Introduction
Gastric cancer (GC) is characterized as the most
common type of the malignant gastric tumor and also one of the most
deadly cancers around the world (1).
Majority of GC patients are often diagnosed at advanced stages for
the reason that clinical symptoms of GC are not obvious at early
stage. The prognosis of advanced GC patients remains extremely low
with the 5-year overall survival rate less than 30%, largely due to
the local and systemic metastasis of GC (2,3).
Therefore, getting a better understanding of the mechanism of GC
tumorgenesis and metastasis can help to improve the therapeutic
treatments for this disease.
Increasing evidence support that long noncoding RNA
(LncRNA), which consists more than 200 nucleotides without
protein-coding capacity, plays a pivotal role in the tumorigenesis
of various types of cancers (4).
Aberrant expression of lncRNAs was often observed in several kinds
of tumors and lncRNAs are also involved in many cellular processes
including cell proliferation, apoptosis, invasion and metastasis
(5–7).
SOX2 is a transcription factor that is essential for the
maintenance of self-renewal and the pluripotency of
undifferentiated embryonic stem cells (8,9). LncRNA
SOX2 overlapping transcript (SOX2OT) is mapped to the human
chromosome 3q26.3 (Chr3q26.3) locus and is transcribed in the same
orientation as SOX2 (10). Liu et
al reported that enhanced expression of lncRNA SOX2OT promoted
colorectal cancer cells proliferation and motility and was
associated with the outcome of colorectal cancer patients (11). SOX2OT could also promote lung cancer
cell proliferation and was a prognostic indicator of poor survival
(12). As in GC, high level of SOX2OT
was reported to contribute to malignant status and poor prognosis
in GC (13). However, the underlying
mechanism about the effect of lncRNA SOX2OT on the GC progression
is still limited.
MicroRNAs (miRNAs/miRs) are a group of
single-stranded RNAs with approximately 22 nucleotides. miRNAs
often bind to 3′-untranslated regions (3′-UTRs) of their target
mRNAs to regulate their expression (14). miR-194-5p was down-regulated in
gallbladder cancer cells and overexpressed miR-194-5p promoted
cells into S-phase and apoptosis, suggesting that miR-194-5p acted
as a tumor suppressor in gallbladder cancer (15). Su's study reported that knockdown of
SOX2OT inhibited the malignant biological behaviors of glioblastoma
stem cells via up-regulating the expression of miR-194-5p and
miR-122 (16). However, the
interaction between SOX2OT and miR-194-5p in the progression of GC
is still unclear.
In our present study, we observed that SOX2OT was
highly expressed in GC tissues and cell lines. Knockdown of SOX2OT
inhibited cell proliferation and mobility of GC cells through
suppressing epithelial-mesenchymal-transition (EMT) via targeting
miR-194-5p. Our results shed light on finding new therapeutic
strategies for GC treatment.
Materials and methods
Tissue samples
GC tumor tissues (n=30) and the same number of
adjacent histological healthy tissues were obtained from GC
patients who underwent surgical treatment without preoperative
radiotherapy and/or chemotherapy. Informed consent was obtained
from all individual participants included in the study. The
specimens were snap-frozen in liquid nitrogen and stored at −80°C
until use. The study was approved by the Medical Ethics Committee
of Universidad de Almería (Almería, Spain).
Cell culture
GC cell lines (MGC-803, SGC-7901, MKN-74) and human
normal gastric epithelium cell line (GES-1) were obtained from
American Type Culture Collection (ATCC) and cultured in RPMI-1640
culture medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in a humidified 5% CO2
incubator.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells with TRIzol
reagent (Thermo Fisher Scientific, Inc.). cDNA was generated using
the M-MLV reverse transcriptase (Clontech, Palo Alto, CA, USA) and
One-Step SYBR PrimeScript RT-PCR kit (Takara Bio, Inc., Otsu,
Japan) was used to detect the expression of SOX2OT. TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) was used for the reverse transcription of
miR-194-5p. The expression of miR-194-5p was detected using TaqMan
Universal Master Mix II. The primers used were as follows: GAPDH:
F: 5′-CGCTGAGTACGTCGTGGAGT-3′ and R: 5′-CGTCAAAGGTGGAGGAGTGG-3′.
SOX2OT: F: 5′-TGCTACAAGACAACACCCTGA-3′ and R:
5′-CCAAAGCCATAACCAGATT-3′. miR-194-5p: F:
5′-GCGGCGGTGTAACAGCAACTCC-3′ and R: 5′-ATCCAGTGCAGGGTCCGAGG-3′. U6:
F: 5′-GCTTCGGCACATATACTAAAAT-3′ and R:
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The amplification protocol included
an initial denaturation step at 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 60 sec. The expression
levels were calculated using the 2−ΔΔCq method with
GAPDH used for the normalization of the mRNA, and U6 for the miRNA
(17).
Cell transfection
Short hairpin RNA (shRNA) against SOX2OT and their
negative control (sh-NC), miR-194-5p mimic or inhibitor and their
negative controls (mimic NC, inhibitor NC) were synthesized
(GenePharma, Shanghai, China). GC cells were seeded into a 6-well
plate to reach 70–80% confluence for transfection. Transfections
were conducted using Lipofectamine 2000 reagent (Thermo Fisher
Scientific, Inc.) according to manufacturer's protocol. The
transfection efficacy was analyzed with RT-qPCR.
Cell proliferation assay
Cell proliferation was measured through Cell
Counting Kit-8 (CCK-8) assay. Cells were seeded in 96-well plates
at a density of 3,000 cells per well in triplicate. Then, 10 µl of
CCK-8 (Beyotime Institute of Biotechnology, Jiangsu, China) was
added to each well at different time points (0–5 days). Cells were
incubated for 2 h at 37°C in dark and the absorbance was recorded
at 450 nm.
Cell migration and invasion assay
For cell migration and invasion assay, chambers of
6.5 mm in diameter with an 8-mm pore size (Corning Incorporated,
Corning, NY, USA) were used. Cells were resuspended in 100 µl of
serum-free medium at a density of 1×105 cells/ml and
seeded into the upper chamber (pre-coated with 50 ng/µl Matrigel
solution for the cell invasion assay and non-coated for the cell
migration assay). Then, 600 µl of 10% FBS medium was placed in the
lower chamber. After incubation at 37°C for 24 h, the cells on the
upper membrane surface were mechanically removed. Cells that
migrated or invaded to the lower surface of the membrane were
stained with 20% Giemsa. Five random fields were chosen to count
and take photos under a microscope (Olympus Corporation, Tokyo,
Japan).
