Introduction
Steroids, including androgens and estrogens
(E2), have been demonstrated to exert multiple effects
not only on reproductive function, but also on numerous other organ
systems, including the liver, in men and women (1). For example, previous human studies have
demonstrated that female menopause has been associated with the
increase in non-alcoholic fatty liver disease, hepatocellular
carcinoma (HCC) and the progression of fibrosis (2). Furthermore, animal studies also revealed
that bilateral ovariectomy increased the risk of HCC (3). There appears to be a sex difference in
the survival of patients with HCC: HCC is a male-dominated cancer,
with men 4–8-fold more likely to develop HCC than women; however,
testosterone may act to protect against hepatic steatosis (4–6).
Additionally, high levels of aromatase, an enzyme catalyzing the
conversion of testosterone into E2, has been detected
within the human liver, and aromatase overexpression has also been
identified in hepatitis and HCC (7,8).
Furthermore, aromatase gene-knockout mice exhibited hepatic glucose
intolerance, which was able to be reversed by E2
administration (9). Recent studies
have identified that high circulating E2 and low
testosterone ratio may be associated with adverse clinical outcomes
in men with advanced liver disease and patients with primary liver
cancer (10,11).
The action of steroids is known to be mediated by
their receptors. Androgen receptor (AR) has been detected in normal
and cancerous liver tissue and cell lines; estrogen receptor (ER) α
and decreased expression of ERβ have also been identified in HCC
(5,12,13). These
receptors have been demonstrated to regulate lipid and glucose
metabolism in the liver. For example, ERs function to decrease
lipogenesis, gluconeogenesis and fatty acid uptake, but enhance
lipolysis, cholesterol secretion and glucose catabolism; AR
functions to increase insulin receptor expression and glycogen
synthesis, decrease glucose uptake and lipogenesis, and promote
cholesterol storage (14).
Additionally, studies have revealed that expression of ERα was
associated with invasion and metastasis in HCC, and AR and ERα, but
not ERβ, gene expression contributed to the prevalence of HCC in
male rats (15,16).
Cholangiocellular carcinoma (CCC) is difficult to
treat due to its chemo-resistance and its inability to be detected
at an early stage of disease (17).
It has been reported that, compared with males, females are more
susceptible to several biliary tract diseases such as primary
biliary cirrhosis, debilitating/symptomatic adult polycystic liver
disease and autoimmune hepatitis (18). The significant increase of estrogen
levels in the serum of patients with CCC has been reported
(19), and has also been demonstrated
to enhance the proliferation and invasiveness of CCC cells in
vitro. Furthermore, the survival time of patients with CCC is
associated with estrogen levels (20). Additionally, different levels of ERα
and ERβ have been detected in CCC cells (18,21), ERα
has been demonstrated to mediate estrogenic stimulation of
interleukin-6 production and thus influence the pathology of CCC
(18), the overexpression of ERβ has
also been demonstrated to exhibit protective abilities against CCC
(21).
Previous studies have demonstrated that steroid
receptor coactivators (SRCs) are required for the transcriptional
activation of target genes by a steroid receptor (22,23). Among
SRCs, the p160 family members SRC-1 and SRC-3 have been
investigated in a number of types of cancer including tissue and
cell lines (24). For example,
overexpression of SRC-1 and SRC-3 has been detected in breast
cancer, non-small cell lung cancer, bone cancer and chondrosarcoma
(25–31). Decreased expression of SRC-3 has been
reported in astrocytic tumors and decreased expression of SRC-1 and
SRC-3 has been identified in meningothelial tumors and
neuroepithelial tumors (32,33). In liver tissue, an early study
demonstrated that SRCs serve a role in the regulation of hepatic
energy homeostasis (34), and further
studies highlighted that SRC-1 was a critical mediator of glucose
homeostasis as it functioned as the integrator of glucose and
oxidized/reduced nicotinamide-adenine dinucleotide homeostasis
(35,36). Thus, hepatic SRC-1 activity may have
potential relevance for human metabolic pathogenesis (37). However, the expression profiles of
SRC-1 and SRC-3 in HCC and CCC have not yet been reported. To
address this question, the present study investigated the
expression and significance of these two coactivators in HCC and
CCC using tissue microarray (TMA) immunohistochemistry.
Materials and methods
Tissue microarray
The two types of hepatic carcinoma and normal TMA
used were purchased from US Biomax, Inc. (cat. no. BC03118;
Rockville, MD, USA). The TMA contained 75 cases of malignant HCC
(65 males and 10 females; mean age, 50.8 years), 15 cases of
malignant CCC (8 males and 7 females; mean age, 48.5 years) and 10
cases of normal hepatic tissue (6 males and 4 females; mean age,
26.8 years). In total, 200 tissue cores featured on a single slide
as 2 cores were punched from each case.
Immunohistochemistry
TMAs were deparaffinized in xylene, rehydrated with
a gradient alcohol series and heat-mediated antigen retrieval (0.01
M sodium citrate buffer, pH 6.0) was performed according to our
previous protocol (32,33). The sections (5-µm thick) were washed
with PBS (0.01 mol/l, pH 7.4) prior to being blocked with 3%
H2O2 for 15 min. TMAs were incubated for 30
min with normal goat serum (2%, v/v) to inhibit non-specific
binding and then incubated with rabbit polyclonal antibody against
SRC-1 (1:200; cat. no. sc-8995; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) or SRC-3 (1:200; cat. no. sc-25742; Santa Cruz
Biotechnology, Inc.) at 4°C for 48 h. Following washing with PBS,
sections were incubated with biotinylated goat-anti-rabbit
secondary antibody (1:200; cat. no. ZB2010; OriGene Technologies,
Inc., Beijing, China) for 1 h at room temperature. The sections
were then washed with PBS, incubated with horseradish
peroxidase-labeled streptavidin (1:200; cat. no. ZB2404; OriGene
Technologies, Inc.) for 1 h at room temperature. Finally, sections
were incubated with a 3,3′-diaminobenzidine-peroxidase substrate
kit (cat. no. ZLI-9018; OriGene Technologies, Inc.) for 5 min at
room temperature. Blank controls were carried out using the same
procedure; however, primary antiserum was replaced with Antibody
Diluent (ZLI-9028, OriGene Technologies, Inc.) according to that
manufacturer's protocol.
Image acquisition and data
analysis
Images of immunohistochemical staining were captured
using a digital camera (DP70; Leica, Germany) equipped with an
Olympus microscope (BX60; Olympus Corporation, Tokyo, Japan; under
×20 or ×40 magnification). The strength of staining was scored in
accordance with a four-point system (0–3) described in a previous
study (32) by a pathologist
double-blindly. A score of 3 indicated visible dark staining of
>50% of cells; a score of 2 indicated either dark focal staining
of <50% of cells or moderate staining of >50%; a score of 1
indicated either moderate focal staining of <50% of cells or
pale staining in any proportion of cells not easily seen at low
power; a score of 0 indicated no positive staining. A high level of
expression was defined as a score of 2 or 3, and a low level of
expression was defined as a score of 0 or 1. Pathologically,
early-stage liver cancer was defined as stage I and II; advanced
stage was defined as stage III and IIIb.
