Introduction
Gastric cancer (GC) is a disease that poses a
serious threat to human health and quality of life globally
(1). In 2015, GC is the third highest
cause of cancer-associated moralities in males and the second
highest in females in China (2).
Currently, patients with GC generally have a poor prognosis, and
early diagnosis is important to improve patient outcomes (3). Treatment primarily comprises of combined
therapy of surgery with chemotherapy and radiotherapy; however,
this treatment regime has limited effectiveness. Due to the
majority of patients with GC being diagnosed at advanced stages,
the resection rate for GC has been reported to be ~50% (4). Chemotherapy is an adjunctive treatment
that is only able to alleviate the symptoms and prolong the
survival of patients with GC, rather than treating the disease
(5). Whether adjuvant radiotherapy
can improve the overall survival of patients with GC remains
controversial (6). Developing
effective therapeutic strategies based on novel targets may help to
improve the overall prognosis of patients with GC. Investigating
the molecular mechanisms underlying GC progression is crucial in
the identification of novel therapeutic targets.
Transmembrane-4 L6 family member 1 (TM4SF1), a low
molecular weight protein consisting of 202 amino acids, is a member
of the transmembrane-4 protein L6 superfamily. This family also
includes TM4SF4, TM4SF5 and TM4SF18 (7). TM4SF1 is highly expressed in activated
endothelial cells and can activate vascular endothelial growth
factor (VEGF)-A or thrombin to stimulate angiogenesis (8). Additionally, TM4SF1 may also promote
cell migration by increasing the formation of filopodia (9). TM4SF1 may therefore be a tumor promoter,
and a number of studies have confirmed the important role of TM4SF1
in tumor progression. Cao et al (10) reported that TM4SF1 regulates
pancreatic cancer migration and invasion in vitro and in
vivo. Huang et al (11)
reported that TM4SF1 promotes proliferation, invasion and
metastasis in human liver cancer cells. Sun et al (12) confirmed that TM4SF1 regulates breast
cancer cell migration and apoptosis; however, the effects of TM4SF1
on GC remain unclear.
A previous study reported that TM4SF1 was
overexpressed in GC (13). Based on
this, it was hypothesized in the present study that TM4SF1 may be
an important regulator of GC development. The aim of the present
study was to assess the effects of TM4SF1 on the proliferation,
migration and invasion of GC cells. These data may improve the
understanding of the molecular mechanisms underlying GC
progression, providing a basis for the development of effective
therapeutic strategies.
Materials and methods
Cell culture and reagents
Human GC cell lines MGC803 and MKN45 were obtained
from the Cell Bank of the Shanghai Institute of Biological
Sciences, Chinese Academy of Sciences (Shanghai, China) and
maintained in RPMI-1640 supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2. Antibodies against B-cell lymphoma 2
(Bcl2; 1:500; ab692), caspase-3 (1:1,000; ab13847), Bcl2-associated
X (Bax; 1:2,000; ab32503) and β-actin (1:2,000; ab8227) were
purchased from Abcam (Cambridge, UK). Bound antibodies were
detected with horseradish peroxidase-conjugated antibody against
mouse (1:10,000; ab6728; Abcam) or rabbit IgG (1:10,000; sc-2357;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), followed by
enhanced chemiluminescence detection (GE Healthcare Life Sciences,
Little Chalfont, UK).
Gene transduction
The TM4SF1 small interfering RNA (siRNA),
TM4SF1-expressing plasmid and their control vectors were obtained
from Shanghai GenePharma Co., Ltd. (Shanghai, China). The sequence
of TM4SF1 siRNA was as follows: Sense,
5′-GGCUCUUGGUCGGAAUUGAATT-3′, and antisense,
5′-UUCAAUUCCACCAAGAGCCTT-3′. The TM4SF1-expressing plasmid was
constructed by subcloning the human TM4SF1 cDNA by Shanghai
GenePharma Co., Ltd.. MGC803 and MKN45 cells were seeded on to a
24-well plate at a concentration of 1×105 cells/well for
transfection. TM4SF1 siRNA (50 nM) and TM4SF1-expressing plasmid (1
µg/well) were transfected into MGC803 and MKN45 cells with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
control group represented MGC803 and MKN45 cells transfected with
their control vectors. The siTM4SF1 group represented MGC803 and
MKN45 cells transfected with TM4SF1 siRNA. The specificity of
TM4SF1 siRNA was verified by a rescue experiment (14). In the rescue experiment, the TM4SF1
siRNA and TM4SF1-expressing plasmid were co-transfected into MGC803
and MKN45 cells, denoted as the siTM4SF1+TM4SF1 group. Changes in
TM4SF1 expression levels were assessed using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) at
48 h after transfection.
RT-qPCR
Total RNA was isolated from MGC803 and MKN45 cells
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.). Purified RNA was reverse transcribed into cDNA with the
M-MLV First Strand kit (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. RT-qPCR reactions
were conducted using the SYBR® mix (Invitrogen; Thermo
Fisher Scientific, Inc.) on an ABI PRISM 7500 Sequence Detection
system (Applied Biosystems; Thermo Fisher Scientific, Inc.)
(15). Briefly, after an initial
denaturation step at 95°C for 30 sec, amplifications were conducted
with 40 cycles of 95°C for 5 sec and 60°C for 34 sec. Human β-actin
was used as the housekeeping gene to normalize the expression
levels of the target genes (16,17).