Western blot assay
Cells were lysed using RIPA (Beyotime Institute of
Biotechnology) buffer and the protein concentrations were analyzed
by the BCA protein assay kit (Beyotime Institute of Biotechnology).
The same amount of proteins were separated by 10% SDS-PAGE gel and
then transferred into PVDF membranes (EMD Millipore, Billerica, MA,
USA). Membranes were blocked by 5% skim milk for 1 h at room
temperature and then incubated with primary antibodies as follows:
anti-E-cadherin (sc-71007), anti-N-cadherin (sc-59987),
anti-Vimentin (sc-80975), anti-MMP-2 (sc-13594), anti-MMP-9
(sc-13520) and anti-GAPDH (sc-32233) (at a dilution 1:1,000; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
Membranes were then washed three times with TBST and incubated with
horseradish peroxidase conjugated secondary antibody for 1 h at
room temperature. Then the antibody-bound proteins were detected
using the ECL system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Bioinformatics prediction
To predict the target miRNAs of LncRNA SOX2OT, the
following prediction algorithms were used: TargetScan (http://www.targetscan.org/vert_71/), PICTAR
(http://www.pictar.org/) and miRDB (http://mirdb.org/miRDB/custom.html) target
prediction algorithms.
Dual luciferase reporter assay
The sequence of SOX2OT was amplified by PCR and
cloned into pmirGLO Dual-luciferase miRNA Target Expression Vectors
along with its mutant sequence of miR-194-5p binding sites
(GenePharma, Shanghai, China). Cells were seeded into 96-well plate
and co-transfected with SOX2OT-WT or SOX2OT-MUT and miR-194-5p
mimics using Lipofectamine 2000 (Thermo Fisher Scientific, Inc.).
The Dual-Luciferase Reporter Assay System (Promega Corporation,
Madison, WI, USA) was used for testing the luciferase activity.
In vivo animal study
