Introduction
Lung cancer is one of the leading causes of
cancer-associated mortality, with a 5-year-survival rate of 17%
worldwide (1). Non-small cell lung
cancer (NSCLC) accounts for 85% all lung cancer cases in China, in
2017 (2). NSCLC can be subcategorized
into adenocarcinoma (LAD), squamous cell carcinoma (SCC) or large
cell carcinoma. Despite improvements in chemotherapy, radiation and
surgical treatments, lung cancer remains accountable for a large
proportion of cancer-associated mortality (3). The average 5-year survival rate of lung
cancer is approximately <15% in the urban areas of China
(4–7).
The increase in incidence of LAD has caused socioeconomic
developmental and environmental concerns (8), and the underlying mechanisms of LAD
remain unclear.
Long non-coding RNAs (lncRNAs) are a class of RNA
transcripts >200 nucleotides in length, with no clear
protein-coding ability (9). Thus far,
>300 lncRNAs have been annotated in the lncRNA database, the
majority of which have been studied in humans (10). Previous studies have demonstrated that
lncRNAs serve important roles in various biological processes in
numerous diseases, including various forms of cancer (9,11–13). LncRNAs function in the regulation of
complicated mechanisms and participate in physiological,
pathological and cytobiological functions, including apoptosis,
cell proliferation and chemoresistance (14–16).
LncRNA cardiac hypertrophy-related factor (CHRF) has recently been
reported to act as an oncogene; Qiuyun Wu et al (17) demonstrated that lncRNA CHRF functions
as an endogenous ‘sponge’ of micro RNA (miR)-489, repressing
miR-489 activity and functioning in pulmonary fibrosis.
In the present study, it was revealed that the
expression of CHRF in LAD tissues and cell lines was increased
compared with the negative controls. Loss-of-function assays were
performed to analyze the effects of CHRF on the proliferation, cell
cycle, apoptosis, migration and invasion of LAD cells. Western
blotting was performed to study the relevance of CHRF in the
phosphoinositide-3-kinase (PI3K)/Akt signaling pathway. These
experiments indicated that CHRF may be considered as a novel
prognostic factor and a therapeutic target for LAD.
Materials and methods
Tissues samples
A total of 80 LAD tissues and matched adjacent
normal tissues were collected from patients treated at the First
Affiliated Hospital of Chinese PLA General Hospital (Beijing,
China) between August 2010 and August 2013. The age of these
patients range from 45 to 75 years (mean age: 55 years). The sex
ratio of these patients is 38 males and 42 females). Inclusion
criteria: Patients with lung adenocarcinoma who have never received
radiotherapy or chemotherapy. Exclusion criteria: Patients who were
diagnosed as pneumonia in addition to LAD or those whose course of
disease was not visited and recorded. The present study was a
retrospective study. The LAD diagnosis of all tissues had been
histopathologically confirmed, and frozen in liquid nitrogen. All
patients provided written informed consent for use of tissue in the
present research. The study protocol was approved by the Ethics
Committee of the First Affiliated Hospital of Chinese PLA General
Hospital (Beijing, China).
Cell culture and transfection
The human LAD cell lines, SPC-A1, NCI-H441 and
NCI-H1975, and the normal lung epithelial cell, BEAS-2B, were
purchased from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). BEAS-2B cells were
incubated in complete medium [RPMI-1640+10% fetal bovine serum
(FBS; Lonza Group, Ltd., Basel, Switzerland)]. LAD cells were
cultured and incubated in medium (DMEM+10% FBS). All mediums were
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
All cell lines were incubated in a humidified atmosphere at 37°C
with 5% CO2. The complete medium was replaced every 2–3
days.
The LAD cell lines (500 cells/well) were transfected
with 50 nM small interfering RNA (si)-CHRF or si-negative control
(NC) using Lipofectamine® 2000 (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The si-CHRF expression vector and matched scrambled control vectors
were synthesized and purchased from GeneCopoeia, Inc. (Rockville,
MD, USA). To obtain the optimal transfection efficiency, we used
two siRNAs to knock down CHRF. They are si-CHRF#1 and si-CHRF#2.
The siRNA sequences used are as follows: si-CHRF#1:
5′-TGCCTCTCTAGAGAGCAGC-3′; si-CHRF#2: (5′-CCGATCTGACATGACTGCG-3′. A
total of 48 h after transfection, the cells were harvested for RNA
extraction and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis. All experiments were performed in
triplicate.
RNA extraction and RT-qPCR
Total RNA was extracted from LAD tissues or cells
using TRIzol (Thermo Fisher Scientific, Inc.), according to
manufacturer's instructions. RNA samples were stored at −80°C. A
One-Step SYBR RT-PCR kit (Takara Bio, Inc., Otsu, Japan) was used
to determine the expression of CHRF using a 7500 Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). Each
reaction contained 2 µl total RNA template, 1 µl forward primer, 1
µl reverse primer and 16 µl reaction mixture. The thermocycling
conditions were as follows: Stage 1 (reverse transcription
reaction), 1 cycle of 42°C for 5 min, 95°C for 10 sec; Stage 2 (PCR
reaction), 40 cycles of 95°C for 5 sec, 60°C for 34 sec; Stage 3
(dissociation curve analysis), 95°C for 15 sec, 40 cycles of 65°C
for 1 min and 95°C for 14 sec. The primers used for CHRF and GAPDH
were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The
primers are as follows: Human CHRF forward primer:
5′-AGATTCACATGGTATCCTGAAC′; reverse: 5′-TAGTCTGGCCACATTTTGTCTC-3′.
