Introduction
Hepatocellular carcinoma (HCC) is a major health
concern, and the second leading cause of cancer-related death
worldwide (1). Japanese surgeons have
made significant contributions towards improvements in the surgery
for HCC (2), and 7,000–8,000 patients
per year undergo liver resection in Japan (3). Moreover, technical improvement in the
safety of less invasive laparoscopic liver resection could change
the practice of treatment for HCC (4,5). The
Barcelona Clinic Liver Cancer (BCLC) classification, which is a
staging system combining tumor status and liver function, has been
widely endorsed by treatment guidelines for HCC in Western
countries (6).
Multinodular HCC beyond the Milan criteria without
vascular invasion is classified as intermediate in the BCLC
classification (BCLC-B), and transarterial chemoembolization (TACE)
is recommended as first-line therapy. However, several reports have
suggested a survival benefit for liver resection over TACE in
patients with HCC in BCLC-B (7–9), and liver
resection for BCLC-B HCC patients has been actively performed,
especially in Asian countries such as Japan. Since patients with
BCLC-B HCC are a heterogeneous group (10), some may indeed benefit from liver
resection compared with TACE. However, it is important to
accurately identify this subgroup of patients.
Several groups have suggested prognostic factors
after resection for BCLC-B HCC; however, many studies included
large solitary tumors (≥5 cm in diameter) (11–15) and
reported pathological factors such as microvascular invasion, tumor
differentiation, and liver cirrhosis as prognostic factors
(12–14,16,17). Since
it is difficult to curatively treat extremely large solitary tumors
with TACE, complete resection is preferred and the indication for
operative treatment is unequivocal. It is impossible to make
decisions on the optimal therapy based solely on preoperative
pathological factors. Therefore, we believe that studies about risk
stratification of BCLC-B HCC should not include patients with
solitary tumors and not use pathological factors as prognostic
factors.
The purpose of this study was to derive a prognostic
model of overall survival based on the pre-treatment tumor
characteristics and patients' statuses, and to identify the
subgroup of patients with multinodular HCC in BCLC-B who could
benefit from liver resection.
Patients and methods
Patients
We retrospectively analyzed 65 patients (training
cohort) with multinodular HCC classified as BCLC-B among the 447
HCC patients who underwent liver resection at Yamaguchi University
Hospital (Yamaguchi, Japan) from January 2000 to December 2014 to
derive a prognostic model. Next, the validity of the model was
assessed in an independent external validation cohort (n=132) that
included 68 patients with multinodular HCC classified as BCLC-B
among the 598 patients who underwent liver resection at Osaka
University Hospital (Osaka, Japan) from May 1992 to December 2008,
as well as 64 patients of 827 patients treated at Osaka
International Cancer Institute (Osaka, Japan) from January 1990 to
August 2013 in the validation cohort. Subsequently, we compared
outcomes of patients with Child Pugh A who underwent liver
resection in the training and validation cohorts (n=186) with those
of patients with Child Pugh A who underwent TACE at Yamaguchi
University Hospital from May 1996 to December 2016 (n=93)
(unpublished data).
HCC was diagnosed using contrast-enhanced computed
tomography (CT) mainly with early enhancement in arterial phase
followed by washout in the portal or late phases, and elevation of
tumor markers, such as α-fetoprotein (AFP) or des-γ-carboxy
prothrombin (DCP). We included the patients with multinodular HCC
in BCLC-B (2 or 3 nodules larger than 3 cm, more than 4 lesions
without macrovascular invasion) diagnosed preoperatively or
intraoperatively.
The present study was approved by the Institutional
Review Boards of each of the three institutes in which data was
obtained (Yamaguchi University Hospital, Osaka International Cancer
Institute and Osaka University Hospital; protocol no. H29-093) and
was conducted in accordance with the ethical standards of the 1964
Declaration of Helsinki. The requirement for informed consent was
waived as this was a retrospective cohort study.
Liver resection
The surgical indication for each patient was
discussed at a multidisciplinary cancer board at each institute
consisting of hepatopancreatobiliary surgeons, hepatologists, and
medical oncologists. Residual liver volume were estimated by CT.
Liver resection was carried out with or without the intermittent
Pringle's maneuver (18) with
intraoperative ultrasonographic guidance. Intraoperative
radiofrequency thermal ablation (RFA) or microwave coagulation
(MCT) with resection was performed in some patients.
Follow-up after liver resection
All patients were followed up in the outpatient
clinic every 3 months after surgery with assessment of liver
function and serum AFP and DCP levels. Enhanced CT or magnetic
resonance images were obtained every 3–4 months and further studies
with bone scintigraphy or positron emission tomography were
performed when extrahepatic recurrence was suspected. For patients
with recurrence, TACE, locoregional therapy, liver resection, or
systemic chemotherapy with sorafenib were administered depending on
tumor spread and liver function.
Statistical analysis
Categorical variables are presented as numbers and
were analyzed by using Fisher exact test or chi-square test as
appropriate. Continuous variables are presented as medians and
ranges and were compared by using the Mann-Whitney U test. The
Kaplan-Meier method was used to calculate recurrence-free and
overall survival, and differences were evaluated using the log-rank
test. Independent prognostic factors for overall survival were
analyzed with the Cox's proportional hazard regression model in a
stepwise manner. Statistical analyses were performed using Statflex
ver. 6 (Artec, Osaka, Japan), and P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of patients in the
training cohort
The median follow-up period after surgery was 37.4
months (range, 4–187 months). The clinicopathologic characteristics
of the 65 patients (51 men, 14 women) are shown in Table I. The median age was 68 years old
(range, 35–83 years old). Laboratory results showed that 13
patients were positive for hepatitis B virus surface antigen
(HBs-Ag), and 42 had antibodies for hepatitis C virus (HCV-Ab). The
median preoperative serum AFP and DCP was 32 ng/ml (range,
0.8–239621 ng/ml) and 160 mAU/ml (range, 12–71200 mAU/ml),
respectively.
 | Table I.Clinicopathological characteristics
and overall survival of the 65 patients in the training cohort. |
Table I.
Clinicopathological characteristics
and overall survival of the 65 patients in the training cohort.