Statistical analysis
All data are expressed as n (%) and compared using a
χ2 test or Fisher's exact test with SPSS software
(version 18.0; SPSS, Inc., Chicago, IL, USA). All P-values were
two-tailed and P<0.05 was considered to indicate a statistically
significant difference.
Results
Subcellular localization of SRCs in
normal and cancerous liver tissue
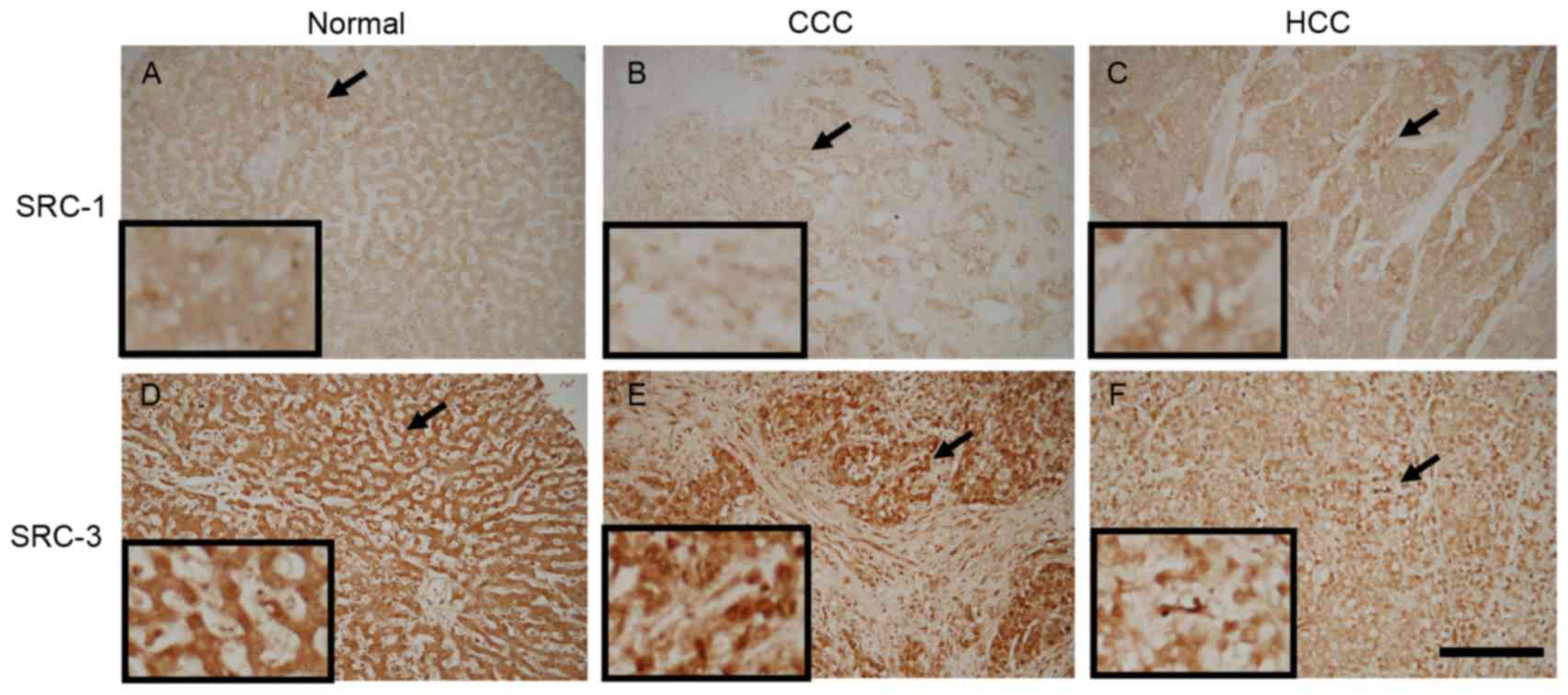
Results presented in Fig.
1 demonstrate the localization patterns of SRC-1- and
SRC-3-immunoreactive materials. In normal liver and HCC tissue,
SRC-1-immunoreactive materials were predominantly detected within
the extra-nuclear component. However, in CCC, SRC-1 materials were
predominantly detected within the cell nuclei. SRC-3-immunoreactive
materials were primarily detected within the cell nuclei.
Expression profiles of SRCs in normal
and cancerous liver tissue
High levels of SRC-1 expression was detected in 30%
(3/10) of the normal liver tissue, 9.3% (7/75) of HCC tissue and
6.7% (1/15) of CCC tissue. The χ2 test indicated no
statistically significant differences in association with the
expression levels of SRC-1 between normal and HCC, normal and CCC,
or HCC and CCC samples (Table I and
Fig. 2A-C). High levels of SRC-3
expression was detected in 40% (4/10) of the normal liver tissue,
36% (27/75) of HCC tissue and 67.7% (10/15) of CCC tissue. The
χ2 test indicated no statistical significance in the
expression levels of SRC-3 between normal and HCC or normal and CCC
samples (Table I, Fig. 2D and E). However, expression of SRC-3
in CCC was significantly increased compared with HCC (67.7 vs.
6.7%; P=0.028) as indicated in Table
I and Fig. 2F.
