Introduction
Lung cancer is one of the most common human cancer
in the world (1). The morbidity and
mortality rate of lung cancer in increasing since 2000 in the world
(2). Non-small cell lung cancer
(NSCLC) is the most common lung cancer, which includes
adenocarcinoma, large cell carcinoma and squamous cell carcinoma
(3–5).
NSCLC is generally resistant to chemotherapy and radiotherapy
(6). Although various treatments
(chemoradiotherapy and targeted therapy) have been developed for
the treatments of patients with NSCLC, the survival rate of
patients remains properly poor (7–9).
Therefore, it is essential to explore the novel clinical treatments
for to improve the therapeutic effects for patients with NSCLC in
clinic.
Gene therapy drug of rAd-p53 is the first generation
gene drug and has been approved for human cancer therapy (10). Previous trials have indicated that the
no serious adverse effect related to rAd-p53 has been reported in
the majority of large intrathoracic malignant and gastric cancer
cases, which is a safe anti-cancer agent (11,12). In
addition, rAd-p53 could enhance the sensitivity of human gastric
cancer cells to chemotherapy, which decreased anti-apoptosis Bcl-2
expression and increased proapoptosis Bax expression in gastric
cancer cells (13). Furthermore, the
combination of recombinant rAd-p53 and adriamycin presented more
efficacy that single treatment and improved drug resistance in
chemotherapy of lung squamous cell cancer (14). However, single treatment of rAd-p53 is
not enough to improve survival of cancer patients (10,11,15).
Currently, target therapy has been widely applied
for the treatment of human cancer, such as lung cancer, liver
cancer and breast cancer (16–18).
Lenvatinib is a target therapy drug, which targets for vascular
endothelial growth factor receptor 1–3 (VEGFR1-3), fibroblast
growth factor receptor 1–4 (FGFR1-4), platelet-derived growth
factor receptor-β (PDGFR-β), RET, and kinase insert domain receptor
(KIT) (19). Study has indicated that
Lenvatinib is beneficial for the treatment of patients with renal
cell carcinoma (RCC) (20). Phase 1
study of Lenvatinib combined with carboplatin and paclitaxel showed
antitumor activity in patients with NSCLC (21). Nagashima et al (22), found that Lenvatinib treatment is
effectiveness for thyroid cancer with lung metastases. However,
combined therapeutic effects of Lenvatinib and rAd-p53 have not
investigated for patients with NSCLC.
In the present study, we investigate the therapeutic
effects of Lenvatinib and rAd-p53 in patients with NSCLC. We
intended to determine the pharmacokinetic (PK) profile of
Lenvatinib and rAd-p53 for NSCLC patients. This study also analyzed
the progression-free survival (PFS) after treated by Lenvatinib and
rAd-p53 in NSCLC patients.
Materials and methods
Ethic statement
The present study was approved by Ethical Committee
of Mudanjiang medical University affiliated HongQi Hospital.
Patients
The phase-I study was performed in Mudanjiang
medical University affiliated HongQi Hospital from May 2011 to
October 2016. All patients provided written informed consent before
any study-related procedures were performed. Eligibility criteria
included age ≥18 years, with a Karnofsky performance status ≥80%;
adequate haematological (platelet count of ≥100×109/l;
absolute neutrophil count of ≥1.5×109/l; and haemoglobin
≥8.5 g/dl), hepatic (serum alanine aminotransferase; bilirubin ≤25
µmol/l and aspartate transaminase ≤3 × the upper limit of normal)
and renal function (a creatinine clearance ≥60 ml/min or serum
creatinine ≤1.5 × the upper limit of normal by Cockcroft-Gault
formula). Patients with cancer history were excluded from this
study.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
NSCLC cells were isolated from NSCLC tissues as
described previously (23) and
cultured in DMEM medium (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Total RNA was extracted from cells (1×106)
using RNAeasy Mini Kit (Qiagen, Inc., Valencia, CA, USA) and RNA (1
µg) was transcribed to cDNA by using an RT kit (Qiagen, Inc.) and
quality was confirmed by electrophoresis. The cDNA (10 ng) was
subjected to qPCR (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
using SYBR-Green Master Mix system (cat. no. 4304886; Applied
Biosystems; Thermo Fisher Scientific, Inc.). All the forward and
reverse primers were synthesized by Invitrogen (P53, forward,
5′-CTATCTTATCTATCTTCTCTATCTTC-3′; reverse,
5′-CTATCTTATCTTCTCTCATCTCTAC-3′, VEGFR, forward,
5′-TTCAGAGCGGAGAAAGCAT-3′; reverse, 5′-TAGTTCCCGAAACCCTGAG-3′;
FGFR, forward, 5′-CGTGGAAAAGAACGGCAGTAAATA-3′; reverse,
5′-GAACTATTTATCCCCGAGTGCTTG-3′; PDGFR-β, forward,
5′-CCATTCCCGAGGAGCTTTATC-3′, reverse, 5′-GGTCATGTTCAGGTCCAACTC-3′;
GAPDH, forward, 5′-AGTGCCAGCCTCGTCTCATAG-3′; reverse,
5′-CGTTGAACTTGCCGTGGGTAG-3′). The reaction conditions were
performed as follows: 95°C for 2 min and 45 cycles of 95°C for 20
sec and 54°C for 1 min and 72°C for 30 sec. Relative mRNA
expression changes were calculated by 2−ΔΔCq (24). The results were presented as the
n-fold change compared with β-actin using Quantiscan2.1 (software
demo of AB QuantStudio™ 12 K Flex System, Thermo Fisher Scientific,
Inc.).
MTT assay
NSCLC cells were cultured in 96-well plates and
incubated with Lenvatinib (2 mg/ml) and/or rAd-p53 (1011
pfu) for 48 h at 37°C. A total of 20 µl MTT (5 mg/ml) in PBS
solution was added to each well and the cells were cultured for 4 h
at 37°C. The medium was removed and 100 µl dimethyl sulfoxide
(DMSO) was added into the wells to solubilize the crystals. The
optical density was measured by a Bio-Rad (ELISA) reader (Bio-Rad
Laboratories, Inc.) at 450 nm.