GAPDH forward primer: 5′-TGTGTCCGTCGTGGATCTGA-3′; reverse:
5′-CCTGCTTCACCACCTTCTTGA-3′. All experiments were performed in
triplicate. The qPCR quantification was conducted according to the
2−∆∆Cq method (18).
Cell proliferation assay
A total of either SPCA-1 or NCI-H441
2×104 cells/well were seeded in 96-well plates. A total
of 20 µl MTT solution (0.5 mg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added to each well, followed by incubation
at 37°C for 4 h. The cell culture medium was then carefully
aspirated, and the formazan crystals were dissolved in 0.2 ml
dimethyl sulfoxide. Absorbance was measured at 490 nm on a
SpectraMax M5 microplate reader. All experiments were performed in
triplicate.
Flow cytometry
Quantification of apoptosis was performed using an
AnnexinV-FITC Apoptosis Detection kit (Beijing Biosea Biotechnology
Co., Ltd., Beijing, China) 48 h after transfection, according to
the manufacturers' protocol. The results were analyzed using
CellQuest software v.0.9.13 (BD, Franklin Lakes, NJ, USA). Cells in
the right lower quadrant were considered to be apoptotic. For cell
cycle analysis, 1×106 cells were fixed. To fix cell, 0.5
ml of cold PBS was used to resuspend cells. Next, the resuspended
cells were added into 1.2 ml of 99.7% absolute ethyl alcohol (The
final concentration is 70%). Finally, the resuspended cells were
fixed at 4°C overnight. The cells were then stained with propidium
iodide (50 µg/ml) at 4°C for 30 min in the dark. The cell cycle
distribution was analyzed by using FlowJo 7.6.1 (FlowJo, LLC,
Ashland, Oregon, USA). All experiments were performed in
triplicate.
Transwell assay
Cell invasion ability of SPCA-1 and NCI-H441 cells
was assessed using Costar transwell chambers (Corning Incorporated,
Corning, NY, USA) containing polycarbonate membranes (6.5 mm in
diameter with a pore size of 8 µm), according to the manufacturer's
protocol. The transwell membranes were each coated with 80 µl
Matrigel (500 ng/µl; BD Biosciences, Franklin Lakes, NJ, USA), and
incubated at 37°C for 4 h. A total of 2×105 cells were
added to the upper compartment of each well in 200 µl of PFHM-II
Protein-Free Hybridoma Medium (Gibco: Thermo Fisher Scientific,
Inc.); supernatant complete medium of cells (0.5 ml) was added to
the bottom chamber. Following incubation for 24 h at 37°C, the
cells which had invaded to the lower chamber were stained with
hematoxylin and eosin (Thermo Fisher Scientific, Inc.). Cells were
fixed with 95% ethanol for 10 min at 37°C and then stained with 19%
hematoxylin for 20 min and 0.5% eosin for 3 min at 37°C., Then it
was counted under a light microscope at a magnification of ×1,000
and the number was counted within randomly nine field for each
experiment. All experiments were performed in triplicate.
Western blot analysis
Cell proteins were isolated using 400 µl of RIPA
buffer (Thermo Fisher Scientific, Inc.). Gels were scanned and
quantified by densitometry using the Quantity-One 4.4 software
(Bio-Rad, Laboratories, Inc., CA, USA). The protein was quantified
by using the Bradford method (Bio-Rad, Laboratories, Inc.). The
sample solution containing 50 µg proteins were separated by 10%
SDS-PAGE, then transferred into nitrocellulose membranes (Merck
KGaA, Darmstadt, Germany). The membranes were blocked with 5%
non-fat dry milk in Tris-buffered saline with 1% Tween (TBS) for 1
h, then incubated with the following primary antibodies overnight
at 4°C: Phosphorylated (p-)PI3K (cat no. ab125633, 1:2,000), total
PI3K (cat no. ab127617, 1:2,000), p-AKT (cat no. ab38449, 1:2,000),
total Akt (cat no. ab126580; 1:2,000) and GAPDH (cat no. ab8245;
1:2,000) (all from Abcam, Cambridge, UK). The secondary antibody
(anti-mouse IgG) conjugated to horseradish peroxidase (cat no.
7076, 1:300; Cell Signaling Technology, Inc., Danvers, MA, USA) was
incubated with the membranes for 1 h at 37°C. Protein binds were
visualized using an enhanced chemiluminescence reagent chromogenic
substrate (Pierce; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. All experiments were performed in
triplicate.