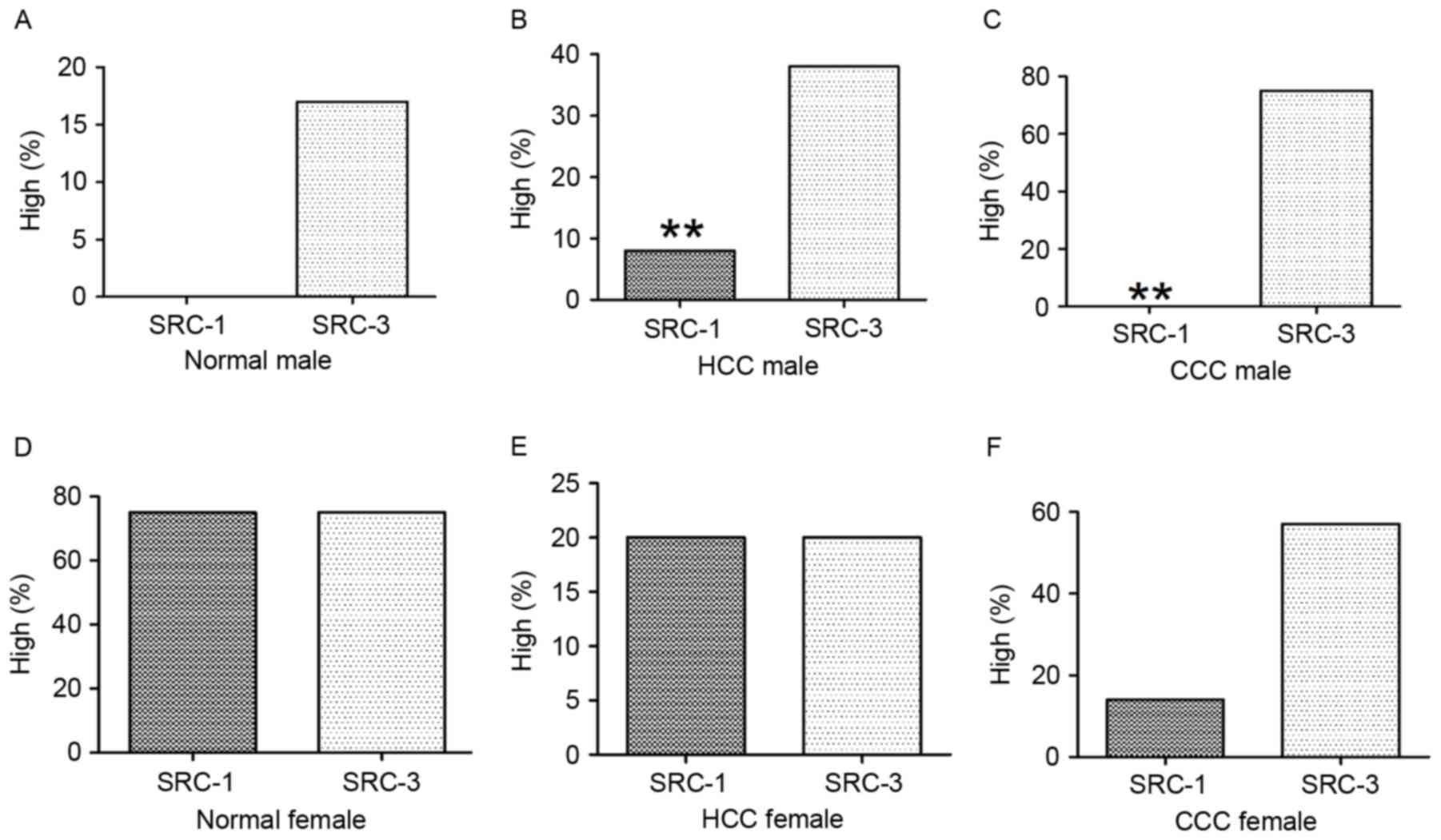
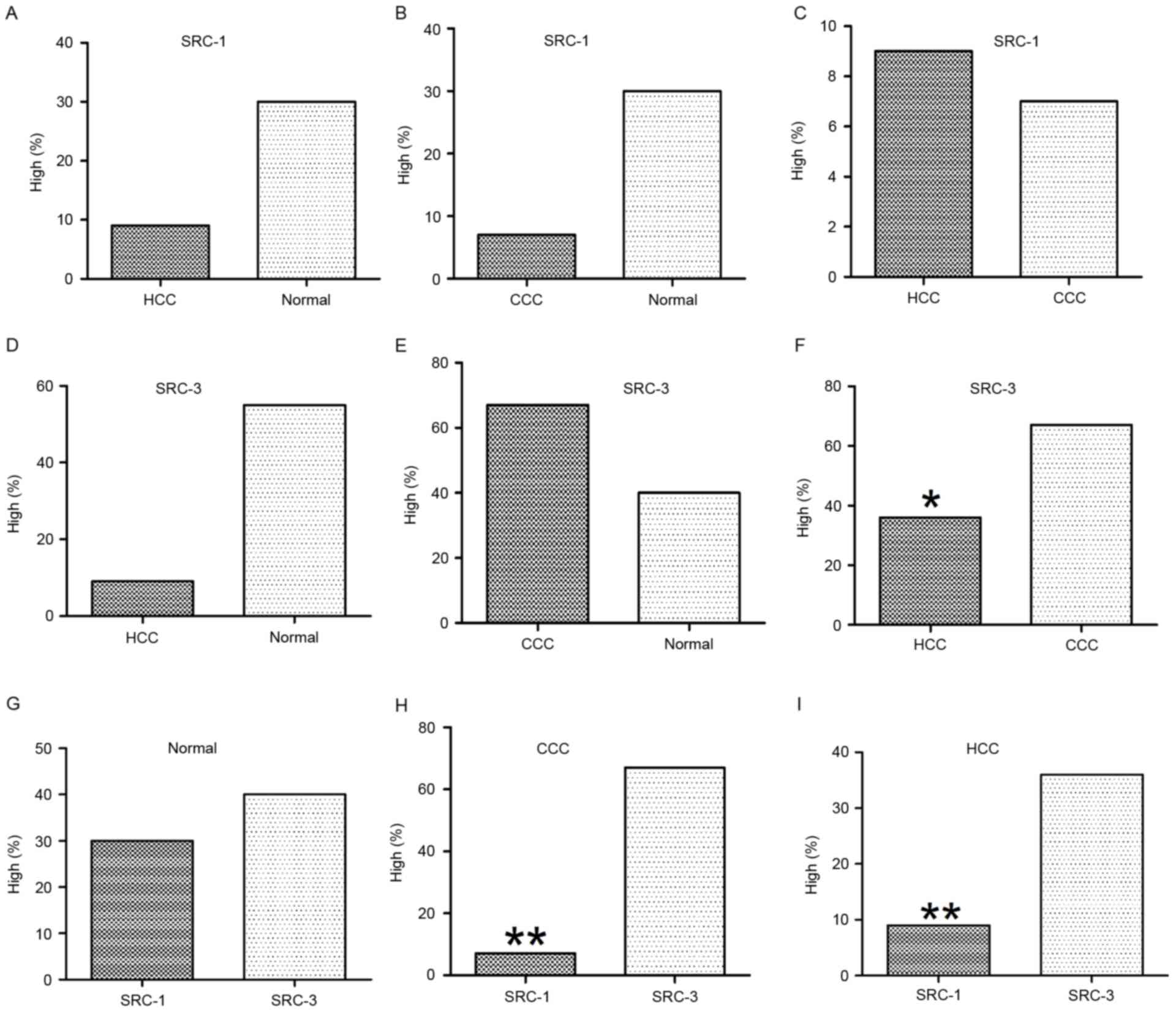 | Figure 2.Statistical analysis of SRC-1 and
SRC-3 immunoreactivities in normal and cancerous liver tissue (HCC
and CCC). The expression of SRC-1 did not reveal significant
differences between (A) HCC and normal, (B) CCC and normal, or (C)
HCC and CCC. Expression of SRC-3 did not reveal significant
differences between (D) HCC and normal, or (E) CCC and normal. (F)
However, SRC-3 was significantly increased in CCC when compared
with that of HCC. Comparison of SRC-1 and SRC-3 in (G) normal, (H)
CCC and (I) HCC tissue. In normal liver tissue, expression of SRC-1
and SRC-3 did not reveal a significant difference. However, in CCC
and HCC, expression of SRC-1 was significantly decreased when
compared with that of SRC-3. *P<0.05; **P<0.01. SRC, steroid
receptor coactivator; CCC, cholangiocellular carcinoma; HCC,
hepatocellular carcinoma. |
 | Table I.Expression of SRC-1 and SRC-3 in
normal, HCC and CCC. |
Table I.
