 are activated by DNA strand breaks and serve an important
role in the repair of single strand breaks and/or double strand
breaks (DSBs) (5–7). PARP-1, the most studied member of the
PARP superfamily, has been reported to be overexpressed in numerous
malignant tumors, including breast cancer, and is associated with
invasiveness and poor clinical outcomes (8–13). In
addition, it is well-known that the a combination of PARP
inhibitors and DNA damaging chemotherapy, can increase tumor
responses and improve the survival of triple-negative patients with
breast cancer compared with chemotherapy alone (14). Most importantly, PARP inhibitors are
particularly efficient against tumors with defects in DNA repair
mechanisms, including tumors with breast cancer gene (BRCA)
mutations (15,16).
Despite having similar functions in the regulation
of cellular responses to DNA damage, PARP-3 exhibits structural and
functional differences from PARP-1 (17). Although PARP-3 shares a conserved
C-terminal region with PARP-1, it has a shorter N-terminal region
than PARP-1. PARP-3 also exhibits different N-terminal functions,
including DNA binding or DNA-dependent activation (18). In addition, it has been reported that
knockdown of PARP3, but not PARP1, results in an increase in the
production of DSB induced by ionizing radiation (19). However, unlike PARP-1, less is known
about the role of PARP-3 in breast cancer. Bieche et al
(20) reported that the mRNA
expression of PARP-3 was under-expressed in 10.4% of patients with
breast cancer and this PARP-3 under-expression was mutually
exclusive with overexpression of PARP-1. To date, the protein
expression of PARP-3 in patients with breast cancer has not yet
been investigated. It remains to be determined whether the protein
level of PARP-3 is consistent with its mRNA level in breast
cancer.
In the present study, the expression of PARP-3 was
investigated in 493 breast cancer samples and 54 tumor-adjacent
control samples using immunohistochemistry. The present study aimed
to analyze the association of PARP-3 expression with
clinicopathological features, chemotherapeutic responses and
prognosis of patients with breast cancer.
Patients and methods
Ethics statement
The present study was approved by the Medical Ethics
Committee of China Medical University (Shenyang, China). Due to the
retrospective nature of the study, the Medical Ethics Committee
waived the requirement of written informed consents by the
patients.
Patients
The present study included human breast tissues from
493 female patients with breast cancer, who underwent surgery at
the First Affiliated Hospital of China Medical University between
January 2005 and October 2010. A total of 54 samples adjacent to
the tumors outside the cancer loci were collected as controls. The
diagnosis of breast cancer was confirmed by pathological staining.
Histological evaluation of 54 samples adjacent to tumors exhibited
no histological tumor-associated features.
The average age of patients with breast cancer was
51.3±10.6 years (range, 20–82 years). The histological grade of the
cancer was determined according to the World Health Organization
grading system (21,22). The stage of the cancer was evaluated
according to the tumor-node-metastasis (TNM) staging system
(22). Clinicopathological data,
including patient age, menopausal status, tumor size, lymph node
metastasis, p53 status and BRCA1 status were retrospectively
retrieved from medical records.
All patients did not undergo radiation therapy and
chemotherapy prior to surgery. Following surgery, 291 patients
received cyclophosphamide/doxorubicin or epirubicin/5-fluorouracil
(CAF/CEF) and 95 patients received CAF/CEF and docetaxel (CAF/CEF
and D). The remaining 107 patients received other chemotherapeutic
regimens containing docetaxel or cisplatin alone or in
combination.
Immunohistochemistry
Tissue sections (4 µm) were fixed with 4% formalin
at room temperature for 48 h and paraffin-embedded tissue blocks
for immunohistochemical staining. Sections were deparaffinized with
xylene, rehydrated in a graded alcohol series of 100 and 95%
(Sinopharm Chemical reagent Co., Ltd., Shanghei, China) at a
concentration of 100, 95, 85, 75, 65% and H2O and
sections were put into 3% citric acid-sodium citrate buffer
(pH=6.0) and heated in a microwave oven at 100°C for 10 min to
retrieve the antigen. Endogenous peroxidase activity was blocked by
incubating the sections in 3% H2O2 at 37°C
for 20 min. Sections were subsequently blocked to avoid nonspecific
binding with 10% normal goat serum (Boster Biological Technology,
Pleasanton, CA, USA) at 37°C for 30 min and incubated at 4°C
overnight with the polyclonal antibody against PARP-3 (dilution,
1:100; cat. no. 96601; rabbit anti-human polyclonal antibodies;
Abcam, Cambridge, UK), followed by incubation with biotinylated
secondary antibodies (secondary antibody A in the kit-0305;
dilution, 1:200; cat. no. kit-0305; Maxim Biotechnologies, Fuzhou,
China) for 30 min at 37°C. Sections were then incubated with
streptavidin horseradish peroxidase (secondary antibody B in the
kit-0305; dilution, 1:200; cat. no. kit-0305; Maxim
Biotechnologies) for an additional 20 min at 37°C and stained with
3,3-diaminobenzidine at room temperature for 1 min (dilution,
1:200; cat. no. LI-9032; OriGene Technologies, Inc, Beijing,
China). Sections were counterstained with hematoxylin, dehydrated
and mounted. For negative controls, the sections were not incubated
with primary antibodies.
Evaluation of
immunohistochemistry
The immunostained sections were examined under the
light microscope (magnification, ×200; select 3 fields/view) by two
pathologists blinded to the experimental conditions. The intensity
of immunoreactivity was scored as follows: 0, no staining; 1, weak
staining; 2, moderate staining and 3, strong staining. A percentage
scoring system was used to assess the number of stained cells and
the scores were assigned by using 5% increments as previously
reported (23,24). The final scores were used to determine
the cutoff value for discriminating tumors with the high expression
of PARP-3 from tumors with the low expression, using receiver
operating characteristic (ROC) curves. The sensitivity and
specificity for the survival of patients with breast cancer was
plotted to generate ROC curves.
Statistical analysis
Analyses were performed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). Pearson's χ2 or Fisher's exact
probability tests were used to evaluate the association between
PARP-3 expression and clinicopathological characteristics of
patients with breast cancer. Survival probabilities were estimated
by the Kaplan-Meier method and assessed by a log-rank test.
Univariate and multivariate Cox proportional hazards regression
models were used for assessing the association between potential
confounding variables and prognosis [overall survival (OS) or
disease-free survival (DFS)]. OS was calculated as the time between
the first day of diagnosis and disease-associated mortality or last
known follow-up. The disease-free survival (DFS) was calculated as
the time between the first day of diagnosis and the occurrence of
local recurrence or distant metastasis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinicopathological characteristics of
patients with breast cancer
Table I summarizes
clinicopathological characteristics of 493 patients with breast
cancer. Of the 493 patients, age, menopausal status, tumor size,
tumor type, histological grade, TNM stage and lymph node metastasis
were recorded in 493, 493, 493, 492, 492, 493 and 493 patients,
respectively. The majority of these patients had a tumor with
invasive ductal carcinoma (92.5%), <2 cm in size (65.9%),
histological grade II (74.6%) or TNM stage I–II (73.8%). Lymph node
metastasis occurred in 225 (45.6%) of 493 patients.
 | Table I.Clinicopathological characteristics
of patients with breast cancer. |
Table I.