Statistical analysis
All statistical analysis was performed using SPSS
software (version 19.0; IBM Corp., Armonk, NY, USA). The data were
analyzed and presented as the mean ± standard deviation. Data
between two groups were analyzed by using the paired-student
t-test. Multiple comparisons were made by one-way ANOVA with the
Least Significant Difference post hoc test. Correlations between
CHRF expression and clinicopathological features of LAD patients
were analyzed by Pearson χ2 test. Survival analysis was
performed using the Kaplan-Meier method and the log-rank test was
used to compare differences between patient groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
CHRF expression is upregulated in lung
adenocarcinoma tissues and cell lines
To determine the role of the lncRNA, CHRF, in the
development and progression of lung adenocarcinoma (LAD), RT-qPCR
was performed to measure the expression of CHRF in 80 pairs of LAD
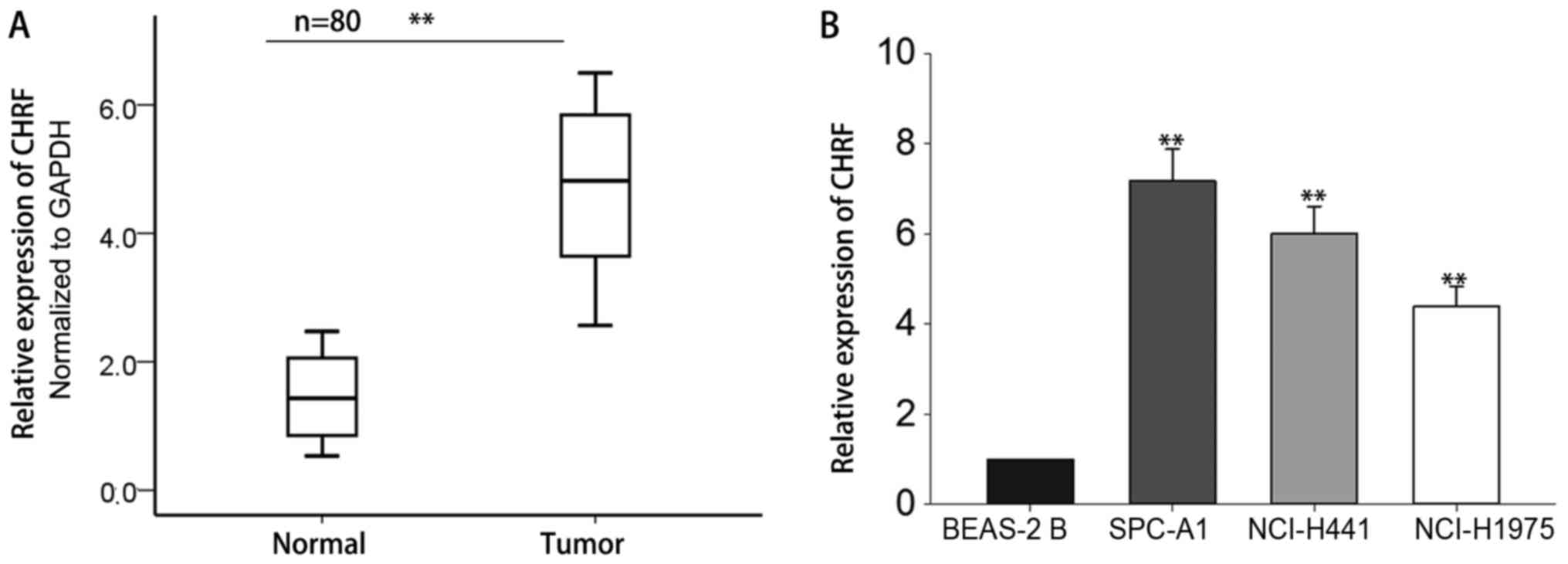
tissues and adjacent normal tissues. The results revealed that CHRF
was significantly increased in LAD tissues compared with adjacent
normal tissues (Fig. 1A, P<0.01).
The expression level of CHRF was also measured in LAD cell lines
and the normal lung epithelial BEAS-2B cells. As demonstrated in
Fig. 1B, the level of CHRF was
increased in LAD cells compared with normal epithelial cells
(P<0.01). The level of CHRF was highest in SPC-A1 and NCI-H441
cells, which were selected for use in subsequent experiments. These
results suggest that CHRF may serve a crucial role in the
development of LAD.
Association between the expression of
CHRF and clinicopathological characteristics of LAD patients