Apoptosis of NSCLC cells
NSCLC cells (A549) were grown at 37°C until 90%
confluences were reached. Cells (1×108) were then
incubated with Lenvatinib (2 mg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and/or rAd-p53 (1011 pfu) for 48 h
at 37°C. After incubation, the tumor cells were trypsinized and
collected. The cells were then washed in cold PBS, adjusted to
1×106 cells/ml with PBS, labeled with Annexin V-FITC and
PI (Annexin V-FITC kit; BD Biosciences, Franklin Lakes, NJ, USA),
and analyzed with a FACScan flow cytometer (BD Biosciences). The
treatments were performed in triplicate, and apoptosis was analyzed
with a FACScan flow cytometer (BD Biosciences). using CellQuest Pro
solfware (v.5.1, BD Biosciences).
Cells invasion and migration
assays
NSCLC cells were cultured in six-well plates with
chamber inserts (BD Biosciences) and incubated with Lenvatinib (2
mg/ml) and/or rAd-p53 (1011 pfu) for 48 h at 37°C. Mg63
cells were cultured in a 24-well culture plate with chamber inserts
(BD Biosciences). For migration assays, 1×106 cells/well
were cultured in DMEM medium (Thermo Fisher Scientific, Inc.)
supplemented with 5% heat-inactivated FBS (Gibco; Thermo Fisher
Scientific, Inc.) and placed into the upper chamber with the
non-coated membrane. For invasion assays, cells (1×106
cells/well) were placed into the upper chamber with a
Matrigel-coated membrane. Procedures were performed according to
the manufacturer's instructions. Cells were fixed in 4%
paraformaldehyde (Sigma-Aldrich; Merck KGaA) and stained with 0.1%
crystal violet (Sigma) to quantify cell migration and invasion for
15 min at 37°C. The tumor cells invasion and migration were counted
in at least three randomly stained microscope fields (Olympus BX51;
Olympus Corp., Tokyo, Japan) for every membrane.
Western blot analysis
NSCLC cells (1×106) were incubated with
Lenvatinib (2 mg/ml) and/or rAd-p53 (1011 pfu) for 48 h
at 37°C and harvested by scraping and lysed in
radioimmunoprecipitation assay buffer (Sigma-Aldrich; Merck KGaA)
followed by homogenization at 4°C for 10 min. Protein concentration
was measured by a BCA protein assay kit (Thermo Fisher Scientific,
Inc.). Protein (10 µg) was separated by 15% SDS-PAGE followed
transfer to PVDF membranes (EMD Millipore, Billerica, MA, USA).
Proteins were blocked with 5% bovine serum albumin (Sigma-Aldrich;
Merck KGaA) for 2 h at 37°C and incubation with primary rabbit
anti-human antibodies: P53 (ab131442), VEGFR (ab36844), FGFR
(ab10646), PDGFR-β (ab220745) and GAPDH (ab9485) (all 1:500
dilutions; Abcam, Shanghai, China) for 12 h at 4°C. Subsequently,
proteins were incubated with the corresponding rabbit horseradish
peroxidase-labeled IgG (1:5,000; Vector Laboratories, Inc.,
Burlingame, CA, USA) for 2 h at 37°C. The proteins expression
levels were detected using a chemi-luminescence detection system
(v.3.0; Sigma-Aldrich; Merck KGaA). The density of the bands was
analyzed by Quantity one software v.4.62 (Bio-Rad Laboratories,
Inc.).
Immunohistochemical staining
NSCLC tissues from patients were fixed using 10%
formaldehyde for 30 at 37°C, washed with PBS (0.01 mmol/l, pH 7.4)
and followed with embedding in paraffin wax. Tissues were
deparaffinized in xylene and rehydrated in grade alcohols. Tissues
were cut into 4-µm thick sections and antigen retrieval was
performed using Antigen Retrieval Reagents (cat. no. CTS015;
Bio-Rad Laboratories, Inc.). The sections were washed with PBS for
10–15 min at 37°C and subsequently blocked using 5% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) for 2 h at 37°C. Tumor sections
were incubated with CD4 (1:1,000 dilutions, Clone 4B12; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA) and CD8 (1:1,000
dilutions, Clone C8/144B; Dako; Agilent Technologies, Inc.), P53
(1:500 dilutions; ab32049), VEGFR (1:500 dilutions; ab36844), FGFR
(1:500 dilutions; ab10646), PDGFR-β (1:500 dilutions, ab220745; all
from Abcam) for 12 h at 4°C. The sections were washed three times
with PBS for 3 min at room temperature and were incubated with
HRP-labeled secondary goat anti-rabbit antibodies (1:2,000,
ab150077; Abcam). Sections were visualized using ZEISS LSM 510
confocal microscope at ×40 magnification.
Treatment administration
Lenvatinib (twice-daily, 32 mg/day) (21) and rAd-p53 (twice-daily,
1×1011 viral units/day) (25) were administered orally and intratumor
injection, respectively. The treatments were continued for a 30-day
administration schedule.
Evaluation of toxicity
Toxicity was graded using the National Cancer
Institute Common Toxicity Criteria (v3.0). Physical examination,
full blood count, biochemical profile measurement of blood pressure
and urinalysis were performed every two days during combined
therapy. Electrocardiograms and biochemical detection were
performed every three days (data not shown).
ELISA
Concentration levels of P53 and Lenvatinib were
analyzed in patients with NSCLC by using commercialized human p53
ELISA kit (DYC1043-2; Bio-Rad Laboratories, Inc.) and human VEGFR
ELASA kit (FAB357P) according to the manufacturer's instructions.
Results were measured at 450 nm in an ELISA reader (Bio-Rad
Laboratories, Inc.).
