Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is a
highly invasive blood cancer and is a serious threat to human
health. As a common T lymph cell malignancy, T-ALL occurs in adults
and children and is characterized by rapid progression and high
mortality (1). Despite the
development of multi-agent chemotherapy, radiotherapy and
hematopoietic stem cell transplantation, ~15% of pediatric and 40%
of adult T-ALL patients relapse due to primary resistance to
treatment (2–4). The 5-year event-free survival rate
increases with age, from 44% for those <12 months to 88% for
those aged 1–9 years (5–7). Babies younger than 6 months have a poor
outcome (8,9); therefore, the search for novel specific
treatment targets to enable a more effective targeted therapy of
T-ALL is urgently required.
Cell migration is a crucial process for the
deterioration of T-ALL. Stromal cell-derived factor-1 (SDF-1) is a
small chemokine that regulates the mobilization, retention,
migration, trafficking and homing of hematopoietic stem cells and
lymphocytes (10,11). An increasing amount of evidence has
indicated that chemotaxis signaling serves a crucial role in the
migration of cancer cells (12–15).
Chemokines serve roles as signal initiators and are involved in
changes of the actin cytoskeleton (16), which are required for cellular
morphological changes and motility (17). SDF-1 can induce changes to cell
behavior, including changes to cellular morphology and the
regulation of cellular motility (18)
by modulating cytoskeletal redistribution and assembly (19).
Nitric oxide (NO) is a multifunctional signaling
molecule that mediates different signaling pathways and regulates
various cellular functions, including vasodilatation,
neurotransmission, macrophage-mediated immunity, chemotaxis and
cell migration (20–23). NO has been demonstrated to regulate
migration in several types of cells, including human neutrophils,
endothelial cells, vascular smooth muscle cells, and T cells
(24–28). However, the mechanism of NO in T-ALL
migration remains poorly understood.
The present study used Jurkat cells, a typical T-ALL
cell line to more carefully investigate the role of NO in T-ALL
migration. An aim of the present study was to provide further
insight into the role of NO in the regulation of chemokine-induced
migration in T-ALL.
Materials and methods
Chemicals and reagents
RPMI-1640 (Gibco, Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 100 U/ml penicillin and 100
µ/ml streptomycin (Beijing Solarbio Science and Technology Co.,
Ltd., Beijing, China), fetal bovine serum (Hangzhou Sijiqing
Biological Engineering Materials Co., Ltd., Hangzhou, China), SDF-1
(R&D Systems, Inc., Minneapolis, MN, USA), and nitric oxide
synthase inhibitor NG-monomethyl-l-arginine monoacetate
salt (L-NMMA, Beyotime Institute of Biotechnology, Haimen, China)
were obtained and used without further modification. Total Nitric
Oxide assay kit was from the Beyotime Institute of Biotechnology
(cat. no. S0021). Anti-NOS2 (Phospho-Tyr151) rabbit polyclonal
antibody (NOS2-pY151; cat. no. D151565) and anti-NOS3
(Phospho-Thr494) rabbit polyclonal antibody (NOS3-pY494; cat. no.
D151279) were obtained from Sangon Biotech Co., Ltd. (Shanghai,
China).
Cell culture
Jurkat cells from Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China) were maintained in
RPMI-1640 medium supplemented with 10% fetal bovine serum, 25 ng/ml
SDF-1 and 1 mM L-NMMA in a humidified incubator with 5%
CO2 at 37°C. A total of 1 mM L-NMMA was dissolved in
double distilled water and cells were pre-treated with L-NMMA for 2
h. Jurkat cells were starved with 0.5% serum overnight in all
experiments.
Total nitric oxide assay
Total nitric oxide production in the culture medium
was determined by measuring the concentration of nitrate and
nitrite, a stable metabolite of NO, by the modified Griess reaction
method (29,30). The procedure was performed according
to the manufacturer's protocol of the Total Nitric Oxide assay kit
(Beyotime Institute of Biotechnology). RPMI-1640 medium was used as
the control for SDF-1.
Western blot assay
Cells were stimulated at 37°C for 0, 1, 2, 3 or 4 h
and lysed in ice-cold lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl,
1 mM EDTA, 1 mM EGTA, 1% Nonidet P-40, 2.5 mM sodium pyrophosphate,
1 mM each NaF, Na3VO4, and β-glycerolphosphate, 10 µg/ml aprotinin
and leupeptin). After 30 min on ice, lysates were centrifuged at
13,000 × g for 30 min. Protein concentration was determined by the
BCA kit (cat. no. P0010) from Beyotime Institute of Biotechnology.
