Introduction
Upper tract urothelial carcinoma (UTUC) originates
from the ureter and renal pelvis. UTUC are rare and account for
only 5–10% of urothelial cancers (1,2). The
incidence of UTUC in western countries is about 2 per 100,000
person-year (1,2). The most common symptoms are hematuria in
70–95% of cases (3). Due to the deep
location of the ureter and kidney, and because the muscle layer is
not as thick as in the bladder, the diagnosis of upper urinary
tract epithelial cancer is often made at a later stage than bladder
cancer, resulting in poor overall prognosis (4). In addition, ~60% of upper urinary tract
epithelial cancers are invasive at diagnosis, compared with 15% for
bladder cancer (5,6). Therefore, the early diagnosis of upper
urinary tract epithelial cancer is an important factor affecting
the survival rate of patients (4).
At present, the diagnosis of UTUC mainly involved
computed tomography urography (CTU), magnetic resonance imaging
(MRI), cystoscopy and urinary cytology, and diagnostic ureteroscopy
(4). Urine cytology is highly
specific and non-invasive, and some authors believe that it is the
gold standard for follow-up of tumor recurrence (7). However, the sensitivity of this method
is poor and specimen collection, inflammation, stones, and foreign
bodies, can affect the results of the examination (4). CTU is preferable to MRI because of
better accuracy and lower costs (4),
but CTU is unable to detect flat lesions unless they create a mass
effect or urothelial thickening (8).
Finally, ureteroscopy is relatively invasive, but may be useful
when diagnosis is uncertain, when conservative treatment is
considered, or when the patient has a single kidney (4). Therefore, traditional diagnosis methods
have a number of limitations.
Novel methods include photodynamic diagnosis of
blood porphyrin derivatives that accumulate in tumor tissue
(9). However, these techniques show
not only UTUC, but also hyperplasia of the transitional epithelium,
squamous metaplasia, inflammation, and granulation tissue (10,11). In
addition, other factors may also affect the efficacy of these
methods including bladder retention time, different display
equipment, facilities, light intensity, cystoscopy observation
angle, physician experience (at least 15 fluorescence cystoscopy
experience) (10), and preoperative
drugs or bladder perfusion therapy (12).
In recent years, near-infrared fluorescence (NIRF)
imaging received more attention from researchers. Light at
600–1,100 nm has a strong tissue penetration, but there is a strong
background of spontaneous fluorescence (13,14).
Upconversion luminescence is a phenomenon that emits visible light
under the excitation of infrared light (13,14). So
far, most of the research on upconversion particles (UCP) was
mainly performed using rare earth metal particles. Novel molecular
imaging probes with high sensitivity, high stability of
fluorescence, good safety, and high energy conversion of light
(known as anti-Stokes effect) are now suitable for NIRF imaging
(13,14). Chatterjee et al (15) published the first report about NaYF4:
YbUCP coated with Er that were used for imaging of tumor cells and
small animals. Their results showed that these UCPs had good
biocompatibility and no toxicity to bone marrow stem cells
(15). However, to be able to detect
cancer cells specifically, surface functionalization of the
nanoparticles is needed, which is usually achieved by coupling
antibodies. Although numerous studies have attempted to create UCPs
with rare earth ions, the feasibility of tumor cells in vivo
imaging still need further study (16–18).
Therefore, the aim of the present study was to
assess the value of a nono-UCP as a diagnostic probe for deep tumor
tissue like UTUC. These results could pave the way for new
technologies for the detection of UTUC.
Materials and methods
Synthesis of polymer-coated
water-soluble UCP
All rare earth reagents used in this study were 5N
(99.999%). In a beaker, a solution containing 1.2 mmol NaCl, 0.48
mmol YCl3, 0.108 mmol YbCl3, 0.012 mmol
HoCl3 (Aladdin Chemicals Co., Ltd., Shanghai, China),
0.15 g of PEI was prepared in 15 ml of ethylene glycol. After
stirring on a magnetic stirrer, 0.4 g of NaF was added, the
solution was stirred for 10 min. The reaction kettle was sealed
into a muffle furnace and heated for 2 h at 200°C. After slow
cooling to room temperature, the precipitate was collected by
centrifugation. The pellet was washed three times with water and
ethanol. After drying, the nanoparticles were dispersed in water.
The particle size of the UCPs was determined by dynamic light
scattering and transmission electron microscopy (TEM) on a
JEOL/JEM-2010F TEM (JEOL, Tokyo, Japan). Infrared spectroscopy was
used to analyze the surface of the UCPs. The luminescence spectra
of the particles were determined by fluorescence spectrometer using
an external 980-nm light source.