Primers used were as follows: TM4SF1, forward,
5′-CTGCTCTCACCAACAGCAAT-3′, and reverse,
5′-TGCCAGTCTTTACAGGCGTT-3′; Bcl2, forward, 5′-CAGGAAACGGCCCGGAT-3′,
and reverse, 5′-CTGGGGCCTTTCATCCTCC-3′; Bax, forward,
5′-GGGTTGTCGCCCTTTTCTAC-3′, and reverse,
5′-CTGGAGACAGGGACATCAGT-3′; caspase-3, forward,
5′-TGCTATTGTGAGGCGGTTGTAG-3′, and reverse,
5′-GGCACACCCACCGAAAAC-3′; and β-actin, forward,
5′-CATTAAGGAGAAGCTGTGCT-3′, and reverse,
5′-GTTGAAGGTAGTTTCGTGGA-3′. The relative expression levels were
quantified with the 2−ΔΔCq method (18).
Western blotting
The protein was extracted from cells using a mixture
of Pierce RAPI Buffer (Thermo Fisher Scientific, Inc.) and Halt
Protease Inhibitor Cocktail (Thermo Fisher Scientific, Inc.) at a
ratio of 100:1. The protein concentration was determined using the
bicinchoninic acid assay (Thermo Fisher Scientific, Inc.). Lysates
of MGC803 and MKN45 cells were centrifuged at 4°C for 10 min at
12,000 × g. Equal amounts of proteins (50 µg) were separated by 10%
SDS-PAGE and transferred onto polyvinylidene fluoride membranes.
Following blocking with 5% bovine serum albumin (Invitrogen; Thermo
Fisher Scientific, Inc.)/PBS with Tween 20 buffer at 37°C for 1 h,
membranes were incubated with primary antibodies at 4°C overnight.
Subsequently, blots were incubated with horseradish
peroxidase-linked secondary antibodies at 37°C for 2–3 h.
Immunoreactive proteins were detected using the SuperSignal West
Pico Chemiluminescent substrate (Thermo Fisher Scientific, Inc.).
Bands were quantified using ImageJ 1.50i (National Institutes of
Health, Bethesda, MD, USA) (19).
Cell apoptosis assay
Apoptosis of MGC803 and MKN45 cells was evaluated
using flow cytometry with an Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) Apoptosis kit (BD Pharmingen; BD
Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's protocol. Cells were washed with cold PBS twice and
resuspended in 100 µl 1X binding buffer, following which 5 µl
Annexin V-FITC and 5 µl PI were added for 10 min at room
temperature in the dark. Finally, 400 µl binding buffer was added
to the cells, which were analyzed by a flow cytometer. All data
were analyzed by FlowJo software version 7.6.1 (FlowJo LLC,
Ashland, OR, USA).
Proliferation assay
An MTT assay was used to measure the viability of
MGC803 and MKN45 cells. A 100 µl GC cell suspension containing
2,000 cells was seeded in each well of a 96-well plate and
incubated for 4 h in an atmosphere containing 5% CO2 at
37°C. Subsequently, 100 µl MTT solution was added to each well and
incubated for 4 h in an atmosphere containing 5% CO2 at
37°C. A total of 100 µl dimethyl sulfoxide was added to each well
and incubated again for 4 h in an atmosphere containing 5%
CO2 at 37°C, in order to dissolve the formazan crystals.
Absorbance was measured at 570 nm using a Multiskan Plate Reader
(Thermo Fisher Scientific, Inc.). Each experiment was repeated
three times.
Transwell assay
Cell migration ability was measured using cell
culture inserts (24-well type, 8-µm pore size; Corning
Incorporated, Corning, NY, USA). Subsequently, 1×105
MGC803 and MKN45 cells were added into the upper chambers with
serum-free RPMI-1640 medium, while the lower chambers were filled
with 500 µl RPMI-1640 supplemented with 10% FBS. Following 16 h of
incubation at 37°C, the cells that had migrated to the lower
chamber were fixed in 100% methanol at room temperature for 30 min
and stained with 0.1% crystal violet at room temperature for 20
min. Cells were visualized and the number in 10 random fields were
counted under a light microscope (Olympus Corporation, Tokyo,
Japan; magnification, ×400).
Wound-healing assay
MGC803 and MKN45 cells were seeded in 6-well plates
(5×105 cells/well) with 2 ml RPMI-1640 medium
supplemented with 10% FBS. Once 80% confluence was attained,
scratches were produced using a 100-µl pipette tip. Wound healing
was observed and images of the migration distance were captured at
room temperature at 0 and 24 h under a light microscope (Olympus
Corporation; magnification, ×100).
Statistical analysis
Statistical analysis was performed using SPSS v21.0
(IBM Corp., Armonk, NY, USA) and GraphPad Prism v5.0 (GraphPad
Software, Inc., La Jolla, CA, USA) software. Differences between ≥2
groups were assessed using one-way analysis of variance followed by
Tukey's post-hoc test. Data are presented as the mean ± standard
error of the mean. All experiments comprised three replicates and
were performed at least twice independently. P<0.05 was
considered to indicate a statistically significant difference,
unless otherwise stated.