A total of 20 µg lysate from each sample were subjected to 10%
SDS-PAGE. Proteins were transferred to the nitrocellulose membranes
using chilled transfer buffer (25 mM Tris, 192 mM glycerin, and 20%
methanol) at 100 V for 1 h. Following protein transfer,
nitrocellulose membranes were washed with TBST (20 mM Tris, 500 mM
NaCl, 0.05% Tween-20, pH 7.5) for ≥3 times, and were immediately
incubated with 3% bovine serum albumin (BSA, cat. no. SW3015) from
Beijing Solarbio Science and Technology Co., Ltd., Beijing, China.
After the incubation for 1 h at room temperature, the membrane was
incubated with primary antibodies NOS2-pY151 and NOS3-pY494
respectively diluted at a ratio of 1:1,000 in TBST at 4°C
overnight, washed with TBST 3 times at room temperature, and
incubated with HRP-conjugated goat anti-rabbit secondary antibody
(cat. no. D110058; Sangon Biotech Co., Ltd., Shanghai, China)
diluted at a ratio of 1:1,000 in TBST at 37°C for 1 h. For the
detection of the reference gene, mouse monoclonal antibody against
actin (cat. no. D190606; Sangon Biotech Co., Ltd., Shanghai, China)
and HRP-conjugated goat anti-mouse IgG (cat. no. D110087; Sangon
Biotech Co., Ltd., Shanghai, China) were used. Chemiluminescent
detection was performed by using ECL plus Western blot reagents
(BeyoECL Plus; cat. no. P0018; Beyotime Institute of
Biotechnology). Western blot assay was repeated three times,
analyzed by image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville,
MD, USA), and expressed as fold increase relative to the basal
level in unstimulated cells.
Cell viability assay
The MTT assay was employed to quantify cell
viability. Jurkat cells were washed once with RPMI-1640, and
~3×105 cells were seeded to each sample. The cells were
then cultured at 37°C for 6 h with different concentrations of
L-NMMA (0, 0.25, 0.5, 1, 2 and 4 mM). At the end of the treatment,
MTT reagent (5 mg/ml, 20 µl) was added to each sample and cells
were incubated at 37°C for 3 h, DMSO (200 µl) was then added to
each sample, in order to dissolve the cell precipitation. A total
of 150 µl liquid was transferred to the well of a96-well plate and
the optical density value of each well was determined using a plate
reader at 570 nm.
Immunofluorescence assay
To detect the distribution of F-actin, Jurkat cells
were pre-treated with L-NMMA (1 mM) at 37°C for 2 h, and then
stimulated with SDF-1 at 37°C for 4 h. At 4 h time, chilled 500 µl
PBS was added to cells to rapidly stop the reaction. Jurkat cells
were then fixed with 4% paraformaldehyde for 20 min at room
temperature. After washing twice with PBS, the Jurkat cells were
permeabilized with 0.2% Triton X-100 in PBS (containing 5mM EDTA
and 2% FBS) for 10 min. Following washing twice with PBS, the cells
were stained with 2×10−7 M Fluorescein isothiocyanate
(FITC)-conjugated phalloidin at room temperature for 20 min, washed
three times with PBS, and then observed under a confocal microscope
at ×600 magnification.
Flow cytometry
The amount of F-actin in cells was examined by flow
cytometry. Following stimulation with SDF-1, Jurkat cells were
fixed with 4% paraformaldehyde at room temperature for 20 min,
permeabilized with 0.2% Triton X-100 in PBS (containing 5 mM EDTA
and 2% FBS) for 10 min, blocked in 1% BSA (cat. no. SW3015) from
Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China)
in 0.05% Tween 20 and PBS for 30 min at room temperature, stained
with 2×10−7 M FITC-conjugated phalloidin (cat. no.
P5282-.1MG; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room
temperature for 20 min, and then washed with PBS. Cells were
examined by flow cytometry (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA), analyzed by FlowJo 7.6 software (TreeStar, Inc.,
Ashland, OR, USA), and the results were expressed as the relative
fluorescence index (RFI) by dividing the fluorescence value of the
experimental groups by that of the control group. RPMI-1640 medium
was used as the control for SDF-1.
