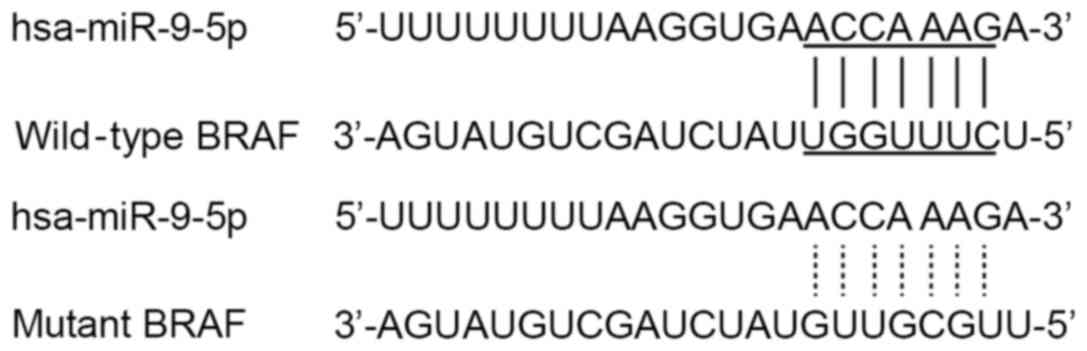Introduction
Globally, thyroid cancer is the eighth leading type
of malignancy in women, accounting for 3% of all cancer types in
women. The worldwide prevalence of thyroid cancer 15 years ago was
1.7%, which ranked 14th among all cancer types, and this value has
now doubled. Each year, there are more incident cases of thyroid
cancer compared with any other type of cancer (3.8%/year between
1992 and 2001) (1). Currently, it is
estimated that there are 5–8 thyroid cancer cases/105 individuals
each year in developed countries (2–5). In
addition, the mortality rate of males with thyroid cancer has risen
faster compared with other types of cancer, following adjustment of
age (2.3% each year between 1992 and 2001), and papillary thyroid
cancer (PTC) accounts for the majority of thyroid cancer types
worldwide (5). In 2013, it was
estimated that 60,000 cases would be newly diagnosed in the USA,
and this number is increasing, which makes thyroid cancer an
escalating and common disease (6,7). Although
conventional options, including surgery and radioactive iodine are
potent for PTC, they are not effective for undifferentiated
(anaplastic) thyroid carcinoma (ATC), and advanced PTC, which are
radioactive iodine-resistant (8).
A previous study reported an association between
BRAF mutation, and the onset and development of PTC, as well as
with the unfavorable prognosis of PTC (9). It has been demonstrated that mutations
of the BRAF gene induce uncontrolled and persistent activation of
kinase signaling pathways, causing over-proliferation, and
differentiation into cancer cells (10). Notably, mutation of BRAF was
identified in PTC progression of all stages. The presence of BRAF
mutation in microcarcinoma or PTC at a very early stage indicates
that this event may serve a role in the development of PTC
tumorigenesis and susceptibility to another genetic variation,
resulting in a more aggressive progression of PTC (9).
MicroRNAs (miRNAs) were identified at the turn of
the 21st century, marking the milestone in cell biology of a new
era. Changing the concept of the association between gene mRNAs and
human disease has extended to contain those sequences in the
residual ~90% of eukaryotic genomes involving generation of
non-coding RNAs. miRNAs, which as meta-controllers of gene
expression, are essential for the cellular alterations required for
development. It is important to have detailed knowledge of the
mechanisms involved in the regulation of mRNA expression,
particularly considering that dysregulation of miRNAs is associated
with several thyroid disorders, including, medullary thyroid
carcinoma, follicular thyroid carcinoma and thyroid adenoma
(11).
It has been previously demonstrated that miR-9-5p is
differentially-expressed in PTC (12), and dysregulation of BRAF has also been
reported to be involved in the molecular mechanism of PTC cell
apoptosis (13,14). By searching the online miRNA database
(www.targetscan.org), BRAF was revealed
to be a virtual target of miR-9-5p with high similarity among
species. In the present study, BRAF was validated as a target of
miR-9-5p, and the involvement of miR-9-5p and BRAF in the
development of PTC was verified.
Materials and methods
Sample collection
A total of 25 female patients (age, 45±0.38 years;
age range 44 years and 7 months-45 years and 4 months old) who were
diagnosed with PTC and underwent surgery were recruited for the
present study between February 2014 and February 2015 at the
Department of Thyroid and Breast Surgery, The Second People's
Hospital of Liaocheng (Liaocheng, China). An additional 25 benign
thyroid nodule tissue samples without PTC were selected as
controls. Subsequent to selecting patients, diagnosis was confirmed
by two independent pathologists blinded to the clinical results.
The protocol of the present study was approved by the Ethics and
Research Committees of The Second People's Hospital of Liaocheng,
and written informed consent was obtained from each patient prior
to their involvement in the study.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The miRNeasy® Mini kit
(TRIzol®; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to extract the total RNA from BCPAP
cells, obtained from the Cell Bank of Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China) in accordance with
manufacturer's protocol. The RNeasy Mini spin column (Qiagen China
Co., Ltd., Shanghai, China) was used to purify the target RNA
according to the manufacturer's protocol, with a P260/280 nm ratio
>1.8 considered acceptable. For subsequent experiments, the RNA
was stored at −80°C in microcentrifuge tubes.
The ThermoScript RT-PCR system (Invitrogen; Thermo
Fisher Scientific, Inc.) was used according to the manufacturer's
protocol to reverse transcribe total RNA into cDNA. Fresh tissues
were used to extract 1 mg RNA for the synthesis of cDNA with a
mixture containing 5X cDNA synthesis buffer (250 mM Tris acetate pH
8.4), 40 nM magnesium acetate and 375 mM potassium acetate, and 10
mM dNTP mix, 15 U thermoscript transcriptase, 1 ml random hexamers
(50 ng/ml), 0.1 M dithiothreitol and 40 U RNaseOUT (Invitrogen;
Thermo Fisher Scientific, Inc.) in a final volume of 20 ml. The
sequences used were as follows: miR-9-5p forward,
5′-CGAGCTCTGTGTGTGTGTGTGTGTG-3′; reverse,
5′-TTCCGCGGCCGCTATGGCCGACGTCGACGGGAATGGGGAAAGGGAA-3′; BRAF forward,
5′-GGCGGCAGCGCTGTGGCGGCG-3′; and reverse,
5′-CGTAGGGTCATACTCATCCAC-3′. The reaction was performed at 18°C for
25 min, 42°C for 30 min and 90°C for 7 min.