CT scan protocol
The CT diagnosis system was used to analyze tumor
volume using preprogrammed setting in clinical trials. The
preprogrammed setting was optimized to reach the best image
formation. NSCLC patients were underwent CT according to instrument
of the manufacture (Philips Medical Systems, Inc., Bothell, WA,
USA). The details of principles and settings of CT were described
in previous study (26). Data of CT
images were analyzed by computerized tomography system (Murphy-M2;
Cook Medical, Bloomington, IN, USA).
Statistical methods
All data were reported as means and SEM and analyzed
using SPSS Statistics v.19.0 (IBM Corp., Armonk, NY, USA).
Statistical significance of differences between mean values was
assessed by Student's t test for unpaired data. Comparisons of data
between multiple groups were performed with analysis of variance
(ANOVA) followed by Tukey's honest significant difference test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 120 NSCLC patients were enrolled and the
mean age of patients was 48.5 years old (average, 33.7–63.3). In
the present study, the numbers of men (n=72) were more than women
(n=48). None of the patients have received any anti-cancer
treatments before this study. All characteristics of NSCLC patients
were summarized in Table I.
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
| Variables | No. of
patients | Percentage, % |
|---|
| Total patients with
NSCLC | 120 | 100.0 |
| Sex |
|
|
|
Female | 72 | 60.0 |
|
Male | 58 | 40.0 |
| Age (years) | 48.5±14.8 |
|
| Performance status
(Karnofsky) |
|
|
|
100 | 60 | 40.1 |
| 90 | 36 | 22.5 |
| 80 | 24 | 37.5 |
| Drugs
treatment |
|
|
|
Lenvatinib | 40 | 33.3 |
|
rAd-p53 | 40 | 33.3 |
|
Combination | 40 | 33.3 |
Expression of p53, VEGFR, FGFR and
PDGFR-β in NSCLC cells and tissues
We investigated expression of p53, VEGFR, FGFR and