In order to explore the association between CHRF and
various clinicopathological characteristics of LAD patients, the
mean value of CHRF expression in LAD tissue was used as a cutoff
value (patients exhibiting expression higher than the cutoff value
were classified into high expression and patients exhibiting
expression lower than or equal to the cutoff value were classified
into low expression) and all patients were divided into a high
expression group (n=41) and a low expression group (n=39). As
presented in Table I, high expression
of CHRF was significantly associated with advanced TNM stage, lymph
node metastasis and large tumor size (P<0.05). However, there
was no evident association between the expression of CHRF and other
characteristics, including age, gender, smoking and differentiation
(P>0.05).
 | Table I.Association between CHRF expression
and clinicopathological characteristics (n=80). |
Table I.
Association between CHRF expression
and clinicopathological characteristics (n=80).
|
| CHRF expression |
|
|---|
|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Age |
| ≤55 | 21 | 15 | 0.177 |
|
>55 | 18 | 26 |
|
| Sex |
| Male | 20 | 18 | 0.655 |
|
Female | 19 | 23 |
|
| Smoking status |
|
Smoking | 24 | 17 | 0.080 |
|
Non-smoking | 15 | 24 |
|
| Differentiation |
| Poor | 17 | 26 | 0.116 |
|
Well/moderate | 22 | 15 |
|
| Tumor size |
| ≤3
cm | 26 | 13 | 0.003b |
| >3
cm | 13 | 28 |
|
| TNM stage |
|
I–II | 23 | 14 | 0.043a |
|
IIIa | 16 | 27 |
|
| Lymph
metastasis |
|
Absent | 24 | 14 | 0.025a |
|
Present | 15 | 27 |
|
Upregulation of CHRF expression is
associated with poor prognosis for patients with LAD
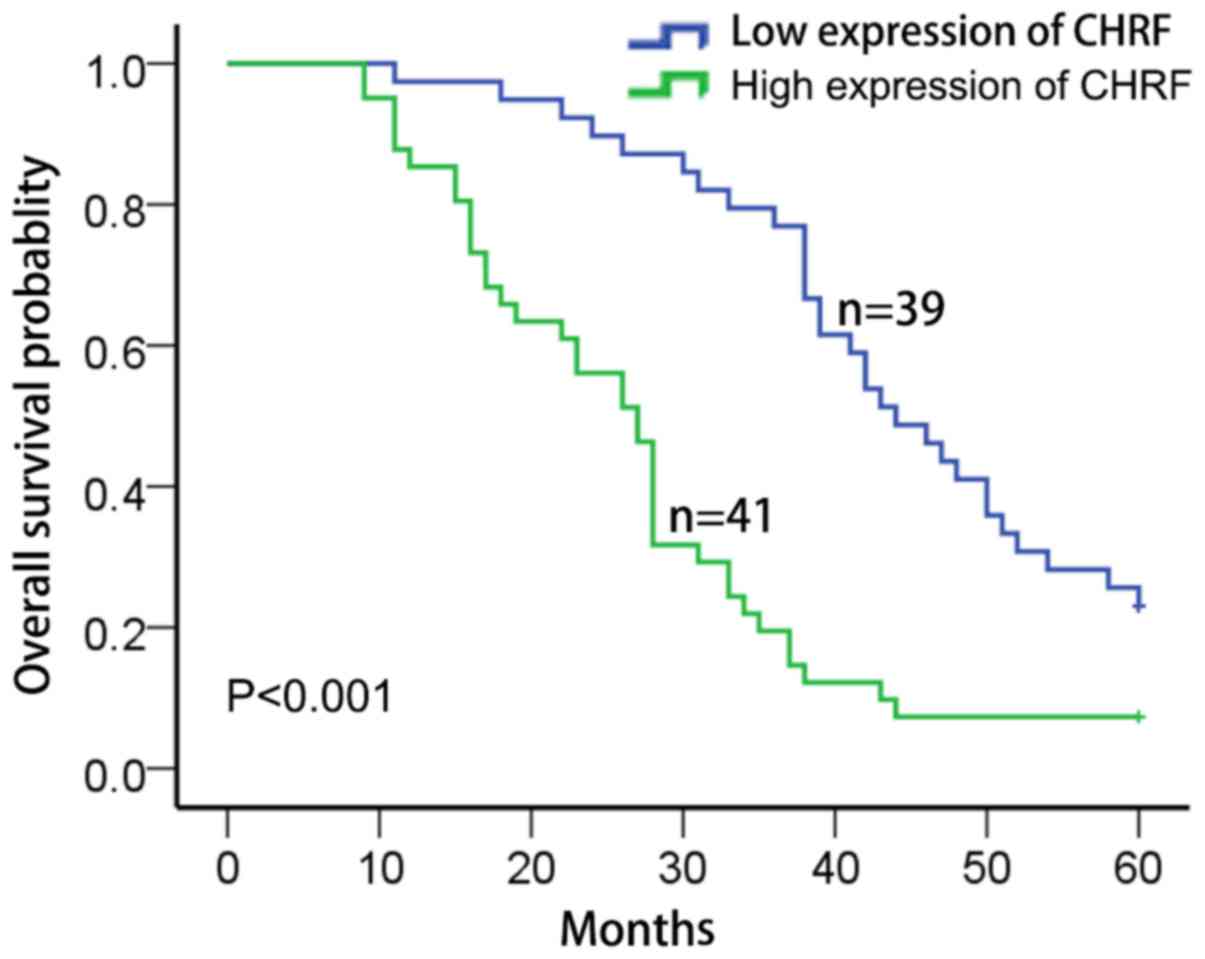
