Introduction
It was found in epidemiological statistics that the
mortality rate of gastric cancer in malignant tumors is only lower
than that of lung cancer, and its incidence rate ranks fourth in
the world (1,2). The clinical treatment means of early
gastric cancer is mainly surgery, but most patients are in the
advanced stage when diagnosed, so the chemotherapy-based
comprehensive treatment is mainly adopted. Unfortunately,
chemotherapy does not significantly improve the survival rate of
patients with advanced gastric cancer, and patients have to face
the adverse effects brought by chemotherapy (3–5). Gastric
cancer does not have typical clinical features in the early stage,
so it is often neglected. There are reports showing that 50–60% of
patients with gastric cancer in China are in the advanced stage,
and the resection rate is only 40% in diagnosed patients. Due to a
high malignant degree of tumor, gastric cancer is still vulnerable
to recurrence and metastasis even after resection, there is a poor
prognosis, and only 20–30% of patients can live for 5 years after
surgery. Therefore, the effect of simple surgical treatment is
often disappointing for patients with advanced gastric cancer,
especially for those who have undergone local tumor spread or
metastasis to other tissues (6).
Currently, the clinical treatment of gastric cancer has been
developed from simple surgical treatment to comprehensive treatment
of surgery and chemoradiotherapy, and individualized and targeted
therapies have become hot spots in treatment (7).
The process in which epithelial cells differentiate
into cells with biological characteristics of mesenchymal phenotype
according to a certain procedure is called epithelial-mesenchymal
transition (EMT). EMT is a key link in invasion and distant spread
of tumor cells (8). Polo-like kinase
(PLK) is a kind of serine/threonine protein kinase, which widely
exists in eukaryotic cells with certain cycle dependence. Its name
is derived from the fact that it can lead to abnormality in the
formation of spindle body in Drosophila (9). Νumerous studies have shown that PLK1
plays an important role in chromosome segregation, promotion of
centrosome maturation, cytokinesis and other processes. However,
the roles of PLK1 in the occurrence and development of cancer have
not been elucidated.
The experiments were carried out to investigate the
effects of PLK1 on the proliferation, migration and invasion of
gastric cancer cells, and to explore the roles of EMT in the
proliferation, migration and invasion processes of gastric cancer
cells.
Materials and methods
Materials and reagents
Human gastric cancer cell lines MGC-803 (Cell Bank
of Chinese Academy of Sciences, Shanghai, China), methyl thiazolyl
tetrazolium (MTT) (Sigma, St. Louis, MO, USA),
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), PLK1 and
E-cadherin human primary antibodies and horseradish peroxidase
(HRP)-labeled second antibodies (all from Wuhan Sanying
Biotechnology, Wuhan, China), primer synthesis (Takara
Biotechnology Co., Ltd., Dalian, China), Dulbecco's modified
Eagle's medium (DMEM) (Gibco Life Technologies, Carlsbad, CA, USA),
ribonucleic acid (RNA) extraction kits, reverse transcription kits
and reverse transcription-polymerase chain reaction (RT-PCR) kits
(all from from Invitrogen; Thermo Fisher Scientific, Inc.,
Carlsbad, CA, USA), bicinchoninic acid (BCA) protein quantification
kits and cell lysis solution (all from Beyotime Institute of
Biotechnology, Nantong, China). The study was approved by the
Ethics Committee of The Second Affiliated Hospital of Zhengzhou
University (Zhengzhou, China) and written informed consents were
signed by the patients and/or guardians.
Design and construction of PLK1
expression vectors
PLK1 and negative control plasmids (siNC) were
designed and synthesized by Shanghai GenePharma Co., Ltd.,
(Shanghai, China). The sense strand of PLK1 small-interfering
ribonucleic acid (siRNA) is 5′-GCAACCUGCAGUGUAAUAATT-3′, and its
antisense strand is 5′-UUAUUACACUGCAGGUUGCTT-3′. The sense strand
of siNC is 5′-GCCTCAACATCCCCTACAAGA-3′, and its antisense strand
is: 5′-CCACGAAGAACAGAAGCACAAA-3′.