| Variable | n | 1-year | 3-year | 5-year | P-value |
|---|
| Age, years |
|
<68 | 29 | 93.1 | 61.7 | 51.7 | 0.4897 |
|
≥68 | 36 | 97.1 | 72.4 | 40.1 |
|
| Sex |
|
Male | 51 | 93.9 | 67.7 | 47.8 | 0.0929 |
|
Female | 14 | 100 | 67.1 | 28 |
|
| HBs-Ag |
|
Negative | 52 | 98 | 69.4 | 44 | 0.4595 |
|
Positive | 13 | 84.6 | 60.6 | 50.5 |
|
| HCV-Ab |
|
Negative | 23 | 90.9 | 72.1 | 65.6 | 0.3263 |
|
Positive | 42 | 97.5 | 65.3 | 35 |
|
| Prothrombin rate,
% |
|
≤70 | 3 | 100 | 66.7 | 33.3 | 0.2581 |
|
>70 | 62 | 95 | 67.6 | 45.4 |
|
| Albumin level,
g/dl |
|
≤3.5 | 24 | 91.3 | 64.1 | 29.4 | 0.1551 |
|
>3.5 | 41 | 97.4 | 69.5 | 53.1 |
|
| Platelet count,
/µl |
|
≤1.0×105 | 18 | 94.1 | 41.2 | 11.8 | 0.0001 |
|
>1.0×105 | 47 | 95.6 | 78.4 | 59.9 |
|
| ICGR15, % |
|
<15 | 28 | 96.3 | 76.8 | 53.3 | 0.1811 |
|
≥15 | 37 | 94.4 | 60.7 | 38.7 |
|
| Child-Pugh |
| 5 | 37 | 97.1 | 68.6 | 53.4 | 0.0873 |
| 6 | 27 | 92.3 | 68.4 | 34.6 |
|
| 7 | 1 | 100 | 0 | 0 |
|
| Tumor size on
image, cm |
|
<5 | 45 | 100 | 74.9 | 54.4 | 0.0563 |
| ≥5 | 20 | 85 | 52.2 | 22.4 |
|
| Number of tumors on
image |
| 2 | 25 | 100 | 73.1 | 48.8 | 0.1636 |
| ≥3 | 40 | 92.4 | 64.4 | 42.8 |
|
| Tumor size + number
of tumor |
| ≤8 | 42 | 100 | 78.3 | 50.9 | 0.0415 |
|
>8 | 23 | 87 | 49 | 35 |
|
| Liver
cirrhosis |
|
Absent | 31 | 93.3 | 79.4 | 54.3 | 0.0307 |
|
Present | 34 | 97 | 56.1 | 35.6 |
|
| AFP, ng/ml |
|
<400 | 50 | 97.9 | 70.2 | 45.2 | 0.5982 |
|
≥400 | 15 | 86.7 | 59.3 | 42.3 |
|
| DCP, mAU/ml |
|
<400 | 39 | 100 | 78 | 58.6 | 0.0047 |
|
≥400 | 26 | 88.5 | 53.1 | 27.2 |
|
| Bilobar
disease |
| No | 30 | 100 | 69.4 | 39 | 0.9002 |
|
Yes | 35 | 91.3 | 66.2 | 50.7 |
|
| Histologic
grade |
|
Well-moderate | 57 | 94.5 | 64.4 | 42 | 0.9984 |
|
Poor | 8 | 100 | 87.5 | 62.5 |
|
| Microscopic
vascular invasion |
|
Absent | 33 | 100 | 79.4 | 61.2 | 0.0400 |
|
Present | 32 | 90.2 | 56 | 30.2 |
|
| Intrahepatic
metastasis |
|
Absent | 35 | 97 | 70 | 46.4 | 0.3759 |
|
Present | 30 | 93.3 | 65.7 | 43.7 |
|
| Residual tumor |
|
Absent | 49 | 97.8 | 66.8 | 47.6 | 0.0590 |
|
Present | 16 | 87.5 | 68.8 | 35.7 |
|
| Preoperative
therapy |
| No | 53 | 96.1 | 70.9 | 47.5 | 0.1821 |
|
Yes | 11 | 90.9 | 54.6 | 34.1 |
|
The median tumor diameter and number was 4 cm
(range, 1.5–17 cm) and 3 (range 2-uncountable), respectively.
Tumors in 13 patients were limited to 1 section, those in 27
patients involved 2 sections, those in 18 patients involved 3
sections and those in 7 patients were involved to 4 sections, and
35 patients (53.8%) had bilobar disease.
Of the 11 patients who underwent pre-operative
therapy, 9 underwent TACE and 2 underwent intra-arterial
chemotherapy with 5-flurouracil and cisplatin. The response to
pre-operative therapy was unsatisfactory in all patients; 2
patients had stable disease while there was disease progression in
9 patients.
Partial resection was performed in 23 patients,
segmentectomy in 15, sectionectomy in 9, hemihepatectomy or
bisectionectomy in 16, and trisectionectomy in 2. Liver resection
with RFA or MCT was performed in 21 patients.
Curative resection without residual tumor was
performed in 49 patients and palliative resection with residual
tumor in 16 patients.
Postoperative survival and recurrence
in the training cohort
The 1-, 3-, and 5-year overall survival rates of all
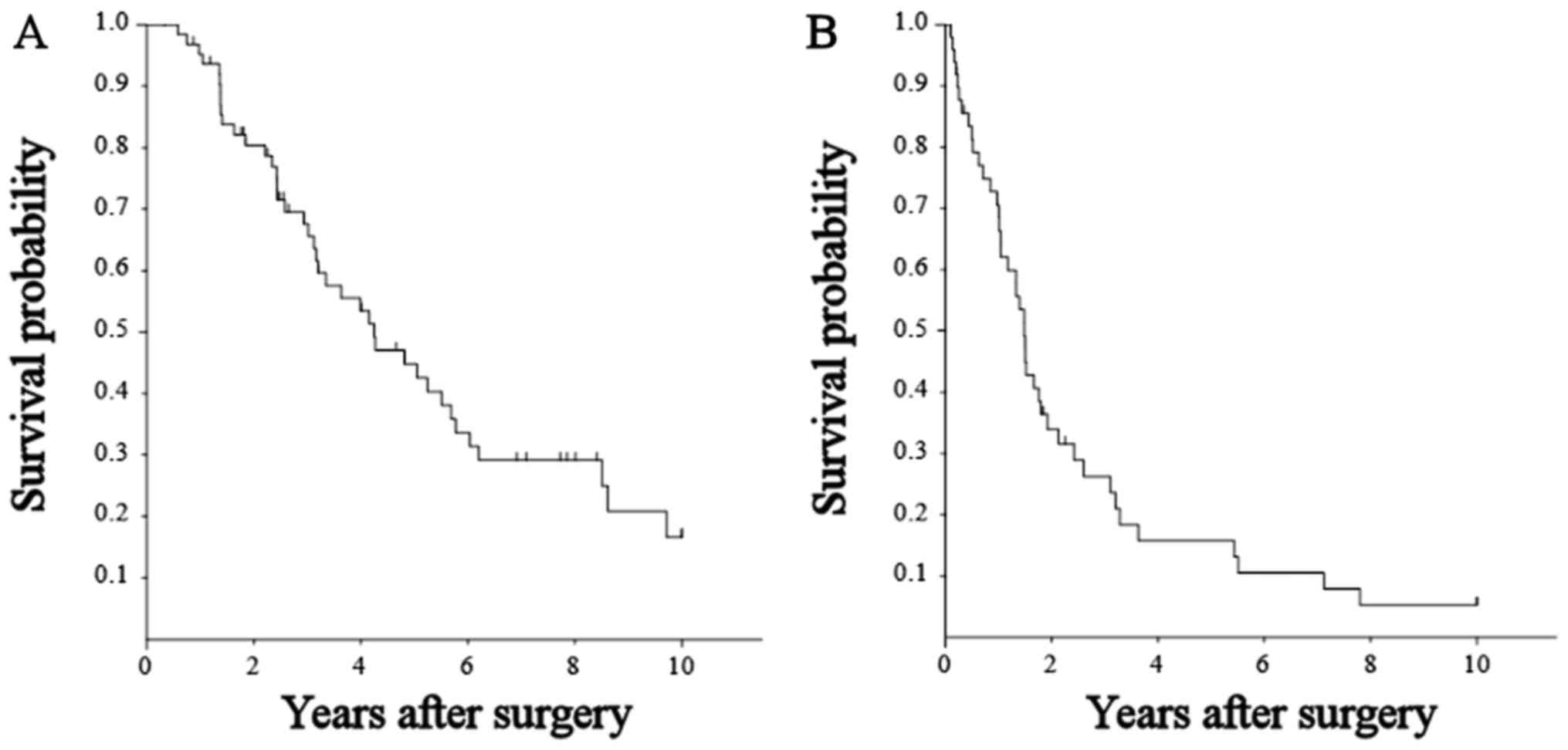
65 patients was 95.2, 67.6, and 44.8%, respectively (Fig. 1A). The median survival was 51.0 months
and 35 patients died in the follow-up period. Cancer progression
(n=19, 54.3%) was the most frequent cause of death, followed by
other diseases (n=8, 22.9%), liver failure (n=4, 11.4%), rupture of
varices (n=1, 2.9%), and unknown causes (n=3, 8.6%). The 1-, 3-,
and 5-year recurrence-free survival rate of the 49 patients without
residual tumor was 70.5, 28.0, and 22.1%, respectively (Fig. 1B).