Coupling of pH Low Insertion Peptide
(pHLIP) with UCP
The pHLIP polypeptide was synthesized by the solid
phase method and using the following sequence: Ala Cys Glu Gln Asn
Pro Ile Tyr Trp Ala Arg Tyr Ala Asp Trp Leu Phe Thr Thr Pro Leu Leu
Leu Leu Asp Leu Ala Leu Leu Val Asp Ala Asp Glu Gly Thr Gly.
Aqueous phase transfer is the first prerequisite for
biomedical applications of UCPs, but ifyou need to use UCPs to
achieve specific functions, for example, targeted imaging or light
detection for specific objects, it is necessary to carry out
surface modification of UCP, which is usually achieved through
coupling antibodies or other targeting molecules. The silane
treatment makes the particles hydrophilic, its surface mainly
presenting hydroxyl groups, which cannot directly link proteins.
Therefore, the silane shell surface was modified to present amino
or carboxyl groups by the crosslinking agent
1-ethyl-3-(3-aminopropyl-methyl-2) carbon imine (EDC) and two N-
hydroxysuccinimide (NHS) reactions.
The linker (SMCC; Pierce; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) was used for the coupling of the
polypeptide to the particles through the Cysthiol group and the
particle surface amino group at the N terminal side of the peptide.
Cy5-labeled pHLIP was used as positive control, and unlabeled UCP
was used as negative control. Anurinary tract epithelial cancer
cell line cultured in vitro was used as a model. The cell
binding reaction of pHLIP-UCP was observed at pH 5–7. The
experiments were repeated to determine the optimal pHLIP-UCP
coupling conditions.
For linking proteins to nanoparticles using SMCC, 50
mg of SMCC was dissolved in 5 ml of DMF to obtain a solution at 10