Expression of SRC-1 and SRC-3 in
normal, HCC and CCC.
|
| SRC-1 | SRC-3 |
|---|
|
|
|
|
|---|
| Tissue | High | Low | χ2 | P-value | High | Low | χ2 | P-value |
|---|
| Normal | 3 | 7 | 1.913 | >0.05 | 4 | 6 | 0.000 | >0.05 |
| HCC | 7 | 68 |
|
| 27 | 48 |
|
|
| Normal | 3 | 7 | 1.004 | >0.05 | 4 | 6 | 0.818 | >0.05 |
| CCC | 1 | 14 |
|
| 10 | 5 |
|
|
| HCC | 7 | 68 | 0.000 | >0.05 | 27 | 48 | 4.856 | 0.028 |
| CCC | 1 | 14 |
|
| 10 | 5 |
|
|
Comparison of the expression profile
of SRCs in normal and cancerous liver tissue
Comparisons of SRC-1 and SRC-3 expression profiles
in normal and liver cancer tissue were performed in order to
determine any statistical significance. Normal liver tissue results
identified that 30% (3/10) exhibited high levels of SRC-1
expression and 40% (4/10) exhibited high levels of SRC-3
expression. The difference in expression profiles was not
statistically significant (P>0.05). In HCC, 9.3% (7/75)
exhibited high levels of SRC-1 expression and 36% (27/75) exhibited
high levels of SRC-3 expression. The HCC results indicated that
SRC-3 expression was significantly increased compared with SRC-1
expression (P<0.01). In CCC results, 6.7% (1/15) exhibited high
levels of SRC-1 expression, whereas SCR-3 expression was
significantly increased in comparison (P=0.02) at 67.7% (10/15).
These results are presented in Table
II and Fig. 2G-I.
 | Table II.Comparison of the expression of SRC-1
and SRC-3 in normal, HCC and CCC. |
Table II.
Comparison of the expression of SRC-1
and SRC-3 in normal, HCC and CCC.
|
| Normal | HCC | CCC |
|---|
|
|
|
|
|
|---|
| SRC | High | Low | χ2 | P-value | High | Low | χ2 | P-value | High | Low | χ2 | P-value |
|---|
| 1 | 3 | 7 | 0.000 | >0.05 | 7 | 68 | 15.213 | <0.01 | 1 | 14 | 9.187 | 0.002 |
| 3 | 4 | 6 |
|
| 27 | 48 |
|
| 10 | 5 |
|
|
Sex-specific analysis of SRCs in normal
and cancerous liver tissue
Male-specific analysis
The normal tissue group revealed that of the 6
cases, none exhibited high levels of SRC-1 and only 1 exhibited
high levels of SRC-3; these results were not statistically
significant (P>0.05). The HCC tissue group revealed that of 65
cases, 7.7% (5/65) exhibited high levels of SRC-1, whereas SCR-3
was significantly increased (P<0.01) at 38.5% (25/65). In CCC,
of 8 cases none exhibited high levels of SRC-1, whereas 75% (6/8)
exhibited high levels of SRC-3. SRC-3 expression was significantly
increased compared with that of SRC-1 (P<0.01). These results
are presented in Table III and
Fig. 3.
 | Table III.Sex-specific comparison of SRC-1 and
SRC-3 in normal, HCC and CCC. |
Table III.
Sex-specific comparison of SRC-1 and
SRC-3 in normal, HCC and CCC.
|
|
| Male | Female |
|---|
|
|
|
|
|
|---|
| Tissue | SRC | High | Low | χ2 | P-value | High | Low | χ2 | P-value |
|---|
| Normal | 1 | 0 | 6 |
0.000 | >0.05 | 3 | 1 | 0.000 | >0.05 |
|
| 3 | 1 | 5 |
|
| 3 | 1 |
|
|
| HCC | 1 | 5 | 60 | 17.333 | <0.01 | 2 | 8 | 0.000 | >0.05 |
|
| 3 | 1 | 5 |
|
| 3 | 1 |
|
|
| CCC | 1 | 0 | 6 |
6.667 |
0.010 | 3 | 1 | 1.244 | >0.05 |
|
| 3 | 1 | 5 |
|
| 3 | 1 |
|
|
Female-specific analysis
The normal tissue group revealed that of the 4
cases, 75% (3/4) exhibited high levels of SRC-1 and 75% (3/4)
exhibited high levels of SRC-3; these results were not
statistically significant (P>0.05). The HCC tissue group
demonstrated that of 10 cases, 20% (2/10) exhibited high levels of
SRC-1 and 20% (2/10) exhibited high levels of SRC-3; these results
were not statistically significant (P>0.05). The CCC tissue
group demonstrated that of 7 cases of CCC, 14.3% exhibited high
levels of SRC-1 (1/7) and 57.1% (4/7) exhibited high levels of
SRC-3; these results were not statistically significant
(P>0.05). These results are presented in Table III and Fig. 3.
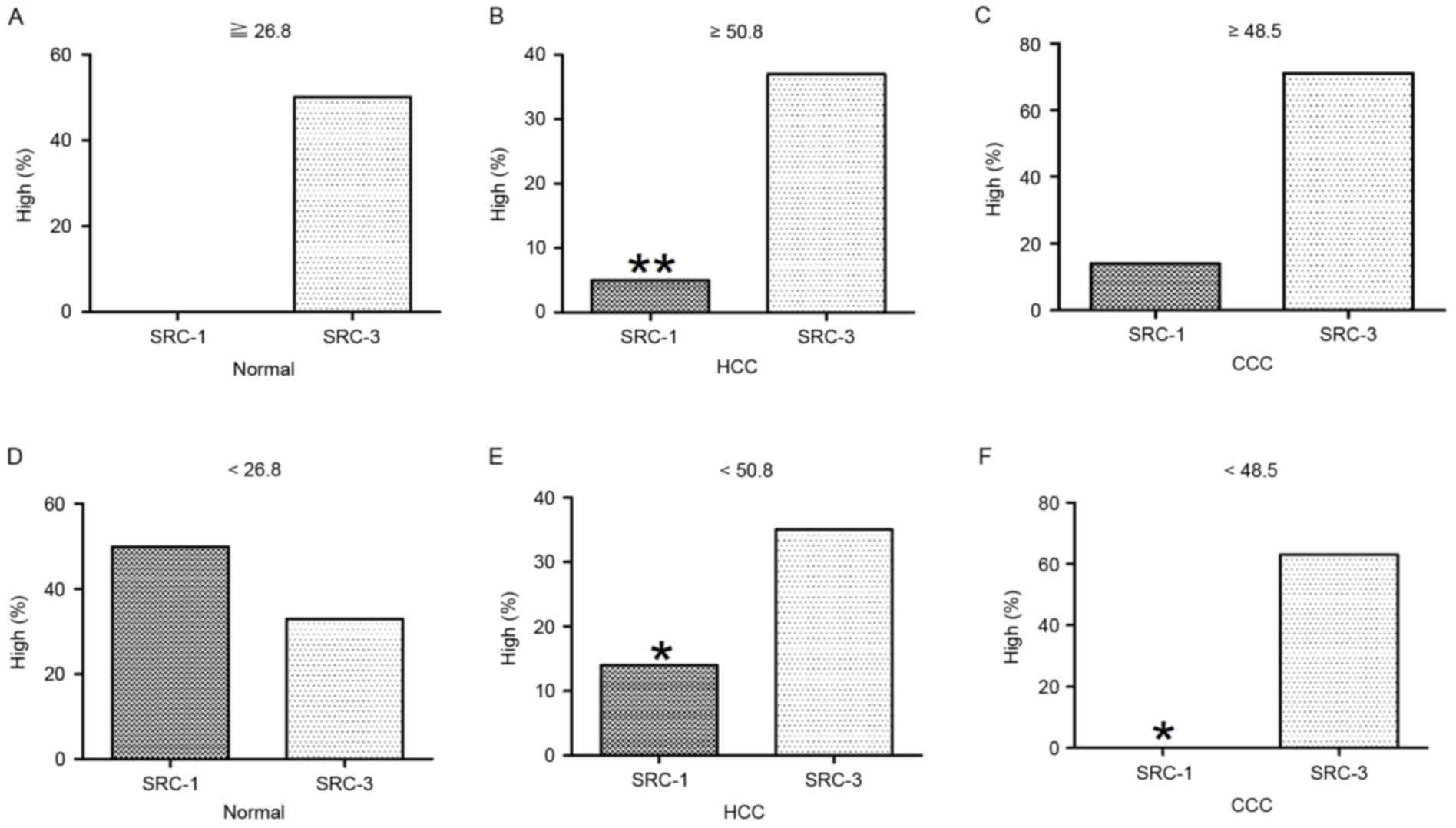
Age-specific analysis of SRCs in
normal and cancerous liver tissue
The mean age of normal cases was 26.8 years (n=10).
