Introduction
Osteosarcoma (OS) is one of the most common primary
bone tumors and accounts for approximately 5–6% in all tumors of
childhood and adolescence (1,2). Recently, larger advanced in therapeutic
methods for the disease including tumor resection, neoadjuvant or
adjuvant chemotherapy and radiotherapy, patients have prolonged the
overall survival time (3). However,
due to the aggressive potential of osteosarcoma, ~40% develop to
metastasis at advanced stages during treatment for patients
(4). Thus, revealing the molecular
mechanisms underlying osteosarcoma development is important for
osteosarcoma treatment.
Circular RNAs are newly discovered endogenous
non-coding RNAs characterized by their covalently closed loop
structures without a 5′cap or a 3′Poly A tail (5). Studies have suggested that circular RNAs
play important roles in various diseases including cancer. CircRNAs
act as oncogenes or tumor suppressors to be involved in
carcinogenesis by regulating gene expression at the transcriptional
or post-transcriptional level (6,7). For
instance, Chen et al reported that CircRNA_100290 is
upregulated and co-expressed with CDK6 in oral cancer and functions
as a competing endogenous RNA to regulate CDK6 expression through
sponging miR-29b family members (8).
Hsa_circ_001569 is higher expression in colorectal cancer and
identified as a sponge of miR-145 targeting E2F5, BAG4 and FMNL2 to
promote cell proliferation and invasion of colorectal cancer
(9). However, the clinical
significance and function of circ_001569 in osteosarcoma remains
larger unknown.
In the present study, we found that circ_001569 was
significantly overexpressed in osteosarcoma and correlated with
distant metastasis, advanced tumor stage, and poor prognosis of
osteosarcoma. Knockdown of circ_001569 significantly inhibited the
proliferation ability of osteosarcoma. Moreover, upregulation of
circ_001569 significantly promoted osteosarcoma cell resistance to
cisplatin by activating Wnt/β-catenin signaling pathway. Thus, our
results indicated that circ_001569 may serve as a novel potentially
therapeutic target of osteosarcoma.
Patients and methods
Patient tissues samples
A total of 36 primary osteosarcoma tissue samples
and adjacent non-cancerous bone tissue samples were obtained from
the Department of Orthopedics, Affiliated Hospital of Weifang
Medical University (Weifang, China) between January 2009 and
February 2013. The patients included 23 males and 13 females, with
a median age of 35.8 years and an age range of 10 to 56 years old.
The histological grade was assessed according to the
tumor-node-metastasis (TNM) system (10). None of the patients received any
treatment prior to surgery. The removed fresh tissues were
immediately placed in liquid nitrogen and then stored at −80°C for
RNA analysis. The clinicopathological data are summarized in
Table I. Written informed consent was
obtained from all patients and the study was approved by the Ethics
Committee of Affiliated Hospital of Weifang Medical University.
 | Table I.Association between circ_001569
expression and clinical characteristics in 36 patients with
osteosarcoma. |
Table I.
Association between circ_001569
expression and clinical characteristics in 36 patients with
osteosarcoma.
|
|
| circ_001569
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
characteristic | Total (n=36) | Lower (n=16) | Higher (n=20) | P-value |
|---|
| Sex |
|
|
| 0.121 |
|
Female | 13 | 8 | 5 |
|
| Male | 23 | 8 | 15 |
|
| Age, years |
|
|
| 0.677 |
| ≤18 | 26 | 11 | 15 |
|
|
>18 | 10 | 5 | 5 |
|
| Site |
|
|
| 0.799 |
|
Femur | 18 | 7 | 11 |
|
|
Tibia | 10 | 5 | 5 |
|
|
Humerus | 8 | 4 | 4 |
|
| Distant
metastasis |
|
|
| 0.009a |
|
Absent | 16 | 11 | 5 |
|
|
Present | 20 | 5 | 15 |
|
| Tumor size, cm |
|
|
| 0.221 |
|
<5 | 14 | 8 | 6 |
|
| ≥5 | 22 | 8 | 14 |
|
| TNM stage |
|
|
| 0.016a |
|
I–IIA | 14 | 10 | 4 |
|
|
IIB-III | 22 | 6 | 16 |
|
Cell line culture
Two human osteosarcoma cell lines (MG-63 and U2OS)
and a normal human osteoblastic cell line hFOB were obtained from
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The hFOB cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The human OS cells were cultured in RPMI-1640 medium
supplemented with 10% FBS (both Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin (Thermo
Fisher Scientific, Inc.). Cells were incubated at 37°C with 5%
CO2 in a humidified chamber.
Cell transfection and plasmid
construct
siRNA targeting has-circ_001569
(5′-GCATCGTGCAGGACTGGAA-3′) and si-negative control (NC;
5′-UUCUCCGAACGUGUCACGUTT-3′) was constructed and purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). Cells were
transiently transfected with 100 nmol/l oligoribonucleotides using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Human hsa_circ_001569 cDNA was
synthesized and cloned into pLVX-IRESneo by GeneCopoeia, Inc.