Cell culture
MGC-803 cells were cultured routinely using DMEM
containing 10% fetal bovine serum (FBS) in an incubator with 5%
CO2 at 37°C. When cells were in the logarithmic growth
phase, they were digested by trypsin, prepared into single-cell
suspension and inoculated into a culture dish at an appropriate
density. Cells in this experiment were divided into 3 groups: Blank
control (no transfection), negative control (transfected with siNC)
and experimental group (transfected with siPLK1).
Detection of cell proliferation
inhibition rate
After transfection, cells were inoculated into a
96-well plate at a density of 1×105/ml (100 µl per
well). After 48 h, 10 µl MTT (5 mg/ml) was added into each well and
cells were cultured for another 4 h. The optical density (OD) value
of each well at a wavelength of 570 nm was detected using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The cell proliferation inhibition rate was calculated as follows:
Inhibition rate (%) = (OD valueblank control group - OD
valueexperimental group/OD valueblank control
group) ×100%.
Detection of PLK1 and E-cadherin mRNA
expression via RT-qPCR
After transfection, cells were inoculated into a
6-well plate (104/well), the supernatant was discarded
after 48 h, and cells in each group were collected. The total RNA
was extracted from tissues according to the instructions of RNA
extraction kit, and the concentration and purity of total RNA were
determined using an ultraviolet-visible spectrophotometer (Hitachi,
Ltd., Tokyo, Japan) (qualified if
A260/A280>1.8). The complementary
deoxyribonucleic acid (cDNA) was obtained via reverse transcription
according to the instructions of reverse transcription kit, and
then the cDNA was used as a template to detect the mRNA expression
of PLK1 and E-cadherin according to the instructions of RT-PCR kit.
The primer sequences are shown in Table
I, and the reaction conditions are as follows: 95°C for 10 min,
95°C for 15 sec, 60°C for 1 min, and amplification for 40 cycles.
The cycle threshold (Cq) value was the output from the instrument
software, and the relative expression level was calculated using
2−ΔΔCq method according to the following formula: ΔΔCq
(target gene) = Cq (target gene) - Cq (control gene) (10).
 | Table I.RT-PCR primer sequences. |
Table I.
RT-PCR primer sequences.
| Genes | Primer sequence |
|---|
|
E-cadherin | F:
5′-GCTTGGAATGAGACTGCTGA-3′ |
|
| R:
5′-CTGGCCATATCCACCAGAGT-3′ |
| PLK1 | F:
5′-CGAGGGTGATGAGAACCTGC-3′ |
|
| R:
5′-CCCATGTGATTCGATGCGT-3′ |
| GAPDH | F:
5′-CAAGGTCATCCATGACAACTTTG-3′ |
|
| R:
5′-GTCCACCACCCTGTTGCTGTAG-3′ |
Detection of protein expression of
PLK1 and E-cadherin via western blot analysis
After transfection, cells were inoculated into the
6-well plate (104/well), and the supernatant was
discarded after 48 h. Then cells in each group were collected,
lysed with the cell lysis solution, and centrifuged at 8,000 × g at
4°C for 15 min, and the supernatant was collected. The protein
concentration was determined using the BCA kit, followed by
degeneration, separation via sodium dodecyl sulfate polyacrylamide
gel electrophoresis (SDS-PAGE), and membrane transfer using the wet
method. Then the membrane was sealed via bull serum albumin (BSA)
for 2 h, and Rabbit anti-human GAPDH, PLK1 and E-cadherin
polyclonal antibodies (cat. nos. 10494-1-AP, 10305-1-AP and
20874-1-AP; 1:1,000; Wuhan Sanying Biotechnology) was added for
incubation at 4°C overnight. After the membrane was washed, goat
anti-rabbit polyclonal secondary antibody (cat. no. SA00001-2;
1:2,000; Wuhan Sanying Biotechnology) was added for incubation at
room temperature for 2 h. After the membrane was washed again, the
image was developed in a darkroom via enhanced chemiluminescence
(ECL), scanned and recorded using a gel imager (Bio-Rad
Laboratories, Richmond, CA, USA). The gray scale was analyzed and
compared with GADPH as internal reference.