Clinicopathological characteristics
of patients with breast cancer.
| Parameters | Total, n | Patients, n
(%) |
|---|
| Age at
diagnosis | 493 |
|
| ≤51
years |
| 279 (56.6) |
| >51
years |
| 214 (43.4) |
| Menopausal
status | 493 |
|
|
Pre-menopause |
| 268 (54.4) |
|
Post-menopause |
| 225 (45.6) |
| Tumor size | 493 |
|
| ≤2.0
cm |
| 325 (65.9) |
|
>2.0, <5.0 cm |
| 146 (29.6) |
| ≥5.0
cm |
| 22 (4.5) |
| Tumor type | 492 |
|
| Ductal
carcinoma |
| 455 (92.5) |
| Lobular
carcinoma |
| 11 (2.2) |
|
Mucinous carcinoma |
| 9 (1.8) |
|
Others |
| 17 (3.5) |
| Histological
grade | 492 |
|
| G1 |
| 83 (16.9) |
| G2 |
| 367 (74.6) |
| G3 |
| 42 (8.5) |
| TNM stage | 493 |
|
|
I–II |
| 364 (73.8) |
|
III–IV |
| 129 (26.2) |
| Lymph node
metastasis | 493 |
|
| No |
| 268 (54.4) |
|
Yes |
| 225 (45.6) |
| p53 | 397 |
|
|
Negative |
| 166 (41.8) |
|
Positive |
| 231 (58.2) |
| BRCA1 | 396 |
|
|
Negative |
| 102 (25.8) |
|
Positive |
| 294 (74.2) |
| Chemotherapy
regimen | 386 |
|
|
CAF/CEF |
| 291 (75.4) |
|
CAF/CEF+T |
| 95 (24.6) |
Follow-up information was available for 493 patients
with breast cancer. During the follow-up period of 9–118 months,
relapses occurred in 85 cases and cancer-associated mortalities
were identified in 55 cases. The 5-year survival rate was 88.0%.
The mean OS and DFS times were 66.3 and 63.7 months,
respectively.
PARP-3 overexpression in breast
cancer
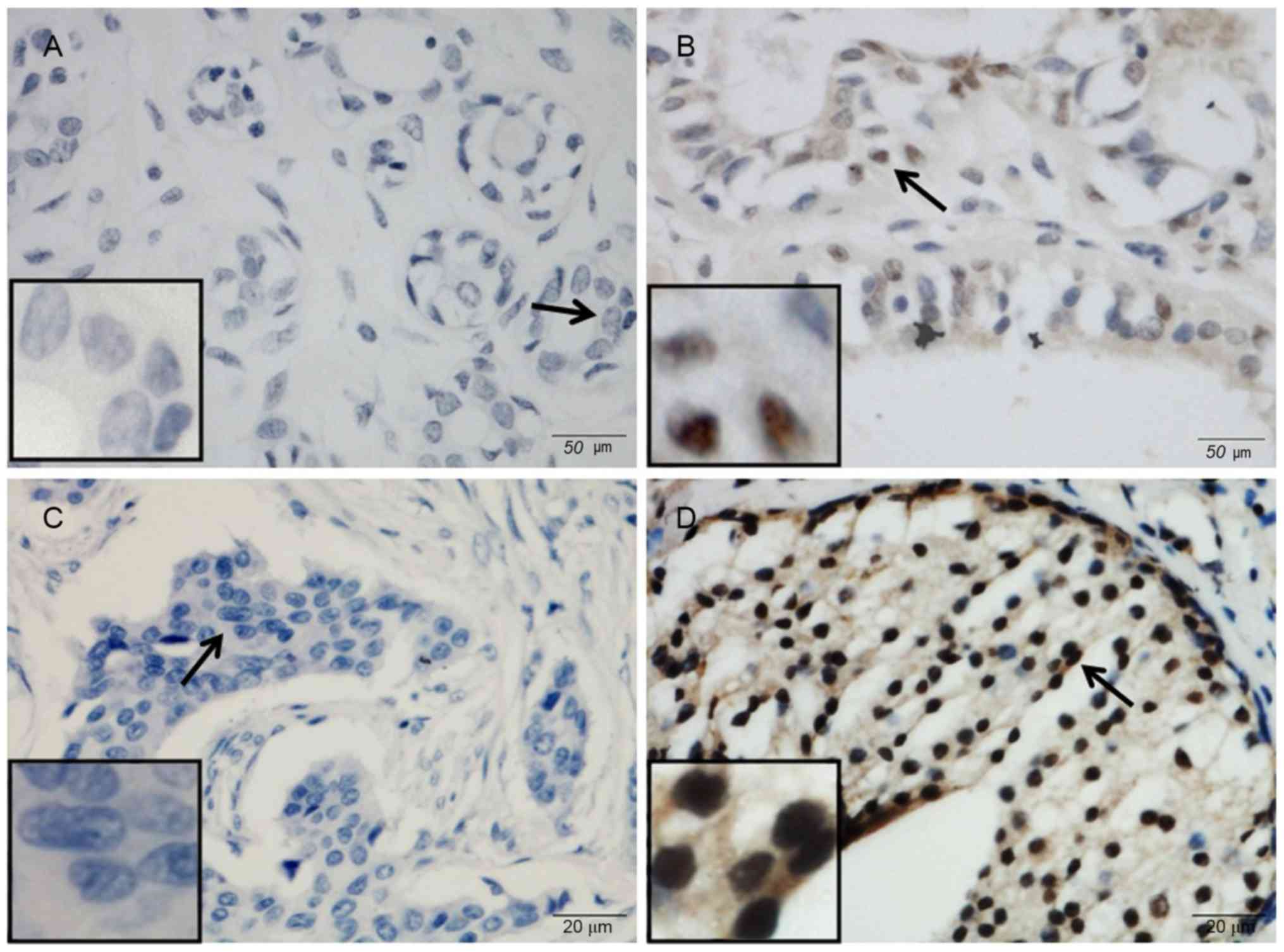
The expression of PARP-3 was studied in 493 breast
cancer samples and 54 tumor-adjacent control samples using
immunohistochemistry (Fig. 1). PARP-3
was mainly expressed in the nucleus. Nuclear expression of PARP-3
was observed in 234 (47.5%) of 493 breast cancer samples and 9
(16.7%) of 54 control samples. PARP-3 immunoreactivity occurred
significantly more frequently in breast cancer samples compared
with control samples (P<0.001).