A total of 4 normal cases were ≥26.8 years, none of which exhibited
high levels of SRC-1 and 50% (2/4) exhibited high levels of SRC-3;
these results were not statistically significant (P>0.05). A
total of 6 normal cases were <26.8 years, 50% (3/6) exhibited
high levels of SRC-1 and 33.3% (2/6) exhibited high levels of
SRC-3; these results were not statistically significant
(P>0.05). The mean age of HCC cases was 50.8 years (n=75). A
total of 38 HCC cases were ≥50.8 years, of which 5.3% (2/38)
exhibited high levels of SRC-1 and 36.8% (14/38) exhibited high
levels of SRC-3. This was significantly increased compared with
that of SRC-1 (P=0.02). The remaining 37 cases were <50.8 years,
13.5% (5/37) exhibited high levels of SRC-1 and 35.1% (13/37)
exhibited high levels of SRC-3; this was also significantly
increased compared with that of SRC-1 (P=0.030). The mean age of
CCC cases was 48.5 years (n=15). A total of 7 CCC cases were ≥48.5
years, 14.3% (1/7) exhibited high levels of SRC-1 and 71.4% (5/7)
exhibited high levels of SRC-3; these results were not
statistically significant (P>0.05). The remaining 8 CCC cases
were <48.5 years, none exhibited high levels of SRC-1 and 62.5%
(5/8) exhibited high levels of SRC-3, which was significantly
increased compared with that of SRC-1 (P=0.031). All results are
presented in Table IV and Fig. 4.
 | Table IV.Age-specific comparison of SRC-1 and
SRC-3 in normal, HCC and CCC. |
Table IV.
Age-specific comparison of SRC-1 and
SRC-3 in normal, HCC and CCC.
| Tissue | High | Low | χ2 | P-value | High | Low | χ2 | P-value |
|---|
| Normal |
| ≥26.8 years |
|
| <26.8 years |
|
| SRC-1 | 0 | 4 | 0.667 | >0.05 | 3 | 3 | 0.000 | >0.05 |
| SRC-3 | 2 | 2 |
|
| 2 | 4 |
|
|
| HCC |
| ≥50.8 years |
|
| <50.8 years |
|
|
SRC-1 | 2 | 36 | 9.579 |
0.002 | 5 | 32 | 4.655 |
0.031 |
|
SRC-3 | 14 | 24 |
|
| 13 | 24 |
|
|
| CCC |
| ≥48.5 years |
|
| <48.5 years |
|
|
SRC-1 | 1 | 6 | 2.625 | >0.05 | 0 | 8 | 4.655 |
0.031 |
|
SRC-3 | 5 | 2 |
|
| 5 | 3 |
|
|
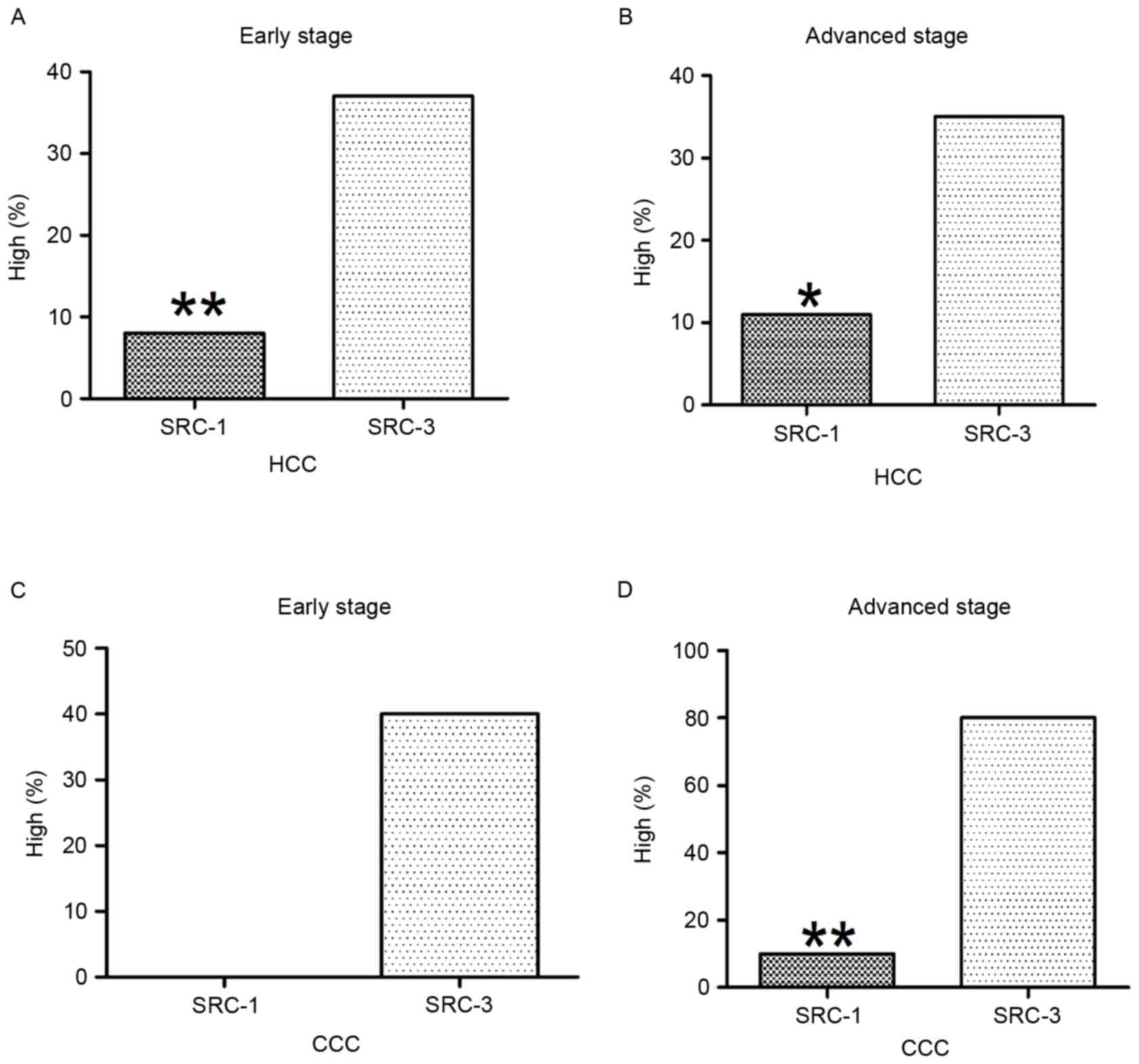
Stage-specific analysis of SRCs in HCC
and CCC
In the early stage of HCC, 7.9% (3/38) exhibited
high levels of SRC-1 and 36.8% (14/38) exhibited high levels of
SRC-3, this was significantly increased compared with that of SRC-1
(P=0.006). In the advanced stage of HCC, 10.8% (4/37) exhibited
high levels of SRC-1 and 35.1% (13/37) exhibited high levels of
SRC-3; this was significantly increased compared with that of SRC-1
(P=0.027). These results are presented in Fig. 5A and B.