For qPCR, a SYBR-Green fluorophore (Applied
Biosystems, Foster City, CA, USA) was used, and the thermocycling
protocol was 10 min at 95°C, followed by 40 cycles of 30 sec at
95°C, 30 sec at 60°C and 30 sec at 72°C. The expression of BRAF
mRNA and miR-9-5p was calculated relative to the expression level
of the endogenous control, β-actin. The 2−ΔΔCq method
(15) was used to analyze the
relative quantification of BRAF mRNA. Expression of miR-9-5p was
normalized to U6, the sequence of primer for U6 is: Forward
5′-CTCGCTTCGGCAGCACA-3′ and Reverse 5′-AACGCTTCACGAATTTGCGT-3′.
Every experiment was performed at least three times.
Cell culture and transfection
Dulbecco's modified Eagle's medium (DMEM;
Invitrogen; Thermo Fisher Scientific, Inc.), supplemented with 50
mg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.),
10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville,
GA, USA) and 50 mg/ml penicillin was used to maintain BCPAP cells
in an atmosphere of 5% CO2 and 95% air at 37°C. The
BCPAP cells were seeded on 6-well plates containing growth medium
and antibiotics at a density of 3–6×105 cells/well for
12 h prior to transfection. When 80% confluence was achieved, 100
pM miR-9-5p mimics; CGAGCTCTGTGTGTGTGTGTGTGTG-3; miR-9-5p
inhibitors; 5′-TTCCGCGGCCGCTATGGCCGACGTCGACGGGAATGGGGAAAGGGAA-3′,
or BRAF small interfering RNA; 5′-GGCGCCTCCCTTCCCCCTCCCCTT-3′
(siRNA; all Invitrogen; Thermo Fisher Scientific, Inc.) were
transfected into the cells using 2 µl Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at 37°C.
The time interval between the completion of transfection and the
use of the transfected cells was 4 h. The sequence of siRNA was:
5′-AAGUGGCAUGGUGAUGUGGCA-3′, and the final concentration used for
transfection was 20 pmol.
Vector construction and
mutagenesis
In order to determine miRNA targeting of the 3′-UTR
region of BRAF, the full length of the BRAF 3′-UTR was amplified
and cloned into a pmiR-REPORT luciferase vector (Promega
Corporation, Madison, WI, USA). DNA was extracted from the tissue
samples using the PicoPure™ DNA Extraction Kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.), and treated with DNA polymerase
(Promega Corporation). The sequences of the primers were as
follows: BRAF forward, 5′-GGCGGCAGCGCTGTGGCGGCG-3′; and reverse,
5′-CGTAGGGTCATACTCATCCAC-3′. The temperature protocol was as
follows: 95°C for 3 min, followed by 30 cycles of 94°C for 40 sec,
56°C for 35 sec and final extension at 72°C for 60 sec. The
Vybrant™ MTT Cell Proliferation Assay kit (Thermo Fisher
Scientific, Inc.) was used to generate the mutated vector by
replacing the miR-9-5p binding site nucleotides, according to the
manufacturer's protocol.
Cell proliferation assay
An MTT assay was performed to analyze the
proliferation of BCPAP cells in accordance with the manufacturer's
protocol (Thermo Fisher Scientific, Inc.). DMEM supplemented with
10% FBS was used to incubate the BCPAP cells on 96-well plates at a
final concentration of 3×104 cells/ml. MTT (10 µl;
Thermo Fisher Scientific, Inc.) was then added to each well, and
the culture medium was incubated for 1, 2 and 3 days at 37°C.
Subsequent to culturing for an additional 48 h, 1% dimethyl
sulfoxide was used to dissolve the formazan crystals, a blood
counting chamber was used to count the number of cells, and an
ELISA plate reader was used to analyze the proliferation of BCPAP
cells based on the absorbance, which was read at 440 nm.
Luciferase assay
The luciferase reaction mixture included
1×106 BCPAP cells, 1 µg of a Renilla luciferase
expression construct pRL-TK (Promega Corporation), 1 µg p-BRAF
(wild-type or mutant) plasmid and 50 pmol miR-9-5p mimics (or
control). The dual-luciferase assay system (Promega Corporation)
was used to detect the luciferase activity according to
Renilla luciferase activity at 36 h post-transfection,
according to the manufacturer's protocol. Each experiment was
performed at least three times, and transfection was performed with
Lipofectamine 2000® (Invitrogen; Thermo Fisher
Scientific, Inc.).
Western blot analysis
For analysis of the expression of BRAF mRNA and
miR-9-5p from tissue samples and BCPAP cells, radio
immunoprecipitation assay lysis buffer (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) containing protease inhibitors (Roche Applied
Science, Madison, WI, USA), 5 g/l sodium deoxycholate, 0.2 g/l
NaN3, 10 ml/l NP-40, 150 mmol/l NaCl 100 µg/ml, 0.1 g/l SDS,
phenylmethylsulfonyl fluoride, 1 µg/ml aprotinin and 50 mmol/l
Tris-HCl (pH 8.5) was used to lyse the BCPAP cells following
transfection, according to manufacturer's protocol, followed by two
washes with PBS (Invitrogen; Thermo Fisher Scientific, Inc.).
Protein determination was performed using a BCA assay (Beyotime
Institute of Biotechnology, Haimen, China). SDS-PAGE (12%) was used
to purify the target proteins, and proteins (35 µg/lane) were then
transferred to a polyvinylidene fluoride membrane (GE Healthcare,
Chicago, IL, USA). Tween-20 or TBS with 5% non-fat milk
(Sigma-Aldrich; Merck KGaA) was used to block the membrane for 2 h
at room temperature. The primary anti-BRAF antibodies in TBS buffer
(dilution, 1:1,000; cat. no., sc-136263; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) were used to detect the target protein via
incubation for 12 h at 4°C. Subsequently, membranes were washed
twice with PBS, followed by incubation with horseradish
peroxidase-conjugated secondary antibody (dilution, 1:3,000; cat.no
7075S Cell Signaling Technologies, Inc., Danvers, MA, USA) for
another 2 h at 25°C. Prior to film exposure, a peroxidase substrate
was used to enhance chemiluminescence (Thermo Fisher Scientific,
Inc.). Amersham ECL Prime Western Blotting Detection reagent (cat.
no. RPN2236; GE Healthcare, Chicago, IL, USA) was used to achieve
visualization of the relative enrichment of proteins. Bio-Rad
Chemi-DocXRS (Bio-Rad Laboratories, Hercules, CA, USA) was utilized
to visualize band of target protein.