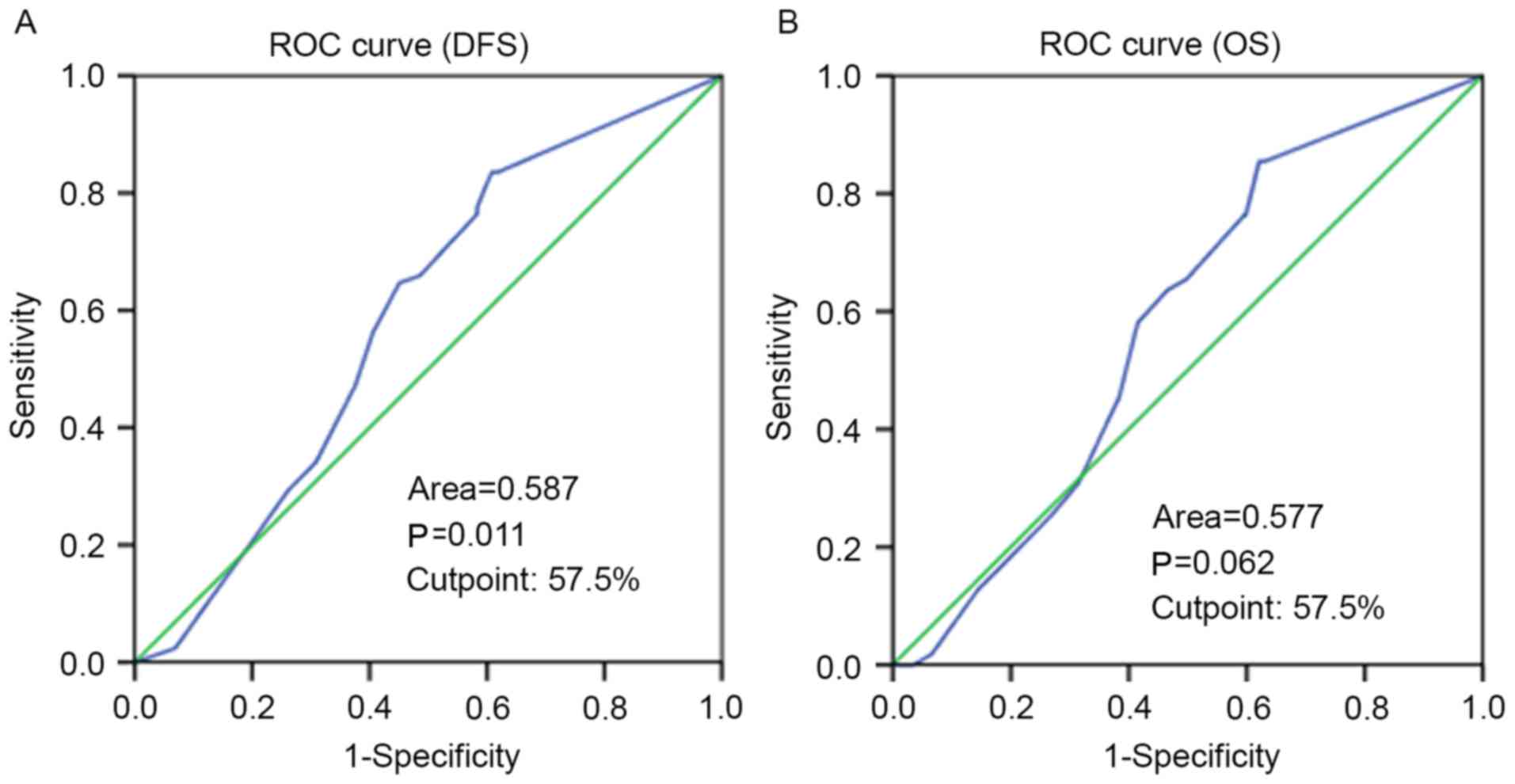
Selection of the cutoff value for
PARP-3 expression
ROC curve analysis was performed to determine an
optimal cutoff score for PARP-3 expression in breast cancer
samples. Based on DFS time data, a cutoff score of 57.5% was
selected for PARP-3 expression (Fig.
2). Tumors with immunohistological scores ≥57.5 and <57.5%
were defined as tumors with high and low PARP-3 expression,
respectively. A total of 234 (47.5%) tumors exhibited high
expression and 259 (52.5%) tumors showed low expression.
Association of PARP-3 expression with
clinicopathological characteristics of patients with breast
cancer
The association between PARP-3 expression and
clinicopathological characteristics of patients with breast cancer
was investigated (Table II). Age,
menopausal status, tumor size, tumor type, TNM stage, lymph node
metastasis, p53 status and BRCA1 status were not significantly
associated with the expression of PARP-3. High PARP-3 expression
level was associated with histological grade II–III (P=0.012) when
compared with PARP-3 low expression level.
 | Table II.Association between PARP-3 expression
and clinicopathological features of patients with breast
cancer. |
Table II.
Association between PARP-3 expression
and clinicopathological features of patients with breast
cancer.