Transwell migration assay
The migration assay was performed using 3.0 µm
Transwell inserts in 24-well culture plates. The Jurkat cells were
resuspended in serum-free RPMI-1640 at a density of
3×106 cell/ml and 100 µl cell suspensions were
pre-treated with L-NMMA (1 mM) at 37°C for 2 h and seeded onto the
upper chambers. Next, 500 µl of RPMI-1640 supplemented with SDF-1
was added to the lower chamber. After 4 h incubation at 37°C, the
migrated cells were imaged at ×100 magnification under a light
microscope, and the relative migration rates were analyzed.
RPMI-1640 medium was used as the control for SDF-1.
Statistical analysis
All experiments were performed in triplicate. The
data were expressed as the mean ± standard deviation and were
analyzed by one-way analysis of variance with Bonferroni's post-hoc
test using the statistical software package SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
NO was produced by SDF-1
stimulation
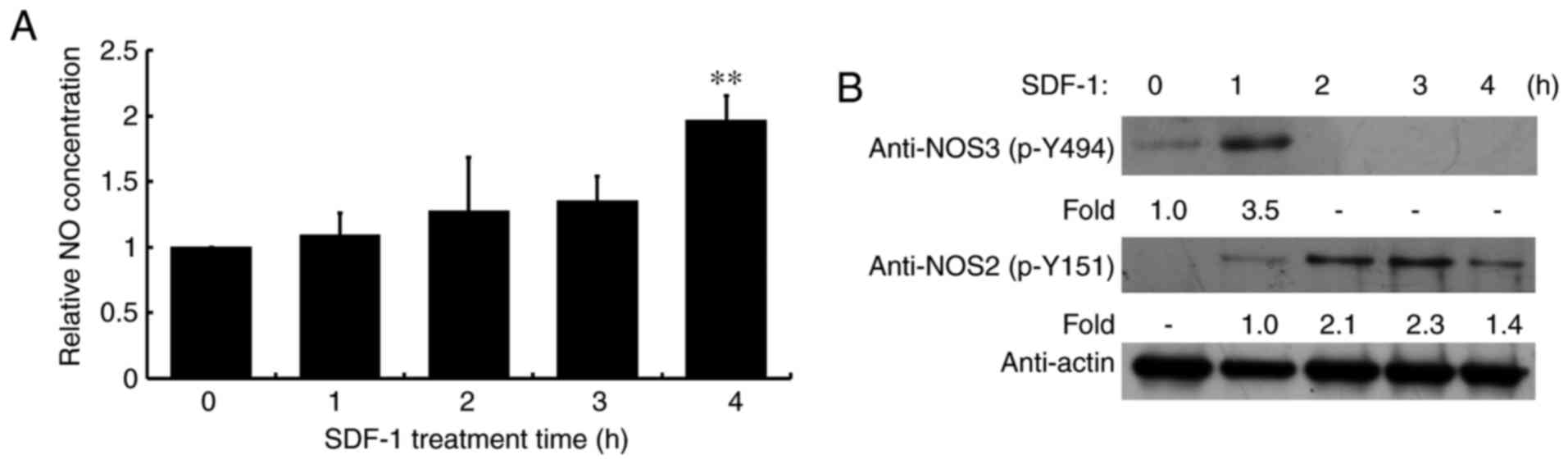
NO serves a critical role in signal transduction.
Therefore, cellular NO levels were analyzed following SDF-1
treatment for 1, 2, 3 or 4 h. NO levels were significantly
increased following SDF-1 stimulation for 4 h compared with the
control group (Fig. 1A).
Additionally, NO synthases 2 and 3 were phosphorylated following
SDF-1 treatment (Fig. 1B),
corresponding to activities, which catalyze the generation of
NO.