Univariate analysis showed liver cirrhosis and
microscopic vascular invasion (mVI) to be associated with poorer
survival, while tumors >5 cm, tumor numbers >3, bilobar
disease, and palliative operations with residual tumors were not
(Table I).
Next, we analyzed the relationship between overall
survival and the sum of the largest tumor's diameter and the number
of tumors (N+S). The overall survival of patients with N+S ≤8 was
significantly better than that of patient with N+S >8.
Cox's regression analysis was used to assess the
relationship between pre-operative factors and overall survival
with stepwise backward elimination. The following variables were
included in the Cox's proportional model: age (≥68 vs. <68
years), sex, preoperative therapy (no vs. yes), positivity for
HBs-Ag, positivity for HCV-Ab, albumin level (>3.5 g/dl vs. ≤3.5
g/dl), prothrombin rate (>70% vs. ≤70%), platelet count (Plt;
>1.0×1010/l vs. ≤1.0×1010/l), indocyanine
green retention rate at 15 min (≥15% vs. <15%), tumor size on
imaging (≥5 cm vs. <5 cm), number of tumors on imaging (≥3 vs.
<3), N+S (>8 vs. ≤8), bilobar disease (no vs. yes), and DCP
(≥400 mAU/ml vs. <400 mAU/ml). Multivariate analysis revealed
that positivity for HCV-Ab, Plt ≤1.0×1010/l, N+S >8,
and DCP >400 mAU/ml were independent prognostic factors for
overall survival (Table II).
 | Table II.Multivariable Cox-regression analysis
of overall survival. |
Table II.
Multivariable Cox-regression analysis
of overall survival.
| Variable | RR | 95% CI | P-value |
|---|
| HCV-Ab |
|
Negative | 1 |
|
|
|
Positive | 3.193 | 1.274–8.000 | 0.0132 |
| Platelet count,
/µl |
|
|
|
|
≤1.0×105 | 1 |
|
|
|
>1.0×105 | 3.785 | 1.764–8.121 | 0.0006 |
| N+S |
|
|
|
| ≤8 | 1 |
|
|
|
>8 | 3.614 | 1.670–7.820 | 0.0011 |
| DCP, mAU/ml |
|
|
|
|
≤400 | 1 |
|
|
|
>400 | 3.556 | 1.664–7.599 | 0.0011 |
Prognostic score and overall survival
in the training cohort
Subsequently, we allocated 1 point to each
prognostic factor, calculated the score for each patient by summing
their points, and analyzed the effect of the score on overall
survival. The 1-, 3-, and 5-year overall rate and median survival
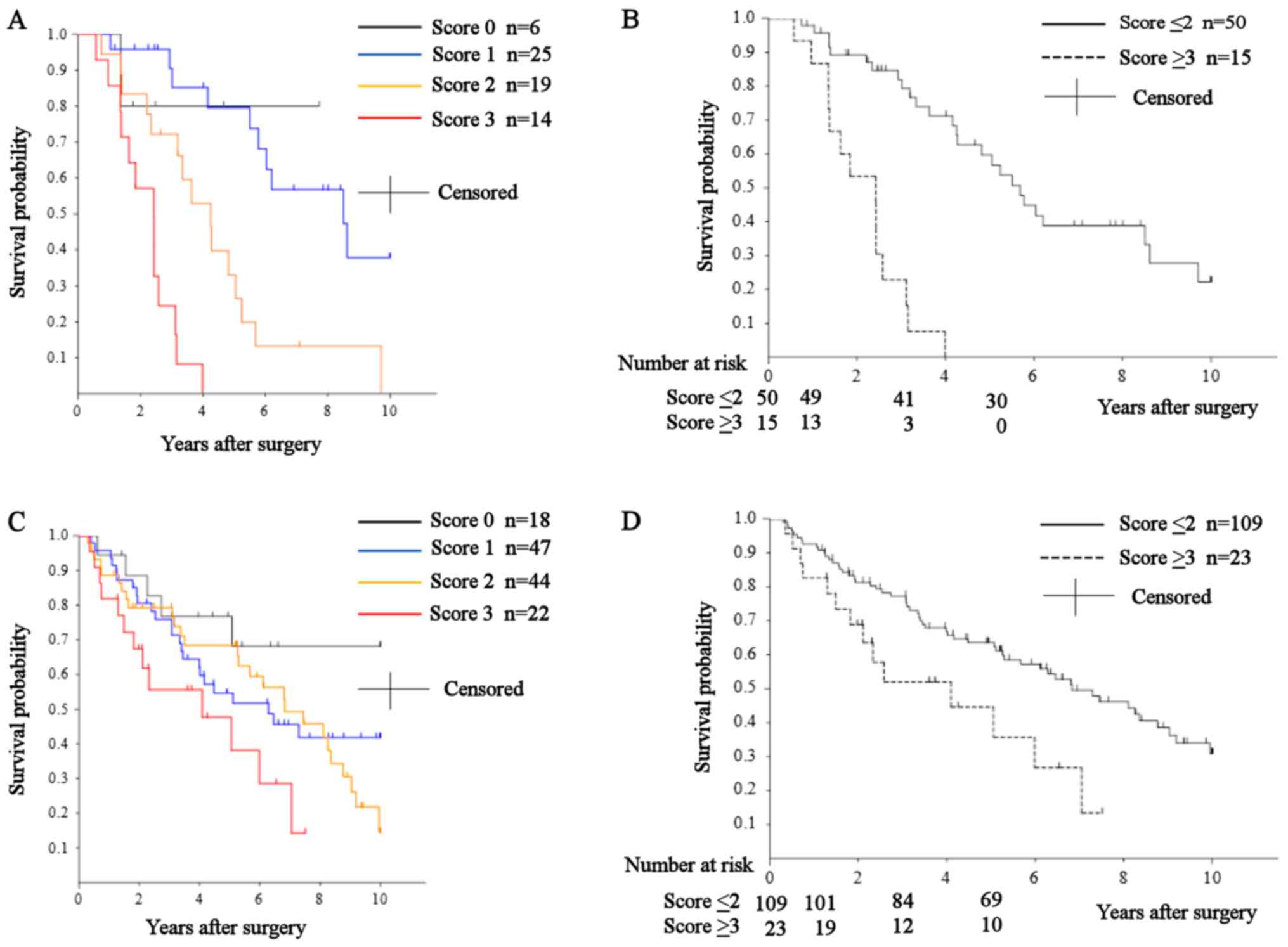
of patients with a score of 0 (n=6) were 100, 80, 80%, and over 10
years (not reached), respectively; for those with a score of 1
(n=25), they were 100, 90.5, 79.5%, and 102.1 months; for those
with a score of 2 (n=19), they were 94.4, 72.2, 33.1%, and 51.0
months; for those with a score of 3 (n=14), they were 85.7, 24.5,
0%, and 29.1 months; and for those with a score of 4 (n=1), they
were 100, 0, 0%, and not available (Fig.
