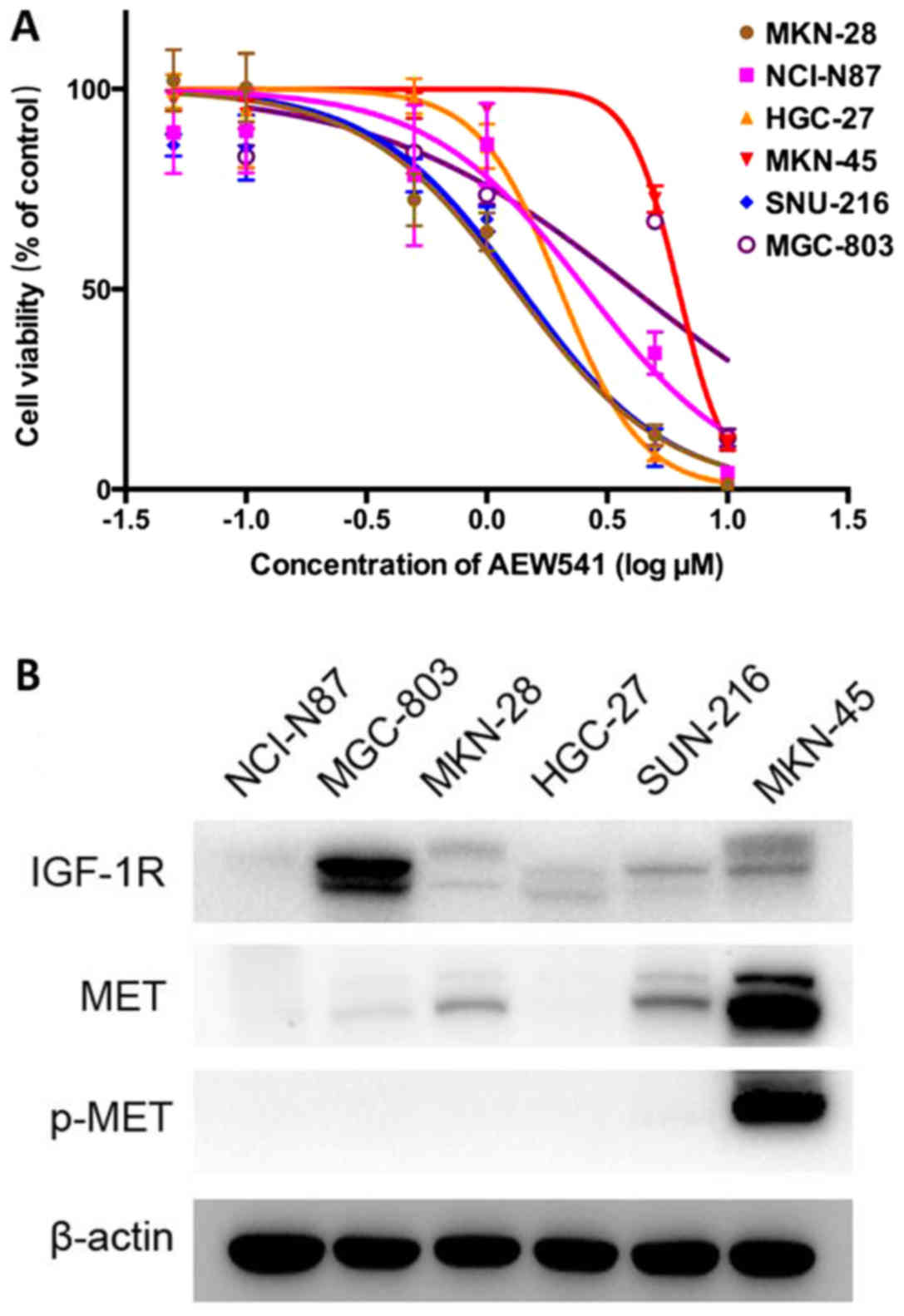
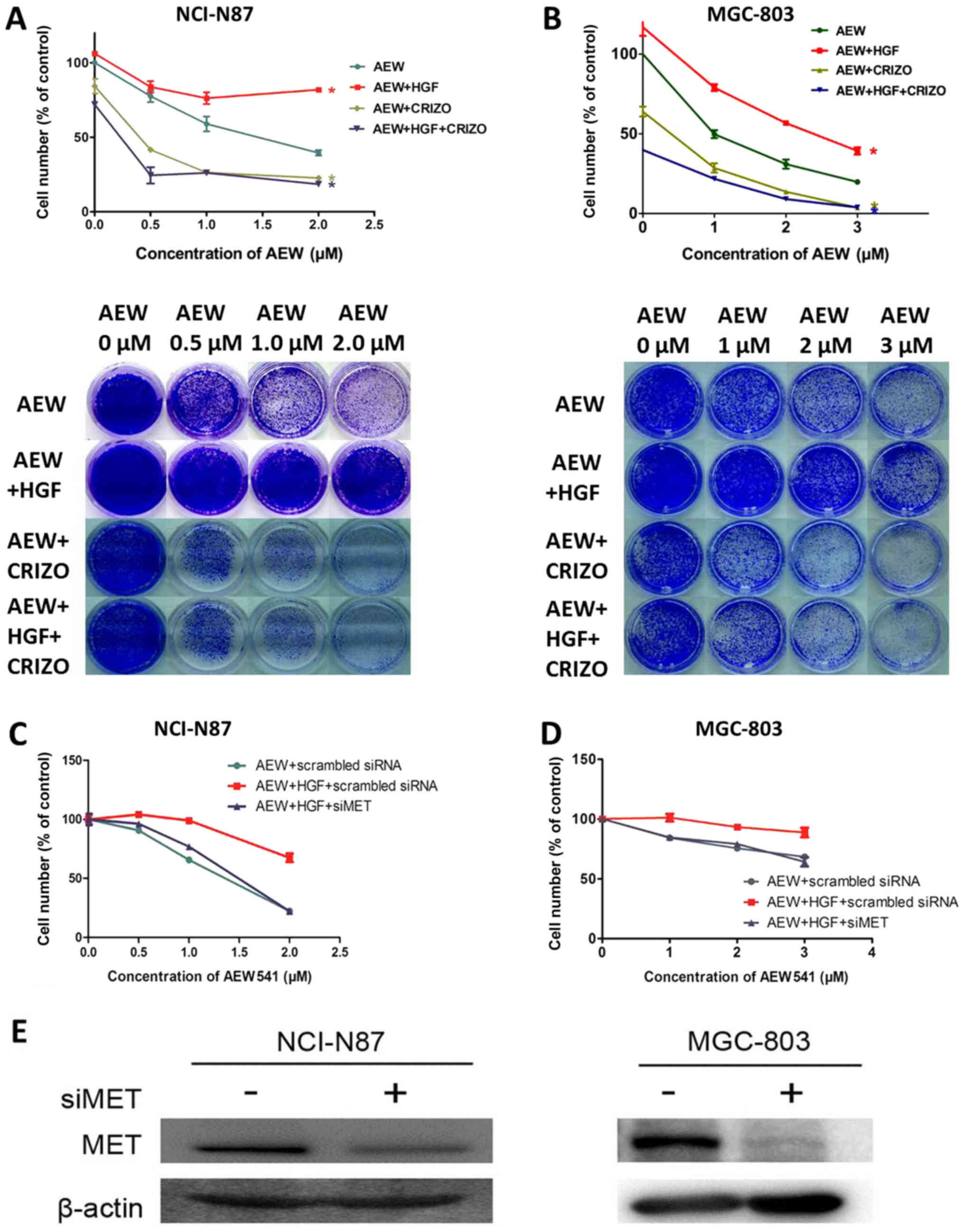
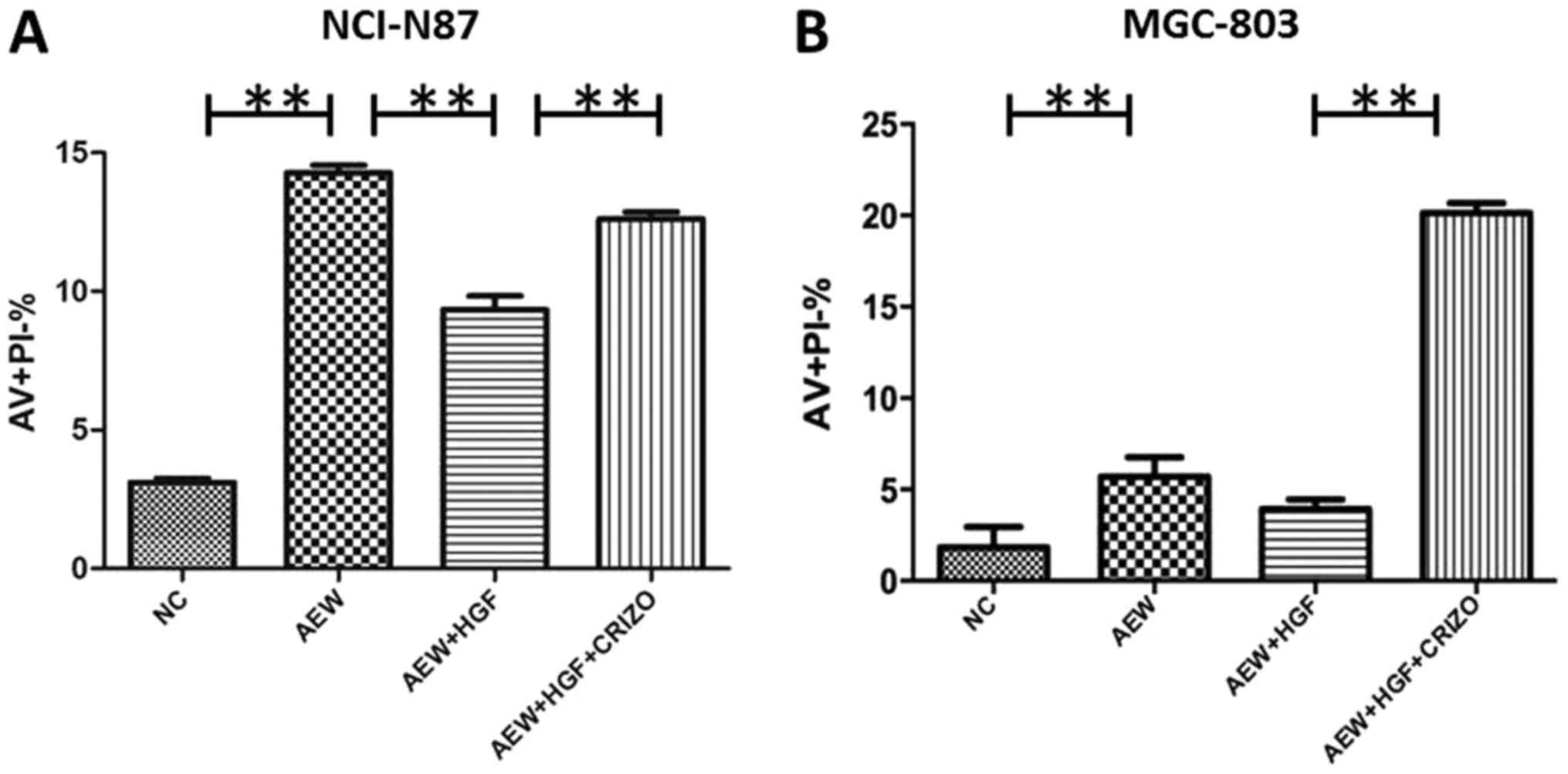
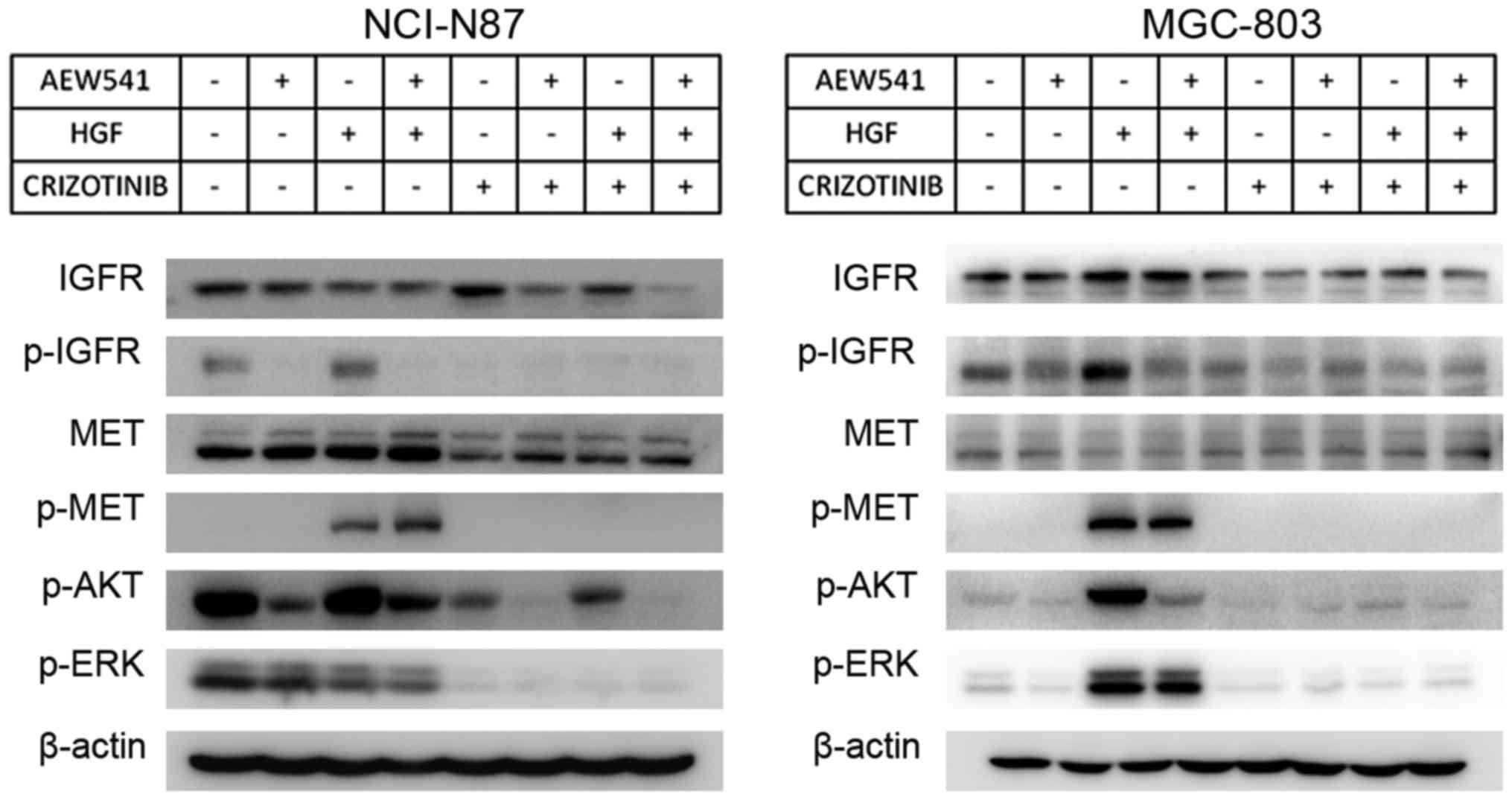
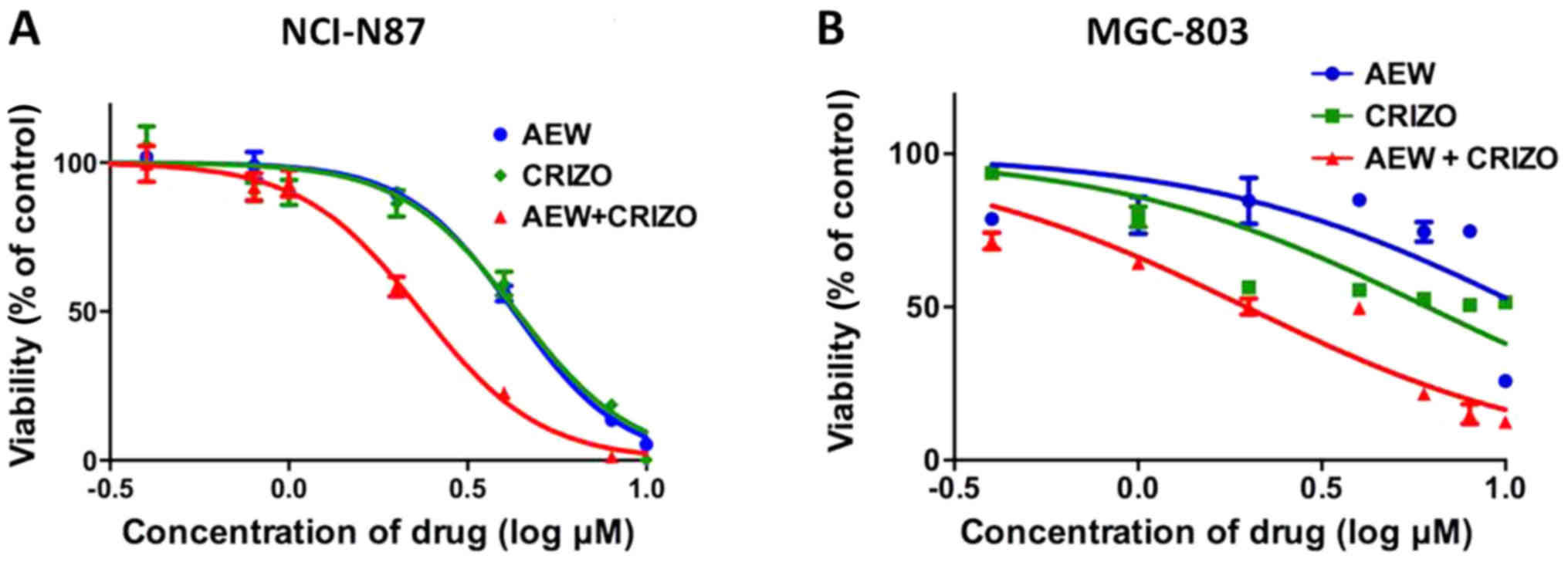
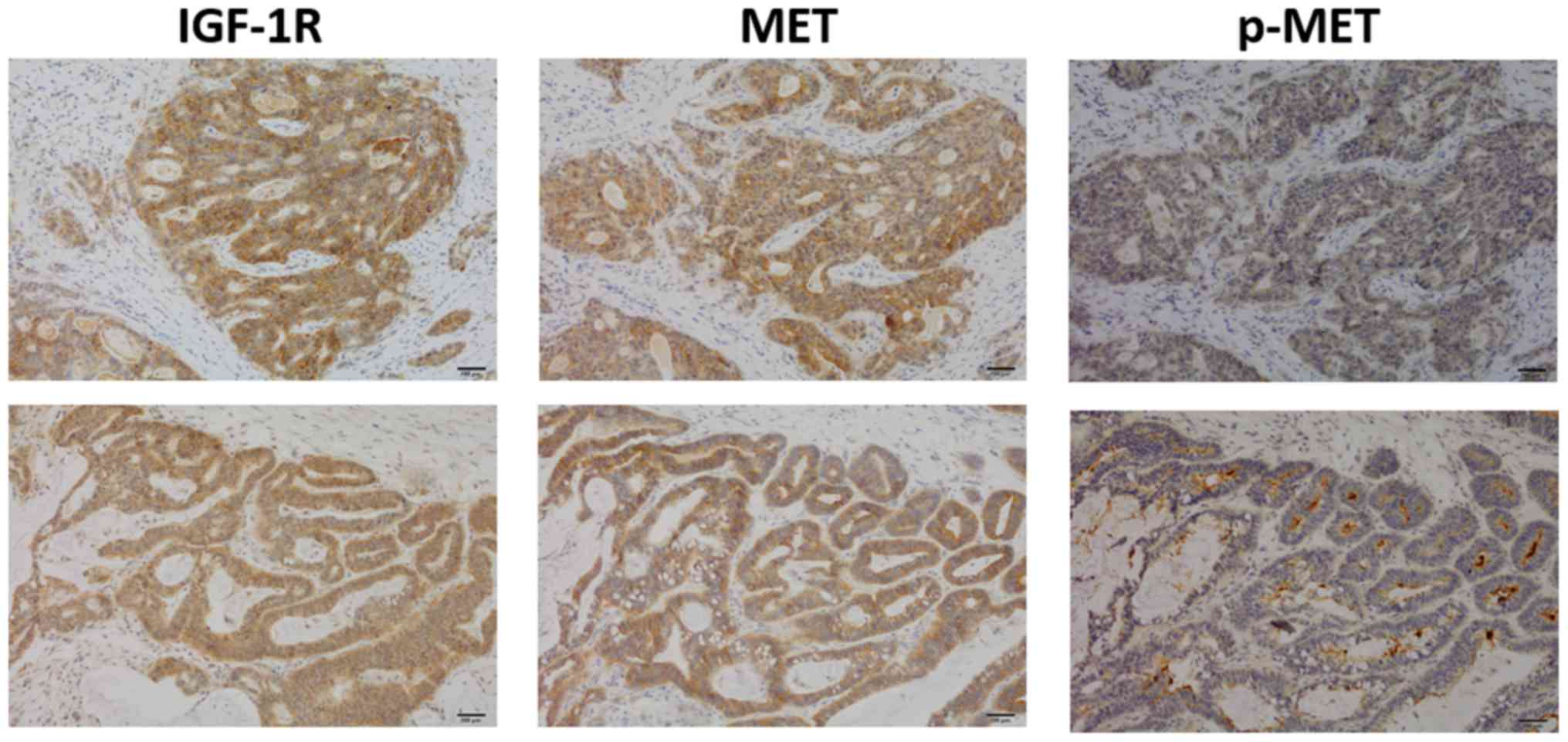