|
| PARP-3 expression,
n (%) |
|
|---|
|
|
|
|
|---|
|
Characteristics | High | Low |
P-valuea |
|---|
| Age at
diagnosis |
|
|
|
| ≤51
years | 138 (49.5) | 141 (50.5) | 0.310 |
| >51
years | 96 (44.9) | 118 (55.1) |
|
| Menopausal
status |
|
|
|
|
Pre-menopause | 133 (49.6) | 135 (50.4) | 0.294 |
|
Post-menopause | 101 (44.9) | 124 (55.1) |
|
| Tumor size |
|
|
|
| ≤2.0
cm | 156 (48.0) | 169 (52.0) | 0.942 |
|
>2.0, <5.0 cm | 68 (46.6) | 78 (53.4) |
|
| ≥5.0
cm | 10 (45.5) | 12 (54.5) |
|
| Lymph node
metastasis |
|
|
|
| No | 122 (45.5) | 146 (54.5) | 0.346 |
|
Yes | 112 (49.8) | 113 (50.2) |
|
| TNM stage |
|
|
|
|
I–II | 175 (48.1) | 189 (51.9) | 0.647 |
|
III–IV | 70 (54.3) | 59 (45.7) |
|
| Histological
grade |
|
|
|
| G1 | 29 (34.9) | 54 (65.1) | 0.012 |
| G2 | 178 (48.5) | 189 (51.5) |
|
| G3 | 26 (61.9) | 16 (38.1) |
|
| Histological
type |
|
|
|
| Ductal
carcinoma | 216 (47.5) | 239 (52.5) | 0.196 |
| Lobular
carcinoma | 7 (63.6) | 4 (36.4) |
|
|
Mucinous carcinoma | 6 (66.7) | 3 (33.3) |
|
|
Other | 5 (29.4) | 12 (70.6) |
|
| p53 status |
|
|
|
|
Negative | 80 (48.2) | 86 (51.8) | 0.650 |
|
Positive | 106 (45.9) | 125 (54.1) |
|
| BRCA1 status |
|
|
|
|
Negative | 52 (51.0) | 50 (49.0) | 0.289 |
|
Positive | 132 (44.9) | 162 (55.1) |
|
Association of PARP-3 expression with
the survival of patients with breast cancer
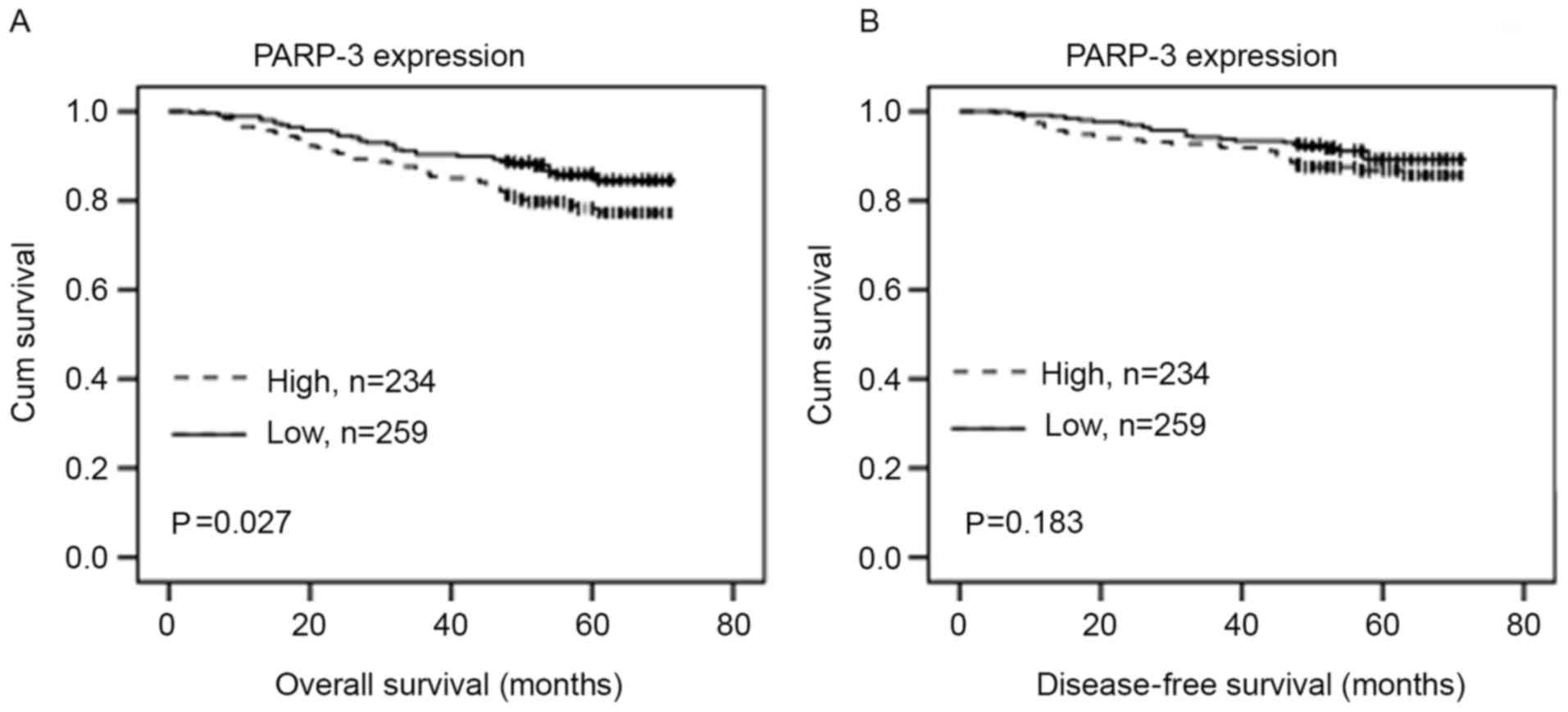
The association of the PARP-3 expression with the OS
or DFS in patients with breast cancer was evaluated using
Kaplan-Meier analysis and log-rank test. PARP-3 overexpression was
significantly associated with shorter DFS time (P=0.027) (Fig. 3A). Although PARP-3 expression
exhibited a tendency toward shorter OS, no statistically
significant difference was observed (P=0.183) (Fig. 3B).
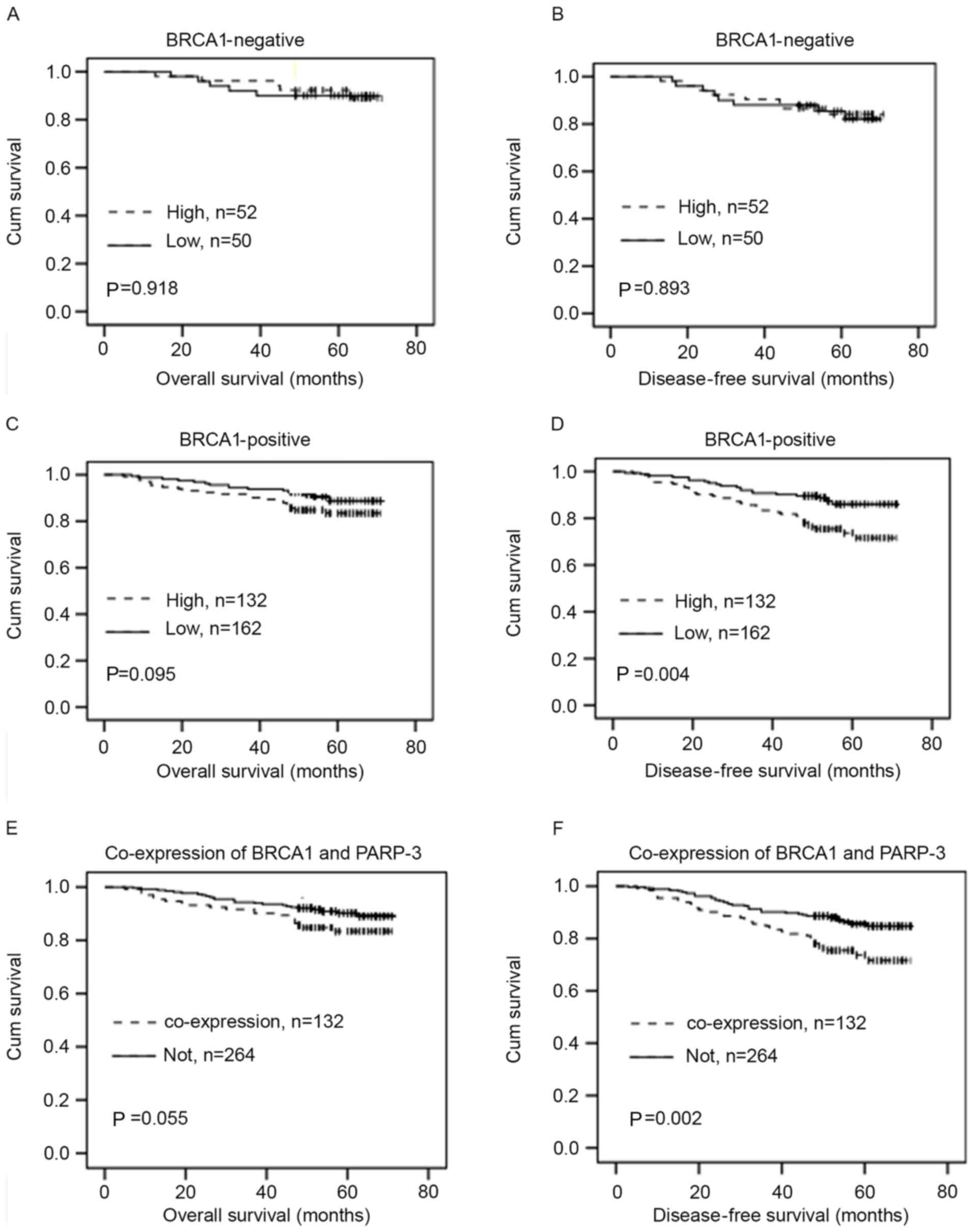
The association of PARP-3 expression with the OS or
DFS in breast cancer patients with different BRCA1 statuses was
then investigated. In BRCA1-negative patients, PARP-3 expression
was not significantly associated with the OS or DFS (P>0.05)
(Fig. 4A and B). However, in
BRCA1-positive patients, PARP-3 expression exhibited a tendency
toward shorter OS, but this association was not statistically
significant (P=0.095) (Fig. 4C).