PDGFR-β in NSCLC cells and tissues. As shown in Fig. 1A and B, gene and protein expression
levels of p53 were down-regulated and VEGFR, FGFR and PDGFR-β were
up-regulated in NSCLC cells compared to adjacent normal cells.
Immunohistochemistry demonstrated that p53 were decreased and
VEGFR, FGFR and PDGFR-β were increased in NSCLC tissues compared to
adjacent normal tissues (Fig. 1C).
These results indicate that changes of p53, VEGFR, FGFR and PDGFR-β
expression levels may be associated with NSCLC cells growth and
metastasis.
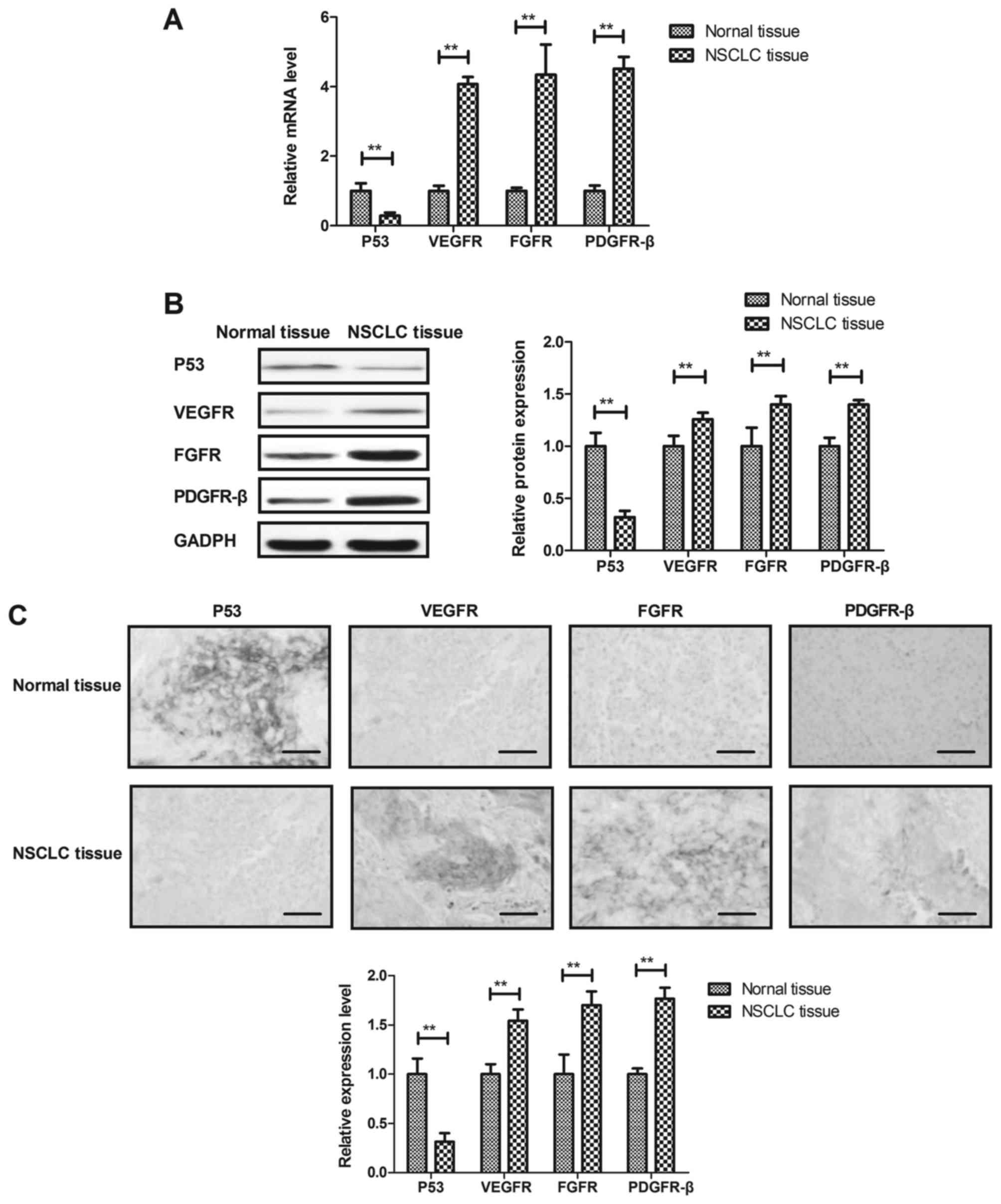 | Figure 1.Expression levels of p53, VEGFR, FGFR
and PDGFR-β in NSCLC cells and tissues. (A and B) Gene and protein
expression levels of p53, VEGFR, FGFR and PDGFR-β in NSCLC cells
and adjacent normal cells. (C) Immunohistochemistry assay analyzes
p53, VEGFR, FGFR and PDGFR-β levels in NSCLC tissues and adjacent
normal tissues (Scale bar=100 µm). **P<0.01. VEGFR, vascular
endothelial growth factor receptor; FGFR, fibroblast growth factor
receptor; PDGFR-β, platelet-derived growth factor receptor- β;
NSCLC, non-small cell lung cancer. |
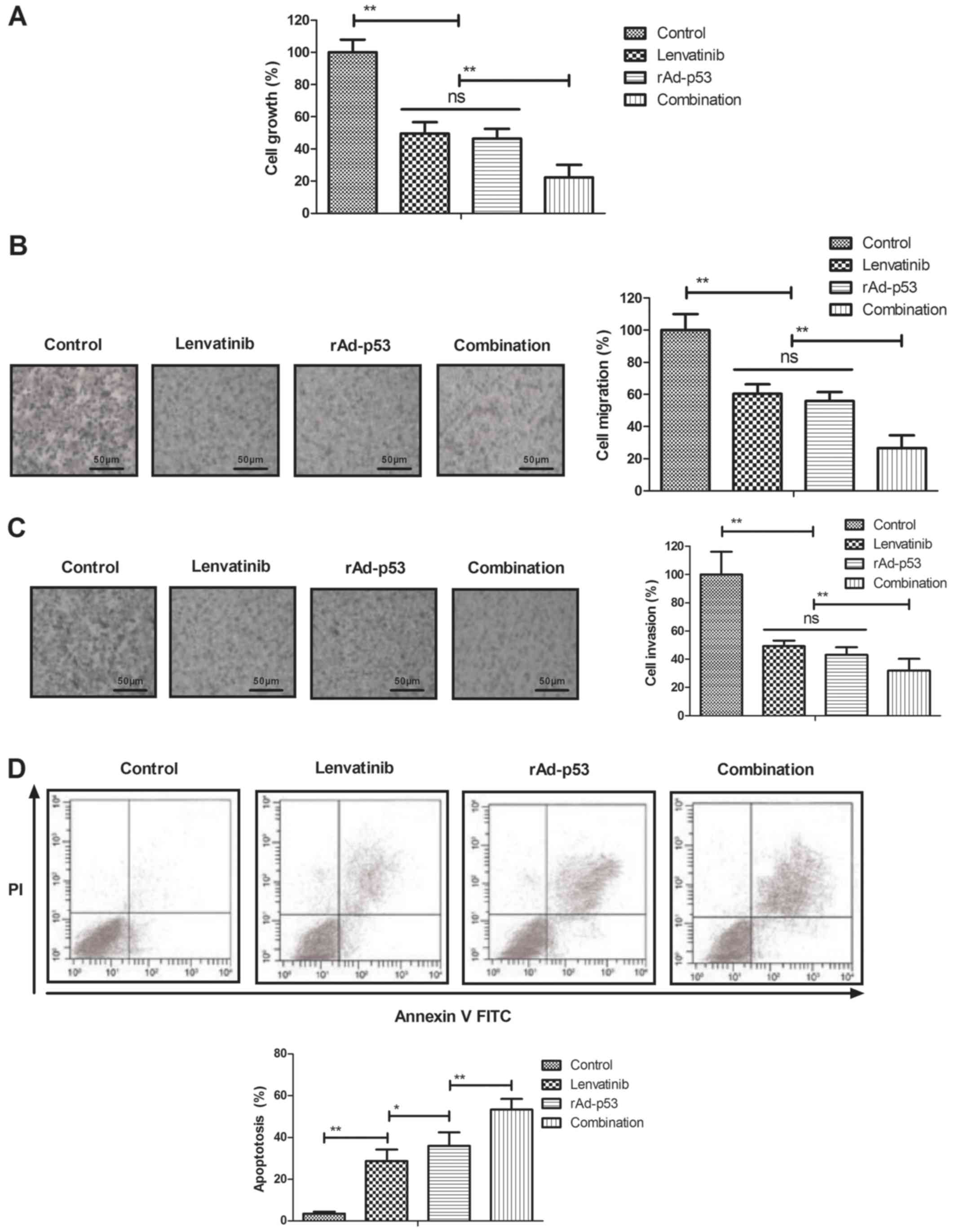
Inhibitory effects of combined
treatment of Lenvatinib and rAd-p53 for NSCLC cells
The inhibitory effects of combined treatment of
Lenvatinib and rAd-p53 on NSCLC cells were analyzed in
vitro. We showed that combined treatment of Lenvatinib and
rAd-p53 significantly inhibited NSCLC cells growth compared to
Lenvatinib and rAd-p53 (Fig. 2A). As
shown in Fig. 2B and C, combined
treatment of Lenvatinib and rAd-p53 inhibited migration and
invasion of NSCLC cells compared either Lenvatinib or rAd-p53. We
also demonstrated that combined treatment of Lenvatinib and rAd-p53
increased apoptosis of NSCLC cells induced by tunicamycin (Fig. 2D). These results indicate that
combined treatment of Lenvatinib and rAd-p53 can inhibit NSCLC
cells growth and aggressiveness.