The Kaplan-Meier and the log-rank test were used to
investigate the clinical relevance of CHRF in LAD. Patients
exhibiting high expression of CHRF had a markedly lower overall
survival probability than those with low expression of CHRF
(Fig. 2). Univariate and multivariate
Cox regression analyses indicated that the expression of CHRF,
tumor size and lymph metastasis were associated with the overall
survival time of LAD patients. Therefore, lncRNA CHRF may act as an
independent prognostic marker for the overall survival time of LAD
patients (Table II).
 | Table II.Multivariate analysis of prognostic
parameters in patients with lung adenocarcinoma by Cox regression
analysis. |
Table II.
Multivariate analysis of prognostic
parameters in patients with lung adenocarcinoma by Cox regression
analysis.
|
| Multivariate | Univariate |
|---|
|
|
|
|
|---|
| Variable | P-value | P-value |
|---|
| Age | 0.944 | 0.940 |
|
≤60 |
|
|
|
>60 |
|
|
| Sex | 0.801 | 0.817 |
|
Male |
|
|
|
Female |
|
|
| Smoking | 0.510 | 0.341 |
|
Smoking |
|
|
| No
smoking |
|
|
|
Differentiation | 0.907 | 0.872 |
|
Poor |
|
|
|
Well/Moderate |
|
|
| Tumor size | 0.001b |
<0.001b |
| ≤3
cm |
|
|
| >3
cm |
|
|
| TNM stage | 0.105 | 0.111 |
|
I–II |
|
|
|
IIIa |
|
|
| Lymph
metastasis | 0.048a | 0.032a |
|
Absent |
|
|
|
Present |
|
|
| CHRF
expression | 0.001b |
<0.001b |
|
Low |
|
|
|
High |
|
|
Knockdown of CHRF represses cell
proliferation by regulating cell cycle and apoptosis
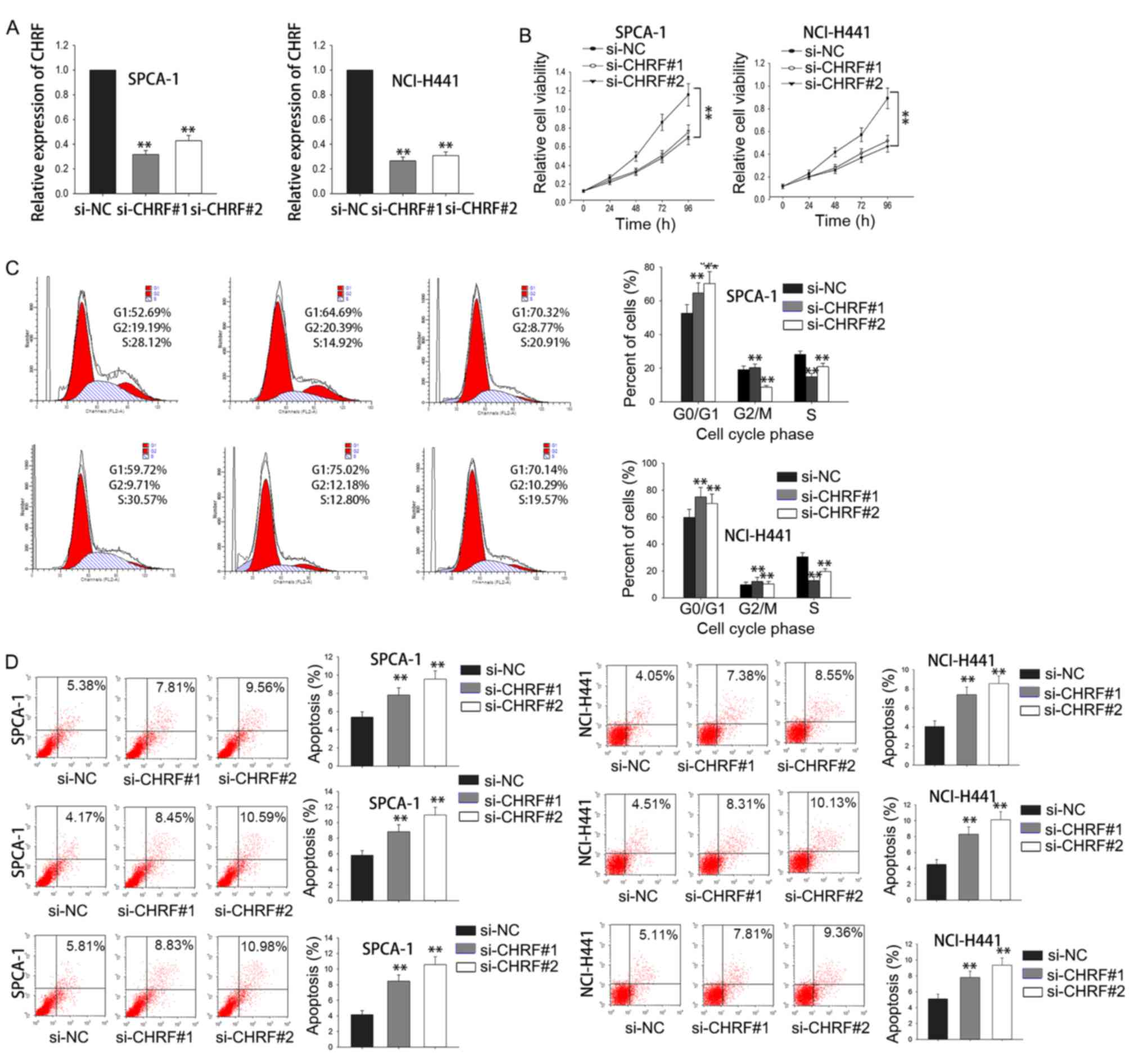
Loss-of-function experiments were performed to
investigate the biological function of CHRF in LAD. The results of
RT-qPCR analysis revealed that the expression of CHRF was