PARP-3 overexpression was significantly associated with shorter DFS
time (P=0.004) (Fig. 4D).
Furthermore, there was a greater association between the
combination of high PARP3 and BRCA1 expression and shorter OS
(P=0.055) (Fig. 4E) and DFS time
(P=0.002) (Fig. 4F) compared with
non-combination of PARP3 and BRCA1.
Univariate Cox regression analysis was performed to
estimate the impact of each clinicopathological variable on OS and
DFS in patients with breast cancer. The univariate analysis
identified that menopausal status [hazard ratio (HR)=1.527;
P=0.044], histological grade (HR=2.674; P=0.012), TNM stage
(HR=4.20; P<0.001) and lymph node metastasis (HR=2.513;
P<0.001) were significantly associated with the OS and DFS of
patients with breast cancer (Table
III). Age (P=0.041) and tumor size (P=0.042) were also
identified to be significantly associated with the OS of patients
with breast cancer. In addition, PARP-3 overexpression was
significantly associated with shorter DFS time of patients with
breast cancer (P=0.029; Table III).
Furthermore, multivariate Cox regression analysis (Table IV) indicated that TNM stage
(RR=5.665; P<0.001), menopausal status (RR=2.535; P=0.045) and
PARP-3 (RR=1.944; P=0.008) were independent prognostic factors for
shorter DFS time. TNM stage (HR=9.75; P<0.001) and histological
grade (HR=2.592; P=0.004) were independent prognostic factors for
shorter OS in patients with breast cancer.
 | Table III.Univariate Cox regression analysis of
overall survival and disease-free survival in patients with breast
cancer. |
Table III.
Univariate Cox regression analysis of
overall survival and disease-free survival in patients with breast
cancer.
|
| DFS | OS |
|---|
|
|
|
|
|---|
| Parameters | HR (95% CI) |
P-valuea | HR (95% CI) |
P-valuea |
|---|
| Age (>51/≤51
years) | 1.352
(0.90–2.04) | 0.148 | 1.702
(1.02–2.84) | 0.041 |
| Menopausal status
(post/pre) | 1.527
(1.01–2.30) | 0.044 | 1.846
(1.10–3.09) | 0.020 |
| Tumor size
(>2.0/≤2.0 cm) | 1.272
(0.83–1.95) | 0.271 | 1.714
(1.02–2.88) | 0.042 |
| Histological grade
(III/II/I) | 2.674
(1.24–5.78) | 0.012 | 6.350
(1.55–25.99) | 0.010 |
| Histological type
(ductal/lobular) | 0.804
(0.53–1.21) | 0.296 | 0.569
(0.26–1.23) | 0.153 |
| TNM stage
(V/IV/III/II/I) | 4.200
(2.78–6.34) | <0.001 | 9.084
(5.12–16.11) | <0.001 |
| Lymph node status
(yes/no) | 2.513
(1.63–3.87) | <0.001 | 4.572
(2.47–8.45) | <0.001 |
| BRCA1 status
(positive/negative) | 1.365
(0.79–2.38) | 0.271 | 1.431
(0.71–2.87) | 0.312 |
| p53 status
(positive/negative) | 1.063
(0.67–1.68) | 0.792 | 1.027
(0.59–1.79) | 0.927 |
| PARP-3
(positive/negative) | 1.619
(1.05–2.49) | 0.029 | 1.434
(0.84–2.44) | 0.186 |
 | Table IV.Multivariate Cox regression analysis
of overall survival and disease-free survival in breast in patients
with breast cancer. |
Table IV.
Multivariate Cox regression analysis
of overall survival and disease-free survival in breast in patients
with breast cancer.