Results
Efficiency of TM4SF1 siRNA and
TM4SF1-expressing plasmid
The efficiency of gene transduction was confirmed by
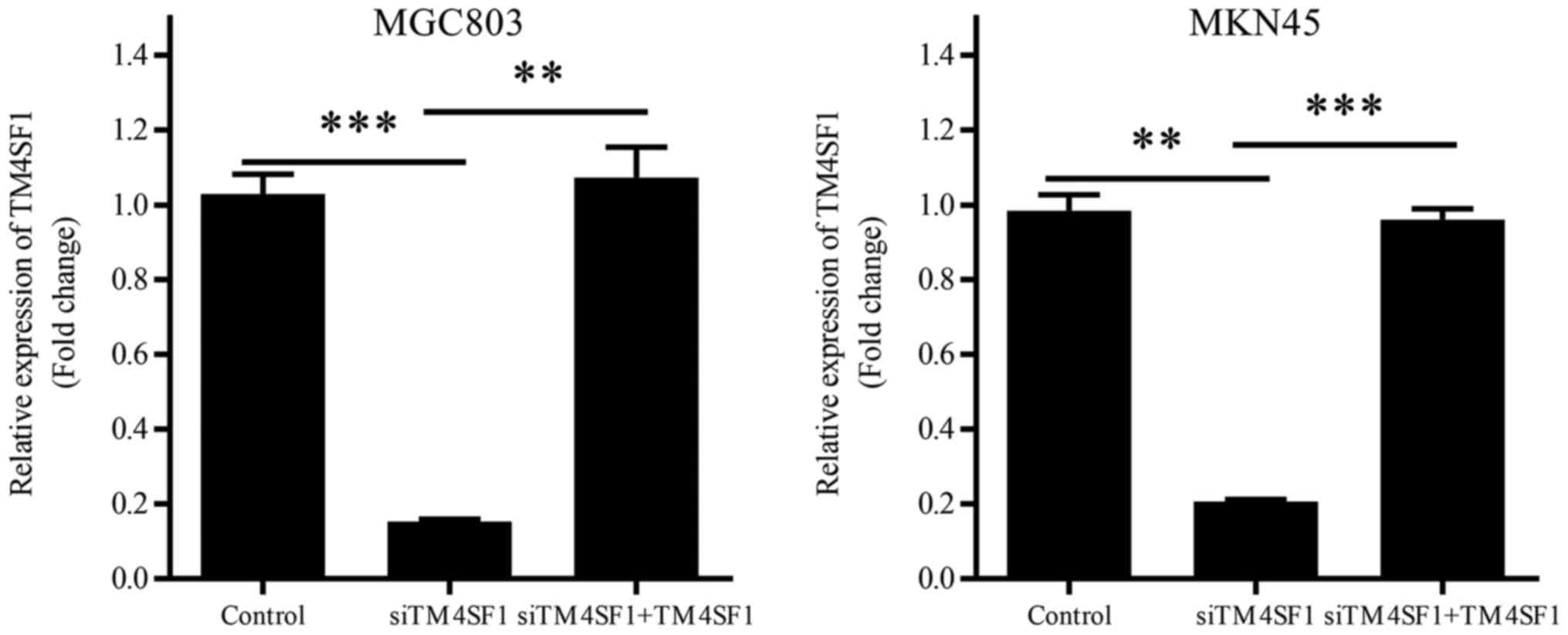
RT-qPCR. The results for MGC803 and MKN45 were consistent with one
another. TM4SF1 mRNA expression was significantly downregulated in
the siTM4SF1 group, compared with the control group (P<0.001 and
P<0.01, respectively; Fig. 1).
Transfection with the TM4SF1-expressing plasmid was demonstrated to
significantly reversed the reduction of TM4SF1 mRNA expression,
compared with the siTM4SF1 group (P<0.01 and P<0.001,
respectively; Fig. 1).
TM4SF1 influences the expression of
apoptotic molecules in MGC803 and MKN45 cells
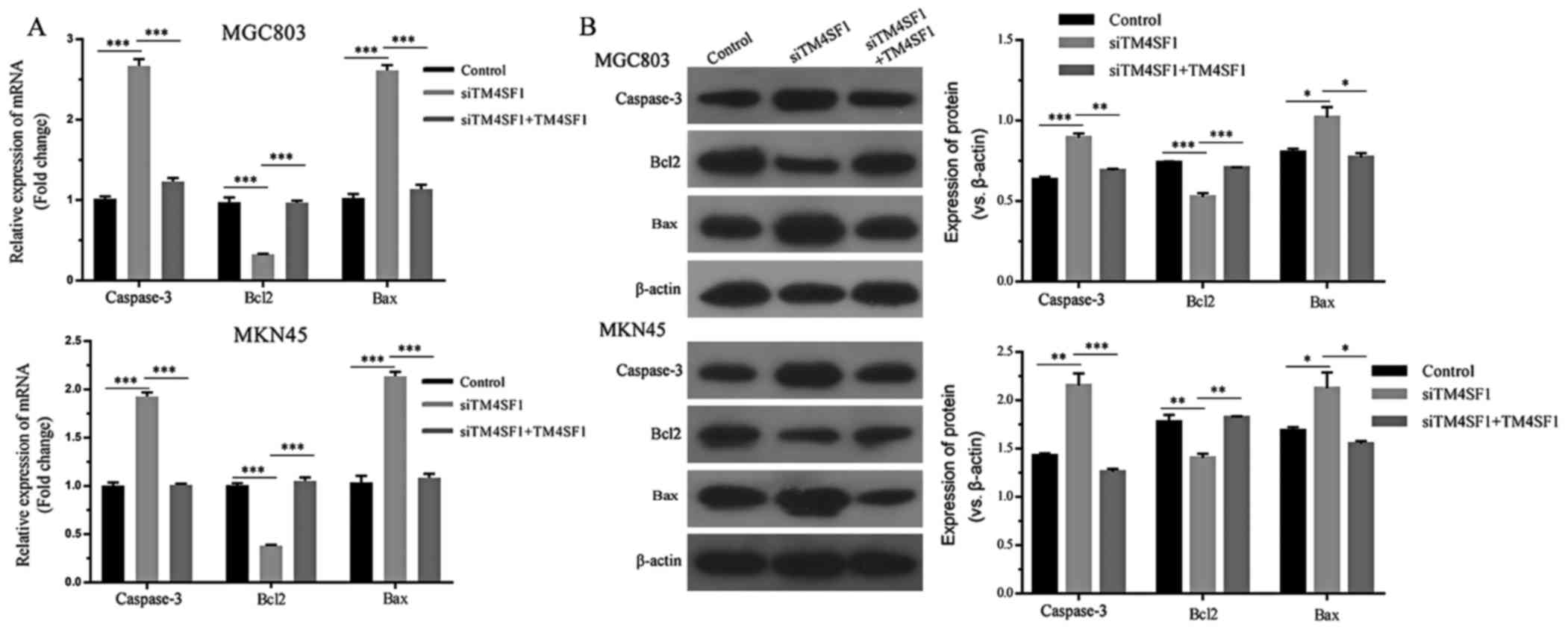
The expression of apoptotic molecules was assessed
using RT-qPCR. By comparing the siTM4SF1 group with the control
group, it was demonstrated that TM4SF1 silencing significantly
decreased Bcl2 mRNA expression, whilst caspase-3 and Bax expression
levels were upregulated (P<0.001; Fig.