Detection of migration capacity of
human gastric cancer MGC-803 cells via wound healing assay
After transfection, cells were inoculated into the
6-well plate (104/well). After about 80% cells were
fused, a 100 µl spearhead was used to scratch at the marker line.
The loose cells were gently rinsed off with phosphate buffered
saline (PBS), and the remaining cells continued to be cultured
using FBS-free DMEM culture solution. After 48 h, the distance
between scratches were observed and photographed.
Detection of invasion capacity of
human gastric cancer MGC-803 cells via Transwell assay
The upper Transwell chamber was evenly added with
100 µl single-cell suspension in a concentration of
4×105/ml and 100 µl serum-free culture solution, while
the lower chamber was added with 500 µl FBS-free culture solution.
Cells were treated according to the above experimental method.
After 48 h, cells were fixed with 4% paraformaldehyde, stained via
crystal violet for 15 min, photographed and analyzed.
Statistical analysis
Data were presented as mean ± standard deviation
(SD) and Statistical Product and Service Solutions (SPSS) 17.0
software (SPSS, Inc., Chicago, IL, USA) was used for data
processing. One-way analysis of variance was used for the
statistical analysis of data obtained and the post hoc test was
Dunnett's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of siPLK1 on MGC-803 cell
proliferation
Results of MTT assay showed that at 48 h after
transfection into MGC-803 cells, the cell inhibition rate in PLK1
siRNA group was significantly increased compared with that in blank
control group, and the difference was statistically significant
(p<0.01) (Table II).
 | Table II.Inhibitory effect of transfection with
PLK1 siRNA for 48 h on MGC-803 cell proliferation (mean ± SD). |
Table II.
Inhibitory effect of transfection with
PLK1 siRNA for 48 h on MGC-803 cell proliferation (mean ± SD).
|
| Proliferation
inhibition rate (%) |
|---|
|
|
|
|---|
| Groups | 24 h | 48 h | 72 h |
|---|
| Blank control | 0 | 0 | 0 |
| Negative control | 0 | 0 | 0 |
| PLK1 siRNA |
11.3±0.32a |
23.32±2.13a |
37.33±2.06a |
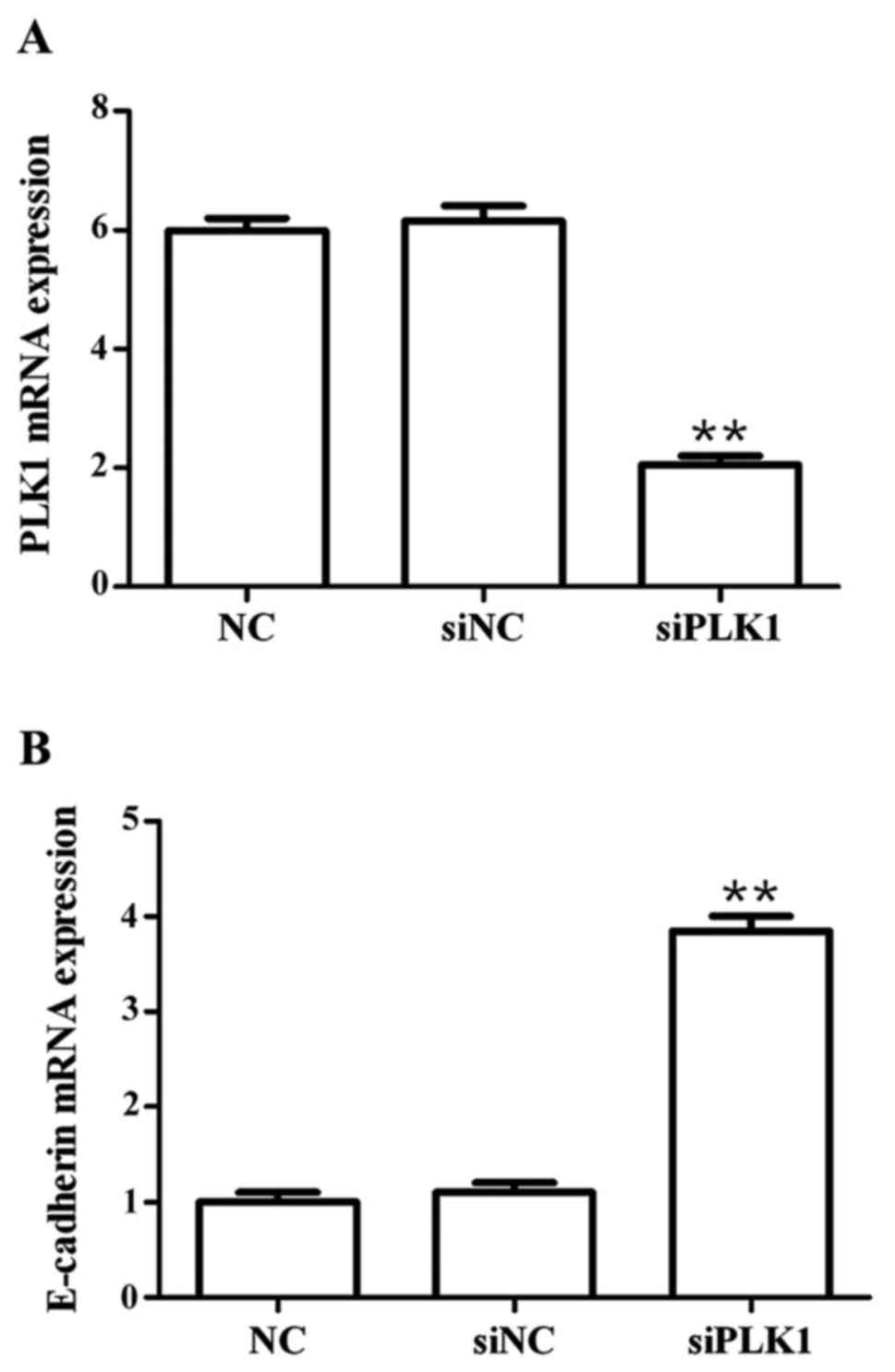
Effects of siPLK1 transfection on mRNA
expression of PLK1 and E-cadherin