(Rockville, MD, USA). Cells were transfected with the
oligonucleotides at room temperature at a final concentration of 20
nmol/l using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Cells were harvested
following cell transfection at 48 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol reagent (Thermo Fisher Scientific, Inc.). Reverse
Transcription kit (Guangzhou RiboBio Co., Ltd.) was used to reverse
RNA to cDNA, according to the manufacturer's protocols. RT-qPCR was
performed using a SYBR Premix Ex TaqTM II kit (Takara Biotechnology
Co., Ltd., Dalian, China) on an ABI7500 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The thermocycling conditions were
as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10
sec, 57°C for 20 sec and 72°C for 15 sec. The mRNA fold-change of
circ_001569 was calculated by the 2−ΔΔCq method
(11) and was normalized to GAPDH
mRNA expression. The primer sequences were as follows: circ_001569
forward, 5′-TCCCCTGAACATTCTCCCCAT-3′ and reverse,
5′-GAAAGCACTTGGTGAAGTCGG-3′; and GAPDH forward,
5′-CACCGTAGCCTTCCGAGTA-3′ and reverse,
5′-GCCCTTGATGAGCTGTTGA-3′.
Cell proliferation assay
Cell proliferation capacity was evaluated using the
Cell Counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) according to the manufacturer's protocol.
Transfected cells were seeded onto 96-well plates (2,000
cells/well) and were incubated overnight at 37°C with 5%
CO2 in a humidified chamber. The proliferation rate of
osteosarcoma cells was detected every 24 h until 72 h. Each well
was supplemented with 10 µl CCK-8 regent and cells were incubated
for 2 h. Next, cell proliferation was measured at 450 nm using a
Multiskan MK3 spectrophotometer.
Cell colony formation assay
The transfected osteosarcoma cells were plated at
500 cells/well onto 6-well plates. After cells were cultured at
37°C with 5% CO2 in a humidified chamber for two weeks,
the cell colonies were fixed with 4% paraformaldehyde at room
temperature for 20 min and stained with 0.1% crystal violet at room
temperature for 20 min, and the images of cell colonies were
captured and cell colonies were calculated.
Western blot analysis
Total protein was extracted using lysis buffer
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). A BCA kit (Tiangen
Biotech Co., Ltd., Beijing, China) was used to detect the
concentration of the proteins. Equal protein (30 µg) was separated
on 8–12% SDS-PAGE gel and transferred onto polyvinylidene
difluoride membranes (Millipore, Billerica, MA, USA). Following
blocking with 5% skimmed milk at room temperature for 1 h, the
membrane was incubated with antibodies including p-GSK-3β (cat. no.
9336; 1:1,000), GSK-3β (cat. no. 9315; 1:2,000), β-catenin (cat.
no. 9562; 1:1,000) and GAPDH (cat. no. 5174; 1:1,000; all Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C and
then incubated with horseradish peroxidase-conjugated goat
anti-rabbit antibody (cat. no. 10285-1-AP; 1:1,000; ProteinTech
Groups, Inc., Chicago, IL, USA) at room temperature for 1 h. The
membrane was detected with an enhanced chemiluminescence detection
system (Nanjing KeyGen Biotech Co., Ltd.). GAPDH was used as an
internal control.
The value of 50% cytotoxic
concentration (IC50) in osteosarcoma cells
The cells were transfected with si-NC and
si-circ_001569 or pLVX-IRESneo-vector (vector) and
pLVX-IRESneo-circ_001569 (circ_001569). Cell viability was detected
using CCK-8, according to the manufacturer's protocol. The IC50
values were defined as the chemotherapeutic agent concentration
producing 50% inhibition of cell proliferation. Absorbance was
detected at 450 nm.