2A). By comparing the siTM4SF1 and siTM4SF1+TM4SF1 groups, it
was demonstrated that TM4SF1 upregulation significantly reversed
alterations in the mRNA expressions profile of MGC803 and MKN45
cells (P<0.001; Fig. 2A). Western
blotting was used to assess these genes at the protein level. The
results indicated that the effects of TM4SF1 on Bcl2, caspase-3 and
Bax protein expression levels were consistent with the mRNA
expression level results, and the comparison was statistically
significant (P<0.05; Fig. 2B).
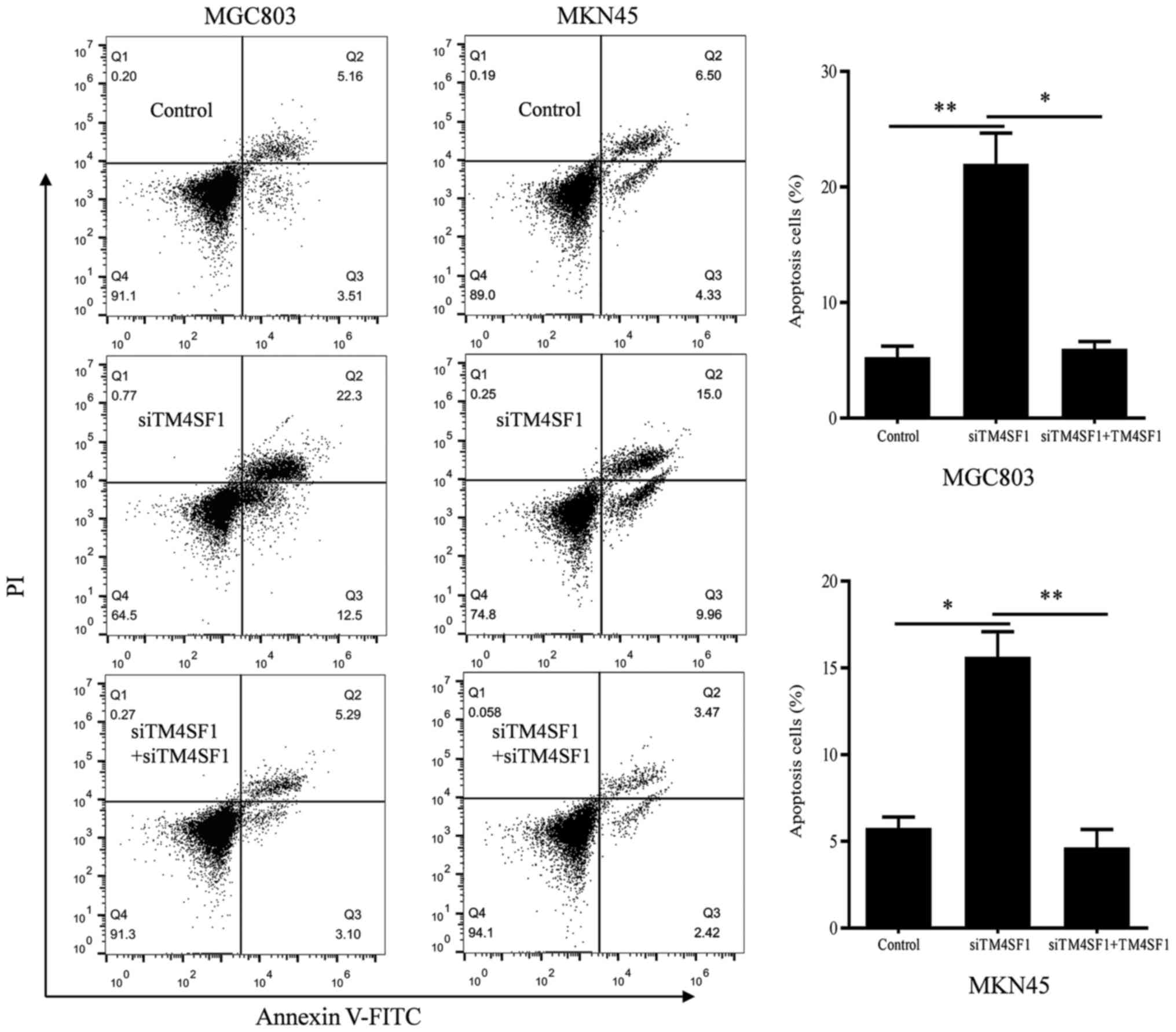
Flow cytometry was used to verify the role of TM4SF1 in the
apoptosis of MGC803 and MKN45 cells. The results demonstrated that
the apoptosis of MGC803 and MKN45 cells in the siTM4SF1 group was
significantly promoted, compared with the control group (P<0.01
and P<0.05, respectively; Fig. 3).