Results showed that compared with those in blank
control group, PLK1 siRNA could significantly decrease the mRNA
expression of PLK1 (p<0.01), but significantly upregulate the
mRNA expression of E-cadherin (p<0.01) at 48 h after
transfection into MGC-803 cells, and the differences were
statistically significant (Fig.
1).
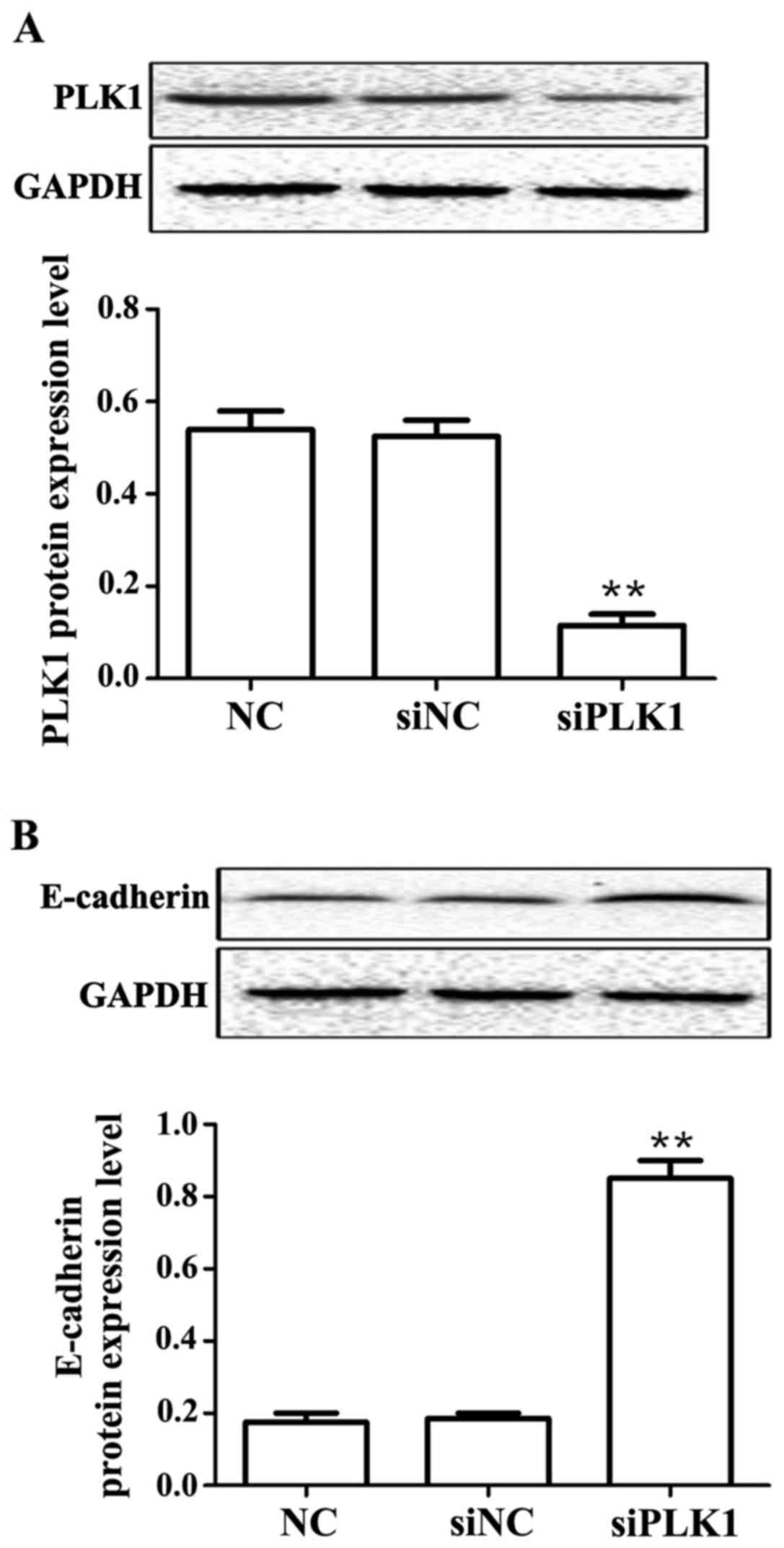
Effects of siPLK1 transfection on
protein expression of PLK1 and E-cadherin
Results showed that compared with those in control
group, PLK1 siRNA could obviously decrease the protein expression
of PLK1 (p<0.01), but obviously upregulate the protein
expression of E-cadherin (p<0.01) at 48 h after transfection
into MGC-803 cells, and the differences were statistically
significant (Fig. 2).
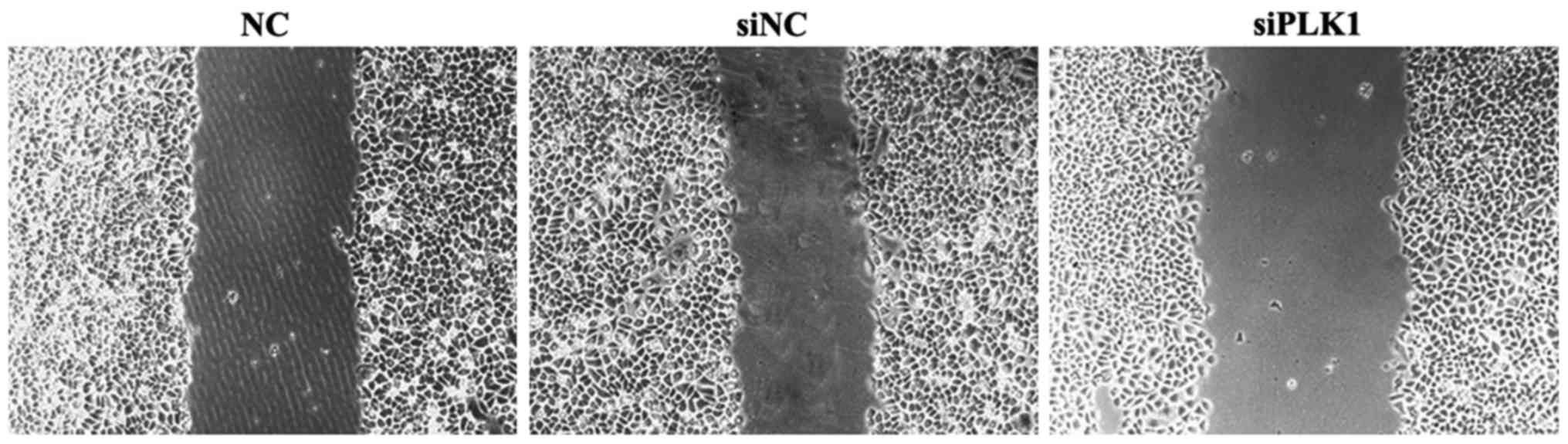
Effect of siPLK1 transfection on
migration capacity of MGC-803 cells
Results of wound healing assay showed that compared
with those in blank control and negative control groups, the space
between wounds in experimental group was wider, the healing was
obviously inhibited, and the cell migration capacitywas weakened.