Statistical analysis
All data in the study are presented as the mean ±
standard deviation and were analyzed using SPSS 19.0 software
package (IBM Corp., Armonk, NY, USA). Student's t-test was applied
to compare the differences between two groups. For comparisons
among 3 or more groups, one-way analysis of variance, followed by
the Student-Newman-Keuls post hoc test, was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of circ_001569 is elevated
in osteosarcoma
To investigate the clinical significance of
circ_001569 expression in osteosarcoma, the present study examined
the expression of circ_001569 by RT-qPCR in 36 primary osteosarcoma
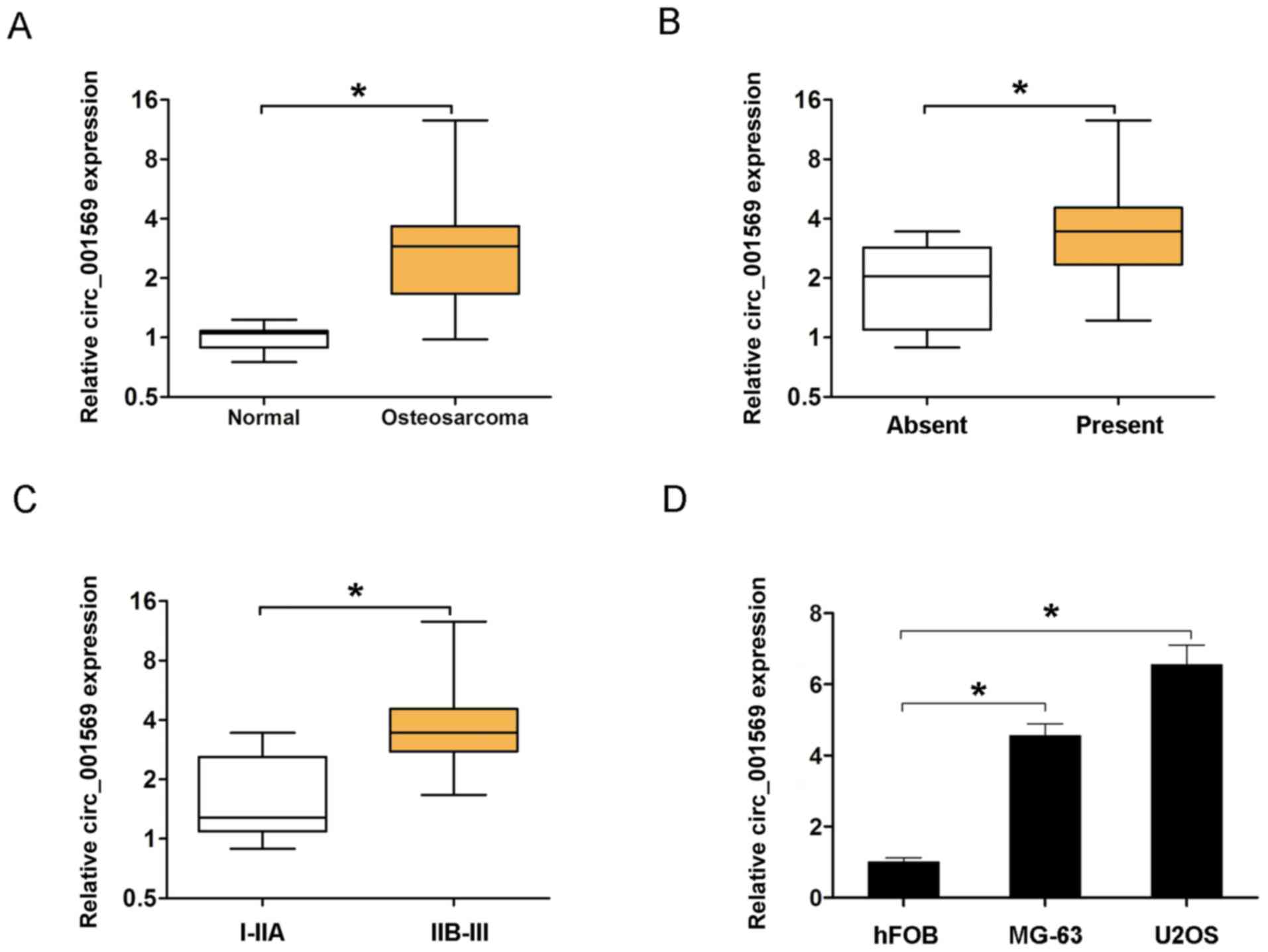
tissues and adjacent non-cancerous bone tissues. As demonstrated in
Fig. 1A, circ_001569 expression was
significantly elevated in osteosarcoma tissues compared with
adjacent non-cancerous bone tissues (P<0.05). Next, the
association between circ_001569 expression and clinicopathological
factors was analyzed. The results of the present study demonstrated
that circ_001569 expression was associated with distant metastasis
(Fig. 1B; Table I, P<0.05) and TNM stage (Fig. 1C; Table
I, P<0.05), but was not associated with any others factors
in osteosarcoma patients (Table I,
P>0.05). In addition, the expression of circ_001569 was detected
in two human osteosarcoma cell lines (U2OS and MG-63) and an
osteoblast cell line (hFOB) by RT-qPCR analyses. The results
revealed that expression of circ_001569 was higher in osteosarcoma
cell lines than that in hFOB cell lines (Fig. 1D). These results indicated that
circ_001569 expression was upregulated in osteosarcoma.