Rescue experiments demonstrated that the apoptosis of MGC803 and
MKN45 cells in the siTM4SF1+TM4SF1 group was significantly
decreased, compared with the siTM4SF1 group (P<0.05 and
P<0.01, respectively; Fig. 3).
These results were consistent with changes in the expression of the
aforementioned apoptotic molecules.
Roles of TM4SF1 in the proliferation,
migration and invasion of MGC803 and MKN45 cells
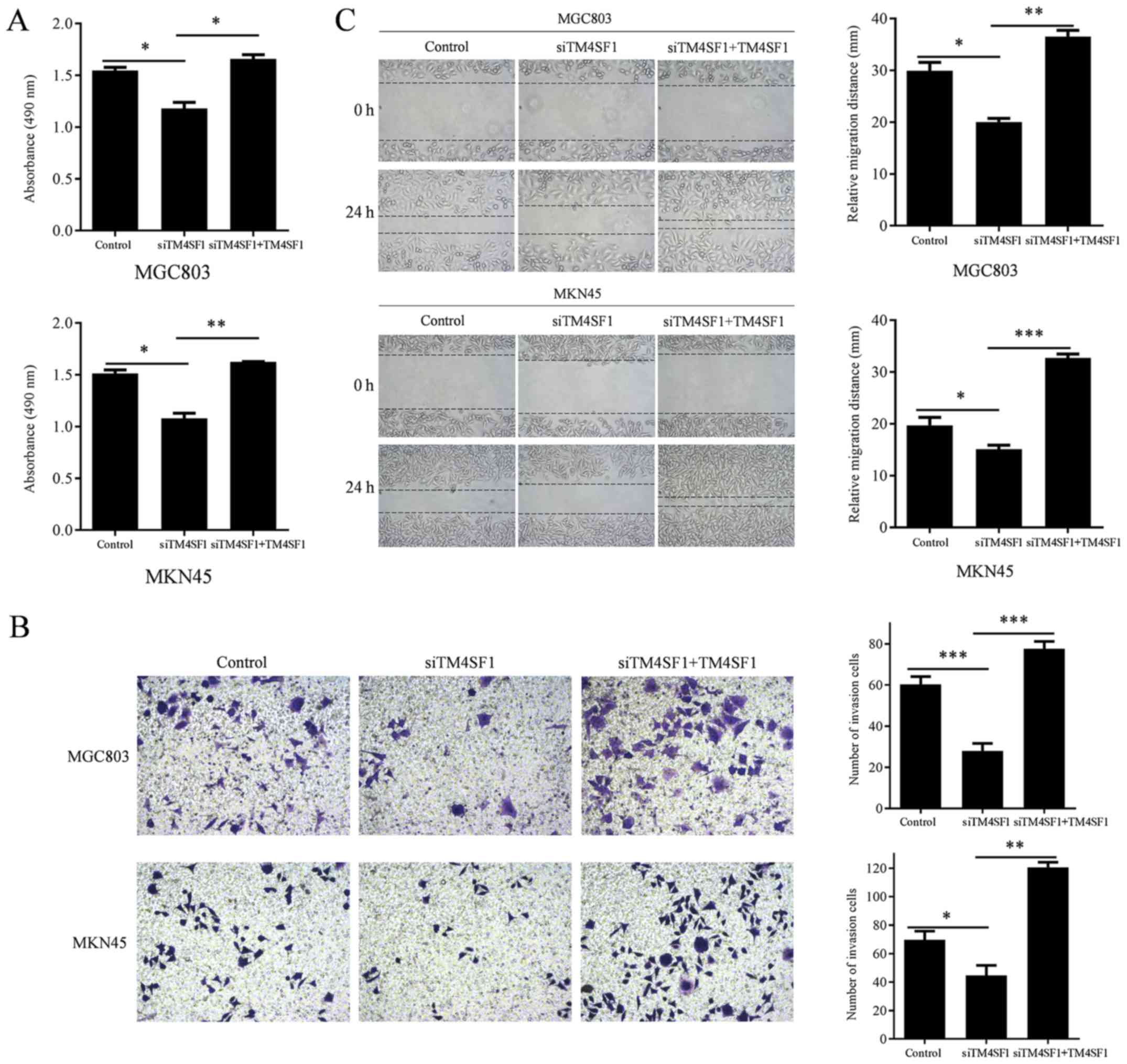
To investigate the biological functions of TM4SF1 in
GC, the proliferation, invasion and migration of MGC803 and MKN45
cells were assessed following the manipulation of TM4SF1
expression. The proliferation of MGC803 and MKN45 cells was
measured using an MTT assay. Wound healing assays were used to
measure the invasion of MGC803 and MKN45 cells. Transwell assays
were used to measure the migration ability of MGC803 and MKN45
cells. The results demonstrated that TM4SF1 knockdown significantly
reduced the proliferation of MGC803 and MKN45 cells, compared with
the control group (P<0.05, and P<0.05, respectively; Fig. 4A), while TM4SF1 upregulation
significantly increased the proliferation of MGC803 and MKN45
cells, compared with the siTM4SF1 group (P<0.05 and P<0.01,
respectively; Fig. 4A). TM4SF1
knockdown significantly reduced the invasion of MGC803 and MKN45
cells, compared with the control group (P<0.05 and P<0.05,
respectively; Fig. 4B), while TM4SF1
upregulation significantly increased the invasion of MGC803 and
MKN45 cells, compared with the siTM4SF1 group (P<0.01 and
P<0.001, respectively; Fig. 4B).
