Introduction
Breast cancer is one of the most common malignant
tumors in women, accounting for 8–12% of all malignancies (1). Coates et al (2) showed that in 2015 breast cancer affected
approximately 1.4 million new cases, and the incidence is rising.
Breast cancer frequently occurs in developed countries in Europe
and North America, and incidence is highest in the United States
(3). Turner et al (4) predict that the incidence of breast
cancer will exceed 50% within the next 50 years and will become the
second most common malignant tumor after gastric cancer. In
addition, breast cancer at early stage usually shows no obvious
symptoms, and can be easily ignored, leading to the high mortality
rate. Hindié and Groheux (5) showed
that the 5-year survival rate of breast cancer patients was only
62.4%. Because of its high incidence and mortality, breast cancer
has long been a hot clinical research topic. MicroRNAs (miRNAs)
have been proven to participate in the development of many types of
tumors (6–8). Among them, miRNA-206 and miRNA-145 were
proved by Sun et al (9) to be
associated with female ovarian cancer. Therefore, we speculate that
miRNA-206 and miRNA-145 may also show unique expression in breast
cancer. Our study investigated the application values of miRNA-206
and miRNA-145 as prognostic or therapeutic indicators for breast
cancer.
Patients and methods
Patient data
Breast cancer specimens and paracancerous tissues
(within 5 cm around the tumor) of 372 breast cancer patients who
underwent surgical resection in the First Affiliated Hospital of
Shantou University Medical College (Shantou, China) from September
2010 to September 2014 were included. Patients were aged 30–75
years with an average age of 45.32±7.21 years (Table I). Pathological classification and
staging were based on the 2007 International Breast Cancer Typing
Guidelines (10).
 | Table I.Basic information of the patients. |
Table I.
Basic information of the patients.
| Cases (n=372) | No. | % |
|---|
| Age (years) |
|
<50 | 152 | 40.9 |
| ≥50 | 220 | 59.1 |
| Body weight (kg) |
|
<60 | 164 | 44.1 |
| ≥60 | 208 | 55.9 |
| Residence |
| City | 254 | 68.3 |
|
Countryside | 118 | 31.7 |
| Ethnicity |
| Han
nationality | 364 | 97.8 |
|
Minority | 8 | 2.2 |
| Types |
|
Non-invasive cancer | 97 | 26.1 |
| Early
invasive cancer | 134 | 36.0 |
| Special
type invasive cancer | 51 | 13.7 |
|
Non-special type invasive
cancer | 90 | 24.2 |
| Pathological
staging |
| I | 46 | 12.4 |
| II | 96 | 25.8 |
| III | 136 | 36.6 |
| IV | 94 | 25.3 |
| T staging |
| Tis | 35 | 9.4 |
| T1 | 94 | 25.3 |
| T2 | 127 | 34.1 |
| T3 | 62 | 16.7 |
| T4 | 54 | 14.5 |
| N staging |
| N1 | 86 | 23.1 |
| N2 | 174 | 46.8 |
| N3 | 112 | 30.1 |
| Distant
metastasis |
| Yes | 243 | 65.3 |
| No | 129 | 34.7 |
Inclusion and exclusion criteria
Inclusion criteria were: Patients confirmed with
breast cancer by pathological biopsy in the First Affiliated
Hospital of Shantou University Medical College. Cancerous tissue
was placed in liquid nitrogen and stored at −80°C immediately after
surgical resection. Before the operation, patients were not treated
with radiotherapy or chemotherapy and patients with complete
medical record. Exclusion criteria were: Combined with other
cardiovascular and cerebrovascular diseases, respiratory tract and
gastrointestinal disease patients, pregnant women, long-term
bedridden patients, patients with physical disabilities,
surgery-intolerant patients, patients transferred to other
hospitals during treatment and patients who received unauthorized
treatment. The study was approved by the Ethics Committee of the
First Affiliated Hospital of Shantou University Medical College.
Signed informed consents were obtained from the patients or
guardians.
Main instruments and reagents
LightCycler real-time PCR instrument (Roche
Diagnostics, Basel, Switzerland), total RNA extraction TRIzol kit
(Invitrogen; Thermo Fisher Scientific, Inc., Carlsbad, CA, USA),
M-MLV reverse transcriptase kit was from Vazyme Biotech Co., Ltd.,
(Nanjing, China). miR-206, miR-145 and real-time PCR kit from
Biomiga China (Shanghai, China). Primers of miR-206, miR-145 and U6
(endogenous control) used in PCR reaction were synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China) (Table II).
 | Table II.Primers of miR-206, miR-145 and
U6. |
Table II.