Upregulation of circ_001569 expression
enhances cell proliferation ability in vitro
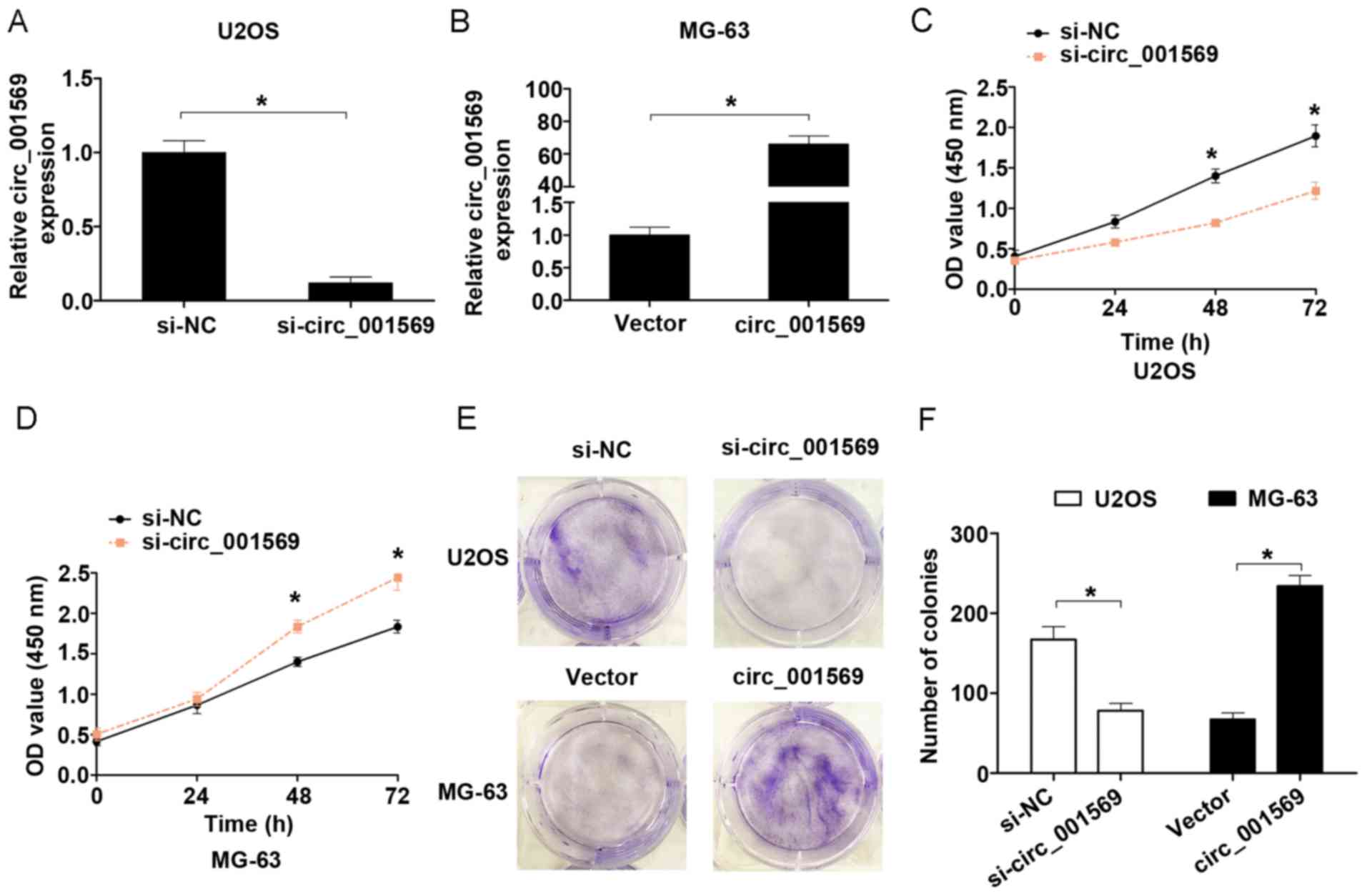
To evaluate the effects of circ_001569 on cell
proliferation, a loss-function assay was performed in U2OS cells
and a gain-function assay was performed in MG-63 cells according to
their expression of circ_001569 (Fig. 2A
and B). CCK-8 assay results revealed that the cell
proliferation capacity was significantly inhibited following
circ_001569 knockdown compared with the negative control group in
U2OS cells, but was notably enhanced following circ_001569
overexpression in MG-63 cells, compared with the control group
(Fig. 2C and D). Cell colony
formation assay revealed that the number of colonies formed in the
circ_001569 knockdown group was smaller than that in the negative
control group in U2OS cells, but was more when circ_001569 was
overexpressed in MG-63 cells (Fig. 2E and
F). Therefore, these results indicated that upregulation of
circ_001569 could enhance cell proliferation in vitro.