TM4SF1 knockdown resulted in significantly reduced migration of
MGC803 and MKN45 cells, compared with the control group (P<0.001
and P<0.05, respectively; Fig.
4C), while TM4SF1 upregulation significantly increased
migration of MGC803 and MKN45 cells, compared with the siTM4SF1
group (P<0.001 and P<0.01, respectively; Fig. 4C).
Discussion
A number of studies have indicated that TM4SF1 may
inhibit tumor apoptosis and promote tumor proliferation (10–12);
however, the underlying mechanisms are notably complex. Sun et
al (12) reported that the
phosphoinositide 3-kinase (PI3K)/AKT/mechanistic target of
rapamycin (mTOR) signaling pathway served a role in TM4SF1
regulation in breast cancer cell apoptosis. The PI3K/AKT/mTOR
signaling pathway has been reported to regulate apoptosis in a
number of tumor types, including breast cancer, glioma and
nasopharyngeal cancer (20–22). The anti-apoptotic effects of the
PI3K/AKT/mTOR signaling pathway are dependent upon downstream
apoptosis-associated proteins, including Bcl2, Bax and caspase-3
(23). Bcl2, as a member of the Bcl2
family, can prevent the release of cytochrome c from the
mitochondria to the cytoplasm, thereby inhibiting apoptosis
(24). The Bcl2 family is comprised
of polarized groups of proteins containing pro-apoptotic proteins
and anti-apoptotic proteins, and cell survival or apoptosis depend
on the balance between these two types (25). Bax, a pro-apoptotic protein belonging
to the Bcl2 family, can form a heterodimer with Bcl2 to inhibit its
function (24). Caspase-3 is the most
critical apoptotic protease involved in apoptosis. DNA-dependent
protein kinase and poly adenosine diphosphate ribose polymerase are
important DNA repair enzymes. Caspase-3 can hydrolysate these two
enzymes to prevent DNA replication, transcription and injury repair
(26). The results of the present
study demonstrated that siTM4SF1 upregulates the expression of
pro-apoptosis proteins Bax and caspase-3 and downregulates the
expression of anti-apoptosis protein Bcl2. This indicates that
TM4SF1 may serve as an anti-apoptotic protein in GC.
The results of the present study demonstrated the
important role of TM4SF1 in tumor migration and invasion, and the
complex mechanisms underlying has been verified in previous studies
(27–29). Lekishvili et al (27) reported that TM4SF1 may be associated
with tetraspanin-enriched microdomains, which are crucial for the
pro-migratory activity of membrane proteins; furthermore, TM4SF1
overexpression downregulated the expression of tetraspanins cluster
of differentiation (CD)63 and CD82, which are associated with the
regulation of surface (28,29). CD82 can promote the proliferation of
epidermal growth factor receptor to activate signaling cascades,
including the FAK-Lyn-p130CAS-CrkII signaling pathway, which
results in reduced cell motility (30). A number of additional molecules are
associated with TM4SF1. A previous study demonstrated revealed an
association between TM4SF1 and CD13 using coimmunoprecipitation
analysis, which can form and enhance cell migration in lung cancer
cells (31). In prostate cancer,
TM4SF1 was reported to directly target the androgen receptor to
promote cell migration (32).
In the present study, functional experiments were
performed to verify the effects of TM4SF1 knockdown and
upregulation in GC cells. The results demonstrated that TM4SF1 may
regulate the apoptosis, proliferation, migration and invasion of GC
cells; however, tumor progression is a complicated process with
numerous contributing factors, relying on not only the changes in
tumor cells but also changes in the tumor microenvironment
(33). It is therefore necessary to
investigate the role of TM4SF1 in the tumor microenvironment.
Xue et al (34)
demonstrated that matrix metallopeptidase (MMP)-2, MMP-9 and VEGF
are the downstream proteins of TM4SF1, and TM4SF1 overexpression in
turn upregulated MMP-2, MMP-9 and VEGF expression. VEGF is known to
be a strong angiogenic factor, and the binding of VEGF and its
receptors may stimulate endothelial cell division, proliferation
and migration, promote physiological and pathological
neovascularization, increase microvascular permeability and promote
embryonic hematopoiesis (35–37). VEGF overexpression promotes the
secretion of MMP-2 and MMP-9, which may serve a crucial role in
tumor invasion and metastasis (28,29).