For the in vivo study, the stably transfected
cells (sh-SOX2OT or sh-NC transfected MKN-74 cells) were used.
shRNA against SOX2OT was constructed in pGPU6/GFP/Neo vector and
then transfected into MKN-74 cells to construct the stably
transfected cells. The 4-week old BALB/c nude mice were supplied by
Animal Center of Universidad de Almería. Experiments with mice were
conducted strictly in accordance with a protocol approved by the
Administrate Panel on Laboratory Animal Care of China Medical
University and the animal experiments were approved by the Medical
Ethics Committee of Universidad de Almería. Mice were divided
randomly into 2 groups: i) sh-NC group and ii) sh-SOX2OT group with
5 in each group. Each mouse was subcutaneously injected with
5×105 transfected cells in the right flank area for
subcutaneous implantation. Tumor volumes (mm3) were
measured every 5 days and calculated according to the following
formula: tumor volumes (mm3)=length ×
width2/2. The mice were sacrificed after 25 days post
injection and tumor tissues were harvested for further
analysis.
Statistical analysis
All results were presented as mean ± standard
deviation (SD). All differences were analyzed by SPSS 19.0
statistical software (SPSS, Inc., Chicago, IL, USA) with the
Student's t-test (two tailed) or one-way ANOVA with
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
SOX2OT is increased while miR-194-5p
is decreased in GC tissues and cell lines
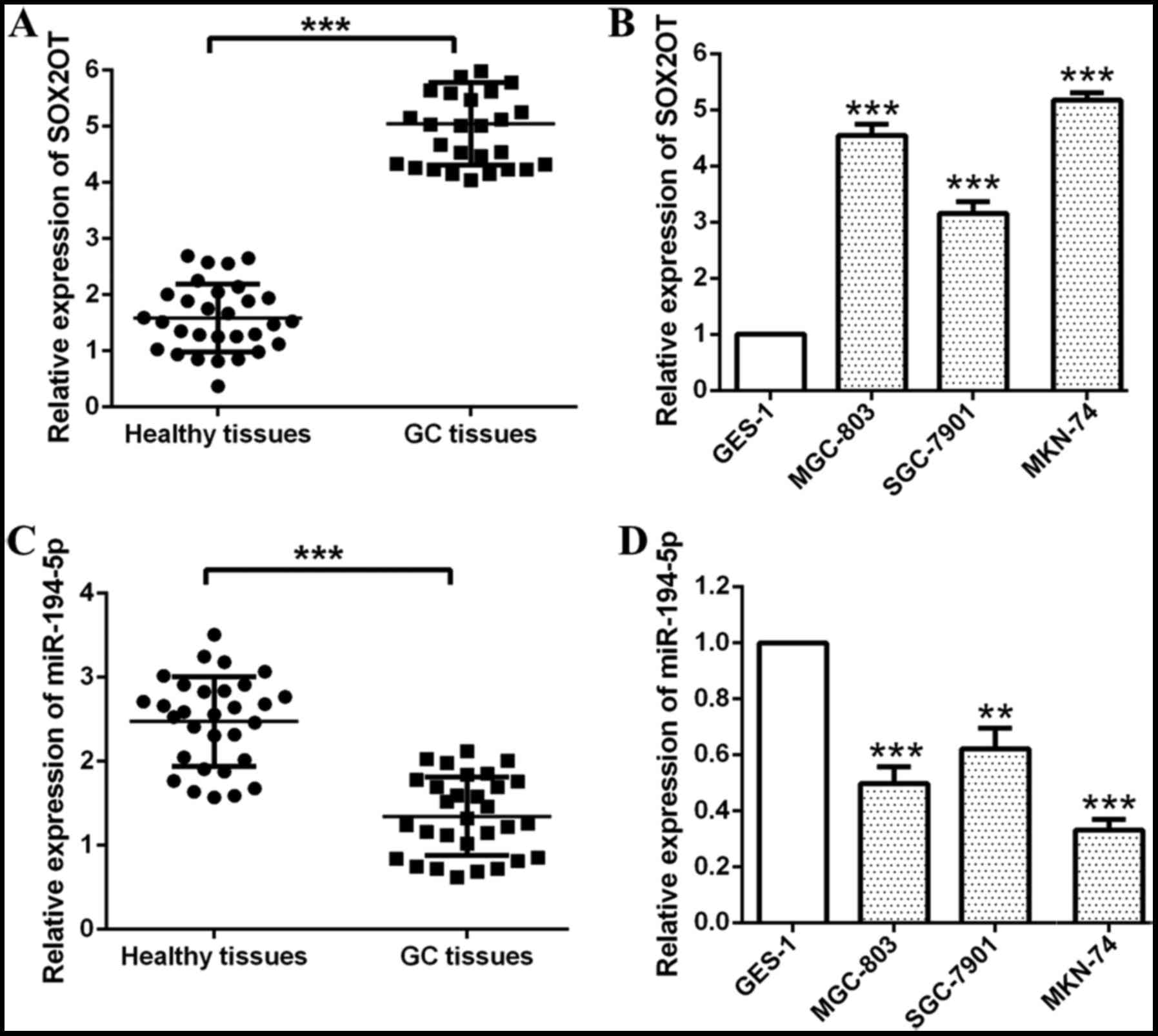
Firstly, we detected relative expression of SOX2OT
and miR-194-5p in GC tissues and cell lines respectively through
RT-qPCR (Fig. 1). Relative expression
of SOX2OT was found significantly up-regulated while the expression
of miR-194-5p was found down-regulated in GC tissues compared with