2A). The overall survival of patients with a score of 0 was
significantly better than those with a score of 3 (P=0.0318).
Overall survival in those with a score of 1 was significantly
better than in those with scores of 2 and 3 (P=0.0014 and
P<0.0001, respectively). Patients with a score of 2 had
significantly better overall survival than those with a score of 3
(P=0.0021). Using a cutoff point of 2, we classified the patients
into two groups: score ≤2 (n=50) and score ≥3 (n=15). The 1-, 3-,
and 5-year overall rates and median survival of patients in the
score ≤2 group were 97.9, 82.0, 59.7%, and 68.3 months,
respectively. In the score ≥3 group, they were 86.7, 22.9, 0%, and
29.1 months, respectively (Fig. 2B).
Overall survival in the score ≤2 group was significantly better
than in the score ≥3 group (P<0.0001).
Prognostic score and overall survival
in the validation cohort
The clinicopathological characteristics of the 132
patients in the validation cohort are shown in Table III. The median follow-up period
after surgery was 49.8 months (range, 3.6–201 months). The median
age was 65 (range, 31–84) years. The median of preoperative serum
AFP and DCP was 28.5 ng/ml (range, 1–33268 ng/ml) and 326 mAU/ml
(range, 10–357456 mAU/ml) mAU/ml, respectively.
 | Table III.Clinicopathological characteristics
and overall survival in the 132 patients of the validation
cohort. |
Table III.
Clinicopathological characteristics
and overall survival in the 132 patients of the validation
cohort.
| Variable | n | 1-year | 3-year | 5-year | P-value |
|---|
| Age, years |
|
<68 | 84 | 91.7 | 74.2 | 60.7 | 0.9019 |
|
≥68 | 48 | 89.6 | 71.4 | 60.4 |
|
| Sex |
|
Male | 110 | 92.7 | 73.4 | 60.9 | 0.7961 |
|
Female | 22 | 81.8 | 71.8 | 59.8 |
|
| HBs-Ag |
|
Negative | 104 | 91.4 | 76.9 | 60.7 | 0.4944 |
|
Positive | 28 | 89.3 | 59.4 | 59.4 |
|
| HCV-Ab |
|
Negative | 56 | 89.3 | 66.7 | 56.3 | 0.8623 |
|
Positive | 76 | 92.1 | 77.9 | 63.5 |
|
| Prothrombin rate,
% |
|
≤70 | 21 | 90.5 | 65.2 | 54.3 | 0.6857 |
|
>70 | 111 | 91 | 74.7 | 61.8 |
|
| Albumin level,
g/dl |
|
≤3.5 | 40 | 85 | 71.5 | 52.2 | 0.2968 |
|
>3.5 | 92 | 93.5 | 74 | 63.9 |
|
| Platelet count,
/µl |
|
≤1.0×105 | 19 | 89.5 | 69.8 | 69.8 | 0.9496 |
|
>1.0×105 | 113 | 91.2 | 73.7 | 59.6 |
|
| ICGR15, % |
|
<15 | 75 | 90.7 | 75.2 | 68.7 | 0.0095 |
|
≥15 | 57 | 91.2 | 70.7 | 50 |
|
| Child-Pugh |
| 5 | 83 | 92.8 | 73.5 | 63.6 |
>0.5 |
| 6 | 39 | 89.7 | 73.8 | 55.6 |
|
| 7 | 10 | 80 | 68.6 | 54.9 |
|
| Tumor size on
image, cm |
|
<5 | 67 | 94 | 84.1 | 69.4 | 0.0462 |
| ≥5 | 65 | 87.7 | 62.2 | 51.5 |
|
| Number of tumor on
image |
| 2 | 89 | 93.3 | 78.2 | 66.7 | 0.0447 |
| ≥3 | 43 | 86.1 | 62.2 | 46.3 |
|
| Tumor size + number
of tumor |
| ≤8 | 85 | 96.5 | 84 | 68.8 | 0.0005 |
|
>8 | 47 | 80.9 | 53.1 | 45 |
|
| AFP, ng/ml |
|
<400 | 106 | 93.4 | 77.5 | 63.4 | 0.1945 |
|
≥400 | 26 | 80.8 | 55.5 | 49.9 |
|
| DCP, mAU/ml |
|
<400 | 69 | 92.8 | 75.6 | 65 | 0.171 |
|
≥400 | 63 | 88.9 | 70.7 | 55.9 |
|
| Histologic
grade |
|
Well-moderate | 91 | 95.6 | 75.7 | 63.9 | 0.6236 |
|
Poor | 41 | 80.5 | 67.6 | 53.1 |
|
| Microscopic
vascular invasion |
|
Absent | 84 | 94.1 | 76.6 | 65.6 | 0.1604 |
|
Present | 48 | 85.4 | 67.2 | 51.6 |
|
The median tumor diameter and number of tumors was
4.6 cm (range, 1.6–17 cm) and 2 (range, 2-uncountable),
respectively.
Partial resection was performed in 44, segmentectomy
in 16, sectionectomy in 9, hemihepatectomy or bisectionectomy in
42, and trisectionectomy in 30.
The 1-, 3-, and 5-year overall rates and median
survival of patients in the validation cohort were 97.0, 75.8,
60.5%, and 77.5 months, respectively. A total of 73 patients died
during the follow-up period. Cancer progression (n=60, 82.2%) was
the most frequent cause of death, followed by other diseases (n=4,
5.5%), liver failure (n=1, 1.4%), and unknown causes (n=8, 11.0%).