Apoptosis analysis
In order to analyze apoptosis, 7-amino-actinomycin D
(7-AAD) was used to dual stain the BCPAP cells after transfecting
for 72 h, and the Annexin V-fluorescein isothiocyanate (FITC)/7-AAD
kit (Beckman Coulter, Inc., Brea, CA, USA) was used to detect the
Annexin V-FITC, according to the manufacturer's protocol. Flow
cytometry (Cell Lab Quanta SC; Beckman Coulter, Inc.) with 585/42
nm (PI) and 530/30 nm (FITC) emission filters was used to analyze
the apoptosis of cells after staining cells according to the
manufacturer's protocol. FCAP Array Software v3.0 was used for
analysis (Cell Lab Quanta SC; Beckman Coulter, Inc.). All
experiments were performed in triplicate.
Statistical analysis
All values are presented as the mean ± standard
deviation. For the bioinformatics analysis, computational
algorithms (www.targetscan.org; access date
02/02/2015) were used to predict putative miR-9-5p targets.
Fisher's exact test was used to analyze the comparisons of
categorical variables. Comparisons among the continuous variables
were performed using Spearman's Rank Correlation and Sign test.
P<0.05 was considered to indicate a statistically significant
difference. StatView Software (v.5, SAS Institute, Inc., Cary, NC,
USA) was used to analyze the statistical analysis.
Results
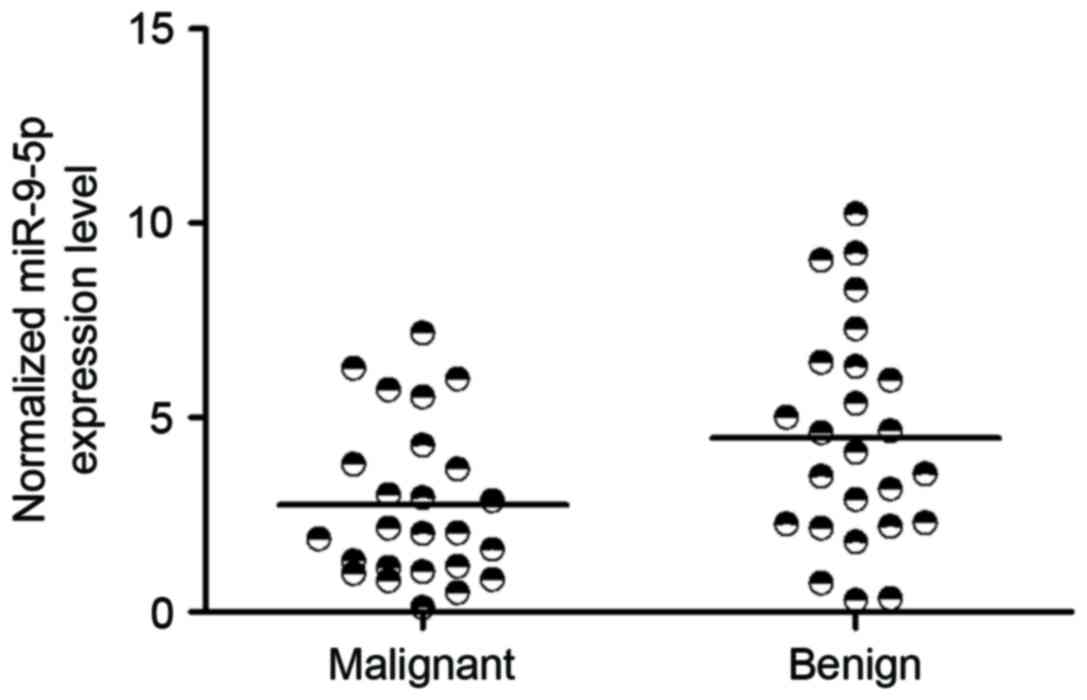
miR-9-5p is downregulated in malignant
PTC
To identify miRNAs regulating malignant PTC, miRNA
expression levels were analyzed between PTC and benign thyroid
nodules. miR-9-5p was consistently downregulated in malignant PTC
compared with benign tumor samples (P<0.05 vs. Thyroid nodule
tissue samples; Fig. 1). Therefore,
the present study focused on the function of miR-9-5p, which was
hypothesized to distinguish the malignant tumors from the benign
ones.
BRAF expression transcripts are
targeted by miR-9-5p
One of the biggest challenges in studying miRNAs is
to identify target genes and associate their downregulation with
cellular properties. Computational algorithms (www.targetscan.org) have been used to predict putative
miR-9-5p targets based on complementarity to the 3′-UTR of the
target mRNA. Through bioinformatics analysis, miR-9-5p was
predicted to target the human BRAF 3′-UTR (Fig. 2). To investigate whether miR-9-5p is a
real BRAF regulator, the following experiments were performed.