healthy tissues (***P<0.001; Fig. 1A
and C). Besides that, we also found GC cell lines (MGC-803,
SGC-7901, MKN-74) expressed higher SOX2OT and lower miR-194-5p
compared with human normal gastric epithelium cell line GES-1
(***P<0.001, **P<0.01; Fig. 1B and
D). MKN-74 and MGC-803 cells were chosen for our following
experiments for they had higher SOX2OT expression and lower
miR-194-5p expression among these GC cell lines. Our data indicated
that SOX2OT was up-regulated while miR-194-5p was down-regulated in
GC tissues and cell lines.
Knockdown of SOX2OT suppresses cell
proliferation and mobility of GC cells through inhibiting EMT
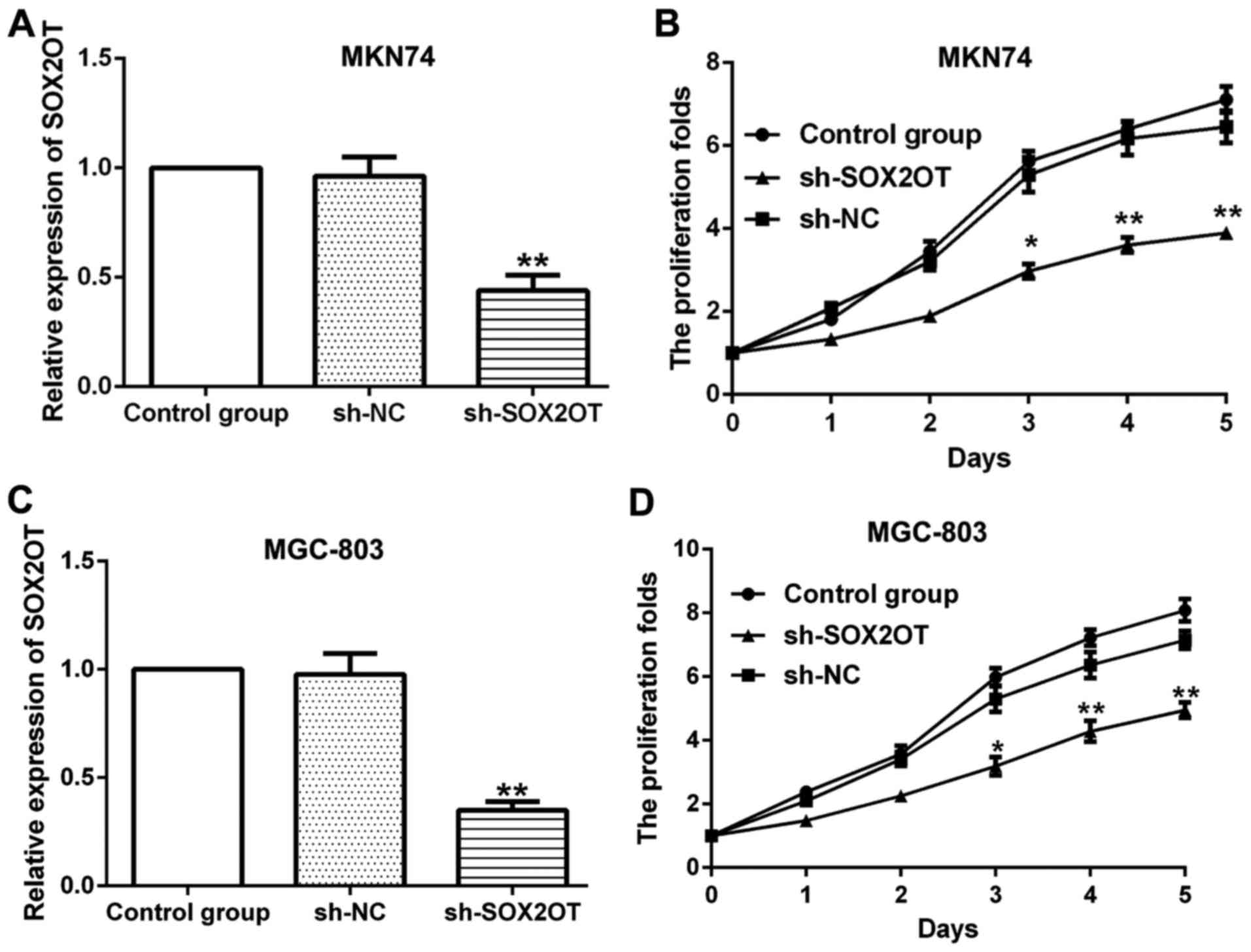
To investigate the role of SOX2OT in GC progression,
sh-SOX2OT and sh-NC were transfected into MKN-74 and MGC-803 cells
respectively (Fig. 2). Sh-SOX2OT
transfection significantly reduced SOX2OT expression compared with
sh-NC group and the transfection efficiency was measured by RT-qPCR
(**P<0.01; Fig. 2A and C). We
observed that knockdown of SOX2OT inhibited cell proliferation
markedly vs. sh-NC group in both MKN74 and MGC-803 cells
(*P<0.05, **P<0.01; Fig. 2B and
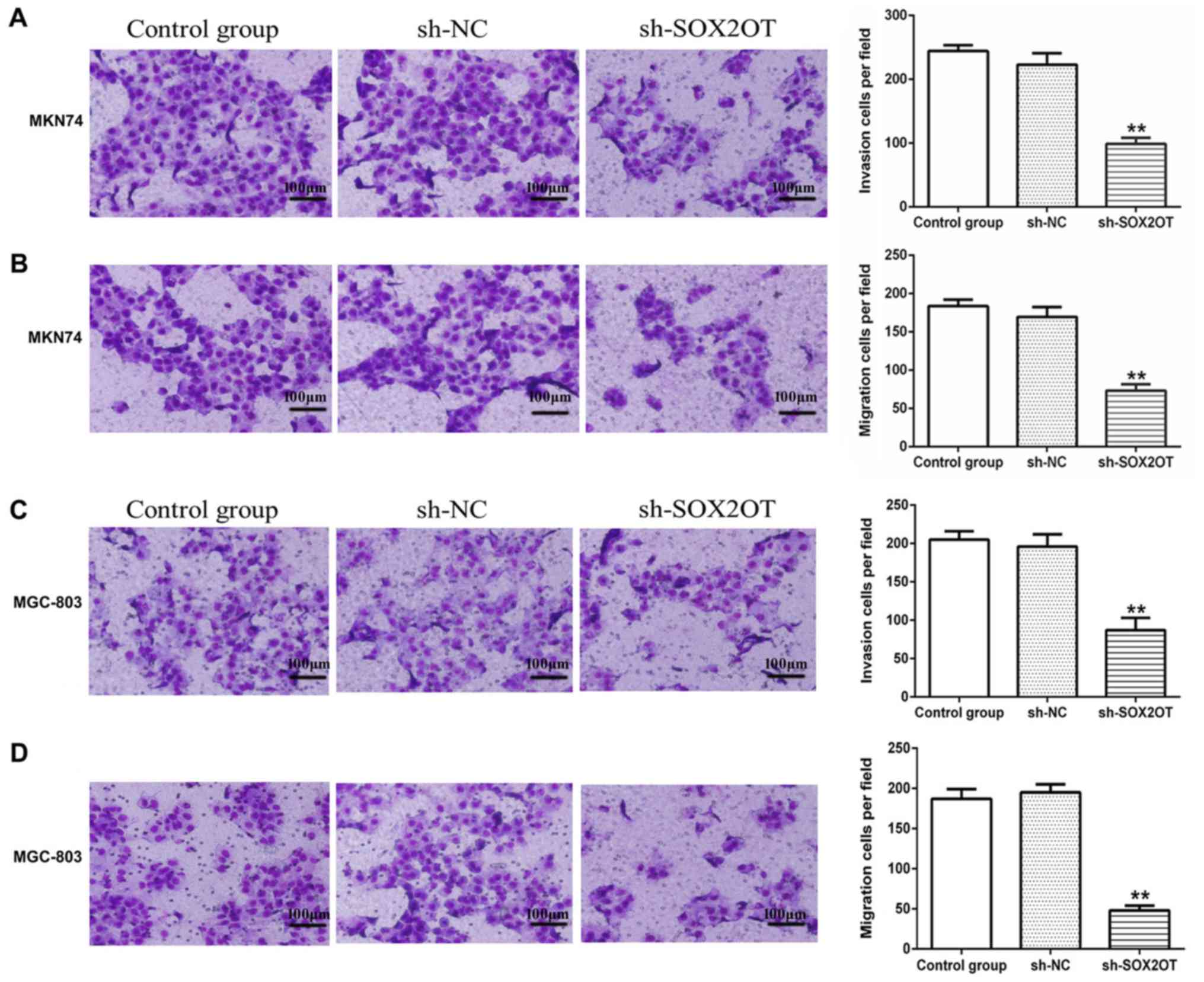
D). Moreover, results from cell invasion and migration assay
showed that sh-SOX2OT significantly hampered invasion and migration
of MKN-74 and MGC-803 cells compared with sh-NC group (**P<0.01;
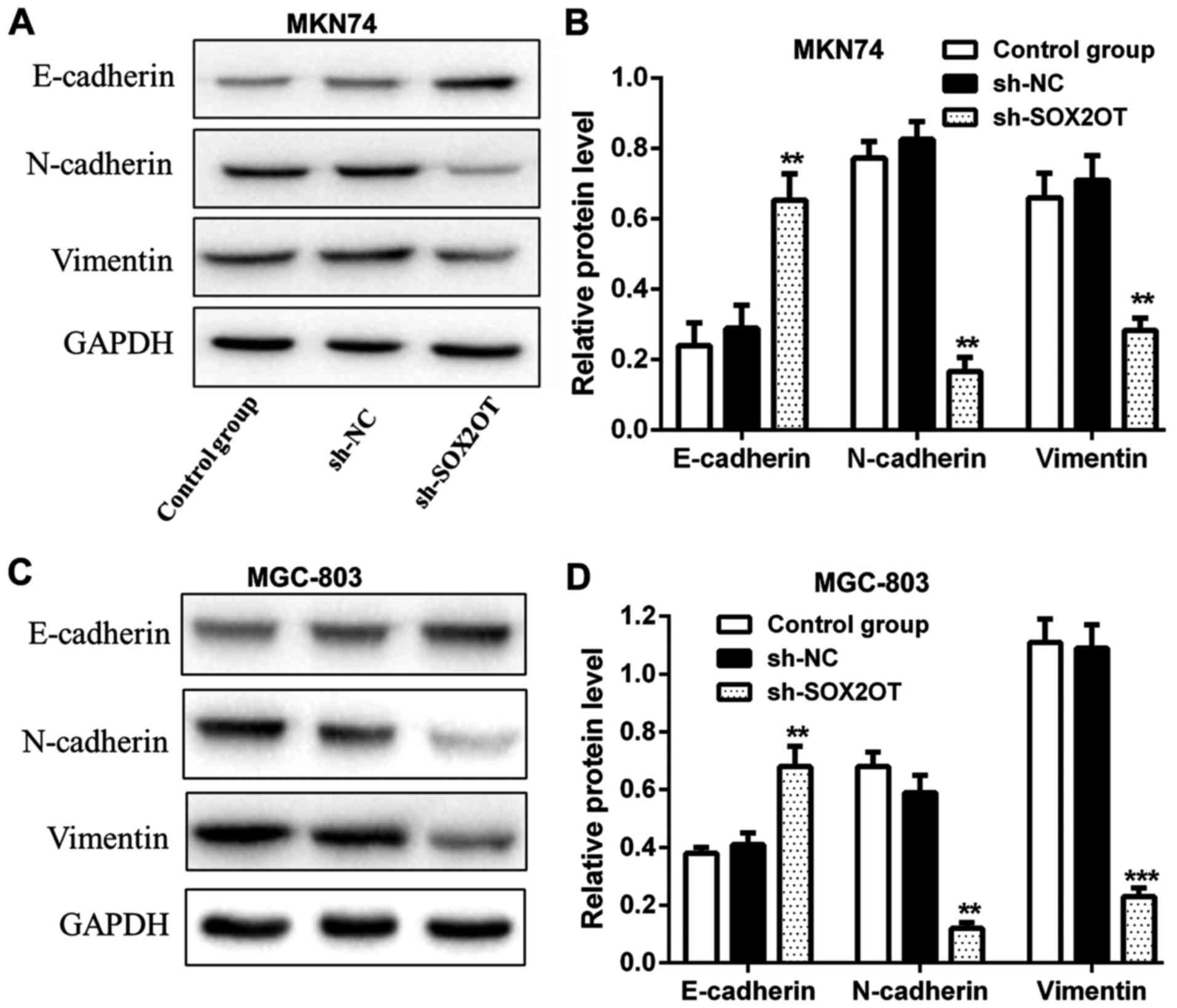
Fig. 3). We also measured relative
expression of EMT-related proteins and found that the expression of
epithelial marker E-cadherin was increased while the expression of
mesenchymal markers N-cadherin and Vimentin was decreased in
sh-SOX2OT group compared with sh-NC group (**P<0.01,
***P<0.001; Fig. 4). These results
suggested that knockdown of SOX2OT suppressed cell proliferation