Primers of miR-206, miR-145 and
U6.
|
| Primer sequences |
|---|
| miR-206 | F |
5′-ATCCAGTGCGTGTCGTG-3′ |
|
| R |
5′-TGCTTGGAATGTAAGGAAG-3′ |
| miR-145 | F |
5′-ACACTCCAGCTGGGCAGGTCAAAAGGGTCC-3′ |
|
| R |
5′-GGTGTCGTGGAGTCG-3′ |
| U6 | F |
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
|
| R |
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
Detection methods
Breast cancer tissue (80 mg) was ground in liquid
nitrogen. TRIzol reagent was added and mixed, and the mixture was
kept at room temperature for 30 min. Total RNA was extracted in
strict accordance with the manufacturer's instructions. The
extracted RNA was tested by ultraviolet spectrophotometer (Bio Rad,
Hercules, CA, USA) and electrophoresis to determine the
concentration and purity. Total RNA was then reverse-transcribed
according to the instructions of reverse transcription kit, and
cDNA samples were stored at −20°C. PCR reaction system was prepared
according to the manufacturer's instructions (10.5 µl), and DEPC
water was added to make a 20 µl volume. PCR reaction conditions:
94°C for 10 min, followed by 40 cycles of 94°C for 45 sec, 60°C for
45 sec and 72°C for 45 sec. Data were analyzed using the software
provided by the manufacturer by 2−ΔΔCq method (11). U6 was used as endogenous control. The
average of three replicates was used as the final result.
Statistical analysis
SPSS 22.0 statistical software was used for data
analysis. Measurement data are expressed as mean × standard
deviation (SD), comparisons between two groups was performed by
t-test. Enumeration data were expressed as rate. Survival curves
were plotted using Kaplan-Meier method and compared by log-rank
test. P<0.05 indicated that the difference was statistically
significant.
Results
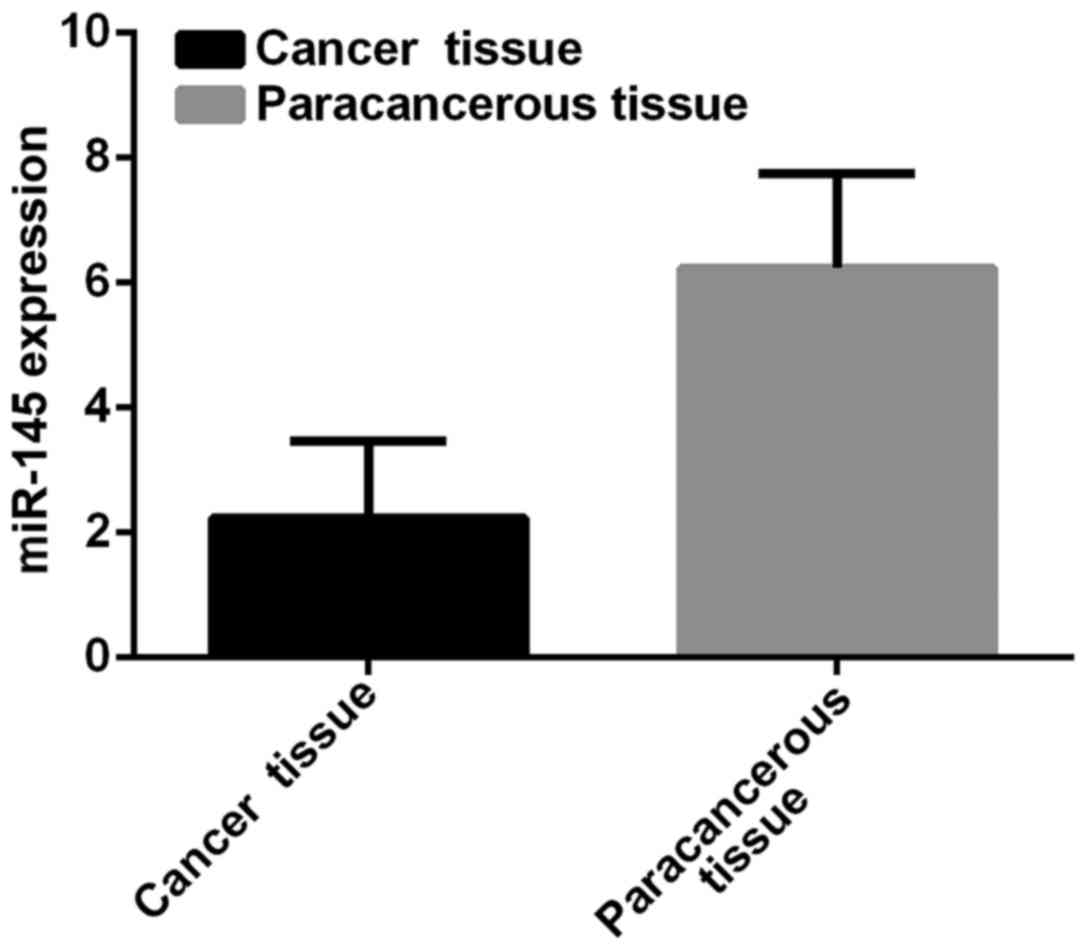
miR-145 expression
Expression level of miR-145 in breast cancer tissues
was 2.24±1.23, and in paracancerous tissues was 6.24±1.51.
Expression level of miR-145 in breast cancer tissues was
significantly lower than that in paracancerous tissues (t=39.61,
p<0.001) (Fig. 1).
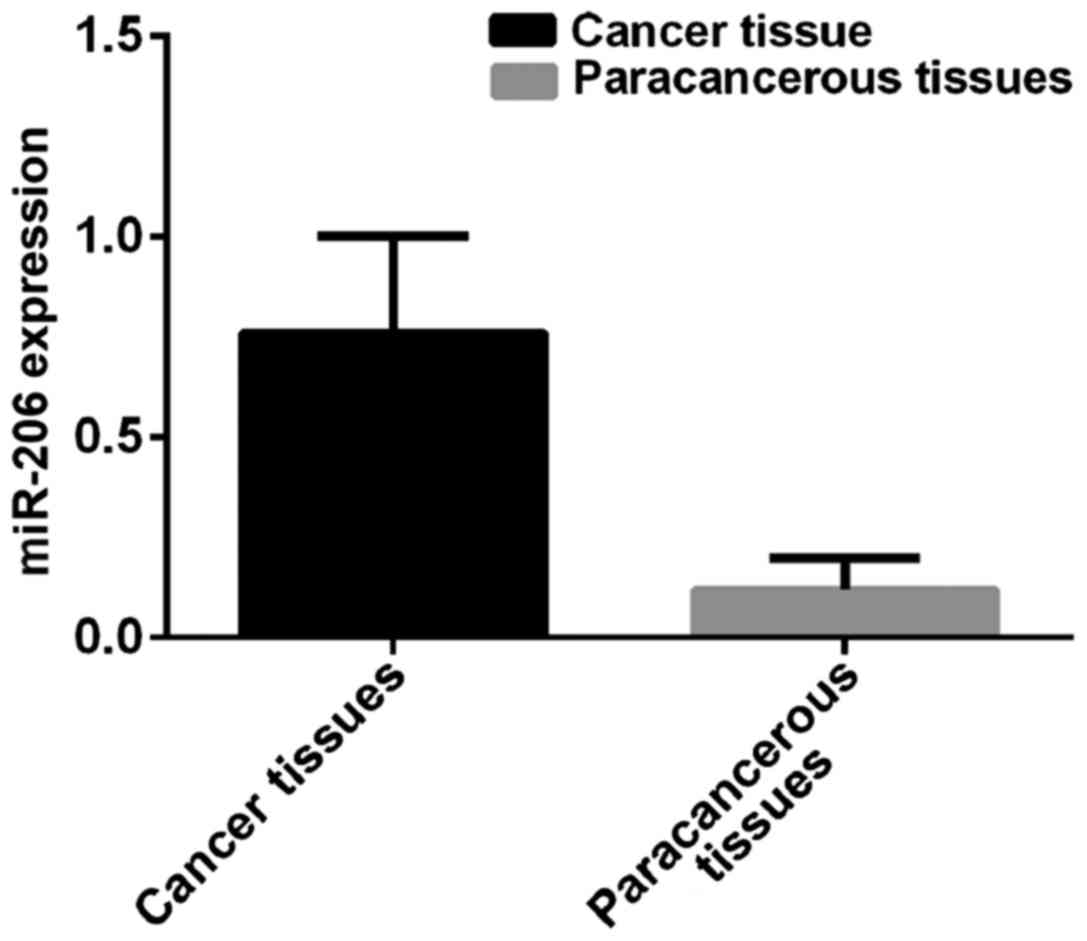
miR-206 expression
Expression level of miR-206 in breast cancer tissues
was 0.76±0.24, and in paracancerous tissues was 0.12±0.08.