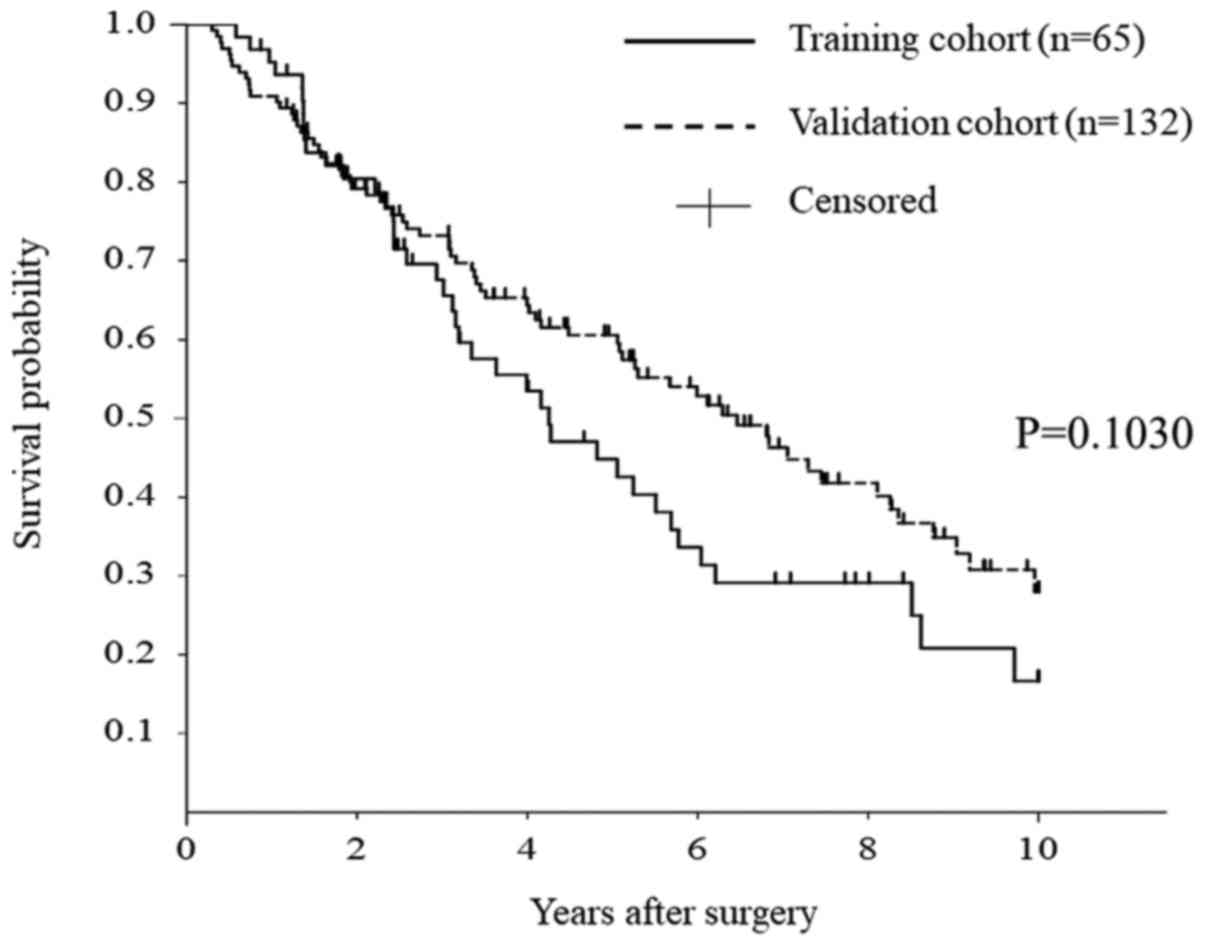
There was no difference in overall survival between the training
and validation cohorts (Fig. 3;
P=0.1030).
The 1-, 3-, and 5-year overall rates and median
survival of patients with a score of 0 (n=18) were 94.4, 76.7,
76.7%, and over 10 years (not reached), respectively. For those
with a score of 1 (n=47), they were 95.7, 75.9, 54.6%, and 75.5
months. For those with a score of 2 (n=44), they were 88.6, 79.3,
68.4%, and 82.0 months. For those with a score of 3 (n=22), they
were 81.8, 55.6, 47.7%, and 49.2 months. For those with a score of
4 (n=1), they were 100, 0, 0%, and not available (Fig. 2C). Overall survival in patients with a
score of 0 was significantly better than in those with a score of 3
(P=0.0243).
Similar to our approach in the training cohort,
patients were classified into two groups: Score ≤2 (n=109) and
score ≥3 (n=23). The 1-, 3-, and 5-year overall rates and median
survival of patients in the score ≤2 group were 92.7, 77.3, 63.5%,
and 82.0 months, respectively. For those in the score ≥3 group,
they were 82.6, 52.0, 44.6%, and 49.2 months (Fig. 2D). Overall survival in the ≤2 group
was significantly better than that in the ≥3 group (P=0.0164).
Relationship between prognostic score
and microvascular invasion
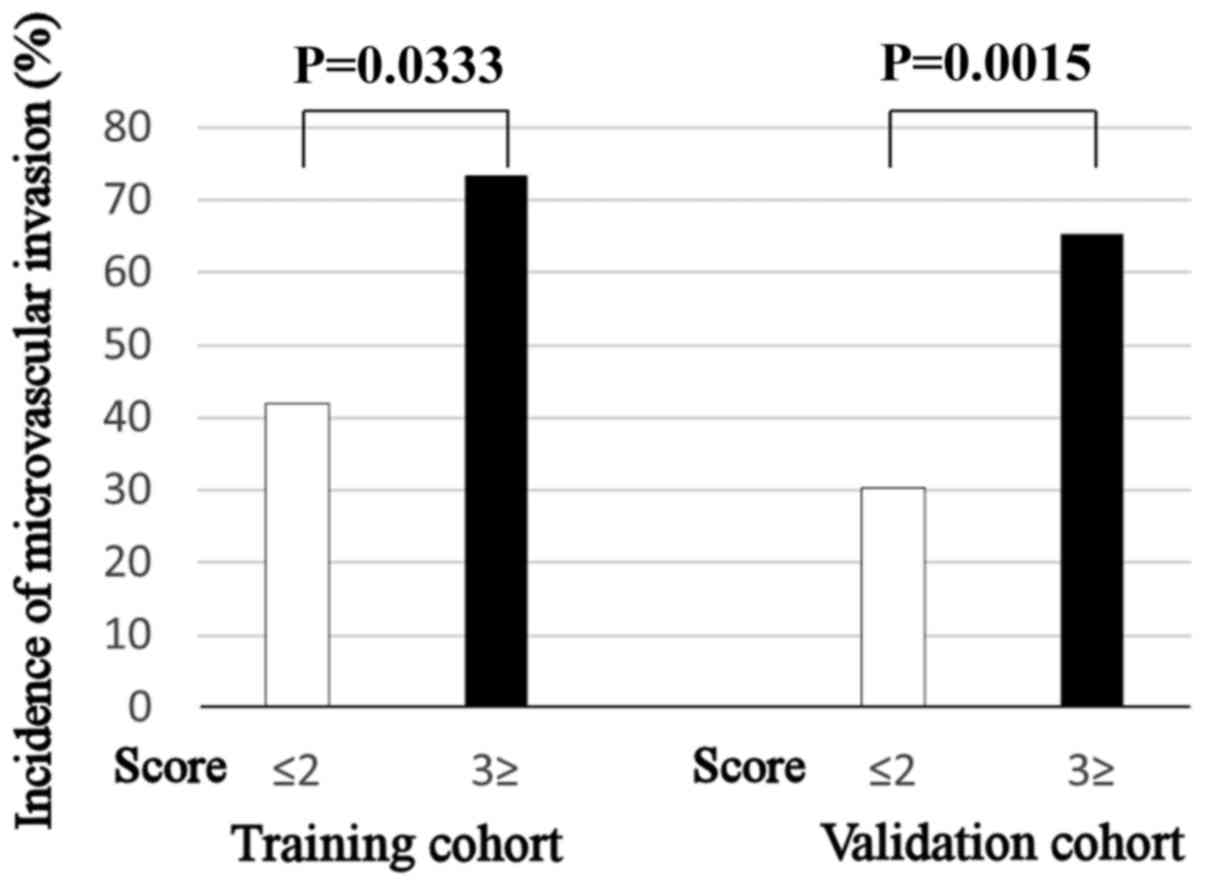
In the training cohort, the incidence of mVI in
patients with a score ≥3 (73.3%) was significantly higher than in
those with a score ≤2 (42.0%; P=0.0333). Similarly, the incidence
of mVI in patients with a score ≥3 (65.2%) was significantly higher
than in those with a score ≤2 (30.3%; P=0.0015) in the validation
cohort (Fig. 4).