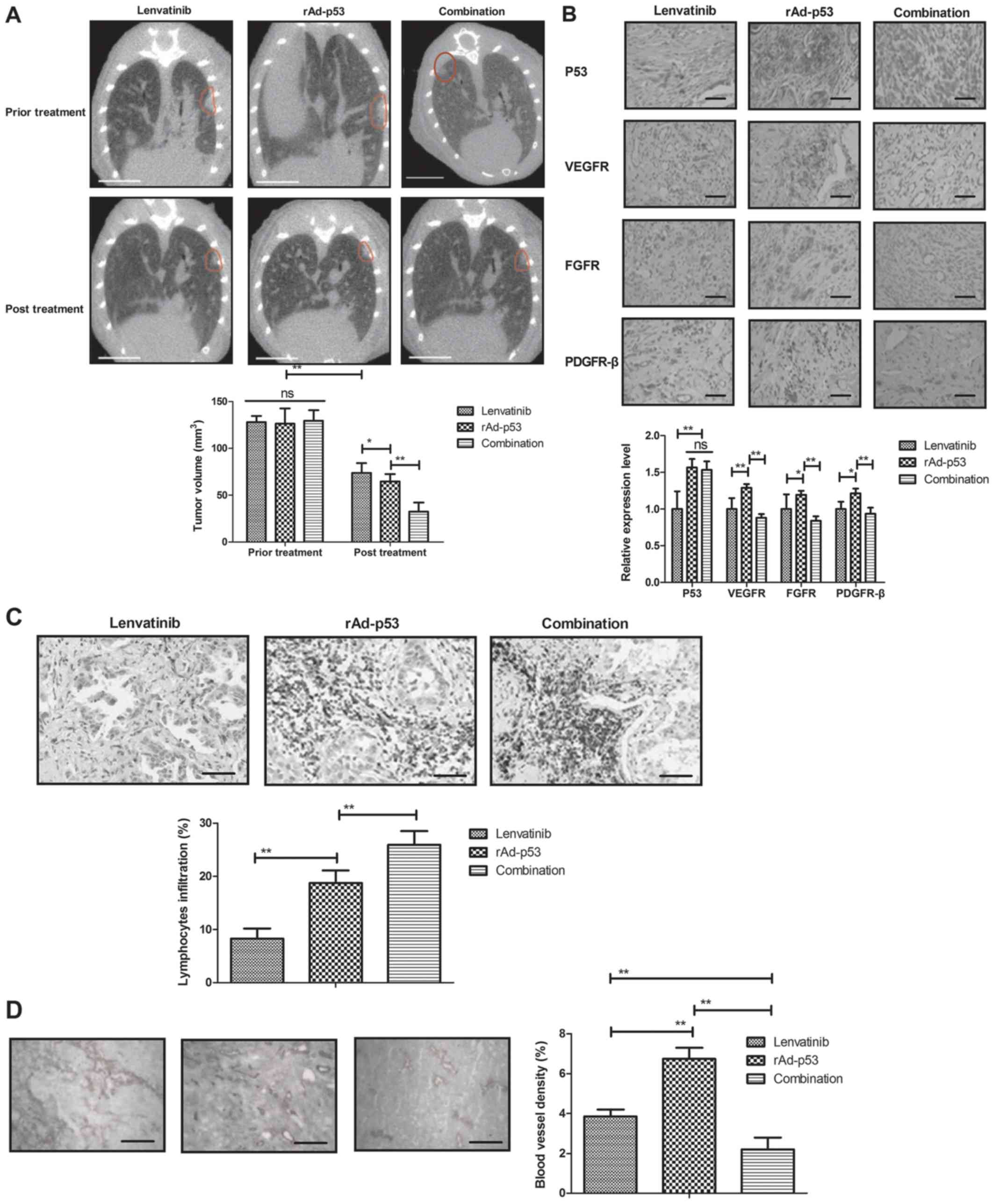
Anti-tumor activity of combined
treatment of Lenvatinib and rAd-p53 for NSCLC patients
The therapeutic effects of combined treatment of
Lenvatinib and rAd-p53 were performed in NSCLC patients. We showed
that combined treatment of Lenvatinib and rAd-p53 markedly
inhibited tumor growth compared to Lenvatinib and rAd-p53 for NSCLC
patients (Fig. 3A). Results found
that expression levels of p53 were increased and VEGFR, FGFR and
PDGFR-β were decreased in NSCLC tissues compared to adjacent normal
tissues after combined treatment of Lenvatinib and rAd-p53
(Fig. 3B). Surgical removal of the
NSCLC tumor tissues presented more lymphocytes infiltration in
rAd-p53 and combined treatment of Lenvatinib and rAd-p53 groups
than Lenvatinib group (Fig. 3C).
Blood vessel density was lower in combined treatment of Lenvatinib
and rAd-p53group than rAd-p53 and Lenvatinib groups (Fig. 3D). These results indicate that
combined treatment of Lenvatinib and rAd-p53 can efficiently
inhibit NSCLC growth.