In the early stage of CCC, no case exhibited high
levels of SRC-1 and 40% (2/5) exhibited high levels of SRC-3; these
results were not statistically significant (P>0.05). In the
advanced stage of CCC, 10% (1/10) exhibited high levels of SRC-1
and 80% (8/10) indicated high levels of SRC-3; this was
significantly increased compared with that of SRC-1 (P=0.007).
These results are presented in Fig. 5C
and D.
Discussion
It is well known that liver disease is a major
health concern worldwide (38).
Statistics published in 2014 estimated that the number of new liver
cancer cases in the USA totaled 33,190 (24,600 male cases), and the
estimated mortality rate was 23,000 (15,870 male cases). Therefore,
these statistics indicate that liver cancer is the fifth leading
cause of male (15,870) and the ninth leading cause of female
(7,130) cancer mortality in the USA in 2014 (39). Similar results were also reported in
China, where liver cancer was ranked within the top five causes of
cancer-associated mortality with clear male predominance (310,600
vs. 111,500) (40). However, the
molecular mechanisms underlying the occurrence and disease
progression, as well as sex differences observed in liver cancers
are poorly understood. Therefore, in the present study tissue
microarray immunohistochemistry was used in order to compare the
expression profiles of SRC-1 and SRC-3 in normal, HCC and CCC. It
was observed that SRC-1-immunopositive materials were predominantly
detected in the extranuclear component in normal and HCC liver
tissue. However, in CCC, SRC-1 was localized in the cell nuclei,
indicating that plasma-nucleus translocations may contribute to the
pathology of CCC. For SRC-3, the immunoreactive materials were
mainly detected within the cell nuclei in all tissue types
examined. The diversity of subcellular localization of
SRC-immunoreactive materials was in general agreement with previous
studies demonstrating that SRC-1 and SRC-3 would be able to be
detected in cell nuclei and cytoplasm (29,41).
Furthermore, results indicated that expression of
SRC-1 did not demonstrate any significant differences among normal,
HCC and CCC liver tissue; similar phenomena for SRC-3 were also
detected; however, significantly increased levels of SRC-3 were
detected in CCC tissue when compared with those in HCC tissue.
These tissue results indicated that there was an unchanged SRC-1
but an evident overexpression of SRC-3 in HCC and CCC when compared
with that detected in the normal liver tissue. Some previous
studies have also reported different changes of SRC-1 or SRC-3 in
specific cancers compared to normal tissue. For example,
overexpression of SRC-1 is present in breast and ovarian cancer
cell lines as well as primary breast cancer, prostate cancer and
endometrial carcinoma (42–44) when compared with normal tissue.
Additionally, overexpression of SRC-3 was identified in CCC, which
was in agreement with previous studies reporting overexpression of
SRC-3 in lung cancer, colorectal carcinoma, endometrial carcinoma,
esophageal squamous cell carcinoma and gastric cancer, ovarian
cancer and pancreatic cancer when compared with normal tissue
(29,45–50).
However, previous studies also demonstrated a decrease in SRC-1
protein in endometrial carcinoma, a decrease in SRC-3 but unchanged
SRC-1 in the high-grade astrocytic tissue and a decrease in SRC-1
and SRC-3 in meningothelial tumor and neuroepithelial tumor when
compared with normal tissue (32,33,51). With
regard to HCC, Martínez-Jiménez et al (52) reported decreased SRC-1 levels in human
hepatomas; by contrast, Tong et al (53) reported SRC-1 overexpression in 62.5%
of HCC tissues using western blot analysis. In the present study,
levels of SRC-1 and SRC-3 remain unchanged in liver cancer
including HCC and CCC when compared with that in normal tissue. The
reasons for these differences are unclear; however, the relatively
small normal sample size (10 cases) in the present study may be a
factor, and thus further examinations are required to confirm these
results.
Owing to a lack of significant differences regarding
the levels of SRC-1 and SRC-3 between normal and cancerous liver
tissue, focus was placed on the increased expression profiles of
SRC-3, and comparisons were made between the levels of SCR-1 and
SRC-3 in normal and cancerous liver tissue. A key point of interest
was the absence of statistical significance regarding the different
levels of SRC-1 and SRC-3; however, in liver cancer, including HCC
and CCC, significantly decreased expression of SRC-1 was detected
when compared with that of SRC-3. Further sex-, age- and
stage-specific analysis revealed that significantly decreased
expression of SRC-1 was detected in the following groups: HCC cases
(male), CCC cases (male), HCC (all ages), CCC cases (below mean
age) and liver cancer stages (all stages). However, levels of SRC-1
and SRC-3 did not exhibit any significant differences in the
following categories: Normal cases (male), all cases (female),
normal cases (all ages) and CCC cases (above or equal to mean age).
The decreased SRC-1/SRC-3 ratio in liver cancer but not normal
liver tissue may be due to the slight decrease in SRC-1 expression
and increase in SRC-3 expression observed. This indicates an
imbalanced expression between these two coactivators, and may
contribute to the occurrence and progression of liver cancer.
Additionally, the loss-of-balance expression pattern of
SRC-1/SRC-3, detected in males caused by their distinct expression
profiles (decreased SRC-1; increased SRC-3), was positively
associated with previous reports demonstrating a high occurrence
and mortality in males with liver cancer (39,40). A
similar imbalanced expression profile was also detected in other
tumors including high-grade astrocytoma, as a decrease in SRC-3 but
unchanged SRC-1 was reported (32).
In summary, although no significant changes in SRC-1
and SRC-3 were identified in liver cancer tissue when compared with
that detected in the normal liver tissue, it was noted that there
was a significantly decreased SRC-1/SRC-3 ratio in liver cancer,
compared with that detected in normal liver tissue. The
significance of this decreased ratio is currently unclear. Louet
et al (35) reported that
SRC-1 is a key coordinator of the hepatic gluconeogenic program and
a critical mediator of liver glucose homeostasis; Ma et al
(54) reported that SRC-3 serves a
crucial role in regulating hepatic lipid metabolism (35,54). Thus,
the imbalanced expression of SRC-1 and SRC-3 in the liver tissue
may induce abnormal hepatic metabolism and finally induce
tumorigenesis. However, further studies are urgently required to
explore the precise roles of these two coactivators in both the
normal liver and liver disease.