MMP-2, a zinc-dependent proteolytic enzyme that is secreted by
tumor and stromal cells in the form of zymogens, has been reported
to be closely associated with the development of tumors. Following
hydrolysis, MMP-2 promotes the transformation of the extracellular
matrix by degrading the main constituents of the basement membrane,
including type IV, V, VI and X collagens and gelatin. This promotes
tumor neovascularization, invasion and metastasis (38). MMP-9 is the enzyme with the largest
molecular weight in the MMP family, and is also secreted in zymogen
form. Following activation, MMP-9 can be transformed into type IV
collagenase, which degrades and destroys type IV and V collagens as
well as gelatin in the extracellular matrix around the tumor
surface. Tumor cells are then able to infiltrate the surrounding
tissue via the deficient basement membrane, which ultimately
results in tumor invasion and metastasis (39).
The present study was not without limitations.
Firstly, experiments were only conducted in vitro. Secondly,
further research is required to identify the signaling pathways
activated by TM4SF1 in GC. Despite these limitations, the results
of the present study indicated that TM4SF1 is an anti-apoptotic
protein that has the ability to promote the proliferation,
migration and invasion of GC cells.
To conclude, the results of the present study
indicated that TM4SF1 may serve an important role in the
progression of GC. In the future, TM4SF1 may be considered as a
novel therapeutic target of GC; however, future in vivo
experiments and clinical trials are required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Public
Welfare Research Project of Huzhou Science and Technology Bureau
(grant no. 2017GY47), Zhejiang Public Welfare Technology Research
Social Development Project (grant no. 2014C33137) and Huzhou
General scientific research project (grant no. 2010YSB08).
Availability of data and materials
All data that were generated or analyzed in this
study are included in this manuscript.
Authors' contributions
YW conceived and designed the study. XS was a major
contributor in writing the manuscript. XS and LL conducted the
majority of the experiments. GC performed the statistical analysis.
XC cultured the cells and drew the figures. YW conducted the data
interpretation. HS performed the literature search and cell
transfection. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
VEGF
|
vascular endothelial growth factor
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
PVDF
|
polyvinylidene fluoride
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lang GD and Konda VJ: Early diagnosis and
management of esophageal and gastric cancer. Minerva Gastroenterol
Dietol. 59:357–376. 2013.PubMed/NCBI
|
|
4
|
Li SC, Lee CH, Hung CL, Wu JC and Chen JH:
Surgical resection of metachronous hepatic metastases from gastric
cancer improves long-term survival: A population-based study. PLoS
One. 12:e01822552017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang JM, Park S and Kim SJ, Kim H, Lee B,
Kim J, Park J, Kim ST, Yang HK, Kim WH and Kim SJ: KIAA1324
suppresses gastric cancer progression by inhibiting the oncoprotein
GRP78. Cancer Res. 75:3087–3097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ng J and Lee P: The role of radiotherapy
in localized esophageal and gastric cancer. Hematol Oncol Clin
North Am. 31:453–468. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wright MD, Ni J and Rudy GB: The L6
membrane proteins-a new four-transmembrane superfamily. Protein
Sci. 9:1594–1600. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shih SC, Zukauskas A, Li D, Liu G, Ang LH,
Nagy JA, Brown LF and Dvorak HF: The L6 protein TM4SF1 is critical
for endothelial cell function and tumor angiogenesis. Cancer Res.
69:3272–3277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zukauskas A, Merley A, Li D, Ang LH,
Sciuto TE, Salman S, Dvorak AM, Dvorak HF and Jaminet SC: TM4SF1: A
tetraspanin-like protein necessary for nanopodia formation and
endothelial cell migration. Angiogenesis. 14:345–354. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao J, Yang JC, Ramachandran V, Arumugam
T, Deng DF, Li ZS, Xu LM and Logsdon CD: TM4SF1 regulates
pancreatic cancer migration and invasion in vitro and in vivo. Cell
Physiol Biochem. 39:740–750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang YK, Fan XG and Qiu F: TM4SF1
promotes proliferation, invasion, and metastasis in human liver
cancer cells. Int J Mol Sci. 17:E6612016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Xu Y, Xu J, Lu D and Wang J: Role
of TM4SF1 in regulating breast cancer cell migration and apoptosis
through PI3K/AKT/mTOR pathway. Int J Clin Exp Pathol. 8:9081–9088.
2015.PubMed/NCBI
|
|
13
|
Kaneko R, Tsuji N, Kamagata C, Endoh T,
Nakamura M, Kobayashi D, Yagihashi A and Watanabe N: Amount of
expression of the tumor-associated antigen L6 gene and
transmembrane 4 superfamily member 5 gene in gastric cancers and
gastric mucosa. Am J Gastroenterol. 96:3457–3458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ke J, Ma P, Chen J, Qin J and Qian H: Lgr6
promotes the progression of gastric cancer through PI3K/AKT/mTOR
pathway. Onco Targets Ther. 11:3025–3033. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahdavinezhad A, Yadegarazari R,
Mousavi-Bahar SH, Poorolajal J, Jafari M, Amirzargar MA, Effatpanah
H and Saidijam M: Evaluation of zinc finger E-box binding homeobox
1 and transforming growth factor-beta2 expression in bladder cancer
tissue in comparison with healthy adjacent tissue. Investig Clin
Urol. 58:140–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Jiang X, Zhang Q, Dong X, Gao Y, He
Y, Qiao H, Xie F, Xie X and Sun X: Neuropilin-1 is associated with
clinicopathology of gastric cancer and contributes to cell
proliferation and migration as multifunctional co-receptors. J Exp
Clin Cancer Res. 35:162016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang M, Gu YY, Peng H, Zhao M, Wang J,
Huang SK, Yuan XH, Li J, Sang JL, Luo Q and Huang C: NAIF1 inhibits
gastric cancer cells migration and invasion via the MAPK pathways.