The difference in the wound space was not significant between blank
control and negative control groups (Fig.
3).
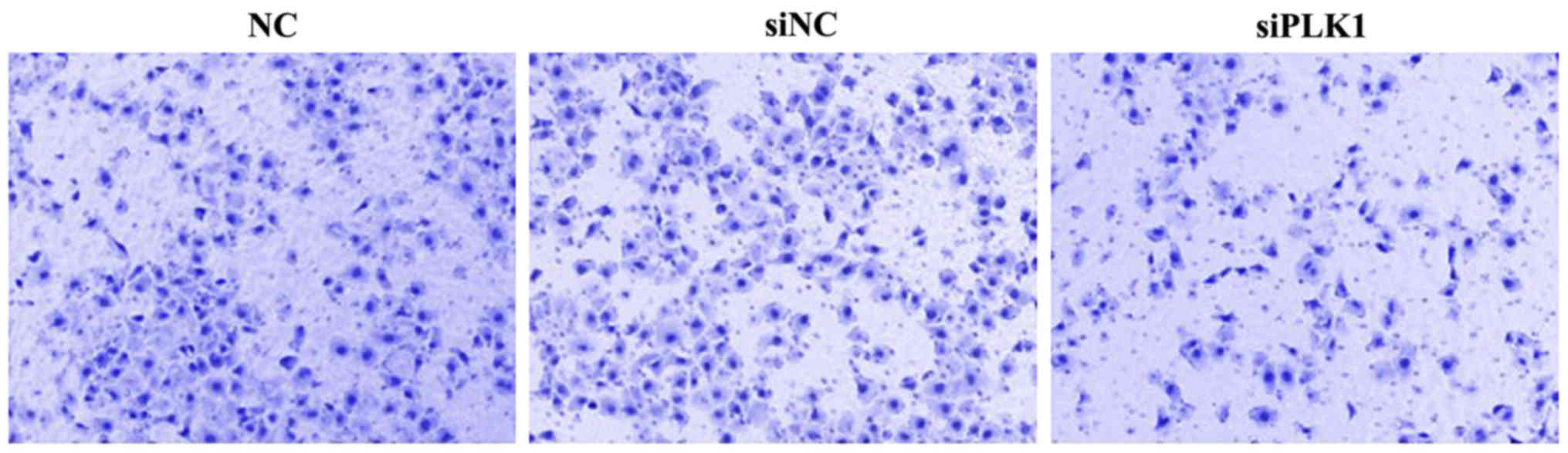
Effect of siPLK1 transfection on
invasion capacity of MGC-803 cells
At 48 h after transfection into MGC-803 cells, cells
were inoculated into the upper Transwell chamber for culture for 48
h. The number of MGC-803 cells passing through Matrigel and
reaching the filter membrane in PLK1 siRNA group was significantly
smaller than those in blank control and negative control groups,
indicating that PLK1 siRNA can inhibit the invasion of MGC-803
cells. The difference was not significant between blank control and
negative control groups (Fig. 4).