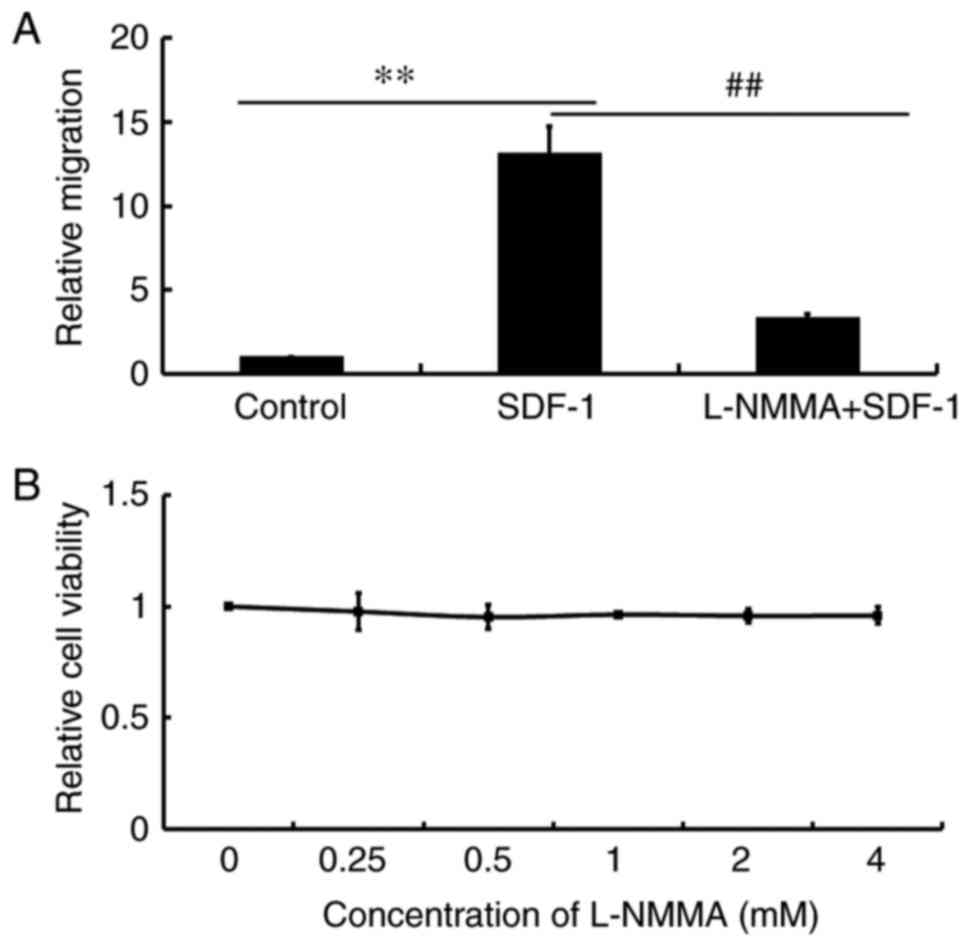
NO was required for cell
migration
To investigate the role of NO in SDF-1-induced cell
migration, the NOS inhibitor, L-NMMA was used. The results
demonstrated that pretreatment of Jurkat cells with L-NMMA led to
the inhibition to cell migration towards SDF-1 (Fig. 2A), which suggested that NO was
required for cell migration. Jurkat cells were then treated with
different concentrations of L-NMMA and found that the different
concentrations of L-NMMA had no significant effect on cell
viability (Fig. 2B), suggesting that
the inhibitory effect of L-NMMA on cell migration was not due to a
reduction in cell viability.
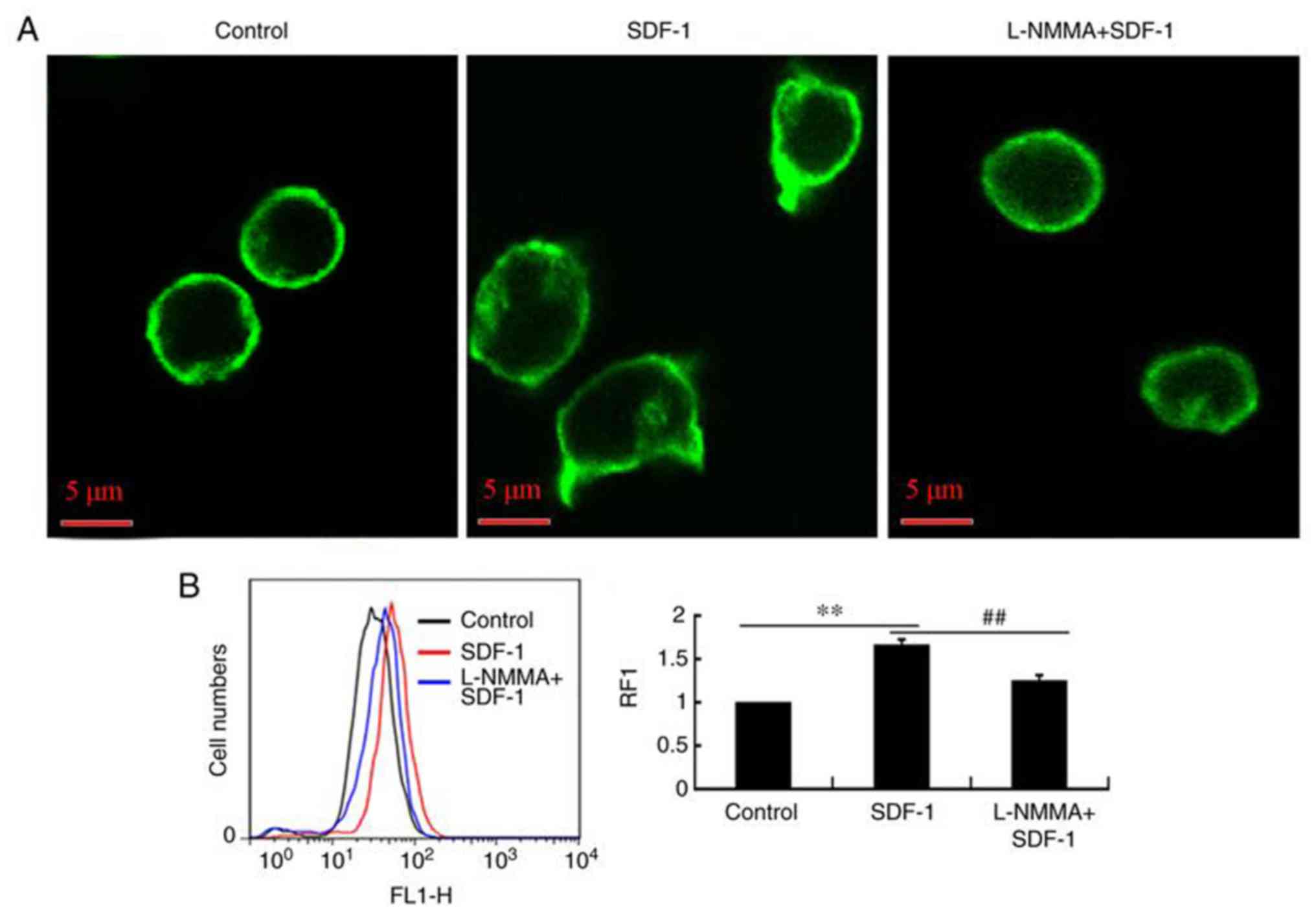
NO is involved in SDF-1-induced
cytoskeletal changes
The mechanism by which NO affects SDF-1-induced
Jurkat cell migration was further tested by observing and measuring
the distribution of the F-actin cytoskeleton, in the presence or
absence of L-NMMA when stimulated by SDF-1. The results showed that
L-NMMA significantly inhibited the F-actin rearrangement induced by
SDF-1 (Fig. 3A) and the F-actin
polymerization-induced by SDF-1 (Fig.
3B). NO was required for F-actin rearrangement and
polymerization in response to SDF-1. This suggests that
SDF-1-induced migration was regulated by NO production and the
subsequent F-actin rearrangement and polymerization.
Discussion
Chemokines mediate cell migration in a key step in
the inflammatory response and the metastasis of cancer (31). SDF-1 is a chemokine that exerts its
effects in a variety of types of cells (31,32). SDF-1
serves a crucial role in lymphocyte trafficking and homing, and is
particularly important in the migration of lymphocytes (33). In Jurkat cells, MAPK/Erk, Akt, in
addition to NO are involved in the process of SDF-1 induced
migration (19). However, the
mechanism by which NO functions in SDF-1 signaling remains unclear.