Firstly, luciferase reporter vectors containing a wild-type or
mutation BRAF 3′-UTR sequence at the downstream of luciferase were
constructed (Fig. 2). miR-9-5p mimics
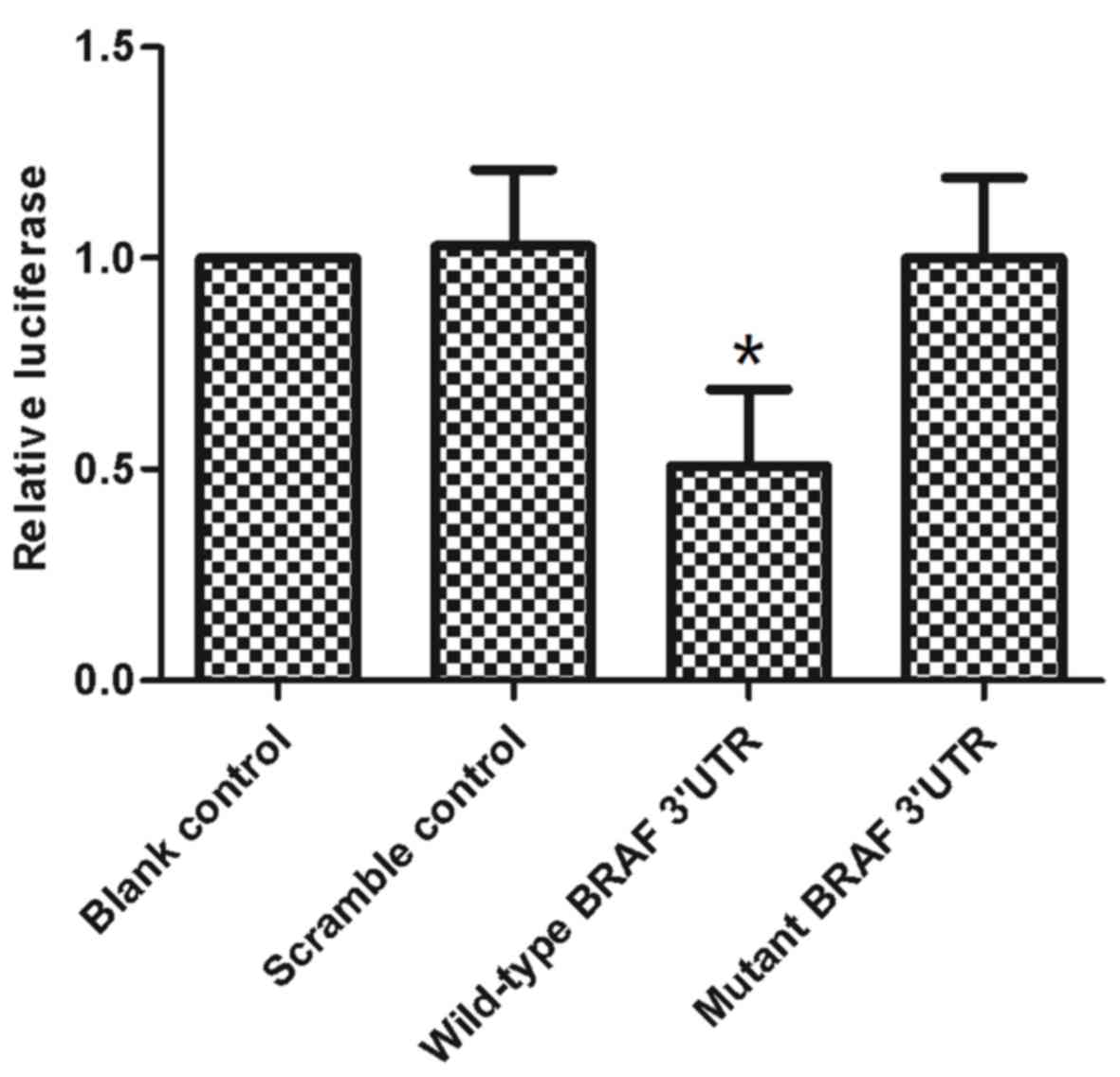
and corresponding reporter vectors were co-transfected into the
BCPAP cell line. A dual-luciferase assay was performed at 48 h
post-transfection. The results of the dual-luciferase assay
revealed that when the reporter vector contained a wild-type BRAF
3′-UTR, miR-9-5p significantly suppressed the luciferase expression
compared with the scramble control group (P<0.05 vs. scramble
control) (Fig. 3). Consistently,
mutation of the target region completely abolished this
observation, returning levels of luciferase activity similar to
that of the scramble control group (Fig.
3).
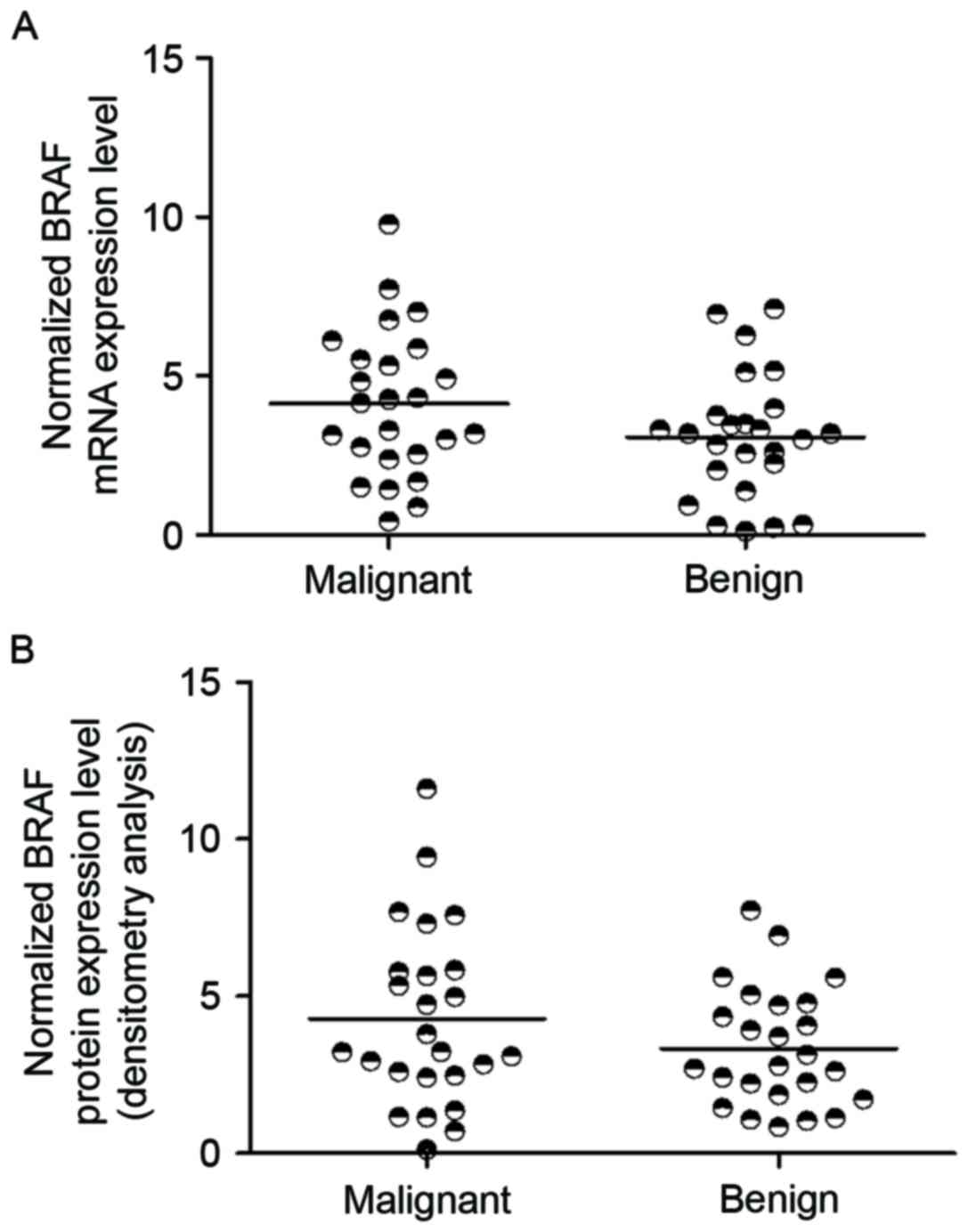
BRAF expression is upregulated in
PTC
It was hypothesized that the reduction of miR-9-5p
leads to the increase of BRAF expression level in malignant
papillary thyroid tumors. To support this hypothesis, RT-qPCR and
western blot analysis was used to determine the expression level of
BRAF mRNA, and protein in the two groups (Fig. 4). The results demonstrated that BRAF
mRNA and protein expression was upregulated in malignant papillary
thyroid tumor compared with that in benign samples (P<0.05 vs.