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsubara J, Yamada Y, Hirashima Y,
Takahari D, Okita NT, Kato K, Hamaguchi T, Shirao K, Shimada Y and
Shimoda T: Impact of insulin-like growth factor type 1 receptor,
epidermal growth factor receptor and HER2 expressions on outcomes
of patients with gastric cancer. Clin Cancer Res. 14:3022–3029.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pollak M: The insulin and insulin-like
growth factor receptor family in neoplasia: An update. Nat Rev
Cancer. 12:159–169. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Samani AA, Yakar S, LeRoith D and Brodt P:
The role of the IGF system in cancer growth and metastasis:
Overview and recent insights. Endocr Rev. 28:20–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karp DD, Paz-Ares LG, Novello S, Haluska
P, Garland L, Cardenal F, Blakely LJ, Eisenberg PD, Langer CJ,
Blumenschein G Jr, et al: Phase II study of the anti-insulin-like
growth factor type 1 receptor antibody CP-751,871 in combination
with paclitaxel and carboplatin in previously untreated, locally
advanced, or metastatic non-small-cell lung cancer. J Clin Oncol.
27:2516–2522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gualberto A and Pollak M: Emerging role of
insulin-like growth factor receptor inhibitors in oncology: Early
clinical trial results and future directions. Oncogene.