mg/ml. High-brightness purified UCPs were added to obtain a
solution at about 0.015 mmol and 5 ml of phosphate-buffered saline
were added and mixed quickly. The solution was left to react for 30
min at 37°C. Excess SMCC was removed by PBS dialysis, 1 ml of 0.15
M of pHLIP polypeptide was added, and the reaction was continued at
37°C for 30 min. After a period of silane reaction, the clear
solution gradually became milky white, indicating that the silane
carried on the coupling reaction on the surface of the particles.
The coated particles gradually precipitated the organic phase. In
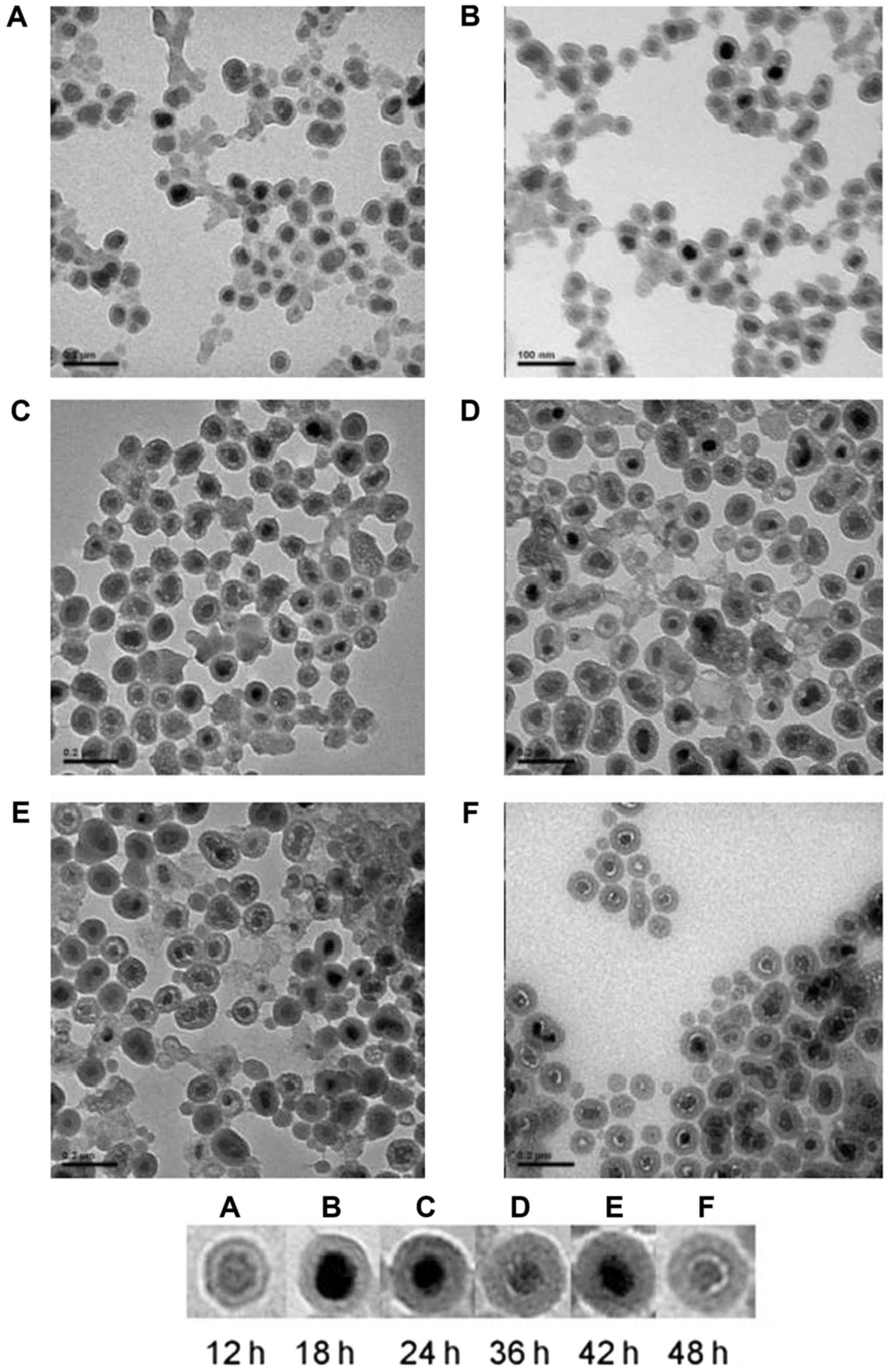
order to observe the continuous process of silane modification to
determine the optimal modification time, samples were observed
after 12, 24, 36, 42, and 48 h by TEM. The nanoparticles were
obtained after centrifugal precipitation and repeated washing.
Nanoparticles were kept at 4°C.
High-sensitivity imaging of early
stage urinary tract cancer tumor model using pHLIP-UCP
An animal model of subcutaneous tumor was
established in 4-week old nude mice using UTUC cells. All
applicable institutional and national guidelines for the care and
use of animals were followed. The tumors were observed from the
first day after inoculation. pHLIP-Cy5.5 was used as the positive
control, prepared as previously described (19). Uncoupled UCPs were used as the
negative control. A foreign body was implanted at the side of the
inoculation site to cause local inflammation and to observe the
effect of inflammation on the sensitivity of the probe.
Cultured urinary tract epithelial cancer cell line
T24 was cultured. About 0.5×106 cells in 0.1
ml were injected in the diaphragm below the heart. PHLIP-UCP