and mobility of GC cells through inhibiting EMT.
miR-194-5p is a target of SOX2OT and
miR-194-5p inhibitor counteracts the effects of SOX2OT shRNA on GC
progression
We then set to explore the relationship between
SOX2OT and miR-194-5p. Through bioinformatics analysis, we found
that miR-194-5p was a predicted target of SOX2OT (Fig. 5A). miR-194-5p mimic or inhibitor was
transfected into MKN-74 cells to increase or decrease miR-194-5p
expression (**P<0.01; Fig. 5B). In
order to verify that miR-194-5p was a target of SOX2OT, luciferase
reporter assay was performed. The results demonstrated the direct
binding relationship between miR-194-5p and SOX2OT (**P<0.01;
Fig. 5C). Relative expression of
miR-194-5p was notably up-regulated in sh-SOX2OT group, indicating
that miR-194-5p expression was negatively regulated by SOX2OT
(**P<0.01; Fig. 5D). In addition,
transfection with miR-194-5p inhibitor enhanced cell proliferation
and mobility of GC cells compared with shRNA + inhibitor NC group,
suggesting that miR-194-5p inhibitor abolished the inhibitory
effects of SOX2OT shRNA on tumor progression of GC (*P<0.05,
**P<0.01, #P<0.05, ##P<0.01,
###P<0.001; Fig. 5E-G).
Besides that, relative expression of E-cadherin was down-regulated
while the expression of N-cadherin and Vimentin was up-regulated
strikingly in shRNA + miR-194-5p inhibitor group compared with
shRNA + inhibitor NC group (**P<0.01, ##P<0.01;
Fig. 5H and I). Results above
demonstrated that miR-194-5p was a target of SOX2OT and was
negatively regulated by SOX2OT. miR-194-5p inhibitor counteracted
the inhibitory effects of SOX2OT shRNA on tumor progression via
enhancing EMT in GC.
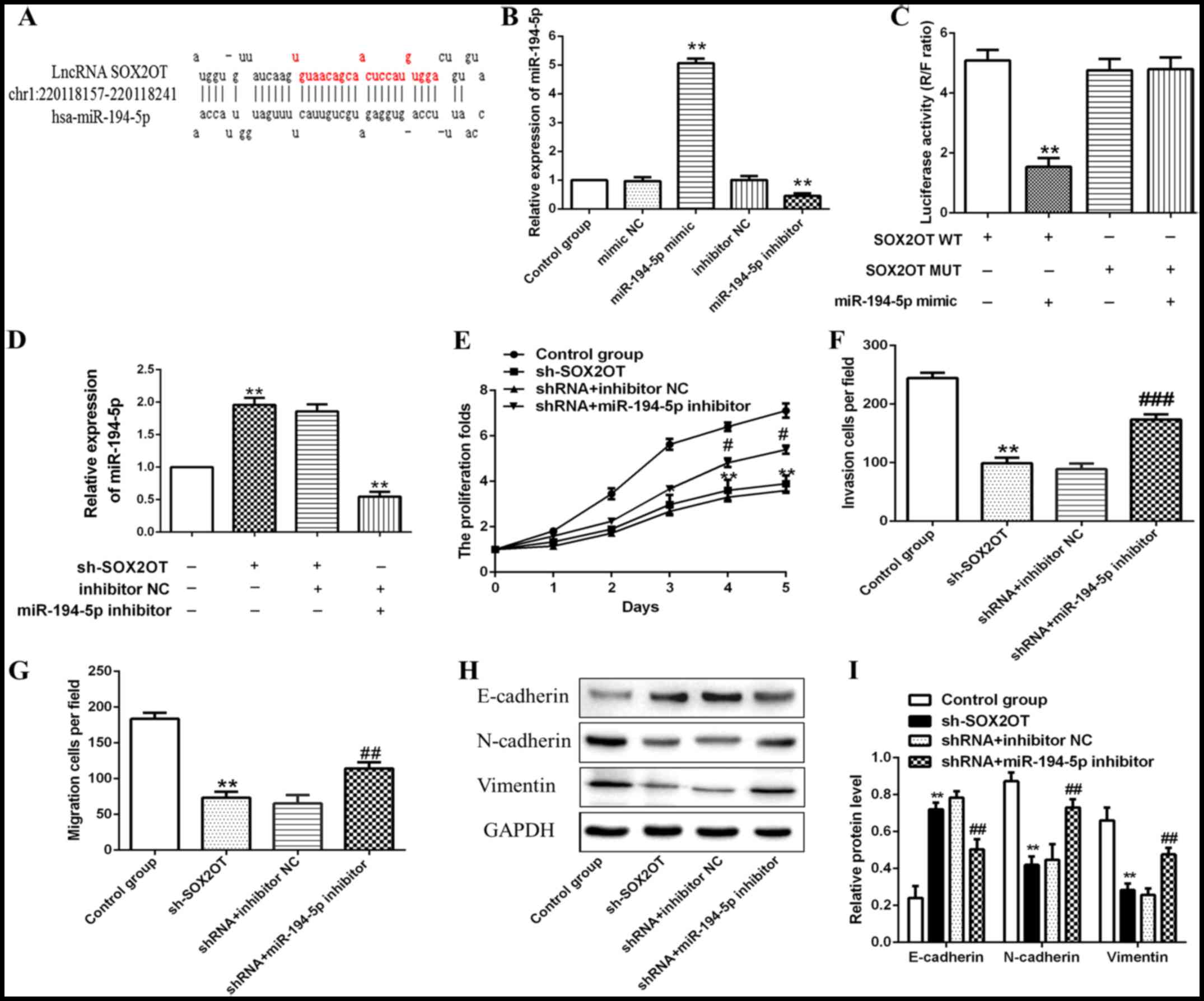 | Figure 5.miR-194-5p is a target of SOX2OT and
miR-194-5p inhibitor counteracts the effects of SOX2OT shRNA on GC