Upregulation of circ_001569 promotes
osteosarcoma cell resistance towards chemotherapy and activates the
Wnt/β-catenin signaling pathway
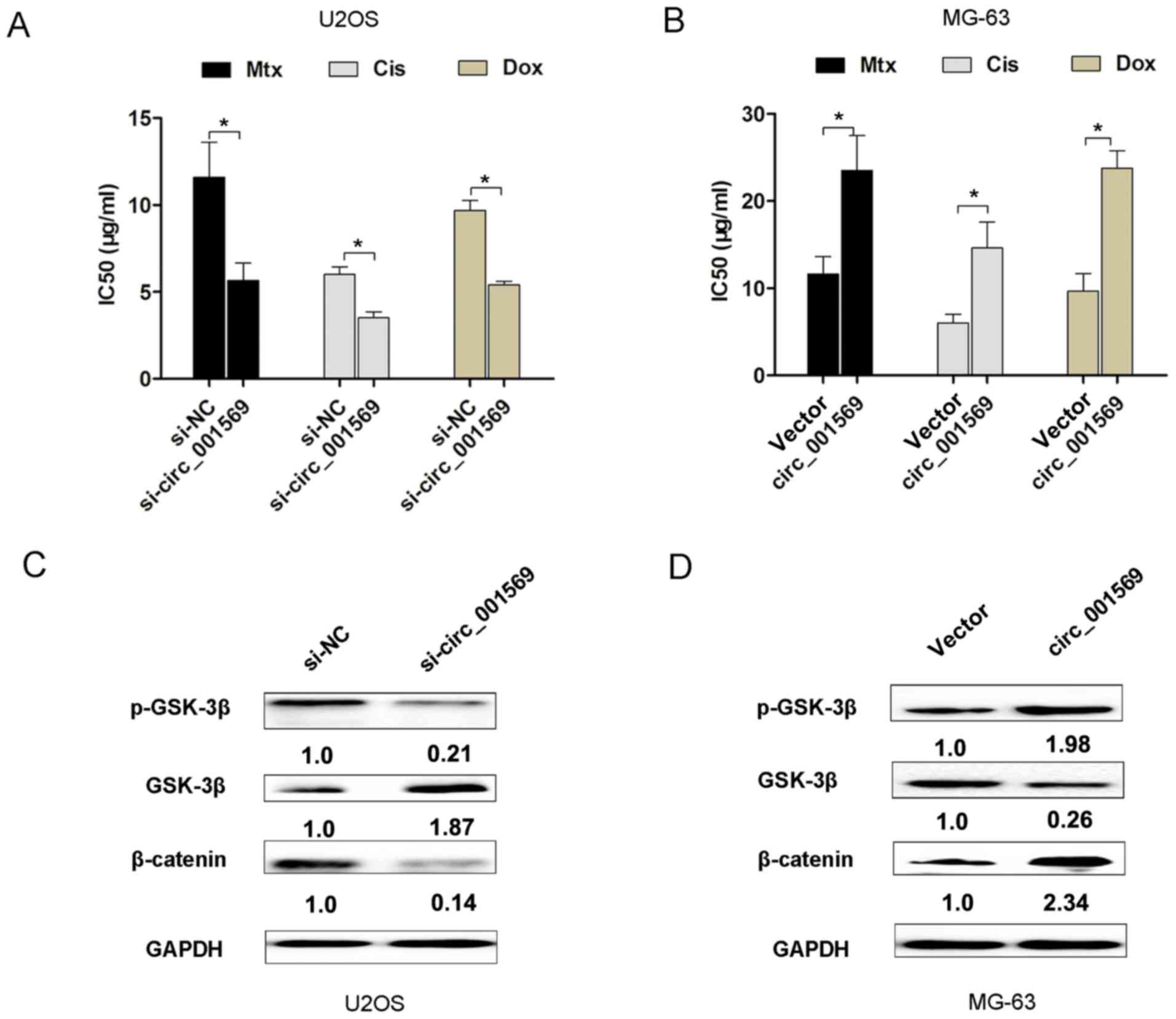
Combination of cisplatin with doxorubicin and
methotrexate is frequently used for osteosarcoma treatment
(12,13). Furthermore, the present study
evaluated whether circ_001569 expression affected conventional
chemotherapy of osteosarcoma. The results indicated that inhibition
of circ_001569 activity in U2OS cells resulted in a significant
decrease in the calculated IC50 value for cisplatin, doxorubicin or
methotrexate, compared with the control group (Fig. 3A). However, overexpression of
circ_001569 activity in MG-63 cells enhanced the IC 50 value for
cisplatin, doxorubicin or methotrexate (Fig. 3B). These results indicated that
upregulation of circ_001569 promoted osteosarcoma cell resistance
towards chemotherapy.
Upregulation of circ_001569 promotes
osteosarcoma cell cisplatin resistance by activating the
Wnt/β-catenin signaling pathway
Activation of Wnt/β-catenin signaling is associated
with chemotherapy resistance in osteosarcoma (14). Furthermore, western blot analysis
revealed that circ_001569 knockdown in U2OS cells decreased the
expression levels of p-GSK-3β and β-catenin, but increased GSK-3β
expression levels (Fig. 3C). However,
circ_001569 overexpression increased the expression levels of
p-GSK-3β and β-catenin in MG-63 cells, but decreased GSK-3β
expression levels (Fig. 3D). These
results indicated that circ_001569 overexpression activated the
Wnt/β-catenin signaling pathway.