benign tumor samples).
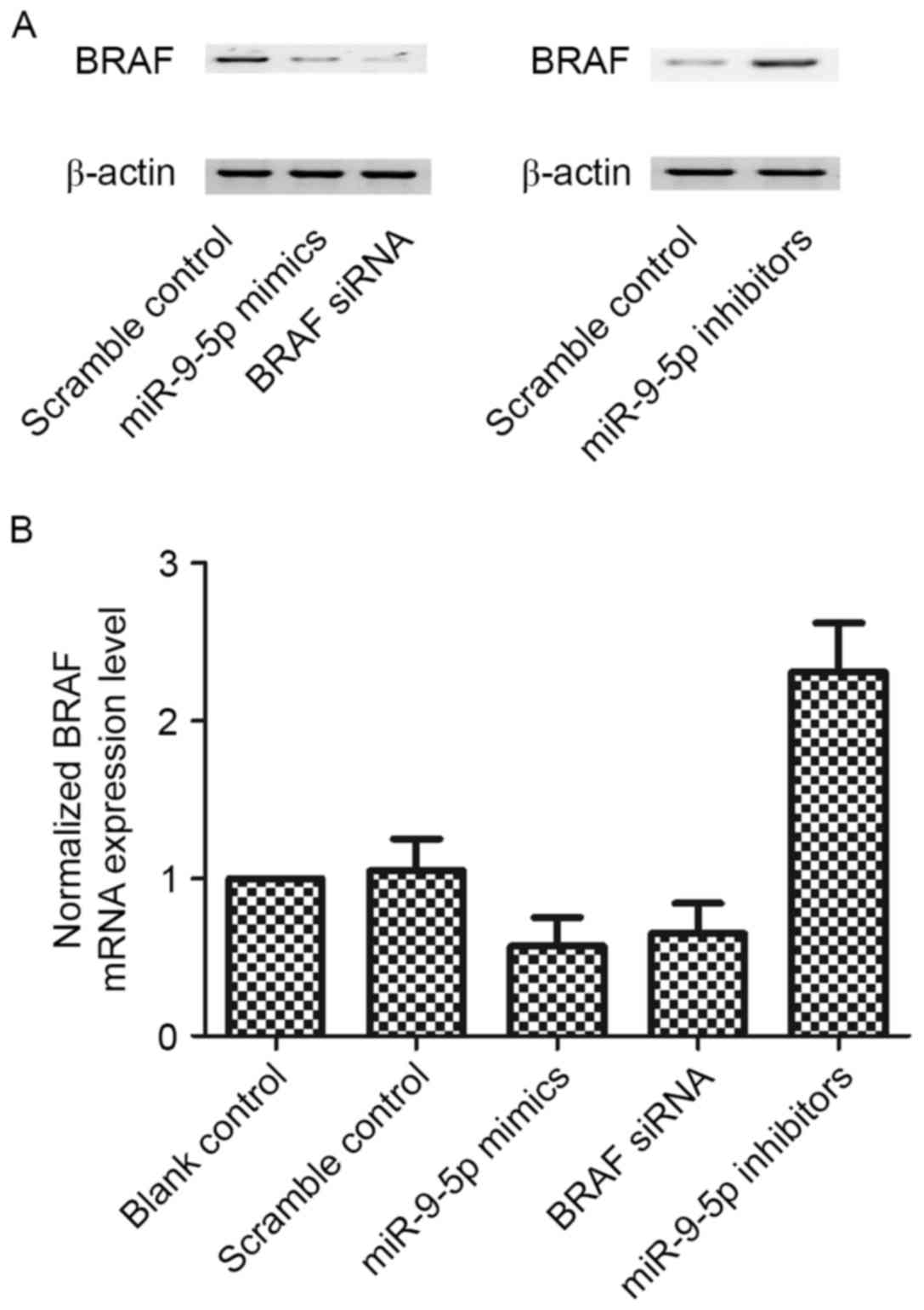
Effect of miR-9-5p upregulation or
downregulation on the expression of BRAF
To determine whether miR-9-5p disrupts BRAF
expression in BCPAP cells, miR-9-5p mimics were transfected into
BCPAP cells. Scramble served as a negative control, while BRAF
siRNA served as a positive control. The data revealed that BRAF
mRNA and protein level could be suppressed by miR-9-5p mimic
(P<0.05 vs. scramble control) (Fig.
5). Furthermore, similar experiments were performed using
miR-9-5p inhibitor treatment and the decrease in miR-9-5p increased
BRAF mRNA and protein levels (P<0.05 vs. scramble control)
(Fig. 5). These results demonstrated
that miR-9-5p targets BRAF 3′-UTR in a direct manner, therefore
suppressing the expression of BRAF.