Expression level of miR-206 in breast cancer tissues was
significantly higher than that in paracancerous tissues (t=48.79,
p<0.001) (Fig. 2).
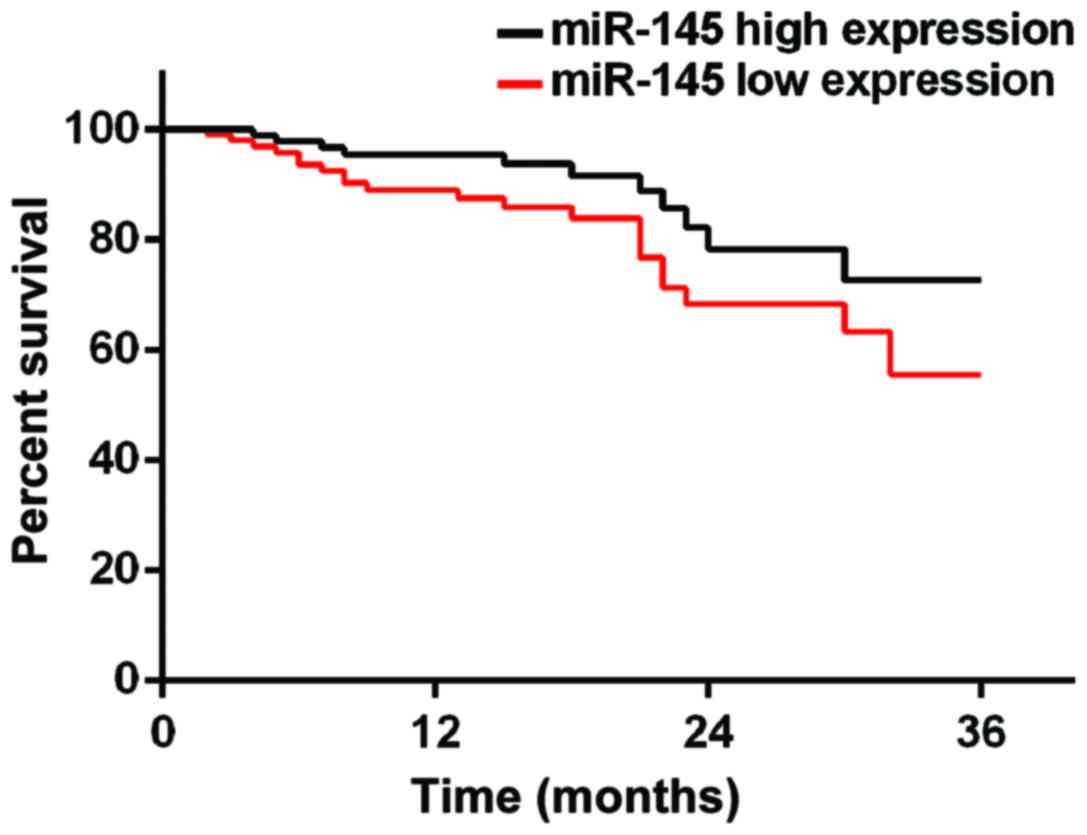
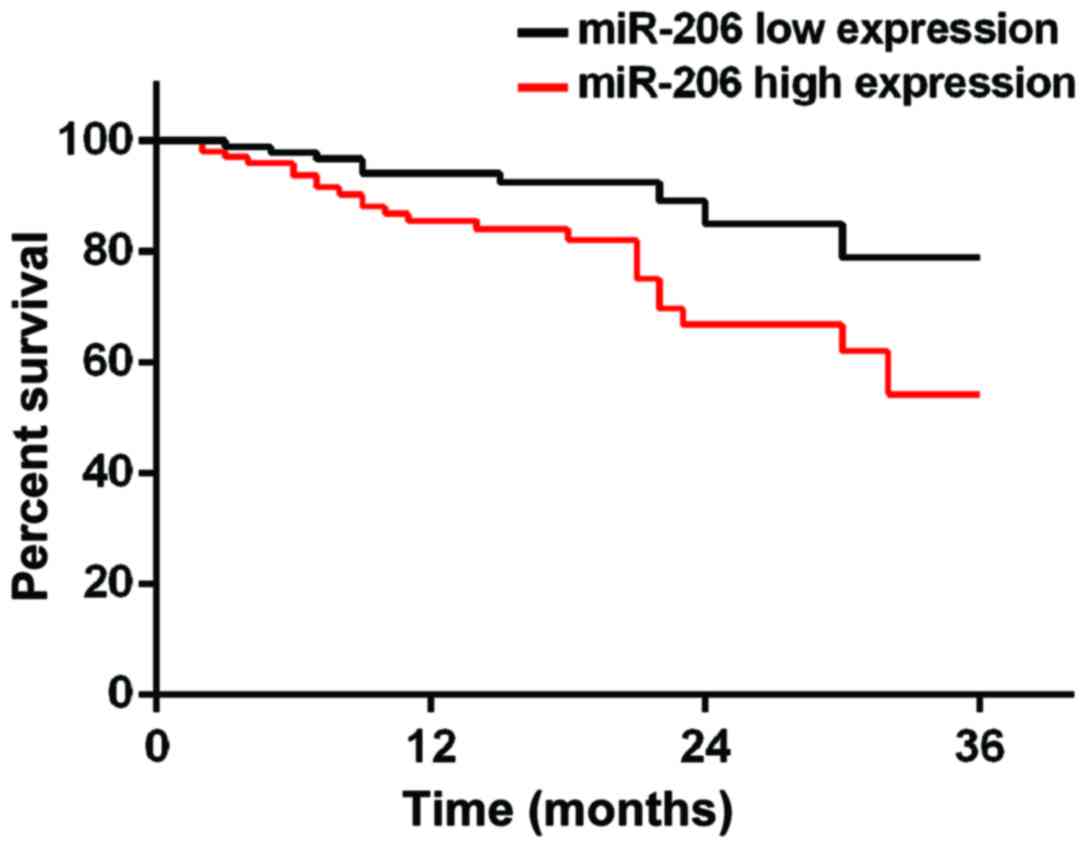
Prognosis of patients
According to the median value of miR-145 and miR-206
expression, patients were divided into miR-145 high expression
group (≥2.24, 219 cases), miR-145 low expression group (<2.24,
153 cases), miR-206 high expression group (≥0.76, 194 cases) and
miR-206 low expression group (<0.76, 178 cases). Patients were
followed up for 3 years by telephone, review and mail. Follow-up
was performed until December 2017 or death of the patients. A total
of 354 patients finished the follow-up, and follow-up success rate
was 95.2%. Survival rates at 1, 2 and 3 years in miR-145 low
expression group were 83.1, 71.2 and 59.8%, respectively, while
survival rates at 1, 2 and 3 years in miR-145 high expression group
were 89.5, 79.1 and 70.6%, which were significantly better than
those in miR-145 low expression group (p=0.028). Survival rates at
1, 2 and 3 years in miR-206 high expression group were 77.3, 67.5
and 55.7%, respectively, while survival rates at 1, 2 and 3 years
in miR-206 low expression group were 89.9, 81.5 and 75.8%,
respectively, which were significantly higher than those in miR-206
high expression group (p=0.034) (Figs.
3 and 4).