Next, the present study demonstrated whether
circ_001569 overexpression enhanced the resistance of osteosarcoma
to cisplatin, doxorubicin or methotrexate by activating the
Wnt/β-catenin signaling pathway. As demonstrated in Fig. 4A and B, the Wnt/β-catenin inhibitor,
XAV939, or Wnt/β-catenin agonist, LiCl, could reduce or increase
the IC50 value for cisplatin, doxorubicin or methotrexate. It was
further demonstrated that circ_001569 knockdown resulted in a
decreased IC50 value for cisplatin, doxorubicin or methotrexate,
compared with the negative control. However, this chemotherapy
sensitivity was only rescued by treatment with the Wnt/β-catenin
agonist, LiCl in the cisplatin-treated group, compared with the
control group (Fig. 4C).
Additionally, it was demonstrated that circ_001569 overexpression
exhibited an increased IC50 value for cisplatin, doxorubicin or
methotrexate, compared with the negative control. However, this
chemotherapy resistance was also rescued by treatment with the
Wnt/β-catenin agonist, LiCl, in the cisplatin-treated group,
compared with the control group (Fig.
4D). Therefore, our aforementioned results indicated that
circ_001569 could enhance cisplatin resistance of OS cells via
activating the Wnt/β-catenin pathway.
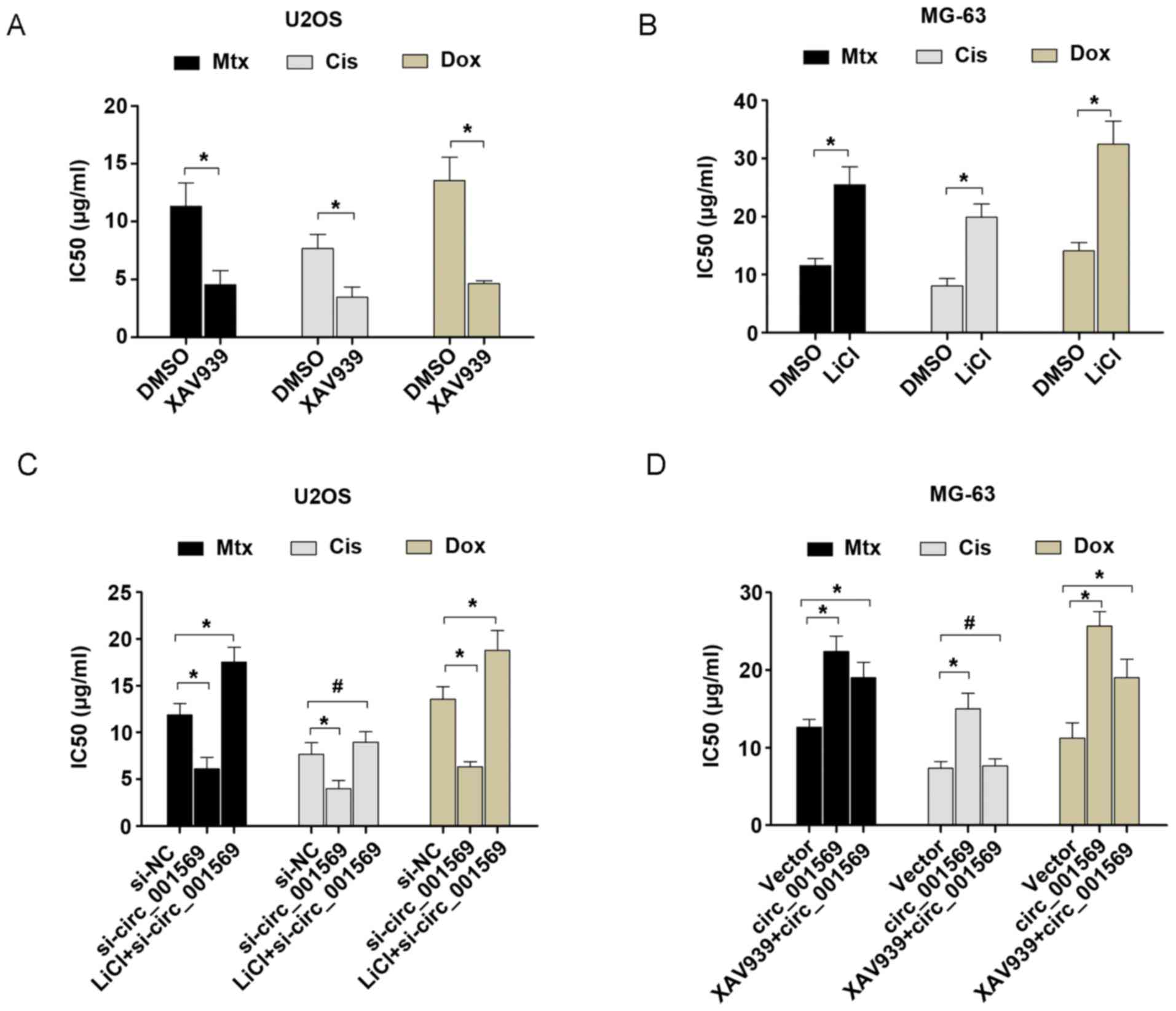 | Figure 4.Upregulation of circ_001569 promotes
resistance of osteosarcoma cells to cisplatin by activating the
Wnt/β-catenin signaling pathway. (A) The Wnt/β-catenin inhibitor,
XAV939, or (B) Wnt/β-catenin agonist, LiCl, could reduce or
increase the IC50 value for cisplatin, doxorubicin or methotrexate.