downregulated in si-SPCA-1 and si-NCI-H441 cells compared with the
si-NC group (P<0.01; Fig. 3A). MTT
assays demonstrated downregulation of CHRF expression significantly
reduced cell proliferation both in si-SPCA-1 and si-NCI-H441 cells
(P<0.01; Fig. 3B). Flow cytometry
revealed that knockdown of CHRF caused cell cycle arrest most often
in the G0/G1 phase, and induced cell
apoptosis (P<0.01; Fig. 3C and D).
These results indicate that knockdown of CHRF reduced cell
proliferation by affecting cell cycle and apoptosis.
Inhibition of CHRF suppresses cell
migration and invasion in LAD
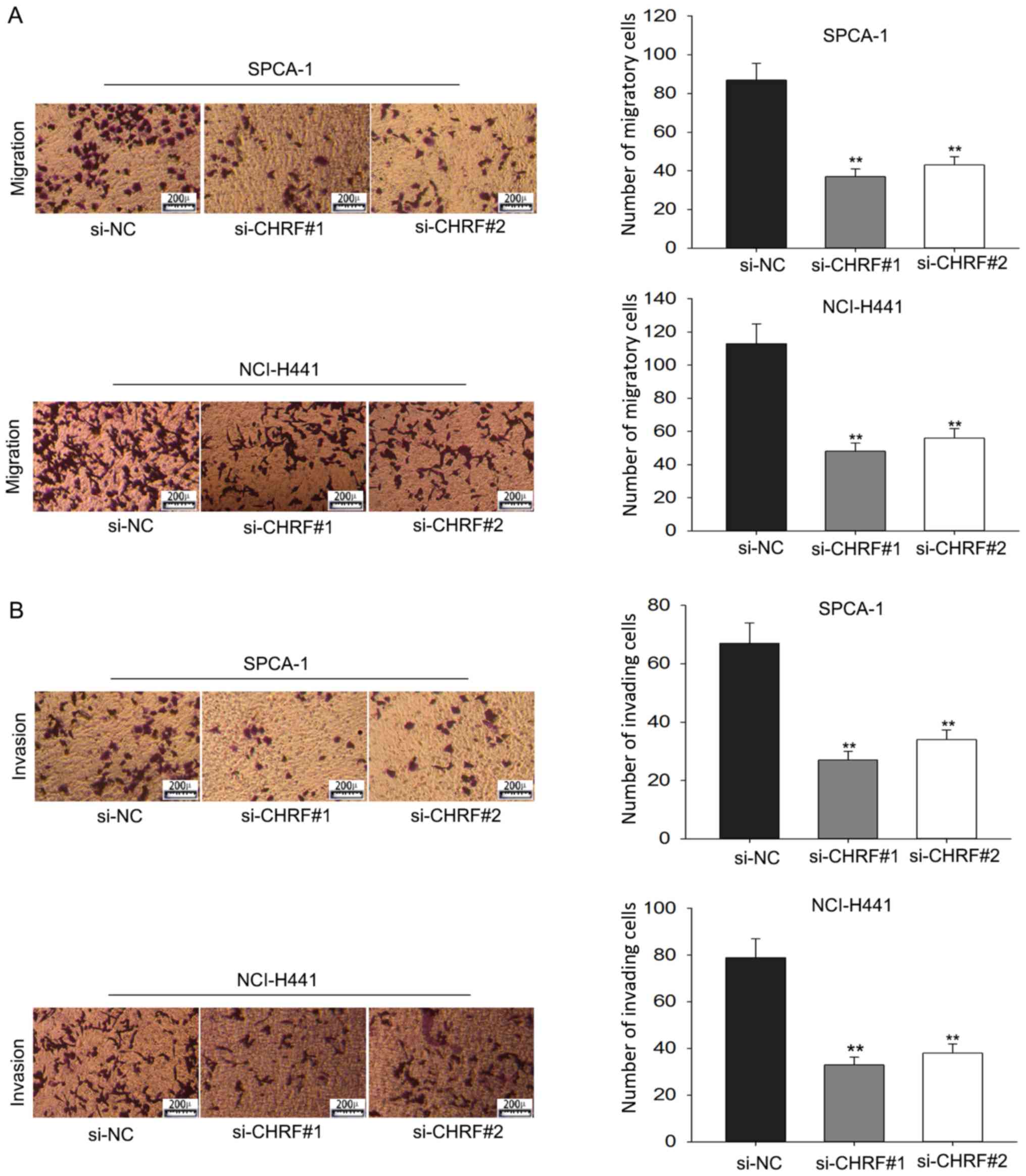
Transwell assays were performed to further explore
the effect of CHRF on the progression of LAD. This demonstrated
that cells transfected with si-CHRF were less migratory compared
with si-NC cells (P<0.01; Fig.
4A). Furthermore, the transwell invasion assay demonstrated
that knockdown of CHRF significantly reduced the invasive ability
of LAD cells (P<0.01; Fig. 4B).
These data suggest that CHRF functioned as an oncogene in the
development and progression of LAD.
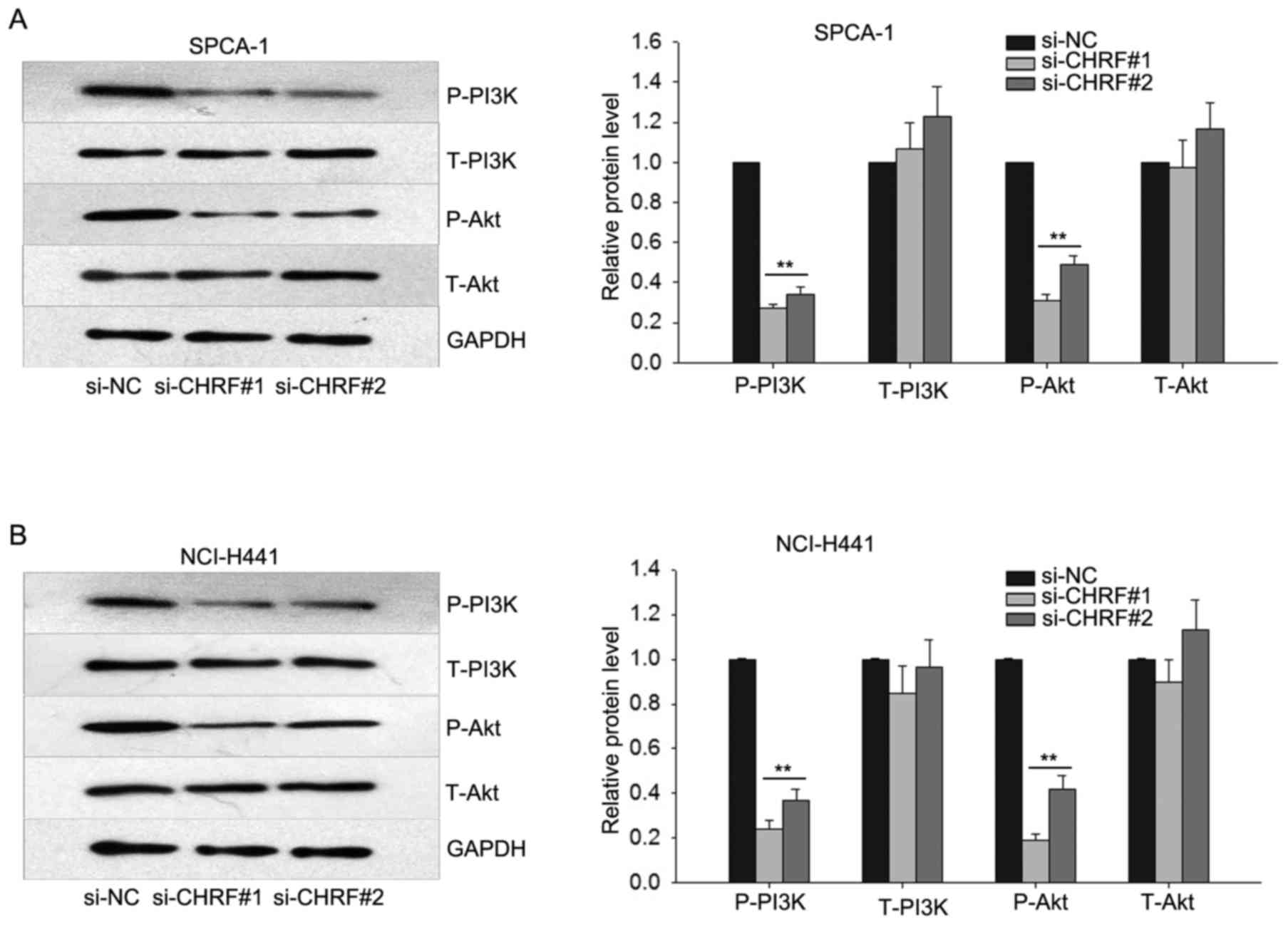
Knockdown of CHRF reduces the
expression level of protein members of the PI3K/Akt signaling
pathway in LAD
To explore the potential mechanism through which
CHRF affects the progression of LAD, western blotting was performed
to determine the effect of downregulated CHRF on the PI3K/Akt
signaling, which has been demonstrated to be ectopically activated
in human cancers, increasing cell proliferation and metastasis
(19). Western blotting revealed that
knockdown of CHRF dramatically reduced the protein level of p-PI3K
and p-Akt in LAD (P<0.01; Fig. 5).