Discussion
Breast cancer is a malignant tumor that seriously
affects life and health of females. Incidence and mortality of
breast cancer rank in the top area among all malignancies (12). Breast cancer at early stage shows no
obvious symptoms and most patients are diagnosed at advanced
stages, and thus missing the best treatment time, leading to poor
prognosis. At present, pathogenesis of breast cancer is still
unclear. Poortmans et al (13)
believed that the occurrence of breast cancer is mainly caused by
genetic factors, while Tutt et al (14) showed that the occurrence of breast
cancer is closely related to cancer stem cells. miRNAs as a group
of endogenous non-protein-coding RNA (15,16) have
been proved to be directly involved in tumorigenesis and
development. Most miRNAs are highly conserved, cell-specific, and
have a strong ability to regulate cell proliferation and apoptosis
(17). Among them, miR-206 plays a
key role in the regulation of cell proliferation, apoptosis,
invasion and migration. miR-206 may play a role as a tumor
suppressor gene and may also have oncogenic functions and has been
proven to promote muscle differentiation by downregulating the P180
subunit of DNA polymerase and muscle transcription factors
(18). miR-145 is expressed in many
eukaryotic organisms and plays a role in regulating gene expression
and has multiple targets that are associated with oncogenes
(19). With the deepening of
research, miR-206 and miR-145 have been proven to be closely
correlated with breast cancer. Therefore, in this study, expression
levels of miR-206 and miR-145 in breast cancer and paracancerous
tissues were measured, and the correlation with prognosis were
analyzed with an expectation of providing references for diagnosis
and treatment of breast cancer.
The results of this study indicate that miR-206 is
upregulated in breast cancer tissues and miR-145 is downregulated
in breast cancer tissues. This is in agreement with the finding by
Kim et al (20) and Oksuz
et al (21) on the role of
miR-206 and miR-145 in ovarian and uterine cancer, suggesting that
miR-206 and miR-145 may be involved in the occurrence and
development of breast cancer. Expression of miR-206 and miR-145 may
be related to the severity of breast cancer, the degree of
differentiation, lymph node metastasis and the depth of invasion.
miR-206 and miR-145 can be used as tumor markers in the early
diagnosis of breast cancer because they can downregulate mRNA
expression of ERα and its coregulatory proteins, inhibit the
proliferation of breast cancer cells and participate in the
development of breast cancer. However, the mechanism of miR-206 and
miR-145 in breast cancer remains unclear.
miR-206 and miR-145 may also inhibit the development
of breast cancer. Because miR-206 is upregulated in breast cancer
and miR-145 is downregulated, and high level of miR-206 expression
and level of miR-145 expression was correlated with poor prognosis,
suggesting that miR-206 and miR-145 can be used as a prognostic
indicator for patients with breast cancer.
There are still some shortcomings in this study. For
example, the sample size was small, and it is not ruled out that
there may be differences in expression levels of miR-206 and
miR-145 among different age groups. We will conduct a longer
follow-up survey of patients in this study to confirm our
conclusions.
In conclusion, miR-206 is upregulated and miR-145 is
downregulated in breast cancer tissues, which may affect the
prognosis of patients. miR-206 and miR-145 may be used as important
prognostic indicators for patients with breast cancer in the
future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQ wrote the manuscript and was responsible for
collecting the tissues. XH contributed to the extraction of RNA. XQ
performed PCR. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Shantou University Medical College
(Shantou, China). Signed informed consents were obtained from the
patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Finn RS, Crown JP, Lang I, Boer K,
Bondarenko IM, Kulyk SO, Etti J, Patel R, Pinter T, Schmidt M, et
al: The cyclin-dependent kinase 4/6 inhibitor palbociclib in
combination with letrozole versus letrozole alone as first-line
treatment of oestrogen receptor-positive, HER2-negative, advanced
breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study.
Lancet Oncol. 16:25–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coates AS, Winer EP, Goldhirsch A, Gelber
RD, Gnant M, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel
Members: Tailoring therapies - improving the management of early
breast cancer: St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2015. Ann Oncol.
26:1533–1546. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swain SM, Baselga J, Kim SB, Ro J,
Semiglazov VM, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, et
al: CLEOPATRA Study Group: Pertuzumab, trastuzumab, and docetaxel
in HER2-positive metastatic breast cancer. N Engl J Med.
372:724–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Turner NC, Ro J, André F, Loi S, Verma S,
Iwata HN, Loibl S, Bartlett Huang C, Zhang K, et al: PALOMA3 Study
Group: Palbociclib in hormone-receptor-positive advanced breast
cancer. N Engl J Med. 373:209–219. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hindié E and Groheux D: Regional nodal
irradiation in early-stage breast cancer. N Engl J Med.