Acknowledgements
The authors would like to thank Dr. Kaifa Wang
(Department of Mathematics, Third Military Medical University,
Chongqing, China) for his help with statistics.
Funding
The present study was supported by the National
Science Foundation of China (grant nos. 81571059 and 81270525).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
SL, HZ and YY conducted the experiments. ML
photographed the pictures. DG conducted the pathological scoring.
SL and HZ prepared the draft of this manuscript. JZ and XZ
conceived this study and finalized this manuscript.
Ethics approval and consent to
participate
Not applicable since the tissue microarray used in
the present study is commercial available.
Pateint consent for publication
Not applicable since the tissue microarray used in
the present study is commercial available.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Simpson E and Santen RJ: Celebrating 75
years of oestradiol. J Mol Endocrinol. 55:T1–T20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brady CW: Liver disease in menopause.
World J Gastroenterol. 21:7613–7620. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGlynn KA, Sahasrabuddhe VV, Campbell PT,
Graubard BI, Chen J, Schwartz LM, Petrick JL, Alavanja MC,
Andreotti G, Boggs DA, et al: Reproductive factors, exogenous
hormone use and risk of hepatocellular carcinoma among US women:
Results from the liver cancer pooling project. Br J Cancer.
112:1266–1272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang D, Hanna DL, Usher J, LoCoco J,
Chaudhari P, Lenz HJ, Setiawan VW and El-Khoueiry A: Impact of sex
on the survival of patients with hepatocellular carcinoma: A
surveillance, epidemiology, and end results analysis. Cancer.
120:3707–3716. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanda T, Jiang X and Yokosuka O: Androgen
receptor signaling in hepatocellular carcinoma and pancreatic
cancers. World J Gastroenterol. 20:9229–9236. 2014.PubMed/NCBI
|
|
6
|
Kelly DM, Nettleship JE, Akhtar S,
Muraleedharan V, Sellers DJ, Brooke JC, McLaren DS, Channer KS and
Jones TH: Testosterone suppresses the expression of regulatory
enzymes of fatty acid synthesis and protects against hepatic
steatosis in cholesterol-fed androgen deficient mice. Life Sci.
109:95–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Biegon A, Alexoff DL, Kim SW, Logan J,
Pareto D, Schlyer D, Wang GJ and Fowler JS: Aromatase imaging with
[N-methyl-11C]vorozole PET in healthy men and women. J Nucl Med.
56:580–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hata S, Miki Y, Saito R, Ishida K,
Watanabe M and Sasano H: Aromatase in human liver and its diseases.
Cancer Med. 2:305–315. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Sinderen ML, Steinberg GR, Jørgensen
SB, To SQ, Knower KC, Clyne CD, Honeyman J, Chow JD, Herridge KA,
Jones ME, et al: Hepatic glucose intolerance precedes hepatic
steatosis in the male aromatase knockout (ArKO) mouse. PloS One.
9:e872302014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sinclair M, Gow PJ, Angus PW, Hoermann R,
Handelsman DJ, Wittert G, Martin S and Grossmann M: High
circulating estrone and low testosterone correlate with adverse
clinical outcomes in men with advanced liver disease. Liver Int.
36:1619–1627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian X, Zhan Q, Lv L, Zhang H, Hong Z, Li
Y, Xu H, Chai Y, Zhao L and Zhang G: Steroid hormone profiles plus
α-fetoprotein for diagnosing primary liver cancer by liquid
chromatography tandem mass spectrometry. Clin Chim Acta. 457:92–98.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei Q, Guo P, Mu K, Zhang Y, Zhao W, Huai
W, Qiu Y, Li T, Ma X, Liu Y, et al: Estrogen suppresses
hepatocellular carcinoma cells through ERβ-mediated upregulation of
the NLRP3 inflammasome. Lab Invest. 95:804–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baldissera VD, Alves AF, Almeida S,
Porawski M and Giovenardi M: Hepatocellular carcinoma and estrogen
receptors: Polymorphisms and isoforms relations and implications.
Med Hypotheses. 86:67–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen M and Shi H: Sex hormones and their
receptors regulate liver energy homeostasis. Int J Endocrinol.
2015:2942782015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheng ML, Xu GL, Zhang CH, Jia WD, Ren WH,
Liu WB, Zhou T, Wang YC, Lu ZL, Liu WF, et al: Aberrant estrogen
receptor alpha expression correlates with hepatocellular carcinoma
metastasis and its mechanisms. Hepatogastroenterology. 61:146–150.
2014.PubMed/NCBI
|
|
16
|
Ahmed HH, Shousha WG, Shalby AB,
El-Mezayen HA, Ismaiel NN and Mahmoud NS: Implications of sex
hormone receptor gene expression in the predominance of
hepatocellular carcinoma in males: Role of natural products. Asian
Pac J Cancer Prev. 16:4949–4954. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang B, Chen L and Chang HT: Potential
diagnostic and prognostic biomarkers for cholangiocarcinoma in
serum and bile. Biomark Med. 10:613–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isse K, Specht SM, Lunz JG III, Kang LI,
Mizuguchi Y and Demetris AJ: Estrogen stimulates female biliary
epithelial cell interleukin-6 expression in mice and humans.
Hepatology. 51:869–880. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hunsawong T, Singsuksawat E, In-chon N,
Chawengrattanachot W, Thuwajit C, Sripa B, Paupairoj A, Chau-in S
and Thuwajit P: Estrogen is increased in male cholangiocarcinoma
patients' serum and stimulates invasion in cholangiocarcinoma cell
lines in vitro. J Cancer Res Clin Oncol. 138:1311–1320. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singsuksawat E, Thuwajit C, Charngkaew K
and Thuwajit P: Increased ETV4 expression correlates with
estrogen-enhanced proliferation and invasiveness of
cholangiocarcinoma cells. Cancer Cell Int. 18:252018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marzioni M, Torrice A, Saccomanno S,
Rychlicki C, Agostinelli L, Pierantonelli I, Rhönnstad P, Trozzi L,
Apelqvist T, Gentile R, et al: An oestrogen receptor β-selective
agonist exerts anti-neoplastic effects in experimental intrahepatic
cholangiocarcinoma. Dig Liver Dis. 44:134–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu J and Li Q: Review of the in vivo
functions of the p160 steroid receptor coactivator family. Mol
Endocrinol. 17:1681–1692. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
York B and O'Malley BW: Steroid receptor
coactivator (SRC) family: Masters of systems biology. J Biol Chem.