(C) The IC50 value for cisplatin, doxorubicin or methotrexate in
U2OS cells was detected following treatment of the cells with
si-NC, si-circ_001569 or Wnt/β-catenin agonist (LiCl) +
si-circ_001569. (D) The IC50 value for cisplatin, doxorubicin or
methotrexate in MG-63 cells was detected following the treatment of
cells with vector, circ_001569, or Wnt/β-catenin inhibitor (XAV939)
+ circ_001569. Data are presented as the mean ± standard deviation.
*P<0.05, #, not statistically significant. IC50,
calculated 50% inhibition of growth; si, small interfering RNA; NC,
negative control. |
Discussion
Recent studies have reported that circRNAs serve as
important regulators in cancer biological functions, including cell
proliferation, migration, invasion and metastasis (15,16). In a
previous study, circ-0016347 was identified as a sponge of miR-214
that upregulates the expression of caspase-1 to promote
proliferation and invasion (17). The
cir-GLI2 exerts tumor-promoting effects on the proliferation,
migration and invasion of osteosarcoma cells via negatively
targeting miR-125b-5p (18). The
expression of hsa_circ_0,01564 is upregulated in osteosarcoma and
hsa_circ_001564 knockdown significantly suppresses proliferation
activity, induces cell-cycle arrest in the G0/G1 phase, and
promotes the apoptosis of HOS and MG-63 cells (19). The present study demonstrated that
circ_001569 was significantly overexpressed in osteosarcoma tissues
compared with adjacent non-cancerous bone tissues. Higher
circ_001569 expression was significantly associated with distant
metastasis and advanced tumor stage in OS patients. Furthermore,
circ_001569 overexpression enhanced cell proliferation and cell
colony formation capacities; however, circ_001569 knockdown
inhibited cell proliferation and cell colony formation abilities.
These findings indicated that circ_001569 serves a promoting role
in osteosarcoma development.
Previous studies have revealed that activation of
Wnt/β-catenin signaling could enhance chemotherapy resistance to
cisplatin, doxorubicin and methotrexate. For example, TWIST
decreases osteosarcoma cell survival against cisplatin by
decreasing the soluble β-catenin level through a PI3K-dependent
manner (14). Hypoxic osteosarcoma
cells can be sensitized to doxorubicin treatment by further
inhibition of the Wnt/β-catenin signaling pathway (20). Overexpression of long non-coding RNA
HOTTIP increases chemoresistance of osteosarcoma cells against
cisplatin, doxorubicin and methotrexate by activating the
Wnt/β-catenin pathway (21). The
present study demonstrated that circ_001569 knockdown decreased the
expression levels of p-GSK-3β and β-catenin, but upregulated GSK-3β
expression levels, while circ_001569 overexpression had an opposite
effect. Furthermore, the present study demonstrated that
osteosarcoma cells treated with Wnt/β-catenin inhibitor, XAV939,
reduced resistance to cisplatin, doxorubicin and methotrexate.
However, osteosarcoma cells treated with the Wnt/β-catenin agonist,
LiCl, promoted cell resistance to cisplatin, doxorubicin and
methotrexate. Furthermore, it was demonstrated that the cell
resistance to cisplatin caused by circ_001569 overexpression could
be partially reversed by the combination treatment of circ_001569
overexpression and the Wnt/β-catenin inhibitor, XAV939. In line
with these results, the enhanced chemotherapy sensitivity induced
by circ_001569 knockdown could be partially reversed by the
combination treatment of circ_001569 knockdown and the
Wnt/β-catenin agonist, LiCl. Therefore, these results indicated
that circ_001569 promoted cell resistance to cisplatin in
osteosarcoma. However, the mechanism by which circ_001569 regulates
Wnt/β-catenin signaling remains unknown. The classic role for
circRNAs is to serve as an miRNA sponge or to interact with
molecular proteins including GFRA1 and Foxo3, and act as ceRNAs in
triple negative breast cancer by regulating miR-34a and Foxo3
activity promoted by non-coding effects of circular RNA and Foxo3
pseudogene in the inhibition of tumor growth and angiogenesis
(22,23). Therefore, revealing the underling
mechanisms of circ_001569 involved in Wnt/β-catenin signaling will
be investigated in the future.