These data indicate that the PI3K/Akt signaling pathway may
function in the proliferation and metastasis of LAD cells induced
by CHRF.
Discussion
Dysregulated expression of lncRNAs has been
demonstrated to contribute to the development and progression of
various types of human cancer, providing novel therapeutic targets
for cancer treatment and drug resistance (20–22).
LncRNA CHRF has been reported to function in numerous human
diseases, including a variety of forms of cancer. LncRNA
CHRF-induced downregulation of miR-489l promotes metastasis of
colorectal cancer via twist family BHLH transcription factor
1/epithelial-mesenchymal transition signaling pathway (23). Previous studies have reported that
miR-489 is regulated by lncRNA CHRF (17,24). CHRF
also serves critical roles in leukemia (25), human erythroleukemia (26) and myeloid leukemia (27), and is a key regulator the pathology of
heart failure (28). However, the
molecular mechanisms underlying the process of LAD tumorigenesis
remain insufficiently characterized.
The present study demonstrated that CHRF was
dramatically overexpressed in LAD tissues and cell lines compared
with normal tissues and cells. Overexpression of CHRF was
associated with advanced TNM stage, lymph node metastasis and large
tumor size. It was also revealed that patients exhibiting high
expression of CHRF had a shorter overall survival time compared
with those exhibiting low expression of CHRF. Furthermore,
loss-of-function assays indicated that knockdown of CHRF inhibited
cell proliferation, migration and invasion of LAD.
The PI3K/Akt pathway is a crucial intracellular
signaling pathway involved in proliferation and EMT in the various
types of cancer (29). It has been
demonstrated that lncRNAs serve important roles in PI3K/Akt
signaling pathway. For example, it has been reported that
downregulation of metastasis associated lung adenocarcinoma
transcript 1 induced EMT via the PI3K/Akt pathway in breast cancer
(30). Downregulation of lncRNA
MALAT1 induces epithelial-to-mesenchymal transition via the
PI3K-AKT pathway in breast cancer (31). In the present study, it was
demonstrated that knockdown of CHRF decreased the protein
expression levels of p-PI3K and p-Akt, suggesting that the PI3K/Akt
pathway may function in the CHRF-induced carcinogenesis of LAD.
Overall the present study indicates that CHRF
expression is enhanced during the progression of LAD.
Overexpression of CHRF was associated with advanced TNM stage,
lymph node metastasis and large tumor size in patients with LAD.
Downregulation of CHRF expression reduced cell proliferation and
metastasis in LAD. Furthermore, knockdown of CHRF resulted in
reduced activity of the PI3K/Akt signaling pathway. Thus, CHRF may
be a novel molecular target for the diagnosis and therapy of
LAD.
Acknowledgements
Not applicable.
Funding
The present study was supported by funding of the
Chinese PLA General Hospital (grant no., 2016FC-304M-TSYS-04).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XXie, WZ, JP, XXio, HW and LM were responsible for
the completion of experiments. XXie and WZ undertook study design
and wrote the manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent for
the use of their tissue in the present research. The study protocol
was approved by the Ethics Committee of the First Affiliated
Hospital of Chinese PLA General Hospital (Beijing, China).
Consent for publication
All patients, researchers and authors participated
in this study have provided written informed consent for the
publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Zhang F, Wang J, Hu L, Chen J, Xu
G and Wang Y: LncRNA LINC01512 promotes the progression and
enhances oncogenic ability of lung adenocarcinoma. J Cell Biochem.
118:3102–3110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gridelli C, Rossi A and Maione P:
Treatment of non-small-cell lung cancer: State of the art and
development of new biologic agents. Oncogene. 22:6629–6638. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen CH, Lai JM, Chou TY, Chen CY, Su LJ,
Lee YC, Cheng TS, Hong YR, Chou CK, Whang-Peng J, et al: VEGFA
upregulates FLJ10540 and modulates migration and invasion of lung
cancer via PI3K/AKT pathway. PLoS One. 4:e50522009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ogawa E, Takenaka K, Katakura H, Adachi M,
Otake Y, Toda Y, Kotani H, Manabe T, Wada H and Tanaka F:
Perimembrane Aurora-A expression is a significant prognostic factor
in correlation with proliferative activity in non-small-cell lung
cancer (NSCLC). Ann Surg Oncol. 15:547–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rachet B, Woods LM, Mitry E, Riga M,
Cooper N, Quinn MJ, Steward J, Brenner H, Estève J, Sullivan R and
Coleman MP: Cancer survival in England and Wales at the end of the
20th century. Br J Cancer. 99 Suppl 1:S2–S10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stewart DJ: Tumor and host factors that
may limit efficacy of chemotherapy in non-small cell and small cell
lung cancer. Crit Rev Oncol Hematol. 75:173–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren K, Xu R, Huang J, Zhao J and Shi W:
Knockdown of long non-coding RNA KCNQ1OT1 depressed chemoresistance
to paclitaxel in lung adenocarcinoma. Cancer Chemother Pharmacol.