Therefore, an aim of the present study was to identify the specific
mechanism of NO that affects SDF-1-induced migration. The present
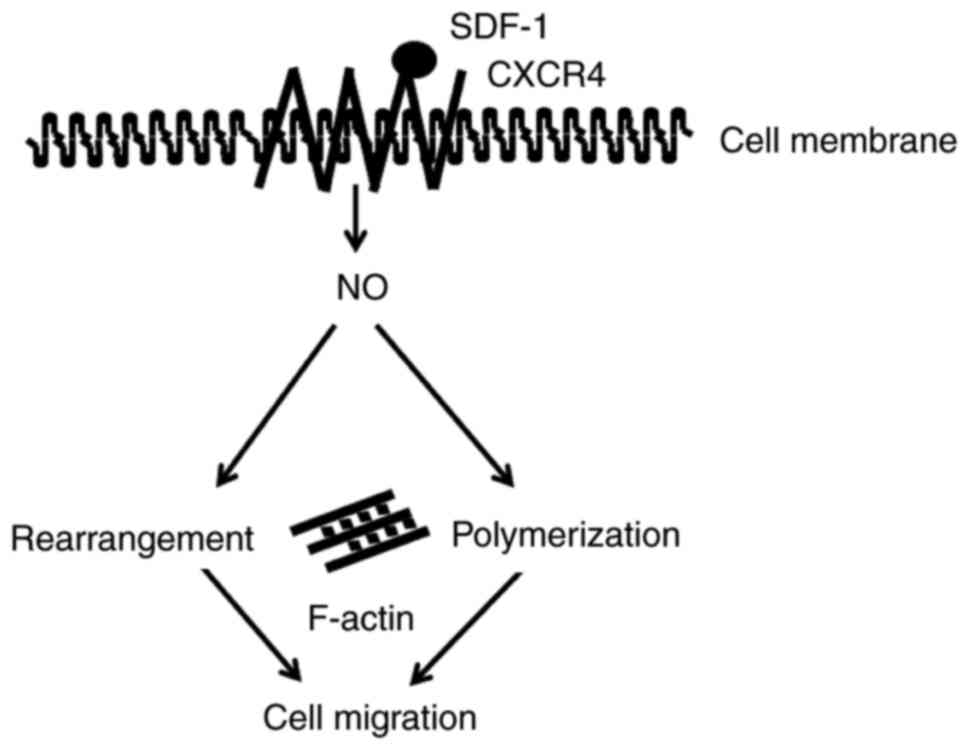
study demonstrated a crucial role of NO in SDF-1-induced
cytoskeleton changes and cell migration. SDF-1 induced the
generation of NO in Jurkat cells. NO signaling is involved in
migration by promoting the rearrangement and polymerization of the
cytoskeleton (Fig. 4). These
important results indicate effective therapeutic targets to prevent
unwanted cell migration.
NO is a potent signaling molecule that contributes
to the migration of cells in human cancers (34). The results of the present study
demonstrated that SDF-1 induced NO production, this is due to
chemokine-induced changes of the cytoskeleton and cell migration by
triggering multiple signaling pathways (35). The change of the actin cytoskeleton is
critical for migrating cells (36).
Here, pre-treatment of Jurkat cells with an NOS inhibitor was
demonstrated to markedly inhibit SDF-1 induced cytoskeletal
rearrangement and polymerization (Fig. 3A
and B). Additionally, NO was involved in SDF-1-induced cell
migration (Fig. 2A). These results
suggest that SDF-1-induced chemotaxis can produce NO and
NO-mediated cytoskeleton changes that are involved in subsequent
cell migration. Similarly, it has been reported that NO modulates
cell migration by the regulation of cytoskeletal proteins (37,38).
Based on the results of the present study, a
mechanism for chemokine-mediated signaling in T-ALL was described,
in which NO signaling regulated SDF-1-induced changes of actin
cytoskeleton and cell migration. Importantly, an inhibitory role of
L-NMMA in the migration of T-ALL was demonstrated, and these
results will contribute to the design of more effective treatment
strategies for T-ALL.
Acknowledgments
The authors would like to thank Professor Shao Chin
Lee, Dr Yingjuan Yang and Dr Pu Wang, (Department of Biology,
School of Life Science, Shanxi University, Taiyuan, Shanxi, China)
for their technical support.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 31401216) and Research
Project Supported by Shanxi Scholarship Council of China (grant no.
2017-012).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Author's contributions
JXL designed some of the experiments and wrote this
manuscript. DW and DYL conducted the experiments. LW designed some
of the experiments and was involved in revising the manuscript.