In conclusion, the results of the present study
demonstrated that circ_001569 expression was higher in
osteosarcoma. Upregulation of circ_001569 promoted cell
proliferation ability. Furthermore, it was demonstrated that
circ_001569 promoted cell resistance to cisplatin in osteosarcoma
by activating Wnt/β-catenin signaling. These results indicated that
circ_001569 may be a potential therapeutic target of
osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and JY conceived and designed the study. JY, XL
and YZ performed the experiments. JY, XL and YZ analyzed and
interpreted the data. YZ wrote the manuscript.
Ethics aproval and consent to
participate
Written informed consent was obtained from all
patients and the study was approved by the Ethics Committee of
Affiliated Hospital of Weifang Medical University.
Patient consent for publication
All patients provided consent for the publication of
their data.
Conflicts of interests
The authors declare that they have no competing
interests.
References
|
1
|
Eppert K, Wunder JS, Aneliunas V, Kandel R
and Andrulis IL: Von Willebrand factor expression in osteosarcoma
metastasis. Mod Pathol. 18:388–397. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
4
|
Thompson LD: Osteosarcoma. Ear Nose Throat
J. 92(288): 290. 2013.
|
|
5
|
Meng S, Zhou H, Feng Z, Xu Z, Tang Y, Li P
and Wu M: CircRNA: Functions and properties of a novel potential
biomarker for cancer. Meng S. 16:942017.
|
|
6
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Z, Xie L, Han L, Qu X, Yang Y, Zhang
Y, He Z, Wang Y and Li J: Circular RNAs: Regulators of
cancer-related signaling pathways and potential diagnostic
biomarkers for human cancers. Theranostics. 7:3106–3117. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L, Zhang S, Wu J, Cui J, Zhong L,
Zeng L and Ge S: circRNA_100290 plays a role in oral cancer by
functioning as a sponge of the miR-29 family. Oncogene.
36:4551–4561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie H, Ren X, Xin S, Lan X, Lu G, Lin Y,
Yang S, Zeng Z, Liao W, Ding YQ and Liang L: Emerging roles of
circRNA_001569 targeting miR-145 in the proliferation and invasion
of colorectal cancer. Oncotarget. 7:26680–26691. 2016.PubMed/NCBI
|
|
10
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 12:3077–3079. 2010.
View Article : Google Scholar
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lindsey BA, Markel JE and Kleinerman ES:
Osteosarcoma overview. Rheumatol Ther. 4:25–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu J, Liao Q, He H, Zhong D and Yin K:
TWIST interacts with β-catenin signaling on osteosarcoma cell
survival against cisplatin. Mol Carcinog. 53:440–446. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He J, Xie Q, Xu H, Li J and Li Y: Circular
RNAs and cancer. Cancer Lett. 396:138–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA: A new star of noncoding
RNAs. Cancer letters. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jin H, Jin X, Zhang H and Wang W: Circular
RNA hsa-circ-0016347 promotes proliferation, invasion and
metastasis of osteosarcoma cells. Oncotarget. 8:25571–25581.
2017.PubMed/NCBI
|
|
18
|
Li JF and Song YZ: Circular RNA GLI2
promotes osteosarcoma cell proliferation, migration, and invasion
by targeting miR-125b-5p. Tumour Biol. 39:10104283177099912017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
WITHDRAWN: Circular RNA hsa_circ_0001564
facilitates tumorigenesis of osteosarcoma via sponging miR-29c-3p.
Tumour Biol. 39:10104283177099892017.
|
|
20
|
Scholten DJ II, Timmer CM, Peacock JD,
Pelle DW, Williams BO and Steensma MR: Down regulation of Wnt
signaling mitigates hypoxia-induced chemoresistance in human
osteosarcoma cells. PLoS One. 9:e1114312014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Zhao L and Wang Q: Overexpression of
long non-coding RNA HOTTIP increases chemoresistance of
osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J
Transl Res. 8:2385–2393. 2016.PubMed/NCBI
|
|
22
|
He R, Liu P, Xie X, Zhou Y, Liao Q, Xiong
W, Li X, Li G, Zeng Z and Tang H: circGFRA1 and GFRA1 act as ceRNAs
in triple negative breast cancer by regulating miR-34a. J Exp Clin
Cancer Res. 36:1452017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang W, Du WW, Li X, Yee AJ and Yang BB:
Foxo3 activity promoted by non-coding effects of circular RNA and
Foxo3 pseudogene in the inhibition of tumor growth and
angiogenesis. Oncogene. 35:3919–3931. 2016. View Article : Google Scholar : PubMed/NCBI
|