solution (1 ml) was injected in the abdominal cavity of each
animal.
Optical detection was performed using a small animal
living body multi spectral imaging system (Maestro; CRi, Inc.,
Woburn, MA, USA). The spectral detection range was 400–950 nm. The
excitation source (infrared laser diode) was set at 980 nm and the
power was 0.2 W (Changchun Mingwan Optics Co., Changchun, China)
(20). The image was captured by a
CCD camera (Andor Clara with Sony ICX285 CCD; Sony, Connecticut,
NE, USA). Images were analyzed using the Maestro software analysis
system 2.4 (CRi, Inc.). Fluorescence intensity was determined to
represent the enrichment degree and sensitivity of the probe to the
tumor cells.
Results
Synthesis of UCP
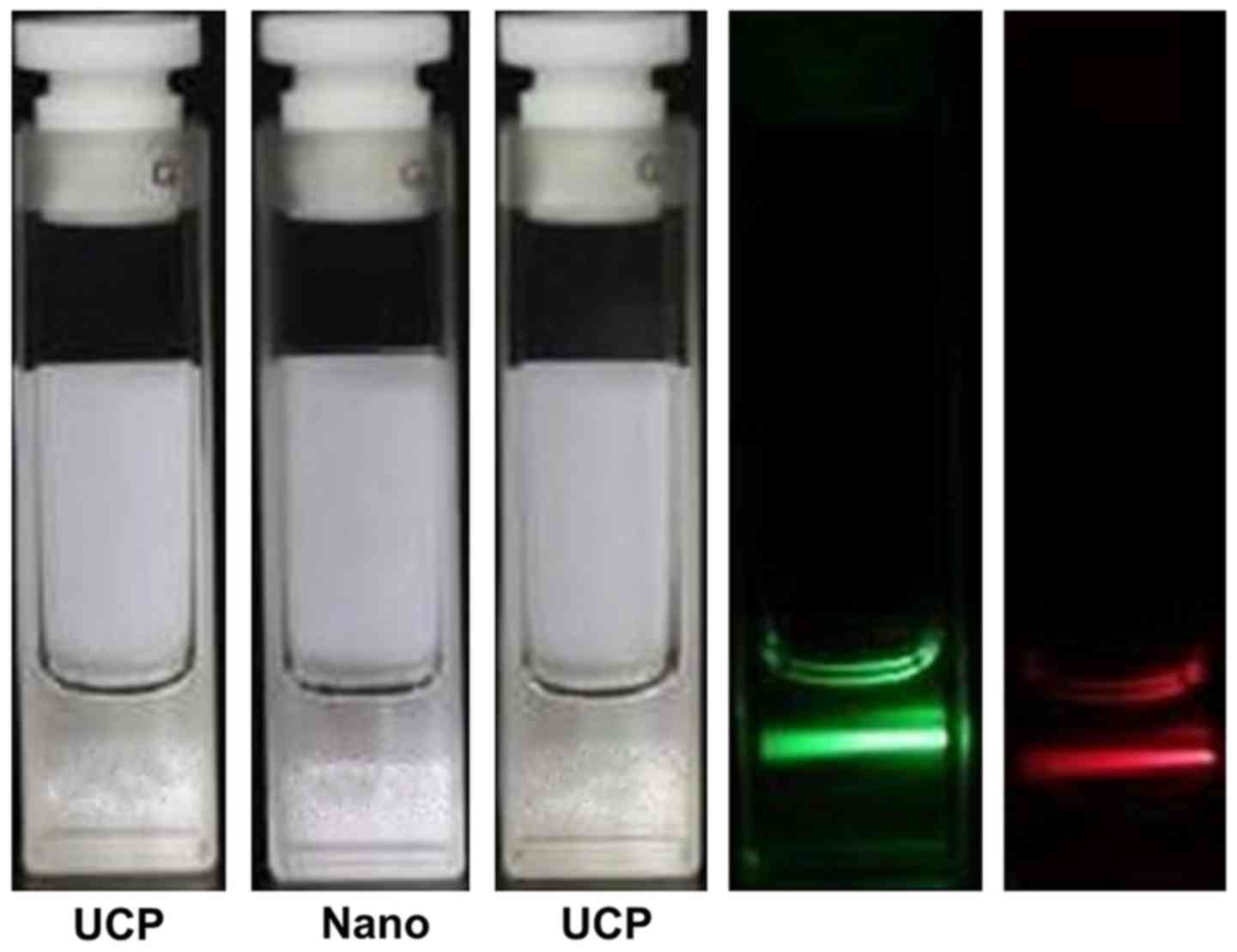
Fig. 1 shows that the
UCPs dispersed in chloroform emitted no light under natural light
(center vial), while they emitted a green light when excited with a
980-nm laser (left vial).
Excitation of Ho3+-doped
UCP
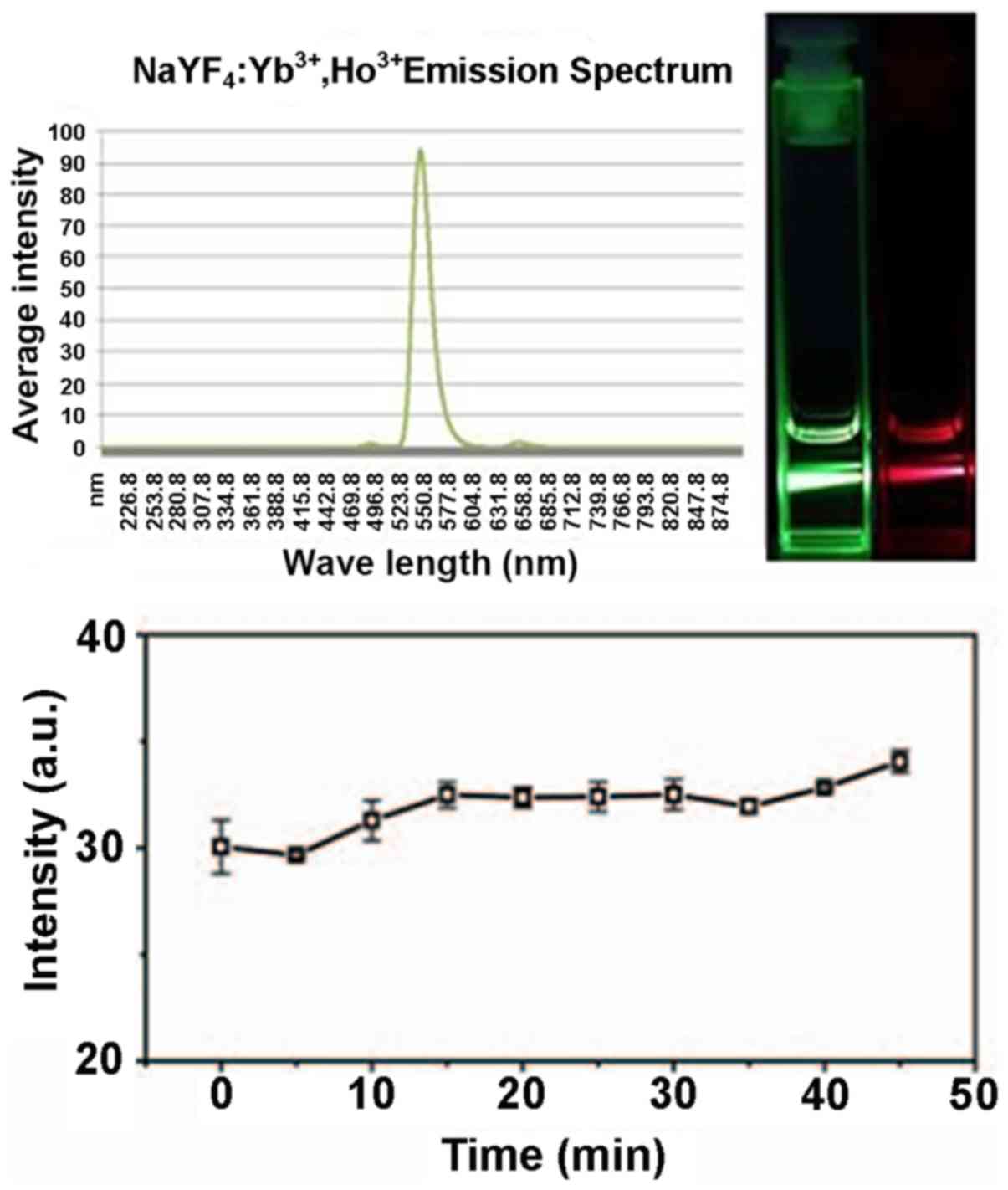
The spectra presented in Fig. 2 were captured using green and red
filters. The graph shows that the emission of the excitation
channel was uniform and transparent and the intensity was high. The
maximum emission wavelength of Ho3+-doped UCPs was about
550 nm and the red emission region was around 650 nm. When the
doping was 2%, the green luminescence dominates and the proportion
of red emission was increased when the proportion of active ions
was increased.
Phase transfer of NaYF4:
Yb, Ho3+UPCs
The diameter of the UCPs was 10.9±1.7 nm. They had a
round shape. The NaYbF4 particles had a crystal
structure of about 0.38 nm composed of a planar hexagon. The
surface of NaYF4: Yb, Ho3+UPCs was coated
with a uniform layer of silica. Fig.