Survival in each subgroup according to
treatment modality
Finally, we compared the overall survival of
patients with Child-Pugh A in the training and validation cohorts
who underwent surgery (n=186) with those of patients with
Child-Pugh A who underwent TACE (n=93) (unpublished data). The
clinicopathological characteristics of patients in each group are
shown in Table IV. Although the
ratio of males to females (surgery: 153 males to 33 females; TACE:
69 males to 24 females; P=0.1153) and positivity of HCV-Ab
(surgery: 114; TACE: 58; P=0.8618) were not different, the median
age was older in the TACE group [surgery: 66.0 (range, 31.0–84.0);
TACE: 74.2 (range, 48.2–87.8); P<0.0001]. Although the median
tumor diameter was larger in the surgery group [surgery: 4.5 cm
(range, 1.5–17.0); TACE: 3.7 cm (range, 1.0–8.9); P=0.0002] and the
median of number of tumors was larger in the TACE group [surgery: 2
(range, 2-uncountable); TACE: 4 (range, 2–11); P<0.0001], the
proportion of N+S >8 in each group (surgery: 64; TACE: 39;
P=0.2194) was similar. The proportion of patients with Plt
≤1.0×1010/l was higher in the TACE group (surgery: 33;
TACE: 29; P=0.0109) but that of patients with DCP >400 mAU/ml
was not different (surgery: 82; TACE: 38; P=0.6079).
 | Table IV.Clinicopathologic characteristics of
patients who underwent surgery or TACE. |
Table IV.
Clinicopathologic characteristics of
patients who underwent surgery or TACE.
| Variable | Surgery
(n=186) | TACE (n=93) | P-value |
|---|
| Age, years | 66.0
(31.0–84.0) | 74.2
(48.2–87.8) | <0.0001 |
| Sex |
|
Male | 153 | 69 | 0.1153 |
|
Female | 33 | 24 |
|
| HCV-Ab |
|
Negative | 72 | 35 | 0.8618 |
|
Positive | 114 | 58 |
|
| Tumor size on
image, cm | 4.5 (1.5–17.0) | 3.7 (1.0–8.9) | 0.00018 |
| Number of tumors on
image | 2
(2-uncountable) | 4 (2–11) | <0.0001 |
| Tumor size + number
of tumor |
| ≤8 | 122 | 54 | 0.2194 |
|
>8 | 64 | 39 |
|
| Platelet count,
/µl |
|
≤1.0×105 | 33 | 29 | 0.0109 |
|
>1.0×105 | 153 | 64 |
|
| DCP, mAU/ml |
|
<400 | 104 | 55 | 0.6079 |
|
≥400 | 82 | 38 |
|
| Prognostic
score |
| ≤2 | 152 | 72 | 0.3946 |
| ≥3 | 34 | 21 |
|
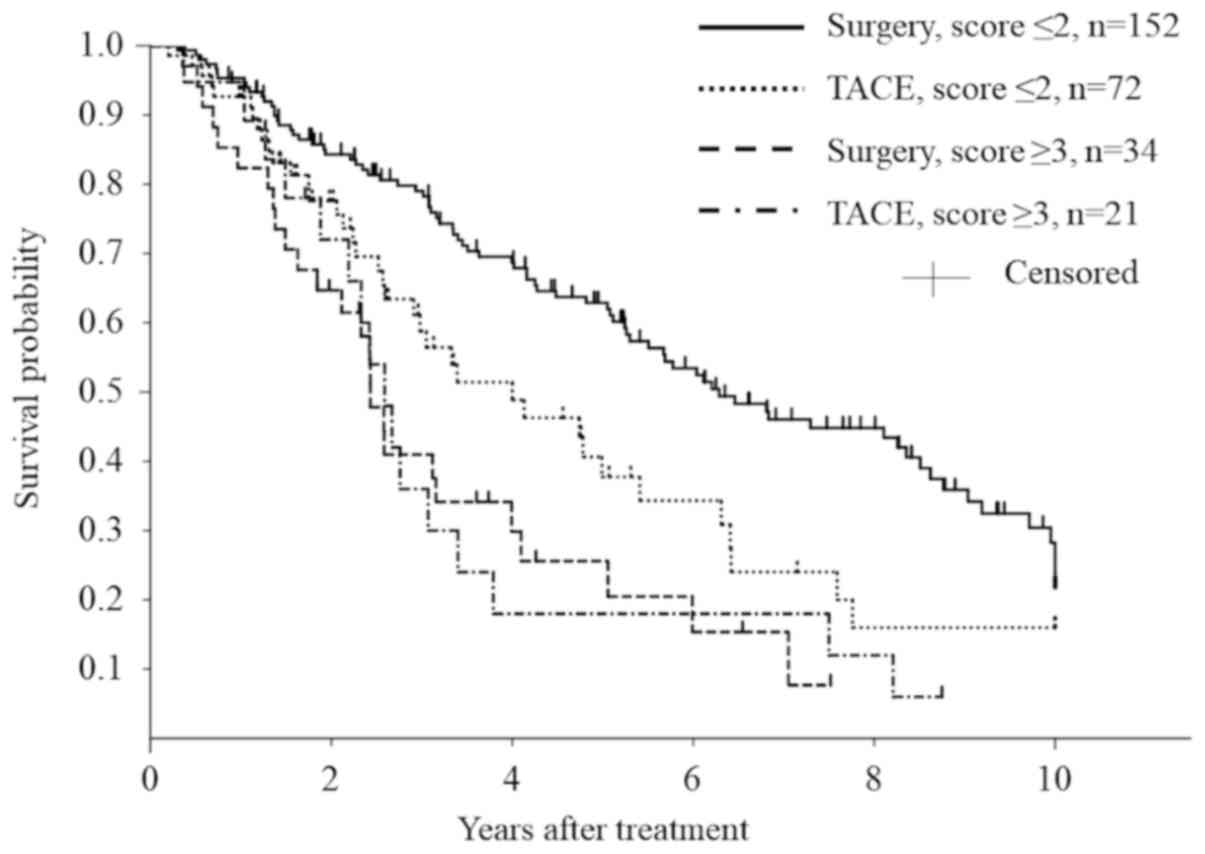
The 1-, 3-, and 5-year overall rates and median
survival of patients who underwent surgery in the score ≤2 group
(n=152) were 95.3, 79.1, 62.9%, and 75.5 months, respectively. For
those who underwent TACE in the score ≤2 group (n=72), they were
92.7, 58.8, 37.8%, and 48.0 months. The survival was significantly
better in patients in the score ≤2 group who underwent surgery
(P=0.0094) (Fig. 5).