progression. MKN-74 cells were transfected with sh-SOX2OT,
sh-SOX2OT + inhibitor NC, sh-SOX2OT + miR-194-5p inhibitor
respectively. (A) Target sequences of miR-194-5p in SOX2OT mRNA
were analyzed through bioinformatics. (B) Relative expression of
miR-194-5p was detected through RT-qPCR (**P<0.01 compared with
mimic NC or inhibitor NC group). (C) The interaction between SOX2OT
and miR-194-5p was confirmed by luciferase reporter assay
(**P<0.01 compared with SOX2OT WT group). (D) Relative
expression of miR-194-5p was detected through RT-qPCR. (E) Cell
proliferation was detected through CCK-8 assay. (F) Cell invasion
and (G) migration abilities were detected using Transwell model
assay. (H) Representative western blot and (I) quantification of
E-cadherin, N-cadherin and Vimentin. All data are presented as the
mean ± standard deviation from three independent experiments.
**P<0.01 compared with control group, #P<0.05,
##P<0.01, ###P<0.001 compared with
shRNA + inhibitor NC group. SOX2OT, SOX2 overlapping transcript;
miR, microRNA; GC, gastric cancer; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; CCK-8, Cell
Counting Kit-8. |
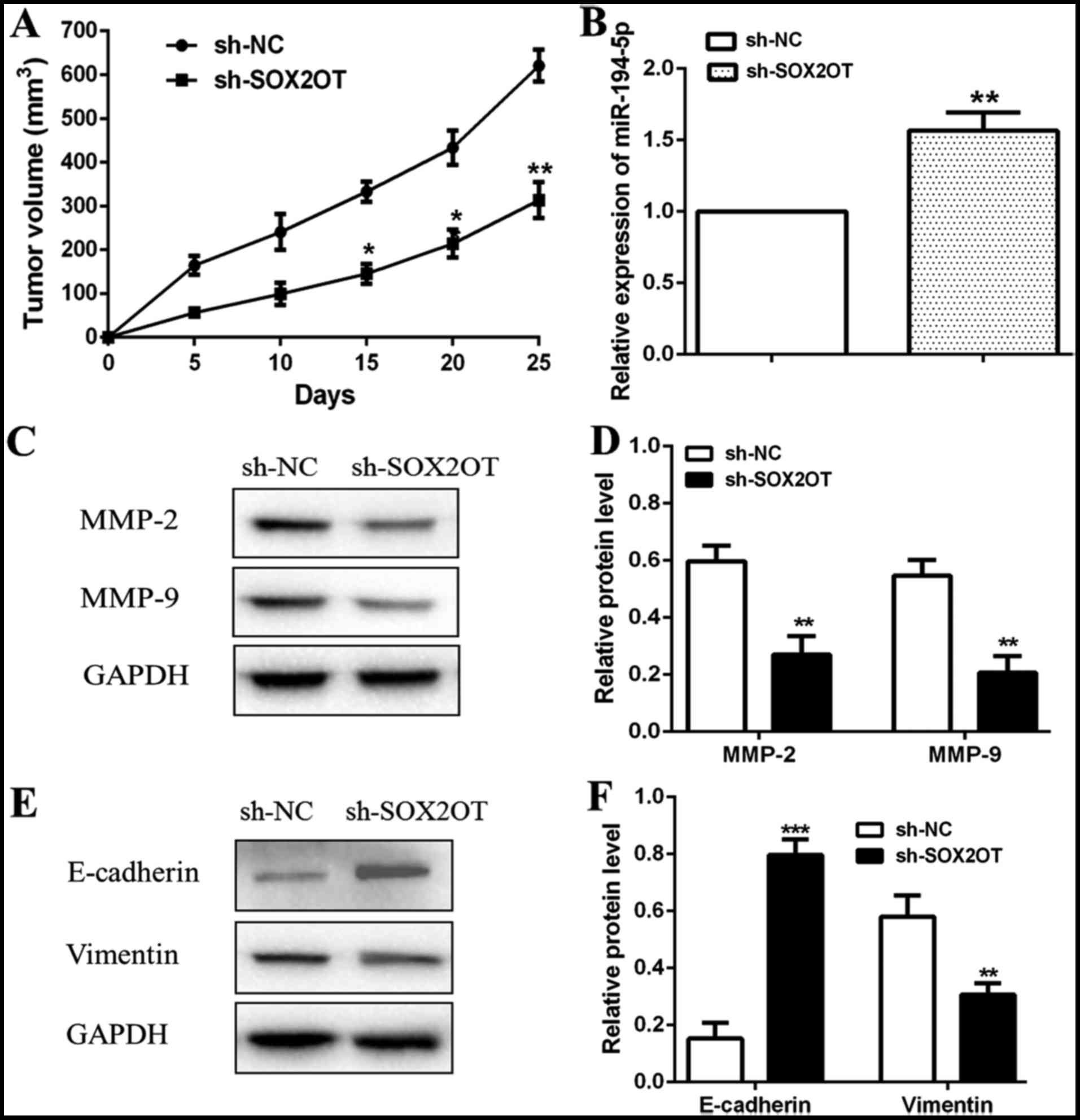
SOX2OT/miR-194-5p axis suppresses GC
tumor growth and metastasis through inhibition of EMT in vivo
Our in vitro experiments suggested that
knockdown of SOX2OT inhibited cell proliferation and mobility
through up-regulating miR-194-5p in GC cells. Then we further
explored the effect of SOX2OT/miR-194-5p axis on GC tumor growth
and metastasis in vivo. BALB/c nude mice were injected with
sh-SOX2OT or sh-NC transfected MKN-74 cells to form animal models
for in vivo researches. Our data showed that tumors in
sh-SOX2OT group grew slower and also smaller than sh-NC group
(*P<0.05, **P<0.01; Fig. 6A).