3 shows that the UCPs dispersed evenly in aqueous solution and
that the surface of the particles was coated with peptides to
achieve the function of the UPCs. A continuous process of silane
reaction is shown in Fig. 3. It can
be seen that the UCPs were uniformly coated with a layer of silane
and that the shell became gradually thicker with elapsing time.
Functional modification of the NaYF4:
Yb, Ho3+ UPCs
As shown in Fig. 4,
the solution is transparent in aqueous solution. Because the coated
UCPs had a tendency to agglomerate and precipitate, the yield of
the UCPs in the aqueous phase was reduced and the overall
fluorescence intensity of the aqueous phase particles was reduced.
Compared with the corresponding organic phase solution, the
fluorescence intensity was decreased.
Imaging of tumor bearing animal model
by UCPs
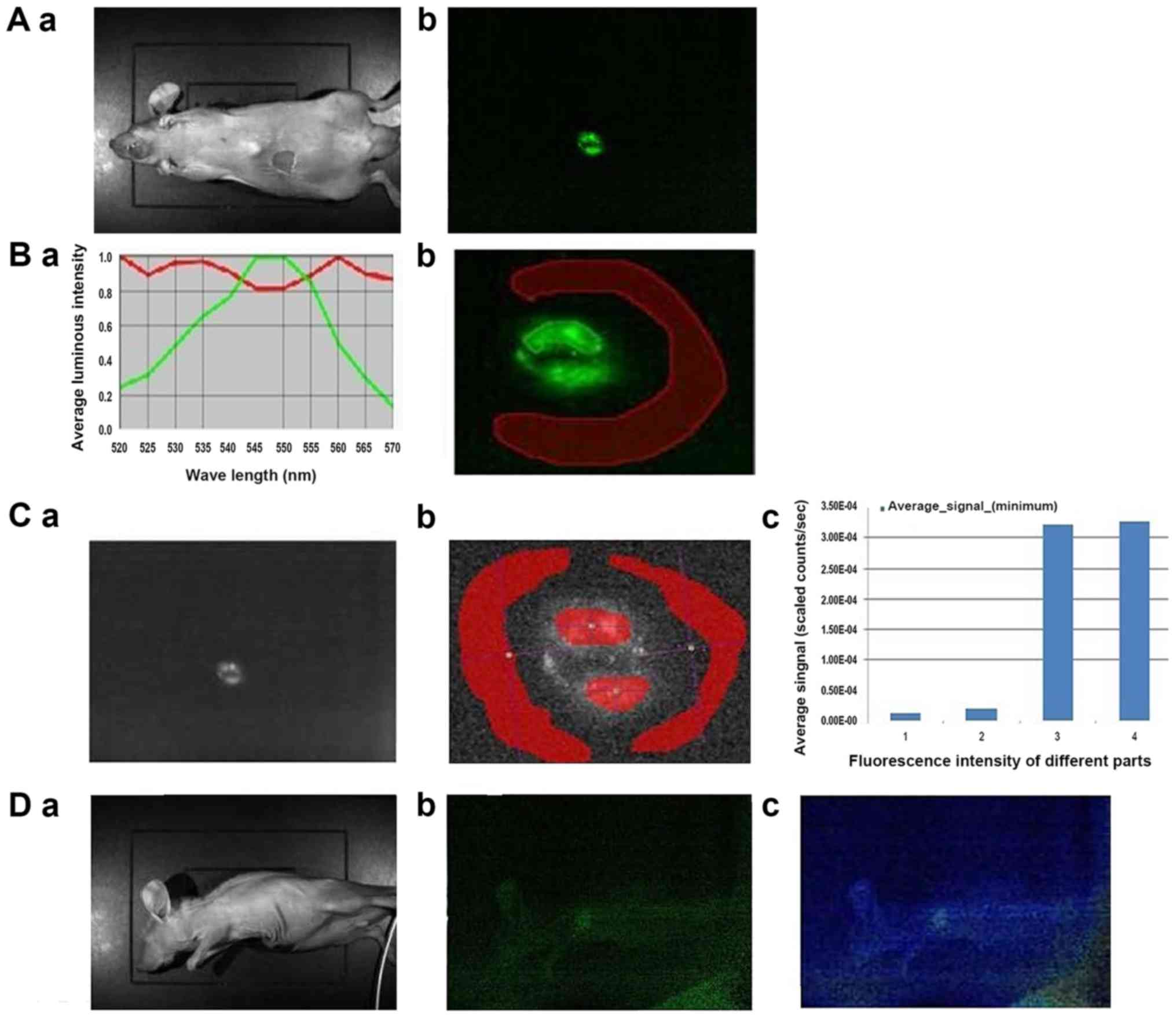
The nanoparticles were injected into the frontal
chest and diaphragm (below the heart) of the mice. Fig. 5A shows the mice and tumor imaging
after UCP injection. Fig. 5B shows
that the tumor is visible with a visible 550 nm upconversion
fluorescence emission. By analyzing the spectral slices at 550 nm,
as shown in Fig. 5C, the fluorescence
intensity measurement is carried out by selecting the fluorescence
emission region and the surrounding background region. The average
counts signal is obtained and the average intensity signal is
plotted over the corresponding region. Regions 3 and 4 represent
the upconversion fluorescence region while regions 1 and 2
represent the background region. Taken from the ventral side, the
image shows a signal-to-noise ratio of 18.5. Fig. 5D shows that the angle of view is