28:3009–3021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Janjigian YY, Tang LH, Coit DG, Kelsen DP,
Francone TD, Weiser MR, Jhanwar SC and Shah MA: MET expression and
amplification in patients with localized gastric cancer. Cancer
Epidemiol Biomarkers Prev. 20:1021–1027. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inoue T, Kataoka H, Goto K, Nagaike K,
Igami K, Naka D, Kitamura N and Miyazawa K: Activation of c-Met
(hepatocyte growth factor receptor) in human gastric cancer tissue.
Cancer Sci. 95:803–808. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wilson TR, Fridlyand J, Yan Y, Penuel E,
Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, et al:
Widespread potential for growth-factor-driven resistance to
anticancer kinase inhibitors. Nature. 487:505–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shattuck DL, Miller JK, Carraway KL III
and Sweeney C: Met receptor contributes to trastuzumab resistance
of Her2-overexpressing breast cancer cells. Cancer Res.
68:1471–1477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yano S, Wang W, Li Q, Matsumoto K,
Sakurama H, Nakamura T, Ogino H, Kakiuchi S, Hanibuchi M, Nishioka
Y, et al: Hepatocyte growth factor induces gefitinib resistance of
lung adenocarcinoma with epidermal growth factor
receptor-activating mutations. Cancer Res. 68:9479–9487. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang F, Greer A, Hurlburt W, Han X,
Hafezi R, Wittenberg GM, Reeves K, Chen J, Robinson D, Li A, et al:
The mechanisms of differential sensitivity to an insulin-like
growth factor-1 receptor inhibitor (BMS-536924) and rationale for
combining with EGFR/HER2 inhibitors. Cancer Res. 69:161–170. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buck E, Gokhale PC, Koujak S, Brown E,
Eyzaguirre A, Tao N, Rosenfeld-Franklin M, Lerner L, Chiu MI, Wild
R, et al: Compensatory insulin receptor (IR) activation on
inhibition of insulin-like growth factor-1 receptor (IGF-1R):
Rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther.
9:2652–2664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varkaris A, Gaur S, Parikh NU, Song JH,
Dayyani F, Jin JK, Logothetis CJ and Gallick GE: Ligand-independent
activation of MET through IGF-1/IGF-1R signaling. Int J Cancer.
133:1536–1546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauer TW, Somcio RJ, Fan F, Liu W, Johnson
M, Lesslie DP, Evans DB, Gallick GE and Ellis LM: Regulatory role
of c-Met in insulin-like growth factor-I receptor-mediated
migration and invasion of human pancreatic carcinoma cells. Mol
Cancer Ther. 5:1676–1682. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bauer TW, Fan F, Liu W, Johnson M, Parikh
NU, Parry GC, Callahan J, Mazar AP, Gallick GE and Ellis LM:
Insulinlike growth factor-I-mediated migration and invasion of
human colon carcinoma cells requires activation of c-Met and
urokinase plasminogen activator receptor. Ann Surg. 241:748–758.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui JJ, Tran-Dubé M, Shen H, Nambu M, Kung
PP, Pairish M, Jia L, Meng J, Funk L, Botrous I, et al: Structure
based drug design of crizotinib (PF-02341066), a potent and
selective dual inhibitor of mesenchymal-epithelial transition
factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med
Chem. 54:6342–6363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iorio F, Knijnenburg TA, Vis DJ, Bignell
GR, Menden MP, Schubert M, Aben N, Gonçalves E, Barthorpe S,
Lightfoot H, et al: A landscape of pharmacogenomic interactions in
cancer. Cell. 166:740–754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang
W, Xiong YQ, Wu WZ, Wang L, Tang ZY and Sun HC: High expression of
macrophage colony-stimulating factor in peritumoral liver tissue is
associated with poor survival after curative resection of
hepatocellular carcinoma. J Clin Oncol. 26:2707–2716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee J, Seo JW, Jun HJ, Ki CS, Park SH,
Park YS, Lim HY, Choi MG, Bae JM, Sohn TS, et al: Impact of MET
amplification on gastric cancer: Possible roles as a novel
prognostic marker and a potential therapeutic target. Oncol Rep.