In contrast, the survival was not different between
surgery (n=34) and TACE (n=21) in the score ≥3 subgroup (median
survival: 29.2 vs. 31.1 months; 5-year survival rate: 25.6% vs.
18.0% for surgery vs. TACE, respectively, P=0.8723).
Discussion
The purpose of the present study was to derive a
prognostic model of overall survival based on pre-treatment tumor
and patient characteristics, and to identify the subgroup of
patients with multinodular BCLC-B HCC who could benefit from liver
resection. Indeed, we developed a prognostic score based on HCV-Ab
status, pre-operative Plt, tumor status (N+S), and DCP level.
According to this prognostic score, patients with a score <2
would benefit more from surgery than from TACE, which provides
5-year survival rate of approximately 30% or median survival of
30–39.7 months for patients with multinodular HCC in Child-Pugh
class A (7,9,19). In
contrast, patients with a score ≥3 would benefit from TACE as its
outcomes were equivalent to those of surgery while being less
invasive. Although mVI is the most widely recognized prognostic
factor after liver resection (13,14,16,17,20),
it is impossible to determine pre-operatively. As our prognostic
score was associated with the frequency of mVI, it could also be
useful for predicting its presence pre-operatively.
Some reports have shown that tumor size and number
are prognostic factors (12,16,21).
Although either of these variables alone tended to be associated
with the outcome of patients in univariate analyses, multivariate
analysis showed that N+S had a stronger effect. Recently, we have
shown that the mathematical product of maximum tumor size and
number of tumors (NxS) was an important prognostic factor after
curative liver resection for HCC (22). The ‘metro ticket’ (23,24) and
up-to-seven criteria (25) for liver
transplantation for HCC, the subclassification (26) and NSP (27) score of BCLC-B HCC for surgery, and the
4 of 7 cm classification (19) of
BCLC-B HCC for TACE all emphasize that both tumor size and number
is a critical prognostic factor for several treatments for HCC.
These results have shown that the sum of maximum tumor size and
number of tumors reflect individual tumor characteristics more
precisely than either variable by itself. We changed the cutoff
point of N+S and analyzed survival using univariate analyses in a
step by step manner (data not shown). Because an N+S >8 had the
strongest effect on survival in this analysis, we selected this
cutoff point as a covariate in the Cox regression analysis. The
up-to-seven criteria have been the most popular cutoff point in
liver transplantation for HCC exceeding the Milan criteria.
Although the optimal cutoff point could change in different
cohorts, N+S could be a stronger prognostic factor than tumor size
or number by themselves.
Some studies have shown that DCP was a prognostic
factor after liver resection for HCC (16,28) and
that it correlated with an aggressive phenotype and mVI (29). Similar to these results, DCP was one
of the independent prognostic factors for survival in this
study.
Liver function is also an important prognostic
factor after hepatic resection (16,22). We
demonstrated here that a Plt count <1010/l was one
such prognostic factor. Plt count and the aspartate
aminotransferase to Plt ratio index have been reported as
independent predictors of survival after liver resection for HCC
(30,31). These results may suggest that multiple
HCCs and poor functional reserve are relative contraindications to
liver resection, as Ishizawa et al (16) had reported earlier.
We acknowledge several limitations to this study.
First, it was a retrospective study with a limited number of
patients. Second, we could not compare the outcomes of patients
underwent surgery and TACE with propensity score matching due to
the small number of cases. Although the outcomes of patients who
underwent liver resection were compared with those that underwent
TACE in this study, bias due to patient selection may have been
present. However, this study is potentially important for
evaluating the effectiveness of liver resection for multinodular
intermediate stage HCC as it excludes patients with large solitary
tumors. Furthermore, the consensus report from the 5th Asia-Pacific
Primary Liver Cancer Expert Meeting stated that randomization of
patients into surgery or TACE is difficult because of patient risks
and ethical reasons (32). In this
situation, clinicians should make their treatment decisions after
referring to previous retrospective analyses. Third, the study
period of this analysis was relatively long. Treatment strategies
and combinations of multimodality therapies could have changed
during this long period; however, this could not be avoided as we
attempted to identify prognostic factors in such a small cohort as
BCLC-B HCC patients. Further investigation is needed to determine
pre-treatment factors that can help clinicians select the
appropriate treatment for each patient.
In conclusion, liver resection for selected patients
with BCLC-B HCC is feasible and can promote long-term survival.
HCV-Ab status, preoperative Plt count, preoperative DCP level, and
N+S could be useful for patient selection.
Acknowledgements
The authors would like to thank Ms. Rie Akiyama
(Department of Gastroenterological, Breast and Endocrine Surgery,
Yamaguchi University Graduate School of Medicine, Yamaguchi, Japan)
for her cooperation during data collection.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM, KS, YoT, YuT, HM, SKa, STo, MI, NS, STa, HW, SKo
and ISaeki collected, analyzed and interpreted the patients' data.
TU, HE, MS, ISakaida and HN conceived and designed the present
study. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Boards of each of the three institutes in which data was
obtained (Yamaguchi University Hospital, Osaka International Cancer
Institute and Osaka University Hospital; protocol no. H29-093) and
was conducted in accordance with the ethical standards of the 1964
Declaration of Helsinki. The requirement for informed consent was
waived as this was a retrospective cohort study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colvin H, Mizushima T, Eguchi H, Takigushi
S, Doki Y and Mori M: Gastroenterological surgery in Japan: The
past, the present and the future. Ann Gastroenterol Surg. 1:5–10.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seto Y, Kakeji Y, Miyata H and Iwanaka T:
National Clinical Database (NCD) in Japan for gastroenterological
surgery: Brief introduction. Ann Gastroenterol Surg. 2:80–81. 2017.
View Article : Google Scholar
|
|
4
|
Kaneko H, Otsuka Y, Kubota Y and
Wakabayashi G: Evolution and revolution of laparoscopic liver
resection in Japan. Ann Gastroenterol Surg. 1:33–43. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kobayashi S, Fukui K, Takeda Y, Nakahira
S, Tsujie M, Shimizu J, Miyamoto A, Eguchi H, Nagano H, Doki Y, et
al: Short-term outcomes of open liver resection and laparoscopic
liver resection: Secondary analysis of data from a multicenter
prospective study (CSGO-HBP-004). Ann Gastroenterol Surg. 2:87–94.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi SH, Choi GH, Kim SU, Park JY, Joo DJ,
Ju MK, Kim MS, Choi JS, Han KH and Kim SI: Role of surgical
resection for multiple hepatocellular carcinomas. World J
Gastroenterol. 19:366–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ho MC, Huang GT, Tsang YM, Lee PH, Chen
DS, Sheu JC and Chen CH: Liver resection improves the survival of
patients with multiple hepatocellular carcinomas. Ann Surg Oncol.