J Cancer Res Clin Oncol. 141:1037–1047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shaik AB, Rao GK, Kumar GB, Patel N, Reddy
VS, Khan I, Routhu SR, Kumar CG, Veena I, Shekar Chandra K, et al:
Design, synthesis and biological evaluation of novel
pyrazolochalcones as potential modulators of PI3K/Akt/mTOR pathway
and inducers of apoptosis in breast cancer cells. Eur J Med Chem.
139:305–324. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao KH, Zhang C, Bai Y, Li Y, Kang X,
Chen JX, Yao K, Jiang T, Zhong XS and Li WB: Antiglioma effects of
cytarabine on leptomeningeal metastasis of high-grade glioma by
targeting the PI3K/Akt/mTOR pathway. Drug Des Devel Ther.
11:1905–1915. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin YT, Wang HC, Hsu YC, Cho CL, Yang MY
and Chien CY: Capsaicin induces autophagy and apoptosis in human
nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR
pathway. Int J Mol Sci. 18:E13432017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wen R, Hu S, Xiao Q, Han C, Gan C, Gou H,
Liu H, Li L, Xu H, He H and Wang J: Leptin exerts proliferative and
anti-apoptotic effects on goose granulosa cells through the
PI3K/Akt/mTOR signaling pathway. J Steroid Biochem Mol Biol.
149:70–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ugarte-Uribe B and García-Sáez AJ:
Apoptotic foci at mitochondria: In and around Bax pores. Philos
Trans R Soc Lond Biol Sci. 372:201602172017. View Article : Google Scholar
|
|
25
|
Henshall DC, Araki T, Schindler CK, Lan
JQ, Tiekoter KL, Taki W and Simon RP: Activation of
Bcl-2-associated death protein and counter-response of Akt within
cell populations during seizure-induced neuronal death. J Neurosci.
22:8458–8465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashimoto S, Setareh M, Ochs RL and Lotz
M: Fas/Fas ligand expression and induction of apoptosis in
chondrocytes. Arthritis Rheum. 40:1749–1755. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lekishvili T, Fromm E, Mujoomdar M and
Berditchevski F: The tumour-associated antigen L6 (L6-Ag) is
recruited to the tetraspanin-enriched microdomains: Implication for
tumour cell motility. J Cell Sci. 121:685–694. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mantegazza AR, Barrio MM, Moutel S, Bover
L, Weck M, Brossart P, Teillaud JL and Mordoh J: CD63 tetraspanin
slows down cell migration and translocates to the
endosomal-lysosomal-MIICs route after extracellular stimuli in
human immature dendritic cells. Blood. 104:1183–1190. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He B, Liu L, Cook GA, Grgurevich S,
Jennings LK and Zhang XA: Tetraspanin CD82 attenuates cellular
morphogenesis through down-regulating integrin alpha6-mediated cell
adhesion. J Biol Chem. 280:3346–3354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang XA, He B, Zhou B and Liu L:
Requirement of the p130CAS-Crk coupling for metastasis suppressor
KAI1/CD82-mediated inhibition of cell migration. J Biol Chem.
278:27319–27328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang YW, Chen SC, Cheng EC, Ko YP, Lin
YC, Kao YR, Tsay YG, Yang PC, Wu CW and Roffler SR: CD13
(aminopeptidase N) can associate with tumor-associated antigen L6
and enhance the motility of human lung cancer cells. Int J Cancer.
116:243–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Allioli N, Vincent S, Vlaeminck-Guillem V,
Decaussin-Petrucci M, Ragage F, Ruffion A and Samarut J: TM4SF1, a
novel primary androgen receptor target gene over-expressed in human
prostate cancer and involved in cell migration. Prostate.
71:1239–1250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan Y, Jiang YC, Sun CK and Chen QM: Role
of the tumor microenvironment in tumor progression and the clinical
applications (Review). Oncol Rep. 35:2499–2515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xue L, Yu X, Jiang X, Deng X, Mao L, Guo
L, Fan J, Fan Q, Wang L and Lu SH: TM4SF1 promotes the self-renewal
of esophageal cancer stem-like cells and is regulated by miR-141.
Oncotarget. 8:19274–19284. 2017.PubMed/NCBI
|
|
35
|
Lin CI, Merley A, Sciuto TE, Li D, Dvorak
AM, Melero-Martin JM, Dvorak HF and Jaminet SC: TM4SF1: A new
vascular therapeutic target in cancer. Angiogenesis. 17:897–907.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mitsutake N, Iwao A, Nagai K, Namba H,
Ohtsuru A, Saenko V and Yamashita S: Characterization of side
population in thyroid cancer cell lines: Cancer stem-like cells are
enriched partly but not exclusively. Endocrinology. 148:1797–1803.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bae S, Shim SH, Park CW, Son HK, Lee HJ,
Son JY, Jeon C and Kim H: Combined omics analysis identifies
transmembrane 4 L6 family member 1 as a surface protein marker
specific to human mesenchymal stem cells. Stem Cells Dev.
20:197–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leppä S, Saarto T, Vehmanen L, Blomqvist C
and Elomaa I: A high serum matrix metalloproteinase-2 level is
associated with an adverse prognosis in node-positive breast
carcinoma. Clin Cancer Res. 10:1057–1063. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ravikumar TS, Steele G Jr, Kane R and King
V: Experimental and clinical observations on hepatic cryosurgery
for colorectal metastases. Cancer Res. 51:6323–6327.
1991.PubMed/NCBI
|