Discussion
Gastric cancer is a common malignant tumor of
digestive tract, and its incidence rate is increasing year by year,
seriously affecting human health (11). At present, clinical treatment means
have not only side effects, but also slow progression, and the
mechanisms of the occurrence and development of gastric cancer are
not clear. Therefore, exploring new mechanisms is of great
significance to find a new breakthrough for gastric cancer
treatment.
EMT is a key link in the metastasis of tumor cells,
involving the activation of multiple factors and different
signaling pathways (12). The
adhesion between epithelial cells with EMT is significantly
decreased, but the cell motility is significantly enhanced, thus
cells further migrate. During the occurrence of EMT in cells,
relevant markers are also changed, such as the deletion of
epithelial phenotype marker E-cadherin. It has been reported that
the typical sign of EMT is the deletion of E-cadherin expression,
and this process involves another important factor, namely the
zinc-finger transcriptional factor Snail that plays a key role in
the EMT process. It is found that Snail can promote the mesenchymal
transition of a variety of tumor cells, and the invasion and
metastasis of tumor cells (13,14).
Zinc-finger transcriptional factor directly binds to the promoter
of E-cadherin, thus leading to the downregulation of E-cadherin
protein expression, further reducing the adhesion between cells and
destroying the physiological tissue structure, thereby promoting
the invasion and metastasis of tumor cells (15). In addition, PLK1, as a kinase, is a
key factor in mitosis, which is closely associated with multiple
processes of mitosis, such as formation of spindle body, centrosome
replication and chromosome segregation (16). A large number of research reports
(17–19) have shown that the PLK1 expression is
increased in various solid tumor tissues (20), such as esophageal cancer, melanoma,
breast cancer, colon cancer and renal cell carcinoma, suggesting
that there is a close relationship between PLK1 and tumor
occurrence. Smith et al transfected the
exogenously-recombinant PLK1 genes into normal fibroblasts,
and results showed that malignant transformation of cells could be
induced, and the tumor could be implanted into nude mice
successfully, indicating that the PLK1 gene can directly
cause malignant transformation of cells (21). In addition, recent reports have
indicated that the high expression of PLK1 gene in liver and
pancreatic cancers occurs mainly in the early stage of tumor cell
progression (22).
In this experiment, siRNA with targeted interference
in PLK1 gene was designed and transfected into gastric
cancer MGC-803 cells via Lipofectamine (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) to inhibit the expression of PLK1
gene in MGC-803 cells. Results of MTT assay showed that compared
with that in control group, the cell proliferation in PLK1 siRNA
group was significantly inhibited. Results of real-time PCR and
western blot analysis showed that compared with those in control
group, the mRNA and protein expression of PLK1 in PLK1 siRNA group
were significantly decreased, but the mRNA and protein expression
of E-cadherin was obviously upregulated. Results of wound healing
assay and invasion assay showed that the migration and invasion of
MGC-803 cells in PLK1 siRNA group were significantly inhibited
compared with those in control group. It is reported (23) that epithelial cancerous cells will
have stronger migration and invasion capacities after EMT. In
addition, it has been reported (24)
that the upregulation of PLK1 expression will lead to the
accumulation of oncogenes in tumor cells and formation of tumors
with migration and invasion capacities in nude mice successfully.
Clinically, Tokumitsu et al (25) studied the PLK1 mRNA expression level
in patients with gastric cancer, and results showed that PLK1 mRNA
was highly expressed in 73% of patients, and the difference was
significant compared with that in normal and atypical hyperplastic
tissues. Besides, the expression level of PLK1 was positively
correlated with the clinical staging and depth of tumor
infiltration. All of these reports indicate that PLK1 plays an
extremely important role in the occurrence and development of human
tumors. Therefore, exploring the mechanism of action of PLK1 in
tumors will provide a new direction for further treatment of
tumors.