16:848–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JY, Sinn DH, Gwak GY, Choi GS, Saleh
AM, Joh JW, Cho SK, Shin SW, Carriere KC, Ahn JH, et al:
Transarterial chemoembolization versus resection for
intermediate-stage (BCLC B) hepatocellular carcinoma. Clin Mol
Hepatol. 22:250–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bolondi L, Burroughs A, Dufour JF, Galle
PR, Mazzaferro V, Piscaglia F, Raoul JL and Sangro B: Heterogeneity
of patients with intermediate (BCLC B) hepatocellular carcinoma:
Proposal for a subclassification to facilitate treatment decisions.
Semin Liver Dis. 32:348–359. 2012.PubMed/NCBI
|
|
11
|
Chang WT, Kao WY, Chau GY, Su CW, Lei HJ,
Wu JC, Hsia CY, Lui WY, King KL and Lee SD: Hepatic resection can
provide long-term survival of patients with non-early-stage
hepatocellular carcinoma: extending the indication for resection?
Surgery. 152:809–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delis SG, Bakoyiannis A, Tassopoulos N,
Athanassiou K, Kelekis D, Madariaga J and Dervenis C: Hepatic
resection for hepatocellular carcinoma exceeding Milan criteria.
Surg Oncol. 19:200–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu W, Wang K, Bao Q, Sun Y and Xing BC:
Hepatic resection provided long-term survival for patients with
intermediate and advanced-stage resectable hepatocellular
carcinoma. World J Surg Oncol. 14:622016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ng KK, Vauthey JN, Pawlik TM, Lauwers GY,
Regimbeau JM, Belghiti J, Ikai I, Yamaoka Y, Curley SA, Nagorney
DM, et al: Is hepatic resection for large or multinodular
hepatocellular carcinoma justified? Results from a
multi-institutional database. Ann Surg Oncol. 12:364–373. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Torzilli G, Belghiti J, Kokudo N, Takayama
T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E,
Donadon M, et al: A snapshot of the effective indications and
results of surgery for hepatocellular carcinoma in tertiary
referral centers: is it adherent to the EASL/AASLD
recommendations?: An observational study of the HCC East-West study
group. Ann Surg. 257:929–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishizawa T, Hasegawa K, Aoki T, Takahashi
M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N and Makuuchi M:
Neither multiple tumors nor portal hypertension are surgical
contraindications for hepatocellular carcinoma. Gastroenterology.
134:1908–1916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim PT, Jang JH, Atenafu EG, Fischer S,
Greig PD, McGilvray ID, Wei AC, Gallinger S and Cleary SP: Outcomes
after hepatic resection and subsequent multimodal treatment of
recurrence for multifocal hepatocellular carcinoma. Br J Surg.
100:1516–1522. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagano H, Kishimoto S, Kobayashi S,
Marubashi S, Eguchi H, Takeda Y, Tanemura M, Tomimaru Y, Noda T,
Umeshita K, et al: A safe protocol of intermittent hilar vascular
clamping for hepatic resection in cirrhosis.
Hepatogastroenterology. 56:1439–1444. 2009.PubMed/NCBI
|
|
19
|
Yamakado K, Miyayama S, Hirota S, Mizunuma
K, Nakamura K, Inaba Y, Yamamoto S, Matsuo K, Nishida N, Aramaki T,
et al: Prognosis of patients with intermediate-stage hepatocellular
carcinomas based on the Child-Pugh score: Subclassifying the
intermediate stage (Barcelona Clinic Liver Cancer stage B). Jpn J
Radiol. 32:644–649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rodríguez-Perálvarez M, Luong TV, Andreana
L, Meyer T, Dhillon AP and Burroughs AK: A systematic review of
microvascular invasion in hepatocellular carcinoma: diagnostic and
prognostic variability. Ann Surg Oncol. 20:325–339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Truant S, Boleslawski E, Duhamel A, Bouras
AF, Louvet A, Febvay C, Leteurtre E, Huet G, Zerbib P, Dharancy S,
et al: Tumor size of hepatocellular carcinoma in noncirrhotic
liver: A controversial predictive factor for outcome after
resection. Eur J Surg Oncol. 38:1189–1196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tokumitsu Y, Tamesa T, Matsukuma S,
Hashimoto N, Maeda Y, Tokuhisa Y, Sakamoto K, Ueno T, Hazama S,
Ogihara H, et al: An accurate prognostic staging system for
hepatocellular carcinoma patients after curative hepatectomy. Int J
Oncol. 46:944–952. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Llovet JM, Schwartz M and Mazzaferro V:
Resection and liver transplantation for hepatocellular carcinoma.
Semin Liver Dis. 25:181–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Majno P and Mazzaferro V: Living donor
liver transplantation for hepatocellular carcinoma exceeding
conventional criteria: Questions, answers and demands for a common
language. Liver Transpl. 12:896–898. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mazzaferro V, Llovet JM, Miceli R, Bhoori
S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi
GL, et al: Predicting survival after liver transplantation in
patients with hepatocellular carcinoma beyond the Milan criteria: A
retrospective, exploratory analysis. Lancet Oncol. 10:35–43. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wada H, Eguchi H, Noda T, Ogawa H, Yamada
D, Tomimaru Y, Tomokuni A, Asaoka T, Kawamoto K, Gotoh K, et al:
Selection criteria for hepatic resection in intermediate-stage
(BCLC stage B) multiple hepatocellular carcinoma. Surgery.
160:1227–1235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang YF, Zhou J, Wei W, Zou RH, Chen MS,
Lau WY, Shi M and Guo RP: Intermediate-stage hepatocellular
carcinoma treated with hepatic resection: The NSP score as an aid
to decision-making. Br J Cancer. 115:1039–1047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shimada M, Takenaka K, Fujiwara Y, Gion T,
Kajiyama K, Maeda T, Shirabe K and Sugimachi K: Des-gamma-carboxy
prothrombin and alpha-fetoprotein positive status as a new
prognostic indicator after hepatic resection for hepatocellular
carcinoma. Cancer. 78:2094–2100. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koike Y, Shiratori Y, Sato S, Obi S,
Teratani T, Imamura M, Yoshida H, Shiina S and Omata M:
Des-gamma-carboxy prothrombin as a useful predisposing factor for
the development of portal venous invasion in patients with
hepatocellular carcinoma: A prospective analysis of 227 patients.
Cancer. 91:561–569. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amano H, Tashiro H, Oshita A, Kobayashi T,
Tanimoto Y, Kuroda S, Tazawa H, Itamoto T, Asahara T and Ohdan H:
Significance of platelet count in the outcomes of hepatectomized
patients with hepatocellular carcinoma exceeding the Milan
criteria. J Gastrointest Surg. 15:1173–1181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng J, Zhao P, Liu J, Liu X and Wu X:
Preoperative aspartate aminotransferase-to-platelet ratio index
(APRI) is a predictor on postoperative outcomes of hepatocellular
carcinoma. Medicine (Baltimore). 95:e54862016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ho MC, Hasegawa K, Chen XP, Nagano H, Lee
YJ, Chau GY, Zhou J, Wang CC, Choi YR, Poon RT and Kokudo N:
Surgery for intermediate and advanced hepatocellular carcinoma: A
consensus report from the 5th asia-pacific primary liver cancer
expert meeting (APPLE 2014). Liver Cancer. 5:245–256. 2016.
View Article : Google Scholar : PubMed/NCBI
|