Relative expression of miR-194-5p was found higher in tumors of
sh-SOX2OT group vs. sh-NC group (**P<0.01; Fig. 6B). We then detected relative
expression of metastasis related proteins (MMP-2 and MMP-9) through
western blot and observed that MMP-2 and MMP-9 expression both
decreased remarkably in sh-SOX2OT group (**P<0.01; Fig. 6C and D). Besides that, relative
protein level of E-cadherin was increased while the level of
Vimentin was decreased in sh-SOX2OT group compared with sh-NC group
(***P<0.001, **P<0.01; Fig. 6E and
F). Taken together, our in vivo experiments determined
that SOX2OT/miR-194-5p axis suppressed GC tumor growth and
metastasis through inhibition of EMT in vivo.
Discussion
GC is a common malignancy with high mortality rate.
Patients with distant metastasis often miss the best time for
operation and also have poor prognosis. Therefore, finding novel
target to suppress GC cells proliferation and metastasis can be
instrumental in improving the 5-year survival rates of GC patients.
Recently, the participation of LncRNA in the tumor progression has
gained much attention of researchers. In our present study, we
indicated that LncRNA SOX2OT was up-regulated while miR-194-5p was
down-regulated in GC tissues and cell lines. Further, SOX2OT was
found to target miR-194-5p in a sequence-specific manner and
negatively regulated the expression of miR-194-5p. Knockdown of
SOX2OT inhibited cell proliferation, invasion and migration of GC
cells through increasing miR-194-5p expression and suppressing EMT
both in vitro and in vivo. This study demonstrated
that the SOX2OT/miR-194-5p axis played a regulatory role in the
proliferation and mobility of GC cells through suppressing EMT.
LncRNA SOX2OT is transcribed in the same orientation
as SOX2 and regulated the transcription of SOX2 which was pivotal
in tumorigenesis, acting as an enhancer (18). Accumulating evidence indicated that
SOX2OT was strikingly elevated in various types of cancers and
served as an oncogenic role. For instance, Liu found that SOX2OT
was increased in colorectal cancer tissues and cell lines.
Decreased SOX2OT expression inhibited cell proliferation, invasion,
migration and EMT in colorectal cancer cells (11). Shi's study reported that SOX2OT
expression level was significantly higher in hepatocellular
carcinoma (HCC) tissues compared with adjacent non-tumor tissues.
In addition, the metastasis ability of HCC cells was significantly
restrained by knocking down lncRNA SOX2OT expression (18). As in GC, it was reported that SOX2OT
overexpression served as a poor biomarker in GC (13). In accordance with these previous
researches, in our present study, SOX2OT expression was also found
highly up-regulated in GC tissues and cell lines. In addition,
knockdown of SOX2OT remarkably impeded cell proliferation, invasion
and migration in GC cells, suggesting the oncogenic role of SOX2OT
in the progression of GC. EMT is a cellular process that epithelial
features are lost and mesenchymal features gradually develop. A
large body of studies determined that EMT can reduce intercellular
adhesion and promote cells metastasis in many types of cancer cells
(19). During EMT, epithelial marker
E-cadherin is down-regulated while the mesenchymal markers
N-cadherin and Vimentin are up-regulated in tumor cells (20). Matrix metalloproteinase (MMP) family
members including MMP-2 and MMP-9 which can degrade extracellular
matrix to promote metastasis of cells were also up-regulated during
EMT (21). In our study, we observed
that knockdown of SOX2OT increased E-cadherin expression while
decreased N-cadherin and Vimentin expression notably, indicating
that knockdown of SOX2OT reduced EMT in GC cells. In summary,
results above elucidated that knockdown of SOX2OT inhibited cell
proliferation and mobility through suppressing EMT in GC cells.
Since we had revealed the role of SOX2OT in the
progression of GC, then we further investigated the underlying
mechanism in detail. Previous researches demonstrated that LncRNAs
could bind to common miRNA binding sites of mRNAs and abolished the
downstream effects of these miRNA (22). Su reported that knockdown of SOX2OT
could inhibit the malignant biological behaviors of glioblastoma
stem cells via up-regulating the expression of miR-194-5p and
miR-122 (16). Here, we also focus on
the relationship between SOX2OT and miR-194-5p in GC. miR-194-5p
expression was suppressed in glioblastoma multiforme (GBM) and
miR-194-5p hampered cell growth and promoted apoptosis of GBM cells
(23). Overexpression of miR-194-5p
increased E-cadherin expression and inhibited invasion and
migration of colorectal cancer cells (24). These results indicated the tumor
suppressive role of miR-194-5p. In consistent with these previous
studies, we also observed decreased miR-194-5p expression in GC
tissues and cell lines. Further, through bioinformatics analysis,
we found that SOX2OT might harbor a binding site for miR-194-5p. To
verify this prediction, dual-luciferase reporter assay was
performed, which demonstrated that SOX2OT could bind to miR-194-5p
directly. Moreover, knockdown of SOX2OT increased the expression of
miR-194-5p, suggesting that miR-194-5p was negatively regulated by
SOX2OT in GC. Besides that, miR-194-5p inhibitor was found to
counteract the inhibitory effects of SOX2OT on cell proliferation
and mobility through enhancing EMT in GC cells. Taken together, our
in vitro experiments revealed that knockdown of SOX2OT
restrained cell proliferation and mobility through suppressing
miR-194-5p and EMT in GC.