|
| Disease-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
| Category | RR (95% CI) |
P-valuea | RR (95% CI) |
P-valuea |
|---|
| Age (>51/≤51
years) | 0.740
(0.30~1.82) | 0.513 | 0.77
(0.215~2.76) | 0.689 |
| Menopausal status
(post/pre) | 2.535
(1.02~6.30) | 0.045 | 3.156
(0.85~11.70) | 0.086 |
| Tumor size (≥5/
2–5/≤2.0 cm) | 1.163
(0.77~1.77) | 0.481 | 1.543
(0.95~2.51) | 0.081 |
| Histological grade
(III/II/I) | 1.681
(0.98~2.90) | 0.062 | 2.592
(1.35~4.97) | 0.004 |
| Histological type
(ductal/ lobular/mucinous/other) | 0.952
(0.52–1.76) | 0.875 | 0.591
(0.14–2.52) | 0.477 |
| TNM stage
(V/IV/III/II/I) | 5.665
(2.56~12.5) | <0.001 | 9.75
(3.12~30.51) | <0.001 |
| Lymph node status
(≥10/4~9/1~3/0) | 0.720
(0.31~1.65) | 0.439 | 0.934
(0.26~3.35) | 0.917 |
| PARP-3
(positive/negative) | 1.944
(1.19~3.19) | 0.008 | 1.716
(0.93~3.15) | 0.082 |
| BRCA1 status
(positive/negative) | 1.167
(0.64~2.12) | 0.612 | 1.078
(0.51~2.29) | 0.846 |
| p53 status
(positive/negative) | 1.130
(0.69~1.87) | 0.632 | 1.116
(0.60~2.08) | 0.730 |
Association of PARP-3 expression with
therapeutic responses in patients with breast cancer
The association between the level of PARP-3
expression and therapeutic responses in patients with breast cancer
receiving chemotherapy was examined. PARP-3 overexpression was
significantly associated with shorter DFS time in patients with
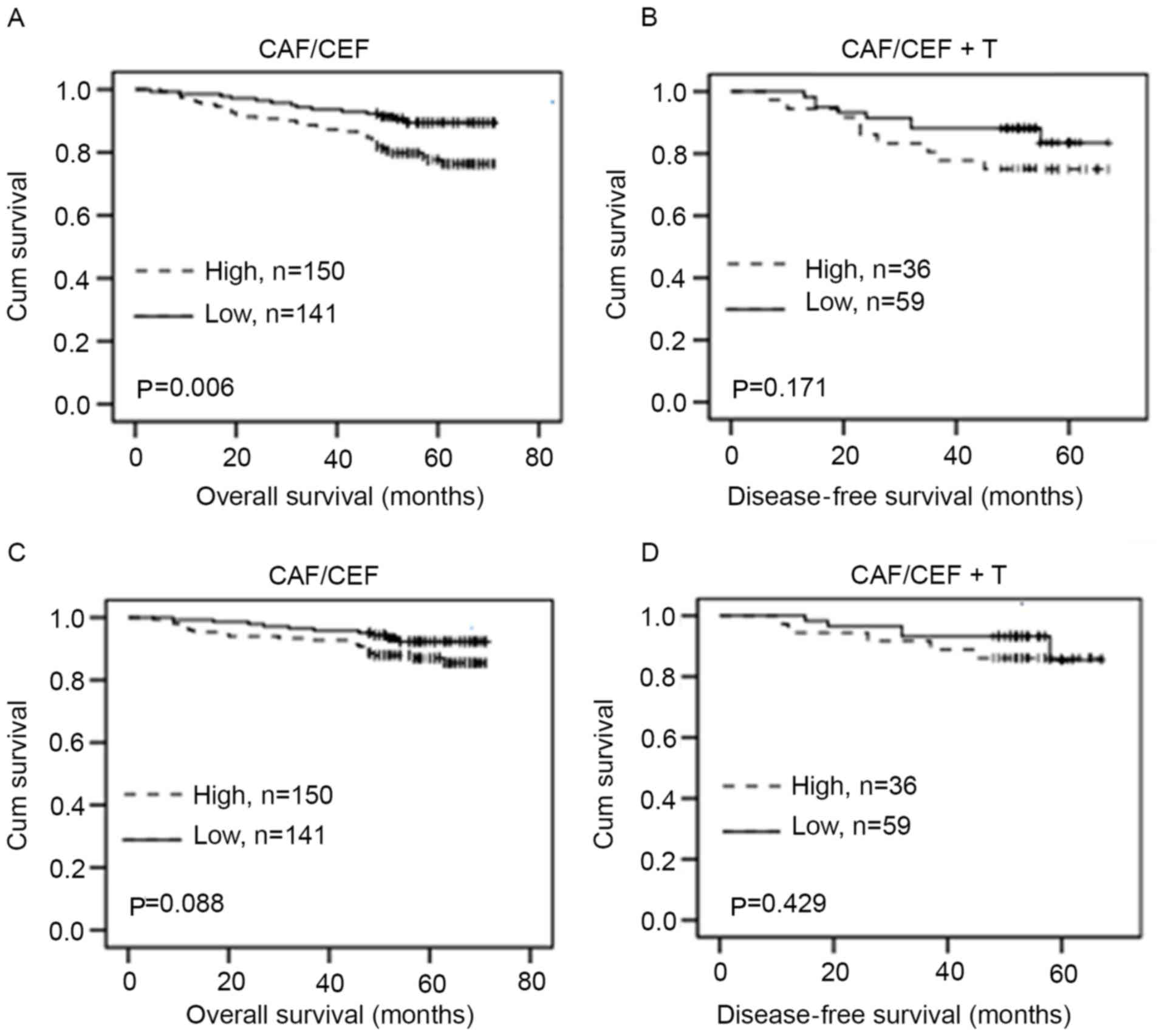
breast cancer with CAF/CEF treatment (P=0.006) (Fig. 5A), but not significantly associated
with DFS (P=0.171) (Fig. 5B) in
patients with CAF/CEF+T treatment. Although PARP-3 overexpression
exhibited a tendency toward shorter OS in patients with breast
cancer with CAF/CEF treatment, no statistically significant
difference was observed (P=0.088) (Fig.
5C). In addition, PARP-3 expression was not significantly
associated with OS (P=0.420) (Fig.
5D) in patients with CAF/CEF+T treatment.
Discussion
It is well known that DNA damage, if not repaired
properly, can lead to genetic instability, which may increase the
development of cancer (25). Previous
studies have shown that PARP-3 serves an important role in DSB
repair (7,19). However, the role of PARP-3 in breast
cancer tumorigenesis remains to be determined. In the present
study, tissue-microarray-based immunohistochemistry was performed
to examine PARP-3 expression in 493 patients with breast cancer and
54 tumor-adjacent control samples. It was revealed that PARP-3
immunoreactivity occurred more frequently in breast cancer samples
compared with control samples, indicating that PARP-3
overexpression may contribute to the development of breast cancer
malignancy. Similarly, several studies have shown that PARP-1, the
most studied member of PARP superfamily that is also involved in
DNA damage repair, is upregulated in numerous tumors, including
breast cancer (8–13). These findings indicated that the DNA
damage repair function of PARP-1 and PARP-3 may be important for
cancer development. However, Bieche et al (20) reported that the mRNA expression of
PARP-3 was under-expressed in 10.4% of patients with breast cancer.
The difference between the study by Bieche et al and the
present study may be due to different methods, since the mRNA
levels of PARP-3 detected by Bieche et al may not reflect
the protein level of PARP-3 examined by the present study.
The association between PARP-3 expression and
clinicopathological features in patients with breast cancer was
analyzed in the present study. It was revealed that PARP-3
overexpression was associated with more differentiated
(histological grade I–II) tumors. It is likely that PARP-3 is
upregulated in response to an increase in DNA breaks during tumor
cell differentiation, and PARP-3 upregulation may promote repair of
DNA damage in tumors, thereby increasing cancer progression and
development. Consistent with this hypothesis, PARP-3 overexpression
was also found to be significantly associated with shorter DFS time
and exhibited a tendency toward shorter OS in patients with breast
cancer. Similarly, PARP-1 overexpression has been reported to be
associated with poor prognosis in patients with breast cancer
(12,26). Furthermore, it was found that PARP-3
overexpression is an independent prognostic factor for shorter DFS
and OS in patients with breast cancer. Therefore, PARP-3 may be
used as a potential biomarker for clinical outcomes of patients
with breast cancer.