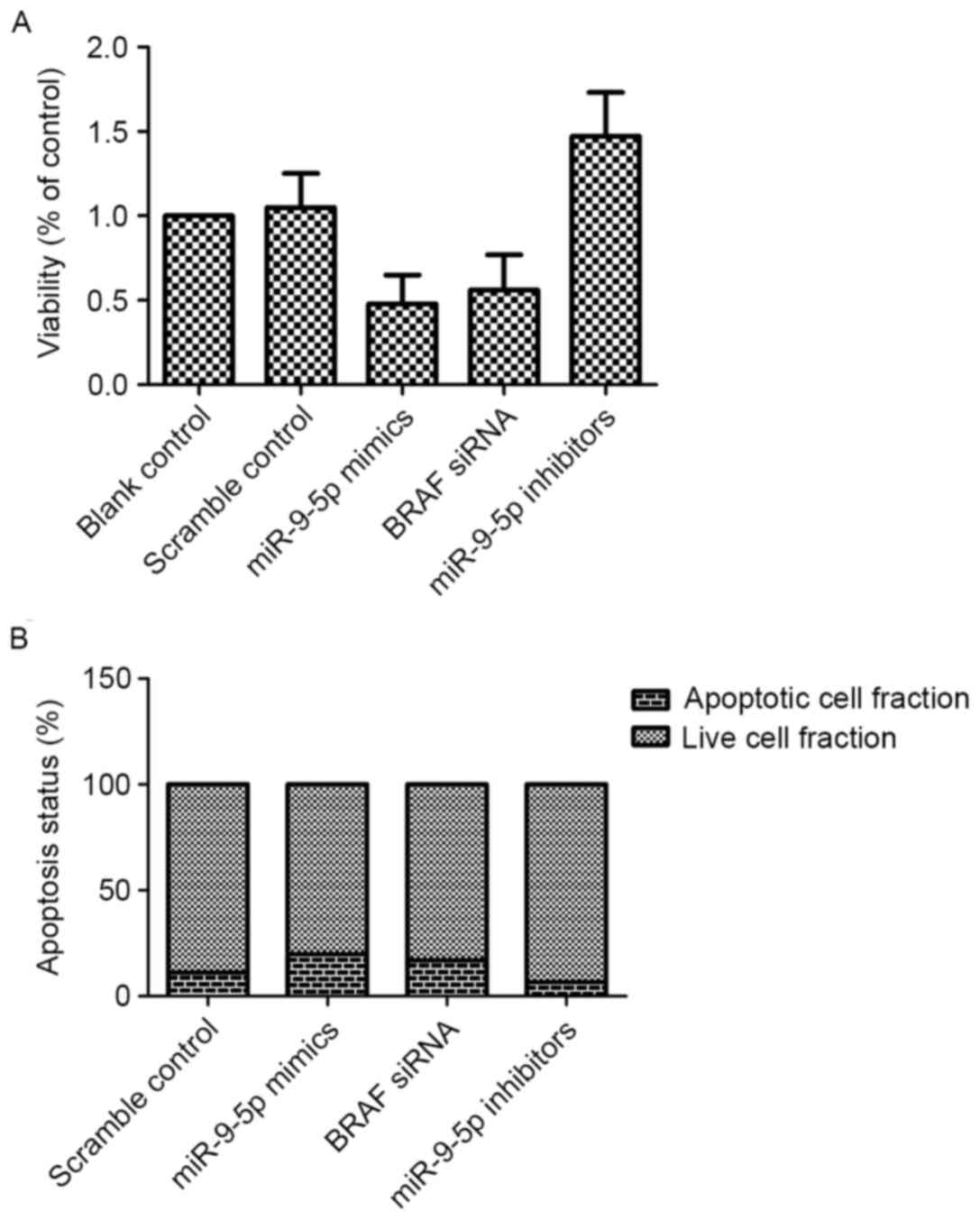
MiR-9-5p affects cell proliferation
and apoptosis by regulating BRAF expression
BRAF performs an important role in regulating cell
proliferation, and high levels of BRAF may promote proliferation of
malignant cells by suppressing apoptosis (16). To verify this, miR-9-5p mimics and
inhibitor were introduced into PTC cells, with scramble serving as
a negative control. As presented in Fig.
6, miR-9-5p and BRAF siRNA suppressed the viability of PTC
cells by inducing apoptosis (P<0.05 vs. scramble control), and
consistently, downregulation of miR-9-5p promoted proliferation of
PTC cells by inhibiting cell apoptosis (P<0.05 vs. scramble
control).
Discussion
MiRNAs have been reported to be functionally
involved in the regulation of biological processes, and may
function as oncogenes or tumor suppressors in the development of
various malignancies (10). The
invasiveness of mesothelial cells (MCs) is markedly reduced when
the levels of miR-9-5p are increased, which reveals a mechanism
involving prevention of build-up of myofibroblasts derived from MCs
in the submesothelial compact zone of the peritoneum, thereby
preventing it from initiating the process of fibrosis (17). Notably, over-expression of miR-9-5p
also reduced NADPH oxidase 4 levels induced by transforming growth
factor-β (TGF-β)1 and TGF-β receptor 2, representing a possible
mechanism involving the anti-fibrotic effect of miR-9-5p in pleural
mesothelial cells (18). The level of
miR-9-5p in human non-epithelial cells was revealed to be higher
compared with that in epithelial MCs, which demonstrates that
miR-9-5p protects against the development of pulmonary fibrosis
(19). In the present study, miRNA
expression levels were analyzed between PTC and benign thyroid
nodule tissue samples. MiR-9-5p was consistently downregulated in
PTC compared with the normal control.
To identify the downstream effector of miR-9-5p,
bioinformatics analysis was performed and revealed that miR-9-5p
was predicted to target the human BRAF 3′-UTR. Furthermore, a
luciferase assay was performed, demonstrating that when the
reporter vector contained a wild-type BRAF 3′-UTR, miR-9-5p
significantly suppressed the luciferase expression. Consistent with
this, mutation of target regions completely abolished this
interaction. In addition, miR-9-5p mimics were transfected into
BCPAP cells, and scramble served as a negative control, while BRAF
siRNA served as a positive control. The data demonstrated that BRAF
mRNA and protein levels were be suppressed by miR-9-5p mimic.
Furthermore, similar experiments were performed using miR-9-5p
inhibitor treatment, and the decrease of miR-9-5p increased BRAF
mRNA and protein levels. These results confirmed that miR-9-5p
targets the BRAF 3′-UTR directly, and therefore suppresses the
expression of BRAF.