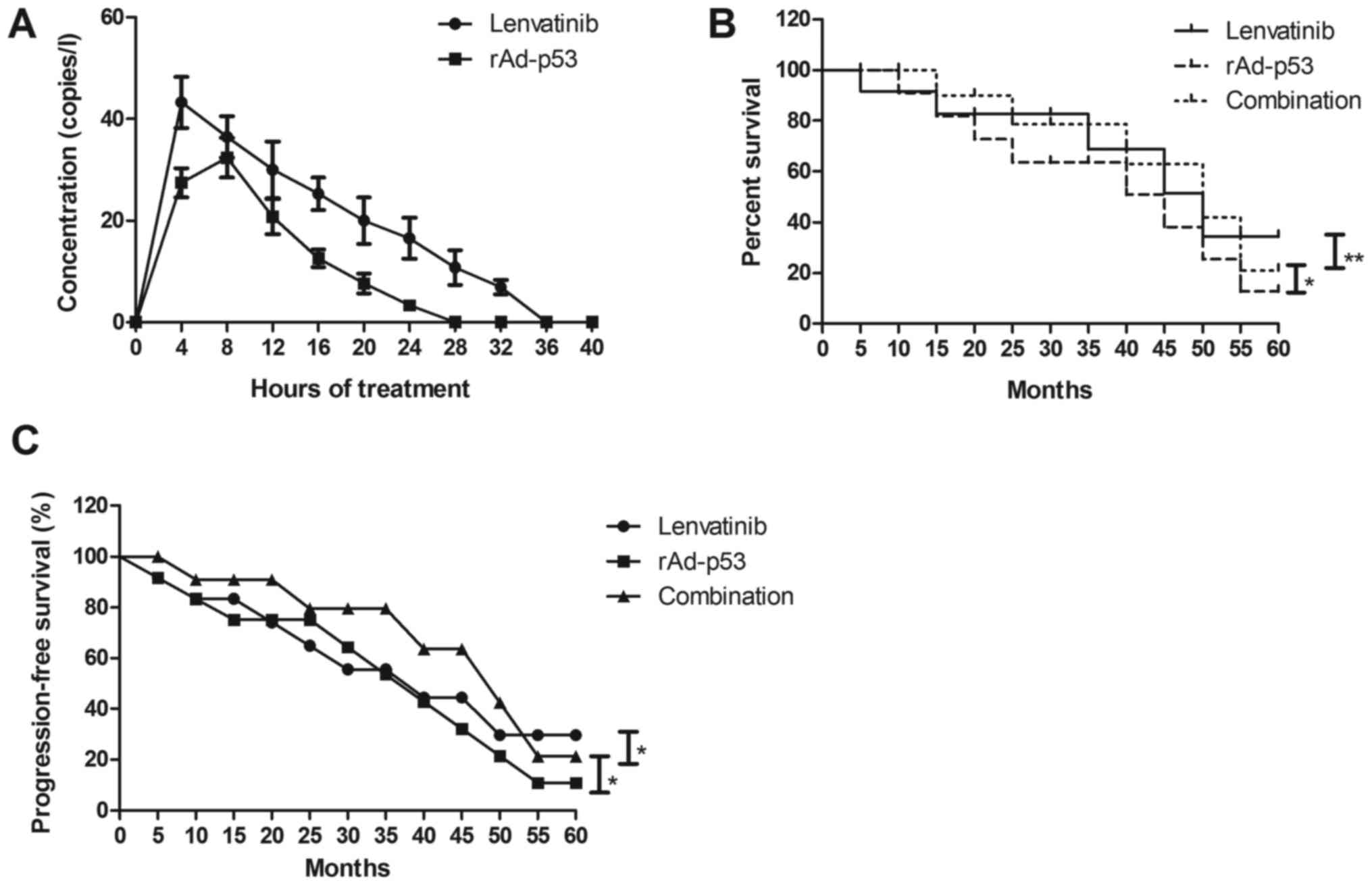
Side effects and pharmacokinetic (PK)
profile of Lenvatinib and rAd-p53 for NSCLC patients
The side effects and PK profile of Lenvatinib and
rAd-p53 were investigated in NSCLC patients. We demonstrated that
the most common treatment-related treatment-emergent adverse events
were hypertension, diarrhea, nausea, proteinuria and body weight
loss in NSCLC patients after treatment with Lenvatinib and/or
rAd-p53 (Table II). Combined
treatment of Lenvatinib and rAd-p53 had more side effects including
stomatitis and vomiting. We observed that Lenvatinib and rAd-p53
could metabolize in blood after 24 h and 36 h, respectively, after
received drugs and rAd-p53 (Fig. 4A).
Notably, outcomes found that combined treatment of Lenvatinib and
rAd-p53 improved survival rate of and progression-free survival
(PFS) compared to either Lenvatinib or rAd-p53 for NSCLC patients
(Fig. 4B and C). These results
indicate that combined treatment of Lenvatinib and rAd-p53 could
improve survival of NSCLC patients.
 | Table II.Treatment-related adverse event of
Lenvatinib and/or rAd-p53 for NSCLC patients. |
Table II.
Treatment-related adverse event of
Lenvatinib and/or rAd-p53 for NSCLC patients.
| Adverse event | Total (n=120)
(%) | Lenvatinib (n=40)
(%) | rAd-p53 (n=40)
(%) | Combination (n=40)
(%) | P-value |
|---|
| Hypertension | 16 (13) | 4 (10) | 5 (13) | 7 (18) | 0.035a, 0.045b |
| Diarrhea | 14 (12) | 3 (8) | 4 (10) | 7 (18) | 0.027a, 0.035b |
| Nausea | 8 (7) | 1 (1) | 2 (1) | 5 (13) | 0.002a, 0.021b |
| Proteinuria | 18 (15) | 5 (13) | 3 (8) | 10 (25) | 0.003a, 0.006b |
| Body weight
loss | 15 (13) | 5 (13) | 3 (8) | 7 (18) |
|
| Stomatitis | 3 (3) | 0 (0) | 0 (0) | 3 (8) |
<0.0001a, <0.0001b |
| Vomiting | 4 (4) | 0 (0) | 0 (0) | 4 (10) |
<0.0001a, <0.0001b |
Discussion
Tumor gene therapy and target therapy has been
widely used for human cancer treatment (27). Evidences have indicated that rAd-p53
is safe, well tolerated, and study has showed that rAd-p53 combined
with platinum-based chemotherapy can be associated with a
significant reduction of ovarian tumor (11). Study has found that Lenvatinib can
improve thyroid cancer patients' survival in clinical trials
(28). In the present study, we
investigated the anti-tumor efficacy of combined treatment of
Lenvatinib and rAd-p53 for NSCLC patients. We reported that
combined treatment of Lenvatinib and rAd-p53 significantly
inhibited NSCLC tumor growth and improved survival rate of NSCLC
patients.