80:243–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carrizosa DR and Gold KA: New strategies
in immunotherapy for non-small cell lung cancer. Transl Lung Cancer
Res. 4:553–559. 2015.PubMed/NCBI
|
|
12
|
Lian Y, Cai Z, Gong H, Xue S, Wu D and
Wang K: HOTTIP: A critical oncogenic long non-coding RNA in human
cancers. Mol Biosyst. 12:3247–3253. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma C, Shi X, Zhu Q, Li Q, Liu Y, Yao Y and
Song Y: The growth arrest-specific transcript 5 (GAS5): A pivotal
tumor suppressor long noncoding RNA in human cancers. Tumour Biol.
37:1437–1444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu L, Jin L, Zhang W and Zhang L: Roles of
long non-coding RNA CCAT2 in cervical cancer cell growth and
apoptosis. Med Sci Monit. 22:875–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pawar K, Hanisch C, Vera Palma SE,
Einspanier R and Sharbati S: Down regulated lncRNA MEG3 eliminates
mycobacteria in macrophages via autophagy. Sci Rep. 6:194162016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shang C, Guo Y, Hong Y and Xue YX: Long
non-coding RNA TUSC7, a target of miR-23b, plays tumor-suppressing
roles in human gliomas. Front Cell Neurosci. 10:2352016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Q, Han L, Yan W, Ji X, Han R, Yang J,
Yuan J and Ni C: miR-489 inhibits silica-induced pulmonary fibrosis
by targeting MyD88 and Smad3 and is negatively regulated by lncRNA
CHRF. Sci Rep. 6:309212016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin J, Sun Z, Yang F, Tang L, Chen W and
Guan X: miR-19b-3p inhibits breast cancer cell proliferation and
reverses saracatinib-resistance by regulating PI3K/Akt pathway.
Arch Biochem Biophys. 645:54–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shang C, Guo Y, Zhang J and Huang B:
Silence of long noncoding RNA UCA1 inhibits malignant proliferation
and chemotherapy resistance to adriamycin in gastric cancer. Cancer
Chemother Pharmacol. 77:1061–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu G, Xiang T, Wu QF and Wang WX: Long
noncoding RNA H19-derived miR-675 enhances proliferation and
invasion via RUNX1 in gastric cancer cells. Oncol Res. 23:99–107.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan J, Dang Y, Liu S, Zhang Y and Zhang G:
LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by
targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumour Biol.
Nov 30–2016.(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Tao Y, Han T, Zhang T, Ma C and Sun C:
LncRNA CHRF-induced miR-489 loss promotes metastasis of colorectal
cancer via TWIST1/EMT signaling pathway. Oncotarget. 8:36410–36422.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang K, Liu F, Zhou LY, Long B, Yuan SM,
Wang Y, Liu CY, Sun T, Zhang XJ and Li PF: The long noncoding RNA
CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res.
114:1377–1388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tytgat GA, Voûte PA, Takeuchi S, Miyoshi I
and Rutgers M: Meta-iodobenzylguanidine uptake in platelets,
megakaryoblastic leukaemia cell lines MKPL-1 and CHRF-28-11 and
erythroleukaemic cell line HEL. Eur J Cancer. 31A:603–606. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schick BP, Petrushina I, Brodbeck KC and
Castronuevo P: Promoter regulatory elements and DNase
I-hypersensitive sites involved in serglycin proteoglycan gene
expression in human erythroleukemia, CHRF 288-11, and HL-60 cells.
J Biol Chem. 276:24726–24735. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scholl S, Kirsch C, Böhmer FD and Klinger
R: Signal transduction of c-Kit receptor tyrosine kinase in CHRF
myeloid leukemia cells. J Cancer Res Clin Oncol. 130:711–718. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen L, Yan KP, Liu XC, Wang W, Li C, Li M
and Qiu CG: Valsartan regulates TGF-β/Smads and TGF- β/p38 pathways
through lncRNA CHRF to improve doxorubicin-induced heart failure.
Arch Pharm Res. 41:101–109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hennessy BT, Smith DL, Ram PT, Lu Y and
Mills GB: Exploiting the PI3K/AKT pathway for cancer drug
discovery. Nat Rev Drug Discov. 4:988–1004. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu S, Sui S, Zhang J, Bai N, Shi Q, Zhang
G, Gao S, You Z, Zhan C, Liu F and Pang D: Downregulation of long
noncoding RNA MALAT1 induces epithelial-to-mesenchymal transition
via the PI3K-AKT pathway in breast cancer. Int J Clin Exp Pathol.
8:4881–4891. 2015.PubMed/NCBI
|
|
31
|
Yang C, Li X, Wang Y, Zhao L and Chen W:
Long non-coding RNA UCA1 regulated cell cycle distribution via CREB
through PI3-K dependent pathway in bladder carcinoma cells. Gene.
496:8–16. 2012. View Article : Google Scholar : PubMed/NCBI
|