The cascade of RAF-mitogen-activated protein kinase
kinase (MEK)-extracellular signal-regulated kinase (ERK) protein
kinases serves a major role in transforming extracellular mitogenic
signals to cell proliferation, as well as other cellular functions
(20,21). The family of RAF Ser/Thr kinases
includes A-, B- and C-RAF, which have similar domain structures. It
has been demonstrated that hetero- and homo-dimerization of RAF
family proteins causes an essential change in MEK phosphorylation
and activation (kinase regulated by extracellular signal/protein
kinase activated by mitogen), and consequently ERK (kinase
regulated by extracellular signal) responding to the activation of
RAS (22). Kinase suppressors of RAS
proteins primarily serve as scaffolds of the signaling cascade and
are also homologs of RAF family members, tethering ERKs, MEKs and
RAFs together. In human cancer, the mutations are commonly present
in the RAF-MEK-ERK signaling pathway (23). A total of ~6% of all human cancers and
50% of melanomas exhibit BRAF mutations (24). In >90% of BRAF mutations, there is
a single base substitution present in a codon of the kinase domain,
contributing to V600E amino acid alteration, constitutive active
BRAF protein kinase, and subsequently downstream signaling of
MEK-ERK (21). In the present study,
it was hypothesized that the reduction of miR-9-5p leads to the
increase of BRAF expression level in malignant papillary thyroid
tumors. To test this hypothesis, RT-qPCR was used to determine the
expression level of BRAF mRNA in two groups, and it was revealed
that BRAF expression is upregulated and miR-9-5p is downregulated
in malignant papillary thyroid tumor.
A transversion point mutation (1799T >A) in the
BRAF gene has been observed in 20–40% of ATCs and 45% of PTCs,
leading to a valine to glutamate substitution at amino acid 600 of
the protein termed BRAFV600E, and finally constitutive activation
of kinase activity (25,26). It is evident that BRAF mutants in
tumors are not always a signal of clinically aggressive thyroid
cancer in patients, although it is true that BRAFV600E has an
important role in tumor behavior (27,28). Other
immune factors and gene pathways are either known or putatively
unknown to interact with mutant BRAF signaling, which involves in
the formation of aggressive features in thyroid tumors (29). Mutations that affect the
phosphoinositide 3-kinase (PI3K)-protein kinase B (AKT) pathway and
tumor suppressor p53 are among other genetic events revealed to
activate dedifferentiation, and tumor progression (27). The majority of ATCs exhibit inactive
p53 (30). The inactivation of the
tumor suppressor phosphatase and tensin homolog is less prevalent
compared with mutations of p53, which are observed in ~15% of cases
of ATC and contributes to the activation of the PI3K-AKT signaling
pathway (31). Furthermore, efforts
will be made to identify other putative relevant drivers of
mutations and signaling pathways, including those made by The
Cancer Genome Atlas (http://tcga-data.nci.nih.gov/tcga/). Thyroid cancer
progression can be well studied using mouse models. Using
orthotopic and genetically engineered approaches that have been
designed more recently, the mouse models of PTC, and the more
aggressive and lethal types of thyroid cancer, ATC, have been
established (32). There are
strengths and drawbacks to each of these approaches. A previous
study identified an association between mortality rate and BRAF
status, previously regarded as additional information to
traditional factors of prognosis (33). The BRAF mutation may also be a
potential marker for predicting the response to targeted treatments
considering the availability of existing targeted treatments,
including a BRAF inhibitor (34).
However, there remains debate about the role of the BRAF mutation
in prognosis, since a prognostic role for the BRAF mutation has not
been identified in numerous studies (35,36). In
the present study, regulation of miR-9-5p was found to suppress the
viability of PTC cells by inducing apoptosis, and consistently,
downregulation of miR-9-5p promoted proliferation of PTC cells by
inhibiting apoptosis of the cells.
In conclusion, the findings of the present study
revealed that BRAF is a direct target of miR-9-5p, and the
miR-9-5p/BRAF signaling pathway may perform an important role in
the tumorigenesis of PTC. Furthermore, miR-9-5p/BRAF may be a
therapeutic target in the treatment of PTC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
FG was responsible for the planning of the present
study, data collection, analysis and interpretation and preparation
of the manuscript. XH also participated in data collection,
analysis and interpretation and was responsible for the collection
of funds. QS was responsible for data analysis and interpretation,
preparation of the manuscript and literature analysis.
Ethics and consent to participate
The protocol of the present study was approved by
the Ethics and Research Committees of The Second People's Hospital
of Liaocheng, and written informed consent was obtained from each
patient prior to their involvement in the study.
Patient consent for publication
The study participants provided consent for the data
in the present study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al-Brahim N and Asa SL: Papillary thyroid
carcinoma: An overview. Arch Pathol Lab Med. 130:1057–1062.
2006.PubMed/NCBI
|
|
2
|
Segovia Gomez I, Gallowitsch HJ, Kresnik
E, Kumnig G, Igerc I, Matschnig S, Stronegger WJ and Lind P:
Descriptive epidemiology of thyroid carcinoma in Carinthia,
Austria: 1984–2001. Histopathologic features and tumor
classification of 734 cases under elevated general iodination of
table salt since 1990: Population-based age-stratified analysis on
thyroid carcinoma incidence. Thyroid. 14:277–286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akslen LA, Haldorsen T, Thoresen SO and
Glattre E: Incidence pattern of thyroid cancer in Norway: Influence
of birth cohort and time period. Int J Cancer. 53:183–187. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colonna M, Grosclaude P, Remontet L,
Schvartz C, Mace-Lesech J, Velten M, Guizard A, Tretarre B, et al:
Incidence of thyroid cancer in adults recorded by French cancer
registries (1978–1997). Eur J Cancer. 38:1762–1768. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Howe HL, Wingo PA, Thun MJ, Ries LA,
Rosenberg HM, Feigal EG and Edwards BK: Annual report to the nation
on the status of cancer (1973 through 1998), featuring cancers with
recent increasing trends. J Natl Cancer Inst. 93:824–842. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Churilla TM, Donnelly PE, Leatherman ER,
Adonizio CS and Peters CA: Total mastectomy or breast conservation
therapy? how radiation oncologist accessibility determines
treatment choice and quality: A SEER Data-base analysis. Breast J.