25:1517–1524. 2011.PubMed/NCBI
|
|
22
|
Ma PC, Tretiakova MS, MacKinnon AC,
Ramnath N, Johnson C, Dietrich S, Seiwert T, Christensen JG,
Jagadeeswaran R, Krausz T, et al: Expression and mutational
analysis of MET in human solid cancers. Genes Chromosomes Cancer.
47:1025–1037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Pelzer AM, Kiang DT and Yee D:
Down-regulation of type I insulin-like growth factor receptor
increases sensitivity of breast cancer cells to insulin. Cancer
Res. 67:391–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wolf S, Lorenz J, Mössner J and Wiedmann
M: Treatment of biliary tract cancer with NVP-AEW541: Mechanisms of
action and resistance. World J Gastroenterol. 16:156–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaulfuss S, Burfeind P, Gaedcke J and
Scharf JG: Dual silencing of insulin-like growth factor-I receptor
and epidermal growth factor receptor in colorectal cancer cells is
associated with decreased proliferation and enhanced apoptosis. Mol
Cancer Ther. 8:821–833. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cunningham MP, Thomas H, Marks C, Green M,
Fan Z and Modjtahedi H: Co-targeting the EGFR and IGF-IR with
anti-EGFR monoclonal antibody ICR62 and the IGF-IR tyrosine kinase
inhibitor NVP-AEW541 in colorectal cancer cells. Int J Oncol.
33:1107–1113. 2008.PubMed/NCBI
|
|
27
|
Bao XH, Takaoka M, Hao HF, Wang ZG,
Fukazawa T, Yamatsuji T, Sakurama K, Sun DS, Nagasaka T, Fujiwara T
and Naomoto Y: Esophageal cancer exhibits resistance to a novel
IGF-1R inhibitor NVP-AEW541 with maintained RAS-MAPK activity.
Anticancer Res. 32:2827–2834. 2012.PubMed/NCBI
|
|
28
|
Min Y, Adachi Y, Yamamoto H, Ito H, Itoh
F, Lee CT, Nadaf S, Carbone DP and Imai K: Genetic blockade of the
insulin-like growth factor-I receptor: A promising strategy for
human pancreatic cancer. Cancer Res. 63:6432–6441. 2003.PubMed/NCBI
|
|
29
|
Jaquish DV, Yu PT, Shields DJ, French RP,
Maruyama KP, Niessen S, Hoover H, Cheresh A D, Cravatt B and Lowy
AM: IGF1-R signals through the RON receptor to mediate pancreatic
cancer cell migration. Carcinogenesis. 32:1151–1156. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Prenen H, Tejpar S and Van Cutsem E: New
strategies for treatment of KRAS mutant metastatic colorectal
cancer. Clin Cancer Res. 16:2921–2926. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai Y, Kim JY, Watters JM, Fang B, Kinose
F, Song L, Koomen JM, Teer JK, Fisher K, Chen YA, et al: Adaptive
responses to dasatinib-treated lung squamous cell cancer cells
harboring DDR2 mutations. Cancer Res. 74:7217–7228. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Topcu-Yilmaz P, Kiratli H, Saglam A,
Söylemezoglu F and Hascelik G: Correlation of clinicopathological
parameters with HGF, c-Met, EGFR, and IGF-1R expression in uveal
melanoma. Melanoma Res. 20:126–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lennerz JK, Kwak EL, Ackerman A, Michael
M, Fox SB, Bergethon K, Lauwers GY, Christensen JG, Wilner KD,
Haber DA, et al: MET amplification identifies a small and
aggressive subgroup of esophagogastric adenocarcinoma with evidence
of responsiveness to crizotinib. J Clin Oncol. 29:4803–4810. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jardim DL, de Melo Gagliato D, Falchook
GS, Janku F, Zinner R, Wheler JJ, Subbiah V, Piha-Paul SA, Fu S,
Murphy MB, et al: MET aberrations and c-MET inhibitors in patients
with gastric and esophageal cancers in a phase I unit. Oncotarget.
5:1837–1845. 2014. View Article : Google Scholar : PubMed/NCBI
|