285:38743–38750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu J, Wu RC and O'Malley BW: Normal and
cancer-related functions of the p160 steroid receptor co-activator
(SRC) family. Nat Rev Cancer. 9:615–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin L, Liu Z, Chen H and Xu J: The steroid
receptor coactivator-1 regulates twist expression and promotes
breast cancer metastasis. Cancer Res. 69:3819–3827. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin L, Chen X, Wu Y, Feng Z, He T, Wang L,
Liao L and Xu J: Steroid receptor coactivator-1 upregulates
integrin α5 expression to promote breast cancer cell
adhesion and migration. Cancer Res. 71:1742–1751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Duan C, Bian C, Xiong Y and Zhang
J: Steroid receptor coactivator-1: A versatile regulator and
promising therapeutic target for breast cancer. J Steroid Biochem
Mol Biol. 138:17–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song X, Zhang C, Zhao M, Chen H, Liu X,
Chen J, Lonard DM, Qin L, Xu J, Wang X, et al: Steroid receptor
coactivator-3 (SRC-3/AIB1) as a novel therapeutic target in triple
negative breast cancer and its inhibition with a phospho-bufalin
prodrug. PLoS One. 10:e01400112015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Zhang D, Wu W, Zhang J, Guo D,
Wang Q, Jing T, Xu C, Bian X and Yang K: Overexpression and
gender-specific differences of SRC-3 (SRC-3/AIB1) immunoreactivity
in human non-small cell lung cancer: An in vivo study. J Histochem
Cytochem. 58:1121–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo F, Li W, Zhang J, Huang K, Fu J and
Xie Z: Overexpression of steroid receptor coactivator-3 in bone
cancers: An in vivo immunohistochemical study with tissue
microarray. Pathol Res Pract. 209:790–796. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li W, Fu J, Bian C, Zhang J and Xie Z:
Immunohistochemical localization of steroid receptor coactivators
in chondrosarcoma: An in vivo tissue microarray study. Pathol Res
Pract. 210:1005–1010. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu C, Zhang Y, Zhang K, Bian C, Zhao Y
and Zhang J: Expression of estrogen receptors, androgen receptor
and steroid receptor coactivator-3 is negatively correlated to the
differentiation of astrocytic tumors. Cancer Epidemiol. 38:291–297.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu M, Zhang K, Zhao Y, Guo Q, Guo D and
Zhang J: Evidence for involvement of steroid receptors and
coactivators in neuroepithelial and meningothelial tumors. Tumour
Biol. 36:3251–3261. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jeong JW, Kwak I, Lee KY, White LD, Wang
XP, Brunicardi FC, O'Malley BW and DeMayo FJ: The genomic analysis
of the impact of steroid receptor coactivators ablation on hepatic
metabolism. Mol Endocrinol. 20:1138–1152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Louet JF, Chopra AR, Sagen JV, An J, York
B, Tannour-Louet M, Saha PK, Stevens RD, Wenner BR, Ilkayeva OR, et
al: The coactivator SRC-1 is an essential coordinator of hepatic
glucose production. Cell Metab. 12:606–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Motamed M, Rajapakshe KI, Hartig SM,
Coarfa C, Moses RE, Lonard DM and O'Malley BW: Steroid receptor
coactivator 1 is an integrator of glucose and NAD+/NADH
homeostasis. Mol Endocrinol. 28:395–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tannour-Louet M, York B, Tang K, Stashi E,
Bouguerra H, Zhou S, Yu H, Wong LJ, Stevens RD, Xu J, et al:
Hepatic SRC-1 activity orchestrates transcriptional circuitries of
amino acid pathways with potential relevance for human metabolic
pathogenesis. Mol Endocrinol. 28:1707–1718. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hansel MC, Davila JC, Vosough M,
Gramignoli R, Skvorak KJ, Dorko K, Marongiu F, Blake W and Strom
SC: The use of induced pluripotent stem cells for the study and
treatment of liver diseases. Curr Protoc Toxicol. 67:14 13 11–14 13
27. 2016.
|
|
39
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang D, Guo Q, Bian C, Zhang J, Lin S and
Su B: Alterations of steroid receptor coactivator-1 (SRC-1)
immunoreactivities in specific brain regions of young and
middle-aged female Sprague-Dawley rats. Brain Res. 1382:88–97.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Anzick SL, Kononen J, Walker RL, Azorsa
DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM and
Meltzer PS: AIB1, a steroid receptor coactivator amplified in
breast and ovarian cancer. Science. 277:965–968. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Culig Z, Klocker H, Bartsch G and Hobisch
A: Androgen receptors in prostate cancer. Endocr Relat Cancer.
9:155–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kershah SM, Desouki MM, Koterba KL and
Rowan BG: Expression of estrogen receptor coregulators in normal
and malignant human endometrium. Gynecol Oncol. 92:304–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie D, Sham JS, Zeng WF, Lin HL, Bi J, Che
LH, Hu L, Zeng YX and Guan XY: Correlation of AIB1 overexpression
with advanced clinical stage of human colorectal carcinoma. Hum
Pathol. 36:777–783. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Glaeser M, Floetotto T, Hanstein B,
Beckmann MW and Niederacher D: Gene amplification and expression of
the steroid receptor coactivator SRC3 (AIB1) in sporadic breast and
endometrial carcinomas. Horm Metab Res. 33:121–126. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu FP, Xie D, Wen JM, Wu HX, Liu YD, Bi J,
Lv ZL, Zeng YX and Guan XY: SRC-3/AIB1 protein and gene
amplification levels in human esophageal squamous cell carcinomas.
Cancer Lett. 245:69–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sakakura C, Hagiwara A, Yasuoka R, Fujita
Y, Nakanishi M, Masuda K, Kimura A, Nakamura Y, Inazawa J, Abe T
and Yamagishi H: Amplification and over-expression of the AIB1
nuclear receptor co-activator gene in primary gastric cancers. Int
J Cancer. 89:217–223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yoshida H, Liu J, Samuel S, Cheng W, Rosen
D and Naora H: Steroid receptor coactivator-3, a homolog of Taiman
that controls cell migration in the Drosophila ovary, regulates
migration of human ovarian cancer cells. Mol Cell Endocrinol.
245:77–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Henke RT, Haddad BR, Kim SE, Rone JD, Mani
A, Jessup JM, Wellstein A, Maitra A and Riegel AT: Overexpression
of the nuclear receptor coactivator AIB1 (SRC-3) during progression
of pancreatic adenocarcinoma. Clin Cancer Res. 10:6134–6142. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Uchikawa J, Shiozawa T, Shih HC, Miyamoto
T, Feng YZ, Kashima H, Oka K and Konishi I: Expression of steroid
receptor coactivators and corepressors in human endometrial
hyperplasia and carcinoma with relevance to steroid receptors and
Ki-67 expression. Cancer. 98:2207–2213. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Martínez-Jiménez CP, Gómez-Lechón MJ,
Castell JV and Jover R: Underexpressed coactivators PGC1alpha and
SRC1 impair hepatocyte nuclear factor 4 alpha function and promote
dedifferentiation in human hepatoma cells. J Biol Chem.
281:29840–29849. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tong Z, Li M, Wang W, Mo P, Yu L, Liu K,
Ren W, Li W, Zhang H, Xu J and Yu C: Steroid receptor coactivator 1
promotes human hepatocellular carcinoma progression by enhancing
Wnt/β-catenin signaling. J Biol Chem. 290:18596–18608. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma X, Xu L, Wang S, Cui B, Li X, Xu J and
Ning G: Deletion of steroid receptor coactivator-3 gene ameliorates
hepatic steatosis. J Hepatol. 55:445–452. 2011. View Article : Google Scholar : PubMed/NCBI
|