Treatment of rAd-p53 has become a leading candidate
for clinical cancer treatment, such as renal cell carcinoma,
hepatic carcinoma and melanomas (11,29,30). Study
has found that expression of P53 is decreased and the potential
chemopreventive effect of pterostilbene dependents on p53-positive
cells during early carcinogenesis (31). We confirmed previous outcomes
(30) and we observed that rAd-p53
enhanced apoptosis of NSCLC cells. Promoting the infiltration and
function of CD8(+) T cells could enhance the antigrowth effects of
cisplatin on lung cancer, which provided new evidence for lung
cancer therapy (32). We showed that
rAd-p53 increased lymphocytic infiltration in NSCLC tissues
compared to Lenvatinib. The combination of rAd-p53 and adriamycin
increased the efficacy of chemotherapy for NSCLC patients, which
overcome resistance of lung squamous cell cancer for chemotherapy
(14). In the present study, we
reported that rAd-p53 treatment inhibited tumor growth and
decreased tumor volume for NSCLC patients.
Currently, target therapy for VEGF has presented
anti-cancer efficacy for human cancers (33–35). We
observed that VEGF is higher expressed in human NSCLC tissues and
cells than normal lung tissue and cells. Lenvatinib is a
multi-targeted tyrosine kinase inhibitor of multi
receptors-mediated angiogenesis, which has been regarded as a
potential drug for human cancer therapy (36). In this study, we observed that
Lenvatinib inhibited NSCLC cells growth and aggressiveness in
vitro and in NSCLC patients. Study has found that surgery
combined with adenoviral p53 gene therapy showed efficacious
effects in preventing recurrence or metastasis and improving
progression free survival and overall survival after a radical
surgery in patients with NSCLC in a phase II study (25). Our outcomes have indicated that
Lenvatinib presented more efficient in inhibiting NSCLC growth than
rAd-p53. In this study, we analyzed the therapeutic effects of
combined treatment of Lenvatinib and rAd-p53 for NSCLC patients.
Outcomes found that the most common treatment-related
treatment-emergent adverse events were hypertension, diarrhea,
nausea, proteinuria and body weight less in NSCLC patients after
treatment with Lenvatinib and/or rAd-p53, which could metabolize
after 48 h drug taken. Notably, combined treatment of Lenvatinib
and rAd-p53 improved survival rate of and progression-free survival
(PFS) compared to either Lenvatinib or rAd-p53 for NSCLC
patients.
In conclusion, the present study indicates that
combined treatment of Lenvatinib and rAd-p53 are well tolerated
when administered to patients with NSCLC. Encouraging anti-tumor
effects were observed for patients with NSCLC after combined
treatment of Lenvatinib and rAd-p53. However, further clinical
trials should be performed in large number NSCLC patients and in
other cancer patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RY performed the experiments. HY designed the
experiments. MW, XZ, ZS and AJ prepared the investigations and
analyzed data.
Ethics approval and consent to
participate
The protocols were approved by the Ethics Committee
of Mudanjiang medical University affiliated HongQi Hospital
(Mudanjiang, China). Informed consent was obtained from patients
before any study-related procedures were performed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Isobe H, Mori K, Minato K, Katsura H,
Taniguchi K, Arunachalam A, Kothari S, Cao X and Kato T: Real-world
practice patterns for patients with advancednon-small cell lung
cancer: Multicenter retrospective cohort study in Japan. Lung
Cancer (Auckl). 8:191–206. 2017.PubMed/NCBI
|
|
2
|
Prince RM, Atenafu EG and Krzyzanowska MK:
Hospitalizations during systemic therapy for metastatic lung
cancer: A systematic review of real world vs clinical trial
outcomes. JAMA Oncol. 1:1333–1339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brody H: Lung cancer. Nature. 513:S12014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moro-Sibilot D, Smit E, de Castro Carpeño
J, Lesniewski-Kmak K, Aerts JG, Villatoro R, Kraaij K, Nacerddine
K, Dyachkova Y, Smith KT, et al: Non-small cell lung cancer
patients with brain metastases treated with first-line
platinum-doublet chemotherapy: Analysis from the European FRAME
study. Lung Cancer. 90:427–432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnett SA, Downey RJ, Zheng J, Plourde G,
Shen R, Chaft J, Akhurst T, Park BJ and Rusch VW: Utility of
routine PET imaging to predict response and survival after
induction therapy for non-small cell lung cancer. Ann Thorac Surg.
101:1052–1059. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cadranel J, Park K, Arrieta O, Pless M,
Bendaly E, Patel D, Sasane M, Nosal A, Swallow E, Galebach P, et
al: Characteristics, treatment patterns and survival among ALK+
non-small cell lung cancer (NSCLC) patients treated with
crizotinib: A chart review study. Lung Cancer. 98:9–14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saadeddin A: Radiotherapy for NSCLC:
Review of conventional and new treatment techniques. J Infect
Public Health. 5 Suppl 1:S45–S49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Meerbeeck JP and Surmont VF: Stage
IIIA-N2 NSCLC: A review of its treatment approaches and future
developments. Lung Cancer. 65:257–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bianco A, Tridico F, Rebecchi F, Contessa
L, Fusca M, Calello G, Monticone C, De Rocca C, Suffat Panier L,
Giaccone C and Suffat Panier P: Adrenal synchronous metastasis from
non small cell lung carcinoma (NSCLC): Combined surgical treatment?
Case report and review of the literature. Minerva chir. 56:535–537.
2001.(In Italian). PubMed/NCBI
|
|
10
|
Li Y, Li LJ, Wang LJ, Zhang Z, Gao N,
Liang CY, Huang YD and Han B: Selective intra-arterial infusion of
rAd-p53 with chemotherapy for advanced oral cancer: a randomized
clinical trial. BMC Med. 12:162014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buller RE, Runnebaum IB, Karlan BY,
Horowitz JA, Shahin M, Buekers T, Petrauskas S, Kreienberg R,
Slamon D and Pegram M: A phase I/II trial of rAd/p53 (SCH 58500)
gene replacement in recurrent ovarian cancer. Cancer Gene Ther.