Ethics approval and consent to
participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no conflict of
interest
References
|
1
|
Pui CH and Evans WE: Treatment of acute
lymphoblastic leukemia. N Engl J Med. 354:166–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Durinck K, Goossens S, Peirs S, Wallaert
A, Van Loocke W, Matthijssens F, Pieters T, Milani G, Lammens T,
Rondou P, et al: Novel biological insights in T-cell acute
lymphoblastic leukemia. Exp Hematol. 43:625–639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Patrick K and Vora A: Update on biology
and treatment of T-cell acute lymphoblastic leukaemia. Curr Opin
Pediatr. 27:44–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vitale A, Guarini A, Chiaretti S and Foà
R: The changing scene of adult acute lymphoblastic leukemia. Curr
Opin Oncol. 18:652–659. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans WE and Relling MV: Moving towards
individualized medicine with pharmacogenomics. Nature. 429:464–468.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kinlen L: Infections and immune factors in
cancer: The role of epidemiology. Oncogene. 23:6341–6348. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hilden JM, Dinndorf PA, Meerbaum SO,
Sather H, Villaluna D, Heerema NA, McGlennen R, Smith FO, Woods WG,
Salzer WL, et al: Analysis of prognostic factors of acute
lymphoblastic leukemia in infants: Report on CCG 1953 from the
Children's Oncology Group. Blood. 108:441–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pieters R, Schrappe M, De Lorenzo P, Hann
I, De Rossi G, Felice M, Hovi L, LeBlanc T, Szczepanski T, Ferster
A, et al: A treatment protocol for infants younger than 1 year with
acute lymphoblastic leukaemia (Interfant-99): An observational
study and a multicentre randomised trial. Lancet. 370:240–250.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobayashi K, Sato K, Kida T, Omori K, Hori
M, Ozaki H and Murata T: Stromal cell-derived factor-1alpha/C-X-C
chemokine receptor type 4 axis promotes endothelial cell barrier
integrity via phosphoinositide 3-kinase and Rac1 activation.
Arterioscler Thromb Vasc Biol. 34:1716–1722. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasuoka H, Tsujimoto M, Yoshidome K,
Nakahara M, Kodama R, Sanke T and Nakamura Y: Cytoplasmic CXCR4
expression in breast cancer: Induction by nitric oxide and
correlation with lymph node metastasis and poor prognosis. BMC
Cancer. 8:3402008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kirui JK, Xie Y, Wolff DW, Jiang H, Abel
PW and Tu Y: Gbetagamma signaling promotes breast cancer cell
migration and invasion. J Pharmacol Exp Ther. 333:393–403. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raman D, Sobolik-Delmaire T and Richmond
A: Chemokines in health and disease. Exp Cell Res. 317:575–589.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drury LJ, Ziarek JJ, Gravel S, Veldkamp
CT, Takekoshi T, Hwang ST, Heveker N, Volkman BF and Dwinell MB:
Monomeric and dimeric CXCL12 inhibit metastasis through distinct
CXCR4 interactions and signaling pathways. Proc Natl Acad Sci USA.
108:17655–17660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Konoplev S, Lin P, Yin CC, Lin E, González
Nogueras GM, Kantarjian HM, Andreeff M, Medeiros LJ and Konopleva
M: CXC chemokine receptor 4 expression, CXC chemokine receptor 4
activation, and wild-type nucleophosmin are independently
associated with unfavorable prognosis in patients with acute
myeloid leukemia. Clin Lymphoma Myeloma Leuk. 13:686–692. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mills SC, Goh PH, Kudatsih J, Ncube S,
Gurung R, Maxwell W and Mueller A: Cell migration towards CXCL12 in
leukemic cells compared to breast cancer cells. Cell Signal.
28:316–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okabe S, Fukuda S and Broxmeyer HE:
Activation of Wiskott-Aldrich syndrome protein and its association
with other proteins by stromal cell-o9 factor-1alpha isassociated
with cell migration in a T-lymphocyte line. Exp Hematol.
30:761–766. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murata K, Kitaori T, Oishi S, Watanabe N,
Yoshitomi H, Tanida S, Ishikawa M, Kasahara T, Shibuya H, Fujii N,
et al: Stromal cell-derived factor 1 regulates the actin
organization of chondrocytes and chondrocyte hypertrophy. PLoS One.
7:e371632012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vicente-Manzanares M, Cruz-Adalia A,
Martín-Cófreces NB, Cabrero JR, Dosil M, Alvarado-Sánchez B,
Bustelo XR and Sánchez-Madrid F: Control of lymphocyte shape and
the chemotactic response by the GTP exchange factor Vav. Blood.