Having understood the regulating role of
SOX2OT/miR-194-5p axis in GC cells in vitro, we then carried
out in vivo experiments for further investigation. Su's
study revealed that sh-SOX2OT reduced tumor volume of glioblastoma
xenograft model (16). Our results
went in line with his study that knockdown of SOX2OT inhibited
tumor growth and metastasis of GC through suppressing miR-194-5p
and EMT in vivo.
In summary, our present study elucidated that SOX2OT
was up-regulated in GC tissues and cell lines. Knockdown of SOX2OT
inhibited cell proliferation and mobility of GC cells through
increasing miR-194-5p expression. The SOX2OT/miR-194-5p axis
participated in the tumor progression of GC through regulation of
EMT both in vitro and in vivo. Hence, targeting the
SOX2OT/miR-194-5p axis can be instrumental in establishing new
strategies for GC therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RQW designed the study and wrote the manuscript. CD,
RAR and MDMRM performed the experiments, and reviewed and edited
the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Universidad de Almería. Informed consent was obtained
from all individual participants included in the study. Animal
experiments with mice were conducted strictly in accordance with a
protocol approved by the Administrate Panel on Laboratory Animal
Care of China Medical University and the animal experiments were
approved by the Medical Ethics Committee of Universidad de
Almería.
Patient consent for publication
Written informed consent was obtained from all
patients for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LncRNAs
|
long noncoding RNAs
|
|
GC
|
gastric cancer
|
|
miRNAs
|
microRNAs
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SOX2OT
|
SOX2 overlapping transcript
|
|
SD
|
standard deviation
|
References
|
1
|
Marqués-Lespier JM1, González-Pons M and
Cruz-Correa M: Current perspectives on gastric cancer.
Gastroenterol Clin North Am. 45:413–428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhong J, Chen Y and Wang LJ: Emerging
molecular basis of hematogenous metastasis in gastric cancer. World
J Gastroenterol. 22:2434–2440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan H, Yuan J, Gao L, Rao J and Hu J: Long
noncoding RNA MEG3 activation of p53 mediates ischemic neuronal
death in stroke. Neuroscience. 337:191–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu X, Hou L, Huang W, Gao Y, Lv X and
Tang J: The mechanism of long non-coding RNA MEG3 for neurons
apoptosis caused by hypoxia: Mediated by miR-181b-12/15-LOX
signaling pathway. Front Cell Neurosci. 10:2012016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kam Y, Rubinstein A, Naik S, Djavsarov I,
Halle D, Ariel I, Gure AO, Stojadinovic A, Pan H, Tsivin V, et al:
Detection of a long non-coding RNA (CCAT1) in living cells and
human adenocarcinoma of colon tissues using FIT-PNA molecular
beacons. Cancer Lett. 352:90–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Avilion AA, Nicolis SK, Pevny LH, Perez L,
Vivian N and Lovell-Badge R: Multipotent cell lineages in early
mouse development depend on SOX2 function. Genes Dev. 17:126–140.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fong H, Hohenstein KA and Donovan PJ:
Regulation of self-renewal and pluripotency by Sox2 in human
embryonic stem cells. Stem Cells. 26:1931–1938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shahryari A, Jazi MS, Samaei NM and Mowla
SJ: Long non-coding RNA SOX2OT: Expression signature, splicing
patterns, and emerging roles in pluripotency and tumorigenesis.
Front Genet. 6:1962015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu S, Xu B and Yan D: Enhanced expression
of long non-coding RNA Sox2ot promoted cell proliferation and
motility in colorectal cancer. Minerva Med. 107:279–286.
2016.PubMed/NCBI
|
|
12
|
Hou Z, Zhao W, Zhou J, Shen L, Zhan P, Xu
C, Chang C, Bi H, Zou J, Yao X, et al: A long noncoding RNA Sox2ot
regulates lung cancer cell proliferation and is a prognostic
indicator of poor survival. Int J Biochem Cell Biol. 53:380–388.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Yang R, Lian J and Xu H: LncRNA
Sox2ot overexpression serves as a poor prognostic biomarker in
gastric cancer. Am J Transl Res. 8:5035–5043. 2016.PubMed/NCBI
|
|
14
|
Meister G: miRNAs get an early start on
translational silencing. Cell. 131:25–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang SH, Wu XC, Zhang MD, Weng MZ, Zhou D
and Quan ZW: Long noncoding RNA H19 contributes to gallbladder
cancer cell proliferation by modulated miR-194-5p targeting AKT2.
Tumour Biol. 37:9721–9730. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su R, Cao S, Ma J, Liu Y, Liu X, Zheng J,
Chen J, Liu L, Cai H, Li Z, et al: Knockdown of SOX2OT inhibits the
malignant biological behaviors of glioblastoma stem cells via
up-regulating the expression of miR-194-5p and miR-122. Mol Cancer.
16:1712017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi XM and Teng F: Up-regulation of long
non-coding RNA Sox2ot promotes hepatocellular carcinoma cell
metastasis and correlates with poor prognosis. Int J Clin Exp
Pathol. 8:4008–4014. 2015.PubMed/NCBI
|
|
19
|
Huber MA, Beug H and Wirth T:
Epithelial-mesenchymal transition: NF-kappaB takes center stage.
Cell Cycle. 3:1477–1480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou X, Yuan P, Liu Q and Liu Z: LncRNA
MEG3 regulates imatinib resistance in chronic myeloid leukemia via
suppressing MicroRNA-21. Biomol Ther (Seoul). 25:490–496. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Lei B, Wu H, Zhang X and Zheng N:
Tumor suppressive role of miR-194-5p in glioblastoma multiforme.
Mol Med Rep. 16:9317–9322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Q, Wei T, Shim K, Wright K, Xu K,
Palka-Hamblin HL, Jurkevich A and Khare S: Atypical role of sprouty
in colorectal cancer: Sprouty repression inhibits
epithelial-mesenchymal transition. Oncogene. 35:3151–3162. 2016.
View Article : Google Scholar : PubMed/NCBI
|