important. Indeed, when imaged from the side, the ribs interfere
with the laser and the emitted light, resulting in weaker signal.
This can be overcome by increasing exposure time, but the
background noise is increased. Spectral analysis of this region, as
shown in Fig. 5E, shows that the
550-nm fluorescence emission is very close to the background. By
analyzing the fluorescence intensity of the 550 nm spectra, as
shown in Fig. 5F, the signal-to-noise
ratio is 2.08, showing that the signal is still positive. Fig. 5G shows the relative location map of
the conversion fluorescence emission for deep injection, which is
expressed in the form of intensity.
Discussion
The imaging of UTUC is presently limited. UCPs could
be used to target tumors for imaging. Therefore, this study aimed
to assess the value of a nano-UCP as a diagnostic probe for deep
tumor tissue like UTUC. Results showed that UCPs dispersed in
chloroform emitted no light under natural light, while they emitted
a green light when excited with a 980-nm laser. The maximum
emission wavelength of Ho3+-doped UCPs was about 550 nm
and the red emission region was around 650 nm. Because the coated
UCPs had a tendency to agglomerate and precipitate, the yield of
the UCPs in the aqueous phase was reduced. Tumors could be
successfully imaged in tumor-bearing mice. These results suggest
that NaYF4: Yb, Ho3+ UPCs could be used for
the detection of UTUC.
For in vivo imaging, the probe has to be
resistant to light, possess a good dispersion, and be
biocompatible. In order to achieve these features, the present
study used a process that first involves the preparation of the
nanoparticles, which are then doped with
Yb3+/Ho3+ doped with NaYF4, as
previously described (21).