105:3026–3034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sessa WC: eNOS at a glance. J Cell Sci.
117:2427–2429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu W, Liu LZ, Loizidou M, Ahmed M and
Charles IG: The role of nitric oxide in cancer. Cell Res.
12:311–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Williams MS, Noguchi S, Henkart PA and
Osawa Y: Nitric oxide synthase plays a signaling role in
TCR-triggered apoptotic death. J Immunol. 161:6526–6531.
1998.PubMed/NCBI
|
|
23
|
Jadeski LC, Chakraborty C and Lala PK:
Nitric oxide-mediated promotion of mammary tumour cell migration
requires sequential activation of nitric oxide synthase, guanylate
cyclase and mitogen-activated protein kinase. Int J Cancer.
106:496–504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beauvais F, Michel L and Dubertret L:
Exogenous nitric oxide elicits chemotaxis of neutrophils in vitro.
J Cell Physiol. 165:610–614. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murohara T, Witzenbichler B, Spyridopoulos
I, Asahara T, Ding B, Sullivan A, Losordo DW and Isner JM: Role of
endothelial nitric oxide synthase in endothelial cell migration.
Arterioscler Thromb Vasc Biol. 19:1156–1161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sarkar R, Meinberg EG, Stanley JC, Gordon
D and Webb RC: Nitric oxide reversibly inhibits the migration of
cultured vascular smooth muscle cells. Circ Res. 78:225–230. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noiri E, Hu Y, Bahou WF, Keese CR, Giaever
I and Goligorsky MS: Permissive role of nitric oxide in
endothelin-induced migration of endothelial cells. J Biol Chem.
272:1747–1752. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cherla RP and Ganju RK: Stromal
cell-derived factor 1 alpha-induced chemotaxis in T cells is
mediated by nitric oxide signaling pathways. J Immunol.
166:3067–3074. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo D, Li JR, Wang Y, Lei LS, Yu CL and
Chen NN: Cyclovirobuxinum D suppresses lipopolysaccharide-induced
inflammatory responses in murine macrophages in vitro by blocking
JAK-STAT signaling pathway. Acta Pharmacol Sin. 35:770–778. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu LY, Lin B, Zhang ZL and Guo LH: Direct
transfer of A20 gene into pancreas protected mice from
streptozotocin-induced diabetes. Acta Pharmacol Sin. 25:721–726.
2004.PubMed/NCBI
|
|
31
|
Bachelerie F, Ben-Baruch A, Burkhardt AM,
Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH,
Locati M, Luster AD, et al: International Union of Basic and
Clinical Pharmacology. (corrected). LXXXIX. Update on the extended
family of chemokine receptors and introducing a new nomenclature
for atypical chemokine receptors. Pharmacol Rev. 66:1–79. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng M, Huang K, Zhou J, Yan D, Tang YL,
Zhao TC, Miller RJ, Kishore R, Losordo DW and Qin G: A critical
role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow
progenitor cell recruitment to the ischemic heart. J Mol Cell
Cardiol. 81:49–53. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Luca A, D'Alessio A, Gallo M, Maiello
MR, Bode AM and Normanno N: Src and CXCR4 are involved in the
invasiveness of breast cancer cells with acquired resistance to
lapatinib. Cell Cycle. 13:148–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim CH and Broxmeyer HE: Chemokines:
Signal lamps for trafficking of T and B cells for development and
effector function. J Leukoc Biol. 65:6–15. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta SK and Vlahakis NE: Integrin
alpha9beta1 mediates enhanced cell migration through nitric oxide
synthase activity regulated by Src tyrosine kinase. J Cell Sci.
122:2043–2054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ambriz-Peña X, Garcia-Zepeda EA, Meza I
and Soldevila G: Jak3 enables chemokine-dependent actin
cytoskeleton reorganization by regulating cofilin and
Rac/RhoaGTPases activation. PLoS One. 9:e880142014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Serrador JM, Nieto M and Sánchez-Madrid F:
Cytoskeletal rearrangement during migration and activation of T
lymphocytes. Trends Cell Biol. 9:228–233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Goligorsky MS, Abedi H, Noiri E, Takhtajan
A, Lense S, Romanov V and Zachary I: Nitric oxide modulation of
focal adhesions in endothelial cells. Am J Physiol.
276:C1271–C1281. 1999. View Article : Google Scholar : PubMed/NCBI
|