373:1877–1878. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narimatsu R and Patterson BK:
High-throughput cervical cancer screening using intracellular human
papillomavirus E6 and E7 mRNA quantification by flow cytometry. Am
J Clin Pathol. 123:716–723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu TP, Liu XX, Xia R, Yin L, Kong R, Chen
WM, Huang MD and Shu YQ: SP1-induced upregulation of the long
noncoding RNA TINCR regulates cell proliferation and apoptosis by
affecting KLF2 mRNA stability in gastric cancer. Oncogene.
34:5648–5661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim KT, Lee HW, Lee HO, Kim SC, Seo YJ,
Chung W, Eum HH, Nam DH, Kim J, Joo KM, et al: Single-cell mRNA
sequencing identifies subclonal heterogeneity in anti-cancer drug
responses of lung adenocarcinoma cells. Genome Biol. 16:1272015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun C, Liu Z, Li S, Yang C, Xue R, Xi Y,
Wang L, Wang S, He Q, Huang J, et al: Down-regulation of c-Met and
Bcl2 by microRNA-206, activates apoptosis, and inhibits tumor cell
proliferation, migration and colony formation. Oncotarget.
6:25533–25574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA
Jr, et al: Prospective validation of a 21-gene expression assay in
breast cancer. N Engl J Med. 373:2005–2014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poortmans PM, Collette S, Kirkove C, Van
Limbergen E, Budach V, Struikmans H, Collette L, Fourquet A,
Maingon P, Valli M, et al: EORTC Radiation Oncology and Breast
Cancer Groups: Internal mammary and medial supraclavicular
irradiation in breast cancer. N Engl J Med. 373:317–327. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tutt A, Ellis P, Kilburn L, Gilett C,
Pinder S, Abraham J, Barrett S, Barrett-Lee P, Chan S and Cheang M:
The TNT trial: A randomized phase III trial of carboplatin (C)
compared with docetaxel (D) for patients with metastatic or
recurrent locally advanced triple negative or BRCA1/2 breast cancer
(CRUK/07/012). Cancer Res. 75 9 Suppl:Abst S3-01. 2015. View Article : Google Scholar
|
|
15
|
Huang X, Yuan T, Liang M, Du M, Xia S,
Dittmar R, Wang D, See W, Costello BA, Quevedo F, et al: Exosomal
miR-1290 and miR-375 as prognostic markers in castration-resistant
prostate cancer. Eur Urol. 67:33–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zargar H, Espiritu PN, Fairey AS, Mertens
LS, Dinney CP, Mir MC, Krabbe LM, Cookson MS, Jacobsen NE, Gandhi
NM, et al: Multicenter assessment of neoadjuvant chemotherapy for
muscle-invasive bladder cancer. Eur Urol. 67:241–249. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gupta Y, Möller S, Witte M, Belheouane M,
Sezin T, Hirose M, Vorobyev A, Niesar F, Bischof J, Ludwig RJ, et
al: Dissecting genetics of cutaneous miRNA in a mouse model of an
autoimmune blistering disease. BMC Genomics. 17:1122016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ge X, Lyu P, Cao Z, Li J, Guo G, Xia W and
Gu Y: Overexpression of miR-206 suppresses glycolysis,
proliferation and migration in breast cancer cells via PFKFB3
targeting. Biochem Biophys Res Commun. 463:1115–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eades G, Wolfson B, Zhang Y, Li Q, Yao Y
and Zhou Q: lincRNA-RoR and miR-145 regulate invasion in
triple-negative breast cancer via targeting ARF6. Mol Cancer Res.
13:330–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim TH, Song JY, Park H, Jeong JY, Kwon
AY, Heo JH, Kang H, Kim G and An HJ: miR-145, targeting
high-mobility group A2, is a powerful predictor of patient outcome
in ovarian carcinoma. Cancer Lett. 356:(2 Pt B). 1–945. 2015.
View Article : Google Scholar
|
|
21
|
Oksuz Z, Serin MS, Kaplan E, Dogen A,
Tezcan S, Aslan G, Emekdas G, Sezgin O, Altintas E and Tiftik EN:
Serum microRNAs; miR-30c-5p, miR-223-3p, miR-302c-3p and miR-17-5p
could be used as novel non-invasive biomarkers for HCV-positive
cirrhosis and hepatocellular carcinoma. Mol Biol Rep. 42:713–720.
2015. View Article : Google Scholar : PubMed/NCBI
|