Eukaryotic cells have two repair pathways to repair
DSB: Homologous recombination and non-homologous end joining (NHEJ)
(27). PARP-3 in combination with
aprataxin and PNKP-like factor accelerates NHEJ (28,29), and
BRCA1 is known to be a central component in homologous
recombination (30–32). Furthermore, Beck et al reported
that PARP-3 performs a key role in determining the choice between
homologous recombination and NHEJ pathways in the repair of DSB
(33). In the present study, it was
indicated that PARP-3 overexpression was significantly associated
with shorter DFS time and exhibited a tendency toward shorter OS in
BRCA1-positive patients with breast cancer. Additionally, the
combined high expression of BRCA1 and PARP-3 was associated with
shorter DFS and OS in patients with breast cancer compared with
non-combined BRCA1 and PARP-3 expression. Therefore, for tumor
cells with high expression of BRCA1, PARP-3-overexpressing cells
may able to repair DNA damage more efficiently compared with PARP-3
deficient cells, thus leading to prolonged survival of the tumor
and poor prognosis of the patients with cancer. However, it was
found that PARP-3 expression was not significantly associated with
the OS or DFS in BRCA1-negative patients with breast cancer. The
findings that PARP-3 overexpression was significantly associated
with the shorter survival time in BRCA1-positive, but not
BRCA1-negative, breast cancer patients suggest that the role of
PARP-3 in breast cancer may depend on BRCA1 status. Therefore,
PARP-3 inhibitor may be a novel strategy for the treatment of
BRAC1-positive breast cancer patients with PARP-3
overexpression.
It is known that chemotherapeutic drugs can induce
DNA damage, and DNA damage repair may affect the outcome of therapy
(34). In the present study, the
association of PARP-3 expression with therapeutic responses in
patients with breast cancer receiving CAF/CEF chemotherapy was
examined. It was revealed that PARP-3 overexpression was
significantly associated with shorter DFS time, and exhibited a
tendency toward shorter OS in patients with breast cancer who
received CAF/CEF treatment. The present findings suggested that
tumors with PARP-3 overexpression exhibited resistance to
chemotherapy, possibly by an increased ability of DNA repair.
Furthermore, PARP-3 overexpression was not significantly associated
with DFS and OS in patients with CAF/CEF+T treatment. It appears
that addition of docetaxel inhibited PARP-3-induced drug resistance
in patients with breast cancer. It has been reported that docetaxel
can cause cleavage of PARP in breast cancer cells, melanoma cells
and ovarian cancer cells (35–37). Taken
together, the present results suggested that docetaxel may induce
cleavage of PARP-3, thereby reducing PARP-3-induced drug resistance
and improving the survival of patients with breast cancer with
PARP-3 overexpression.
In summary, PARP-3 expression was investigated in
493 patients with breast cancer, and the association of PARP-3
expression with the clinicopathological feature, therapeutic
responses and prognosis of patients with breast cancer was
analyzed. It was found that PARP-3 expression was significantly
increased in breast cancer tissues compared with tumor-adjacent
tissues. PARP-3 overexpression was associated with poor outcome of
patients with breast cancer, particularly in BRCA1-positive
patients. Furthermore, it was found that PARP-3 overexpression was
associated with shorter survival time in patients with CAF/CEF
chemotherapy, but not in patients with CAF/CEF+T chemotherapy,
indicating that inhibition of PARP-3 by docetaxel may increase the
survival of patients with breast cancer. PARP-3 may be used as a
biomarker for predicting the clinical outcome of patients receiving
chemotherapy, and targeting PARP-3 may be a potential therapeutic
strategy for the treatment of breast cancer with PARP-3
overexpression.
Acknowledgements
The authors would like to acknowledge Xuefeng Bai
for providing technical help and Xiaosong Yu for assisting in
writing the manuscript.
Funding
The present study was supported by grants from the
National Natural Science Foundation of the People's Republic of
China (grant no. 71273279; Beijing China), Program for Liaoning
Innovative Research Team in University, (grant no. LT2014016;
Shenyang, China) and Shenyang Science and Technology Projects
(grant no. F14-232-6-05; Shenyang, China).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
MW, ZS, LZ and HW assisted in the design and
conception of the present study. ZS, YW, QX, MS and ZY analyzed and
interpreted the data of patients with breast cancer. ZS, WL and XG
performed the histological examination of the breast cancer
tissues. LZ, HW, MS contributed in drafting the manuscript. QX and
MW were major contributor in revising the manuscript. ZC, PH
collected the patients' clinical data and performed the follow-up
study. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The Institute Research Medical Ethics Committee of
China Medical University approved the consent procedure.
Patient consent for publication
The patient, or parent, guardian or next of kin (if
patient is deceased) provided verbal informed consent for the
publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bertucci F and Birnbaum D: Reasons for
breast cancer heterogeneity. J Biol. 7:62008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patani N, Martin LA and Dowsett M:
Biomarkers for the clinical management of breast cancer:
International perspective. Int J Cancer. 133:1–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hakmé A, Wong HK, Dantzer F and Schreiber
V: The expanding field of poly(ADP-ribosyl)ation reactions.
‘Protein modifications: Beyond the usual suspects’ review series.
EMBO Rep. 9:1094–1100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hottiger MO, Hassa PO, Lüscher B, Schüler
H and Koch-Nolte F: Toward a unified nomenclature for mammalian
ADP-ribosyltransferases. Trends Biochem Sci. 35:208–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Megnin-Chanet F, Bollet MA and Hall J:
Targeting poly(ADP-ribose) polymerase activity for cancer therapy.
Cell Mol Life Sci. 67:3649–3662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cepeda V, Fuertes MA, Castilla J, Alonso
C, Quevedo C, Soto M and Pérez JM: Poly(ADP-ribose) polymerase-1
(PARP-1) inhibitors in cancer chemotherapy. Recent Pat Anticancer
Drug Discov. 1:39–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boehler C and Dantzer F: PARP-3, a
DNA-dependent PARP with emerging roles in double-strand break
repair and mitotic progression. Cell Cycle. 10:1023–1024. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miwa M and Masutani M:
PolyADP-ribosylation and cancer. Cancer Sci. 98:1528–1535. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimizu S, Nomura F, Tomonaga T, Sunaga M,
Noda M, Ebara M and Saisho H: Expression of poly(ADP-ribose)
polymerase in human hepatocellular carcinoma and analysis of biopsy
specimens obtained under sonographic guidance. Oncol Rep.