Thereafter, the surface is coated with a non-porous silica shell.
Finally, the UCPs were coupled with peptides specific to UTUC
cells. The final characteristics of the UCPs was similar to
previously published results (22).
At room temperature, using an excitation wavelength
of 980 nm, the strongest emission wavelength was at ~550 nm, which
may be due to the generation of the Ho3+ to the 5I8
transition of 5S2 (23). At the same
time, results showed that the particles emitted a strong signal for
as long as 40 min. These properties make these particles suitable
for use in a clinical setting.
Nanoparticles may enter the cells via passive
absorption and active uptake pathway. Unfortunately, these entry
patterns have great limitations in the diagnosis of cancers because
of the lack of specificity of the nanoparticles to cancer cells.
Therefore, the nanoparticles have to be coupled with molecules that
target cancer cells. In the present study, the UCPs were coupled to
pHLIP, which is a peptide that can recognize acidic cancer cells
(24–26). Therefore, after coupling the molecular
probe with the nanoparticles, the biological activity of the probe
is changed. A tumor host interface microenvironment is formed after
the interaction between tumor cells and adjacent host components
(27). The growth rate of tumor cells
is fast and the metabolism rate is high, which leads to an acidic
microenvironment, which is an important feature of tumor biology
(28).
The results of the present study showed that
pHLIP-bound UCPs could be used successfully to image tumors in
mice. However, mice are small animals that are easy to image using
NIRF imaging. Future studies will have to test this modality in
larger animals, in which the tumors will be deeper. Indeed, the
main obstacle to optical imaging for biological and medical
applications isthat most biological tissues can absorb or scatter
light. NIRF imaging, which includes upconversion luminescence,
needs to pay more attention to better tissue penetration and low
background fluorescence. This problem directly affects the quality
of the detected signal and the accuracy of the image. Further study
is necessary to improve this technology before its clinical
applications.
In this study, upconversion luminescence was used to
establish nanoprobes for imaging of living animals, without
background fluorescence. The probe had a high signal-to-noise ratio
and could achieve tissue imaging. NaYF4: Yb,
Ho3+ UPCs could be used for the detection of UTUC.
Further studies are necessary to determine if it could be used in
larger animals with deeper tumors.
Acknowledgements
The present study was supported by the Public
Welfare Technology Application Research Projects of Zhejiang
Provincial Science and Technology Agency (no. 2014C33129).
Competing interests
The authors declare that they have no conflict of
interest.
Glossary
Abbreviations
Abbreviations:
|
CTU
|
computed tomography urography
|
|
EDC
|
1-ethyl-3-(3-aminopropyl-methyl-2)
carbon imine
|
|
MRI
|
magnetic resonance imaging
|
|
NHS
|
N-hydroxysuccinimide
|
|
NIRF
|
near-infrared fluorescence
|
|
TEM
|
transmission electron microscopy
|
|
UCP
|
upconversion particles
|
|
UTUC
|
upper tract urothelial carcinoma
|
References
|
1
|
Munoz JJ and Ellison LM: Upper tract
urothelial neoplasms: Incidence and survival during the last 2
decades. J Urol. 164:1523–1525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cowan NC: CT urography for hematuria. Nat
Rev Urol. 9:218–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rouprêt M, Zigeuner R, Palou J, Boehle A,
Kaasinen E, Sylvester R, Babjuk M and Oosterlinck W: European
guidelines for the diagnosis and management of upper urinary tract
urothelial cell carcinomas: 2011 update. Eur Urol. 59:584–594.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A, Palou-Redorta J and Rouprêt M; European
Association of Urology (EAU), : EAU guidelines on
non-muscle-invasive urothelial carcinoma of the bladder, the 2011
update. Eur Urol. 59:997–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Margulis V, Shariat SF, Matin SF, Kamat
AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD and Wood CG;
The Upper Tract Urothelial Carcinoma Collaboration, : Outcomes of
radical nephroureterectomy: A series from the Upper Tract
Urothelial Carcinoma Collaboration. Cancer. 115:1224–1233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oosterlinck W: Guidelines on diagnosis and
treatment of superficial bladder cancer. Minerva Urol Nefrol.
56:65–72. 2004.PubMed/NCBI
|
|
8
|
Xu AD, Ng CS, Kamat A, Grossman HB, Dinney
C and Sandler CM: Significance of upper urinary tract urothelial
thickening and filling defect seen on MDCT urography in patients
with a history of urothelial neoplasms. AJR Am J Roentgenol.