9:553–566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu K, Zhao J, Jiang H, Ma J, Tan J, Pei Y
and Chen J: A patient with a large intrathoracic malignant
schwannoma who showed a complete clinical response to
rAd-p53-combined with radiotherapy. Anticancer Drugs. 26:902–906.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen GX, Zheng LH, Liu SY and He XH:
rAd-p53 enhances the sensitivity of human gastric cancer cells to
chemotherapy. World J Gastroenterol. 17:4289–4297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du CH, Wu Z and Xu J: The combination of
recombinant rAd-p53 and adriamycin for management of primary drug
resistance in chemotherapy of lung squamous cell cancer. Zhonghua
Jie He He Hu Xi Za Zhi. 29:622–624. 2006.(In Chinese). PubMed/NCBI
|
|
15
|
Guan YS, Liu Y, Zou Q, He Q, La Z, Yang L
and Hu Y: Adenovirus-mediated wild-type p53 gene transfer in
combination with bronchial arterial infusion for treatment of
advanced non-small-cell lung cancer, one year follow-up. J Zhejiang
Univ Sci B. 10:331–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takasu S, Mutoh M, Takahashi M and
Nakagama H: Lipoprotein lipase as a candidate target for cancer
prevention/therapy. Biochem Res Int. 2012:3986972012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miyazono K: Ectodomain shedding of HB-EGF:
A potential target for cancer therapy. J Biochem. 151:1–3. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sebens S and Schafer H: The tumor stroma
as mediator of drug resistance-a potential target to improve cancer
therapy? Curr Pharm Biotechnol. 13:2259–2272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O'Reilly A and Larkin J: Lenvatinib for
use in combination with everolimus for the treatment of patients
with advanced renal cell carcinoma following one prior
anti-angiogenic therapy. Expert Rev Clin Pharmacol. 10:251–262.
2017.PubMed/NCBI
|
|
20
|
Kuznar W: Lenvatinib extends survival in
metastatic renal-cell carcinoma. Am Health Drug Benefits.
8:182015.PubMed/NCBI
|
|
21
|
Nishio M, Horai T, Horiike A, Nokihara H,
Yamamoto N, Takahashi T, Murakami H, Yamamoto N, Koizumi F, Nishio
K, et al: Phase 1 study of lenvatinib combined with carboplatin and
paclitaxel in patients with non-small-cell lung cancer. Br J
Cancer. 109:538–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagashima S, Matsuo S, Takahashi M,
Umemoto Y, Hirano T, Enomoto K, Sakurai K and Amano S:
Effectiveness of lenvatinib for thyroid cancer with lung Metastases
-report of a case. Gan To Kagaku Ryoho. 43:2121–2123. 2016.(In
Japanese). PubMed/NCBI
|
|
23
|
Halim NHA, Zakaria N, Satar NA and Yahaya
BH: Isolation and characterization of cancer stem cells of the
non-small-cell lung cancer (A549) cell line. Methods Mol Biol.
1516:371–388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deng B, Sun T, Tang B, Tao S, Kang P, Qian
K, Jiang B, Li K, Li K, Zhou J, et al: Surgery combined with
adenoviral p53 gene therapy for treatment of non-small cell lung
cancer: A phase II study. Oncotarget. 8:107089–107095. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghantous Y, Naddaf R, Barak M, Abd-Elraziq
M and AbuEln-Naaj I: The role of fine needle aspiration in the
diagnosis of parotid gland tumors: Correlation with preoperative
computerized tomography tumor size. J Craniofac Surg. 27:e192–e196.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Solbach C, Roller M, Peters S, Nicoletti
M, Kaufmann M and Knecht R: Pituitary tumor-transforming gene
(PTTG): A novel target for anti-tumor therapy. Anticancer Res.
25:121–125. 2005.PubMed/NCBI
|
|
28
|
Mayor S: Lenvatinib improves survival in
refractory thyroid cancer. Lancet Oncol. 16:e1102015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie Q, Liang BL, Wu YH, Zhang J, Chen MW,
Liu HY, Gu XF and Xu J: Synergistic anticancer effect of rAd/P53
combined with 5-fluorouracil or iodized oil in the early
therapeutic response of human colon cancer in vivo. Gene.
499:303–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo SH, Zheng CS, Feng GS, Sun XM, Zhou
GF, Liang HM, Xia XW and Fang JL: Experimental studies of rAd-p53
injection by interventional approach for the treatment of rabbit
VX2 liver cancer. Zhonghua Gan Zang Bing Za Zhi. 18:502–505.
2010.(In Chinese). PubMed/NCBI
|
|
31
|
Lee H, Kim Y, Jeong JH, Ryu JH and Kim WY:
ATM/CHK/p53 pathway dependent chemopreventive and therapeutic
activity on lung cancer by pterostilbene. PLoS One.
11:e01623352016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li W, Yang Y, Ouyang Z, Zhang Q, Wang L,
Tao F, Shu Y, Gu Y, Xu Q and Sun Y: Xiao-Ai-Ping, a TCM injection,
enhances the antigrowth effects of cisplatin on lewis lung cancer
cells through promoting the infiltration and function of CD8(+) T
lymphocytes. Evid Based Complement Alternat Med. 2013:8795122013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jia J, Dellinger AE, Weiss ES, Bulusu A,
Rushing C, Li H, Howard LA, Kaplan N, Pang H, Hurwitz HI and Nixon
AB: Direct Evidence of Target Inhibition with Anti-VEGF, EGFR and
mTOR Therapies in a Clinical Model of Wound Healing. Clin Cancer
Res. 21:3442–3452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shibuya M: Vascular endothelial growth
factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A
crucial target for anti- and pro-angiogenic therapies. Genes
Cancer. 2:1097–1105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Canavese M, Altruda F, Ruzicka T and
Schauber J: Vascular endothelial growth factor (VEGF) in the
pathogenesis of psoriasis-a possible target for novel therapies? J
Dermatol Sci. 58:171–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|