12:821–825. 2004.PubMed/NCBI
|
|
10
|
Staibano S, Pepe S, Lo Muzio L, Somma P,
Mascolo M, Argenziano G, Scalvenzi M, Salvatore G, Fabbrocini G,
Molea G, et al: Poly(adenosine diphosphate-ribose) polymerase 1
expression in malignant melanomas from photoexposed areas of the
head and neck region. Hum Pathol. 36:724–731. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brustmann H: Poly(adenosine
diphosphate-ribose) polymerase expression in serous ovarian
carcinoma: Correlation with p53, MIB-1, and outcome. Int J Gynecol
Pathol. 26:147–153. 2007.PubMed/NCBI
|
|
12
|
Rojo F, García-Parra J, Zazo S, Tusquets
I, Ferrer-Lozano J, Menendez S, Eroles P, Chamizo C, Servitja S,
Ramírez-Merino N, et al: Nuclear PARP-1 protein overexpression is
associated with poor overall survival in early breast cancer. Ann
Oncol. 23:1156–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ossovskaya V, Koo IC, Kaldjian EP, Alvares
C and Sherman BM: Upregulation of Poly(ADP-Ribose) Polymerase-1
(PARP1) in triple-negative breast cancer and other primary human
tumor types. Genes Cancer. 1:812–821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Shaughnessy J, Osborne C, Pippen JE,
Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM and Bradley C:
Iniparib plus chemotherapy in metastatic triple-negative breast
cancer. N Engl J Med. 364:205–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fong PC, Boss DS, Yap TA, Tutt A, Wu P,
Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et
al: Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tutt A, Robson M, Garber JE, Domchek SM,
Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler
RK, et al: Oral poly(ADP-ribose) polymerase inhibitor olaparib in
patients with BRCA1 or BRCA2 mutations and advanced breast cancer:
A proof-of-concept trial. Lancet. 376:235–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Vos M, Schreiber V and Dantzer F: The
diverse roles and clinical relevance of PARPs in DNA damage repair:
Current state of the art. Biochem Pharmacol. 84:137–146. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langelier MF, Riccio AA and Pascal JM:
PARP-2 and PARP-3 are selectively activated by 5′ phosphorylated
DNA breaks through an allosteric regulatory mechanism shared with
PARP-1. Nucleic Acids Res. 42:7762–7775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boehler C, Gauthier LR, Mortusewicz O,
Biard DS, Saliou JM, Bresson A, Sanglier-Cianferani S, Smith S,
Schreiber V, Boussin F and Dantzer F: Poly(ADP-ribose) polymerase 3
(PARP3), a newcomer in cellular response to DNA damage and mitotic
progression. Proc Natl Acad Sci USA. 108:2783–2788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bieche I, Pennaneach V, Driouch K, Vacher
S, Zaremba T, Susini A, Lidereau R and Hall J: Variations in the
mRNA expression of poly(ADP-ribose) polymerases, poly(ADP-ribose)
glycohydrolase and ADP-ribosylhydrolase 3 in breast tumors and
impact on clinical outcome. Int J Cancer. 133:2791–2800.
2013.PubMed/NCBI
|
|
21
|
Bansal C, Pujani M, Sharma KL, Srivastava
AN and Singh US: Grading systems in the cytological diagnosis of
breast cancer: A review. J Cancer Res Ther. 10:839–845. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cowherd SM: Tumor staging and grading: A
primer. Methods Mol Biol. 823:1–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang Y, Wei J, Cao J, Zhao H, Liao B, Qiu
S, Wang D, Luo J and Chen W: Protein expression of ZEB2 in renal
cell carcinoma and its prognostic significance in patient survival.
PLoS One. 8:e625582013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ,
Liao YJ, Bian XW, Lin MC, Kung HF, Zeng YX, et al: Overexpression
of EIF5A2 promotes colorectal carcinoma cell aggressiveness by
upregulating MTA1 through C-myc to induce
epithelial-mesenchymaltransition. Gut. 61:562–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khanna KK and Jackson SP: DNA
double-strand breaks: Signaling, repair and the cancer connection.
Nat Genet. 27:247–254. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goncalves A, Finetti P, Sabatier R,
Gilabert M, Adelaide J, Borg JP, Chaffanet M, Viens P, Birnbaum D
and Bertucci F: Poly(ADP-ribose) polymerase-1 mRNA expression in
human breast cancer: A meta-analysis. Breast Cancer Res Treat.
127:273–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chapman JR, Taylor MR and Boulton SJ:
Playing the end game: DNA double-strand break repair pathway
choice. Mol Cell. 47:497–510. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rulten SL, Fisher AE, Robert I, Zuma MC,
Rouleau M, Ju L, Poirier G, Reina-San-Martin B and Caldecott KW:
PARP-3 and APLF function together to accelerate nonhomologous
end-joining. Mol Cell. 41:33–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fenton AL, Shirodkar P, Macrae CJ, Meng L
and Koch CA: The PARP3- and ATM-dependent phosphorylation of APLF
facilitates DNA double-strand break repair. Nucleic Acids Res.
41:4080–4092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J: The role of BRCA1 in homologous
recombination repair in response to replication stress:
Significance in tumorigenesis and cancer therapy. Cell Biosci.
3:112013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prakash R, Zhang Y, Feng W and Jasin M:
Homologous recombination and human health: The roles of BRCA1,
BRCA2, and associated proteins. Cold Spring Harb Perspect Biol.
7:a0166002015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Willis NA, Chandramouly G, Huang B, Kwok
A, Follonier C, Deng C and Scully R: BRCA1 controls homologous
recombination at Tus/Ter-stalled mammalian replication forks.
Nature. 510:556–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beck C, Boehler C, Barbat Guirouilh J,
Bonnet ME, Illuzzi G, Ronde P, Gauthier LR, Magroun N, Rajendran A,
Lopez BS, et al: PARP3 affects the relative contribution of
homologous recombination and nonhomologous end-joining pathways.
Nucleic Acids Res. 42:5616–5632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Casorelli I, Bossa C and Bignami M: DNA
damage and repair in human cancer: Molecular mechanisms and
contribution to therapy-related leukemias. Int J Environ Res Public
Health. 9:2636–2657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang Q and Wieder R: All-trans retinoic
acid potentiates Taxotere-induced cell death mediated by Jun
N-terminal kinase in breast cancer cells. Oncogene. 23:426–433.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mhaidat NM, Wang Y, Kiejda KA, Zhang XD
and Hersey P: Docetaxel-induced apoptosis in melanoma cells is
dependent on activation of caspase-2. Mol Cancer Ther. 6:752–761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kolfschoten GM, Hulscher TM, Duyndam MC,
Pinedo HM and Boven E: Variation in the kinetics of caspase-3
activation, Bcl-2 phosphorylation and apoptotic morphology in
unselected human ovarian cancer cell lines as a response to
docetaxel. Biochem Pharmacol. 63:733–743. 2002. View Article : Google Scholar : PubMed/NCBI
|