195:959–965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stepp H, Baumgartner R, Kriegmair M and
Hofstetter A: Fluorescence diagnosis of bladder tumor using
5-Aminolevulinic acid-fundamentals and results. Verlag Endo-Press;
Berlin: 2000
|
|
10
|
De Dominicis C, Liberti M, Perugia G, De
Nunzio C, Sciobica F, Zuccalà A, Sarkozy A and Iori F: Role of
5-aminolevulinic acid in the diagnosis and treatment of superficial
bladder cancer: Improvement in diagnostic sensitivity. Urology.
57:1059–1062. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zaak D, Kriegmair M, Stepp H, Stepp H,
Baumgartner R, Oberneder R, Schneede P, Corvin S, Frimberger D,
Knüchel R and Hofstetter A: Endoscopic detection of transitional
cell carcinoma with 5-aminolevulinic acid: Results of 1012
fluorescence endoscopies. Urology. 57:690–694. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grimbergen MC, van Swol CF, Jonges TG,
Boon TA and van Moorselaar RJ: Reduced specificity of 5-ALA induced
fluorescence in photodynamic diagnosis of transitional cell
carcinoma after previous intravesical therapy. Eur Urol. 44:51–56.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soukka T, Kuningas K, Rantanen T,
Haaslahti V and Lövgren T: Photochemical characterization of
up-converting inorganic lanthanide phosphors as potential labels. J
Fluoresc. 15:513–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vennerberg D and Lin Z: Upconversion
nanocrystals: Synthesis, properties, assembly and applications. Sci
Adv Mater. 3:pp26–40. 2011. View Article : Google Scholar
|
|
15
|
Chatterjee DK, Rufaihah AJ and Zhang Y:
Upconversion fluorescence imaging of cells and small animals using
lanthanide doped nanocrystals. Biomaterials. 29:937–943. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng L, Yang K, Zhang S, Shao M, Lee S
and Liu Z: Highly sensitive multiplexed in vivo imaging using
pegylated upconversion nanoparticles. Nano Res. 3:pp722–732. 2010.
View Article : Google Scholar
|
|
17
|
Cheng L, Yang K, Shao M, Lee ST and Liu Z:
Multicolor in vivo imaging of upconversion nanopaticles with
emissions tuned by luminescence resonance energy transfer. J Phys
Chem. 115:pp2686–2692. 2011.
|
|
18
|
Liu Q, Chen M, Sun Y, Chen G, Yang T, Gao
Y, Zhang X and Li F: Multifunctional rare-earth self-assembled
nanosystem for tri-modal upconversion
luminescence/fluorescence/positron emission tomography imaging.
Biomaterials. 32:8243–8253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hahn CD, Riener CK and Gruber HJ: Labeling
of antibodies with Cy3-, Cy3.5-, Cy5- and Cy5.5-monofunctional dyes
at defined dye/protein ratios. Single Mol. 2:1492001. View Article : Google Scholar
|
|
20
|
Yi GS and Chow GM: Water-soluble NaYF4:
Yb, Er(Tm)/NaYF4/polymer core/shell/shell nanoparticles
with significant enhancement of upconversion fluorescence. Chem
Mater. 19:pp341–343. 2007. View Article : Google Scholar
|
|
21
|
Shan J and Ju Y: A single-step synthesis
and the kinetic mechanism for monodisperse and hexagonal-phase
NaYF4: Yb, Er upconversion nanophosphors. Nanotechnology.
20:2756032009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L and Li Y: Na(Y1.5 Na0.5)F6
single-crystal nanorods as multicolor luminescent materials. Nano
Lett. 6:1645–1649. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Wang F, Xu J, Wang Y, Liu Y, Chen
X, Chen H and Liu X: Lanthanide-doped LiYF4
nanoparticles: Synthesis and multicolor upconversion tuning.
Comptes Rendus Chimie. 13:731–736. 2010. View Article : Google Scholar
|
|
24
|
Tapmeier TT, Moshnikova A, Beech J, Allen
D, Kinchesh P, Smart S, Harris A, McIntyre A, Engelman DM, Andreev
OA, et al: The pH low insertion peptide pHLIP variant 3 as a novel
marker of acidic malignant lesions. Proc Natl Acad Sci USA.
112:9710–9715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimbrough CW, Khanal A, Zeiderman M,
Khanal BR, Burton NC, McMasters KM, Vickers SM, Grizzle WE and
McNally LR: Targeting acidity in pancreatic adenocarcinoma:
Multispectral optoacoustic tomography detects pH-low insertion
peptide probes in vivo. Clin Cancer Res. 21:4576–4585. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wagner E: Tumor-targeted delivery of
anti-microRNA for cancer therapy: pHLIP is Key. Angew Chem Int Ed
Engl. 54:5824–5826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raghunand N, Gatenby RA and Gillies RJ:
Microenvironmental and cellular consequences of altered blood flow
in tumours. Br J Radiol 76 Spec No. 1:S11–S22. 2003. View Article : Google Scholar
|