In conclusion, this study proved that PLK1 can
enhance the proliferation, migration and invasion of gastric cancer
MGC-803 cells through affecting EMT.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RS and GH designed the study, JUY and JIY collected
and analysed the data, CW helped with Transwell assay. TC and ZL
were responsible for PCR and western blot analysis. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Second Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) and written informed consents were signed by the patients
and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brenner H, Rothenbacher D and Arndt V:
Epidemiology of stomach cancer. Methods Mol Biol. 472:467–477.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ryu MH and Kang YK: ML17032 trial:
Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as
first-line therapy in advanced gastric cancer. Expert Rev
Anticancer Ther. 9:1745–1751. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Vita F, Vecchione L, Galizia G, Di
Martino N, Fabozzi T, Catalano G, Ciardiello F and Orditura M:
Perspectives in adjuvant therapy of gastric cancer. Oncology. 77
Suppl 1:38–42. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mlkvý P: Multimodal therapy of gastric
cancer. Dig Dis. 28:615–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mello BS, Lucena AF, Echer IC and Luzia
MF: Patients with gastric cancer submitted to gastrectomy: An
integrative review. Rev Gaúcha Enferm. 31:803–811. 2010.(In
Portuguese). View Article : Google Scholar
|
|
7
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
de Cárcer G, Escobar B, Higuero AM, García
L, Ansón A, Pérez G, Mollejo M, Manning G, Meléndez B,
Abad-Rodríguez J, et al: Plk5, a polo box domain-only protein with
specific roles in neuron differentiation and glioblastoma
suppression. Mol Cell Biol. 31:1225–1239. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak and Schmittgen: Analysis of relative
gene expression data using real-time quantitative PCR and the
2-ΔΔCq method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crew KD and Neugut AI: Epidemiology of
upper gastrointestinal malignancies. Semin Oncol. 31:450–464. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spiegel S and Milstien S: Functions of the
multifaceted family of sphingosine kinases and some close
relatives. J Biol Chem. 282:2125–2129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ando K, Ozaki T, Yamamoto H, Furuya K,
Hosoda M, Hayashi S, Fukuzawa M and Nakagawara A: Polo-like kinase
1 (Plk1) inhibits p53 function by physical interaction and
phosphorylation. J Biol Chem. 279:25549–25561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steinhauser I, Langer K, Strebhardt K and
Spänkuch B: Uptake of plasmid-loaded nanoparticles in breast cancer
cells and effect on Plk1 expression. J Drug Target. 17:627–637.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim SA, Kwon SM, Yoon JH and Ahn SG: The
antitumor effect of PLK1 and HSF1 double knockdown on human oral
carcinoma cells. Int J Oncol. 36:867–872. 2010.PubMed/NCBI
|
|
19
|
Takai N, Hamanaka R, Yoshimatsu J and
Miyakawa I: Polo-like kinases (Plks) and cancer. Oncogene.
24:287–291. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang G, Zhang Z and Liu Z: Polo-like
kinase 1 is overexpressed in renal cancer and participates in the
proliferation and invasion of renal cancer cells. Tumour Biol.
34:1887–1894. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith MR, Wilson ML, Hamanaka R, Chase D,
Kung H, Longo DL and Ferris DK: Malignant transformation of
mammalian cells initiated by constitutive expression of the
polo-like kinase. Biochem Biophys Res Commun. 234:397–405. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petrelli A, Perra A, Schernhuber K,
Cargnelutti M, Salvi A, Migliore C, Ghiso E, Benetti A, Barlati S,
Ledda-Columbano GM, et al: Sequential analysis of multistage
hepatocarcinogenesis reveals that miR-100 and PLK1 dysregulation is
an early event maintained along tumor progression. Oncogene.
31:4517–4526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
24
|
Smits VA, Klompmaker R, Arnaud L, Rijksen
G, Nigg EA and Medema RH: Polo-like kinase-1 is a target of the DNA
damage check point. Nat Cell Biol. 2:672–676. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tokumitsu Y, Mori M, Tanaka S, Akazawa K,
Nakano S and Niho Y: Prognostic significance of polo-like kinase
expression in esophageal carcinoma. Int J Oncol. 15:687–692.
1999.PubMed/NCBI
|