21:473–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schechter RB, Nagilla M, Joseph L, Reddy
P, Khattri A, Watson S, Locati LD, Licitra L, Greco A, Pelosi G, et
al: Genetic profiling of advanced radioactive iodine-resistant
differentiated thyroid cancer and correlation with axitinib
efficacy. Cancer Lett. 359:269–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knauf JA, Ma X, Smith EP, Zhang L,
Mitsutake N, Liao XH, Refetoff S, Nikiforov YE and Fagin JA:
Targeted expression of BRAFV600E in thyroid cells of transgenic
mice results in PTCs that undergo dedifferentiation. Cancer Res.
65:4238–4245. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kundu A, Quirit JG, Khouri MG and
Firestone GL: Inhibition of oncogenic BRAF activity by
indole-3-carbinol disrupts microphthalmia-associated transcription
factor expression and arrests melanoma cell proliferation. Mol
Carcinog. 56:49–61. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saugstad JA: MicroRNAs as effectors of
brain function with roles in ischemia and injury, neuroprotection,
and neurodegeneration. J Cereb Blood Flow Metab. 30:1564–1576.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nalysnyk L, Cid-Ruzafa J, Rotella P and
Esser D: Incidence and prevalence of idiopathic pulmonary fibrosis:
Review of the literature. Eur Respir Rev. 21:355–361. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sondermann A, Andreghetto FM, Moulatlet
AC, da Silva Victor E, de Castro MG, Nunes FD, Brandão LG and
Severino P: MiR-9 and miR-21 as prognostic biomarkers for
recurrence in PTC. Clin Exp Metastasis. 32:521–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoon S, An YS, Lee SJ, So EY, Kim JH,
Chung YS and Yoon JK: Relation between F-18 FDG uptake of PET/CT
and BRAFV600E mutation in PTC. Medicine (Baltimore). 94:e20632015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–8. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen CH, Yuan P, Perez-Lorenzo R, Zhang Y,
Lee SX, Ou Y, Asara JM, Cantley LC and Zheng B: Phosphorylation of
BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation.
Mol Cell. 52:161–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rothenberg SM, Daniels GH and Wirth LJ:
Redifferentiation of iodine-refractory BRAF V600E-Mutant metastatic
papillary thyroid cancer with dabrafenib-response. Clin Cancer Res.
21:5640–5641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noble PW, Barkauskas CE and Iang D:
Pulmonary fibrosis: Patterns and perpetrators. J Clin Invest.
122:2756–2762. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amara N, Goven D, Prost F, Muloway R,
Crestani B and Boczkowski J: NOX4/NADPH oxidase expression is
increased in pulmonary fibroblasts from patients with idiopathic
pulmonary fibrosis and mediates TGFbeta1-induced fibroblast
differentiation into myofibroblasts. Thorax. 65:733–738. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Udell CM, Rajakulendran T, Sicheri F and
Therrien M: Mechanistic principles of RAF kinase signaling. Cell
Mol Life Sci. 68:553–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Osborne JK, Zaganjor E and Cobb MH: Signal
control through Raf: In sickness and in health. Cell Res. 22:14–22.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Roberts PJ and Der V: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matallanas D, Birtwistle M, Romano D,
Zebisch A, Rauch J, von Kriegsheim A and Kolch W: Raf Family
Kinases: Old dogs have learned new tricks. Genes Cancer. 2:232–260.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer, . Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL,
Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al: Revised
American Thyroid Association management guidelines for patients
with thyroid nodules and differentiated thyroid cancer. Thyroid.
19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tuttle RM, Ball DW, Byrd D, Dilawari RA,
Doherty GM, Duh QY, Ehya H, Farrar WB, Haddad RI, Kandeel F, et al:
Thyroid carcinoma. J Natl Compr Canc Netw. 8:1228–1274. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brown RL, de Souza JA and Cohen EE:
Thyroid cancer: Burden of illness and management of disease. J
Cancer. 2:193–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cohen Y, Xing M, Mambo E, Guo Z, Wu G,
Trink B, Beller U, Westra WH, Ladenson PW and Sidransky D: BRAF
mutation in papillary thyroid carcinoma. J Natl Cancer Inst.
95:625–627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ
and Qu JM: The BRAF mutation is predictive of aggressive
clinicopathological characteristics in papillary thyroid
microcarcinoma. Ann Surg Oncol. 1:3294–3300. 2010. View Article : Google Scholar
|
|
30
|
Kimura ET, Nikiforova MN, Zhu Z, Knauf JA,
Nikiforov YE and Fagin JA: High prevalence of BRAF mutations in
thyroid cancer: Genetic evidence for constitutive activation of the
RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma.
Cancer Res. 63:1454–1457. 2003.PubMed/NCBI
|
|
31
|
Soares P, Trovisco V, Rocha AS, Lima J,
Castro P, Preto A, Máximo V, Botelho T, Seruca R and
Sobrinho-Simões M: BRAF mutations and RET/PTC rearrangements are
alternative events in the etiopathogenesis of PTC. Oncogene.
22:4578–4580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vanden Borre P, McFadden DG, Gunda V,
Sadow PM, Varmeh S, Bernasconi M, Jacks T and Parangi S: The next
generation of orthotopic thyroid cancer models: immunocompetent
orthotopic mouse models of BRAF V600E-positive papillary and
anaplastic thyroid carcinoma. Thyroid. 24:705–714. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xing M, Alzahrani AS, Carson KA, Viola D,
Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, et al:
Association between BRAF V600E mutation and mortality in patients
with papillary thyroid cancer. JAMA. 309:1493–1501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang HC and Chen YC: Insight into
molecular dynamics simulation of BRAF(V600E) and potent novel
inhibitors for malignant melanoma. Int J Nanomedicine.
10:3131–3146. 2015.PubMed/NCBI
|
|
35
|
Eloy C, Santos J, Soares P and
Sobrinho-Simões M: The preeminence of growth pattern and
invasiveness and the limited influence of BRAF and RAS mutations in
the occurrence of papillary thyroid carcinoma lymph node
metastases. Virchows Arch. 459:265–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng S, Serra S, Mercado M, Ezzat S and
Asa SL: A high-throughput proteomic approach provides distinct
signatures for thyroid cancer behavior. Clin Cancer Res.
17:2385–2394. 2011. View Article : Google Scholar : PubMed/NCBI
|