Introduction
There are approximately 63,990 new cases of kidney
cancer and an estimated 14,400 cancer associated deaths in the
United States in 2017 (1). Renal cell
carcinoma (RCC) represents one of the most common type of kidney
cancer, accounting for 85–90% of renal malignancies (2). Nephrectomy and nephron-sparing surgery
are effective clinical management for localized RCC. However, Only
30% of patients are diagnosed on the basis of symptoms, and up to
17% of all RCC patients present with distant metastases at the time
of diagnosis (3). Metastatic RCC is
highly resistant to current available therapies including
chemotherapy, immunotherapy using interferon-a (IFN-a) and targeted
therapy using tyrosine kinase inhibitors, while the underlying
mechanisms of how these metastasis occur are largely
uncharacterized. It is needed to identify novel molecular targets
for the better management of RCC.
Leucine zipper-EF-hand containing transmembrane
protein 1 (LETM1), first reported in 1999 and is deleted in nearly
all Wolf-Hirschhorn syndrome (WHS) patients (4), is a mitochondrial inner membrane protein
and plays an important role in mitochondrial ATP production and
biogenesis (5,6). Piao et al (6), first reported the role of LETM1 in
tumorigenesis in 2009, they showed that dysregulation of LETM1 is a
key feature of tumorigenesis by altering cancerous metabolic
reactions. Recently, several studies reported that high expression
of LETM1 predicts poor prognosis (7)
and associated with the clinicopathological parameters of human
cancers (8–10). However, the role of LETM1 in RCC has
not yet been determined.
In the present study, we demonstrated that LETM1
overexpression significantly correlated with poor prognosis of RCC
patients and knockdown of LETM1 markedly decreased the
proliferation, migration and invasion of RCC cells.
Materials and methods
Cell lines and cultures
Human RCC cell lines (Caki-1, 786-O, OS-RC-2, A498
and ACHN) and human normal renal tubular epithelial cell line HK-2
were obtained from the cell bank of type culture collection of the
Chinese Academy of Sciences (Shanghai, China). Caki-1, A498 and
ACHN were cultured in Dulbecco's Modified Eagle's Medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). 786-O and
OS-RC-2 cells were maintained in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.). HK-2 cells were cultured in DMEM/F12
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 5 mM
glucose. These media were supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA). All cells were incubated in a humidified incubator
with 5% CO2 at 37°C.
Xena public data hubs
UCSC Xena browser (https://xenabrowser.net/heatmap/) was used to obtain
the Cancer Genome Atlas (TCGA) Kidney Clear Cell Carcinoma data and
to evaluate the overall survival.
Cell transfection
RCC cells 786-O and A498 were transfected with
double-stranded short interfering RNA (siRNA) (Shanghai GenePharma
Co., Ltd., Shanghai, China) with Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. At 72 h after transfection, the gene silencing effect
was measured using western blot analysis. The target sequence of
LETM1 siRNA is: 5′-CCACAGAAUCGUGUCUGGAUCCACA-3′ and the sequence of
negative control siRNA is: 5′-CGAGCAGAGACTCTAACATTCTCGC-3′.
Cell proliferation assay
Cell proliferation was assessed using Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies Inc., Kumamoto,
Japan). Briefly, 1,000 cells/100 µl/well in 96-well plate were
seed, and then 10 µl of CCK-8 was added into each well at indicated
time-points (24, 48, 72 and 96 h) and incubation followed for 2 h
at 37°C. The absorbance at 450 nm was detected on a microplate
spectrophotometer (BioTek Instruments, Inc., Winooski, VT,
USA).
Colony formation assay
For colony forming assay, 1,000 cells were seeded in
six-well plates and cultured at 37°C in a humidified incubator.
After two-weeks' incubation, the cells were fixed with 4%
paraformaldehyde, then stained with 0.5% gentian violet and
observed under a digital camera.
Transwell migration and invasion
assays
Cell migration and invasion assays were performed
using transwell chambers (BD Biosciences, San Jose, CA, USA). For
the invasion assay, chambers were precoated with 25 µl of Matrigel
(BD Biosciences) at 37°C for 2 h, whereas the chambers used for
migration assay were not precoated with Matrigel. 5 ×104
transfected cells in 200 µl serum-free medium were plated in the
upper well of the, and 600 µl of medium containing 10% FBS were
added into the lower chambers as chemoattractant. After incubated
at 37°C for 10 h, cells on the upper surface of the insert were
removed with a cotton swab, the cells migrating to the lower
surface of the chamber were fixed with 95% ethanol for 20 min,
stained with 0.1% crystal violet solution for 20 min. The migrated
cells were counted under using light microscope (Olympus
Corporation, Tokyo, Japan).
Western blot analysis
Cells were washed with cold phosphate-buffered
saline (PBS) and lysed in ice-cold RIPA buffer (Sigma-Aldrich;
Merck KGaA) containing protease inhibitor. BCA protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China) was used to
determine the concentration of total cellular protein. Then equal
amounts of protein were loaded into 10% SDS-PAGE gel and after
separation transferred to nitrocellulose membranes, the membranes
were blocked in 5% non-fat milk for one hour and then incubated
with primary antibodies: Mouse anti-LETM1 (sc-271234, 1:500; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), mouse anti-β-Actin
(sc-130300, 1:2,000; Santa Cruz Biotechnology, Inc.), rabbit
anti-β-Catenin (no. 8480, 1:1,000; Cell Signaling Technology),
rabbit anti-Cyclin D1 (ab134175, 1:2,000; Abcam, Cambridge, MA,
USA), and rabbit anti-c-Myc (no. 5605, 1:2,000; Cell Signaling
Technology) overnight at 4°C. After washing with PBST three times,
the membranes were incubated with the corresponding secondary
antibodies (Goat anti-Rabbit IgG, 926-32211; LI-COR Biosciences,
Lincoln, NE, USA; Goat anti-Mouse IgG, 926-32210; LI-COR
Biosciences) at 1:2,000 dilution ratio at room temperature for one
hour. The protein bands were visualized using the Odyssey two-color
infrared laser imaging system (LI-COR Biosciences).
Statistical analysis
The SPSS v.16.0 statistical software (IBM SPSS,
Armonk, NY, USA) was used for statistical analysis. All data are
presented as means ± standard deviation (SD) from at least three
independent experiments. Survival analysis was evaluated by
Log-rank test, other experiment results were calculated using
Student's t-test or one-way analysis of variance followed by
post-hoc LSD test. P<0.05 was considered to indicate a
statistically significant difference. The diagrams were drawn by
GraphPad Prism v.5 software (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
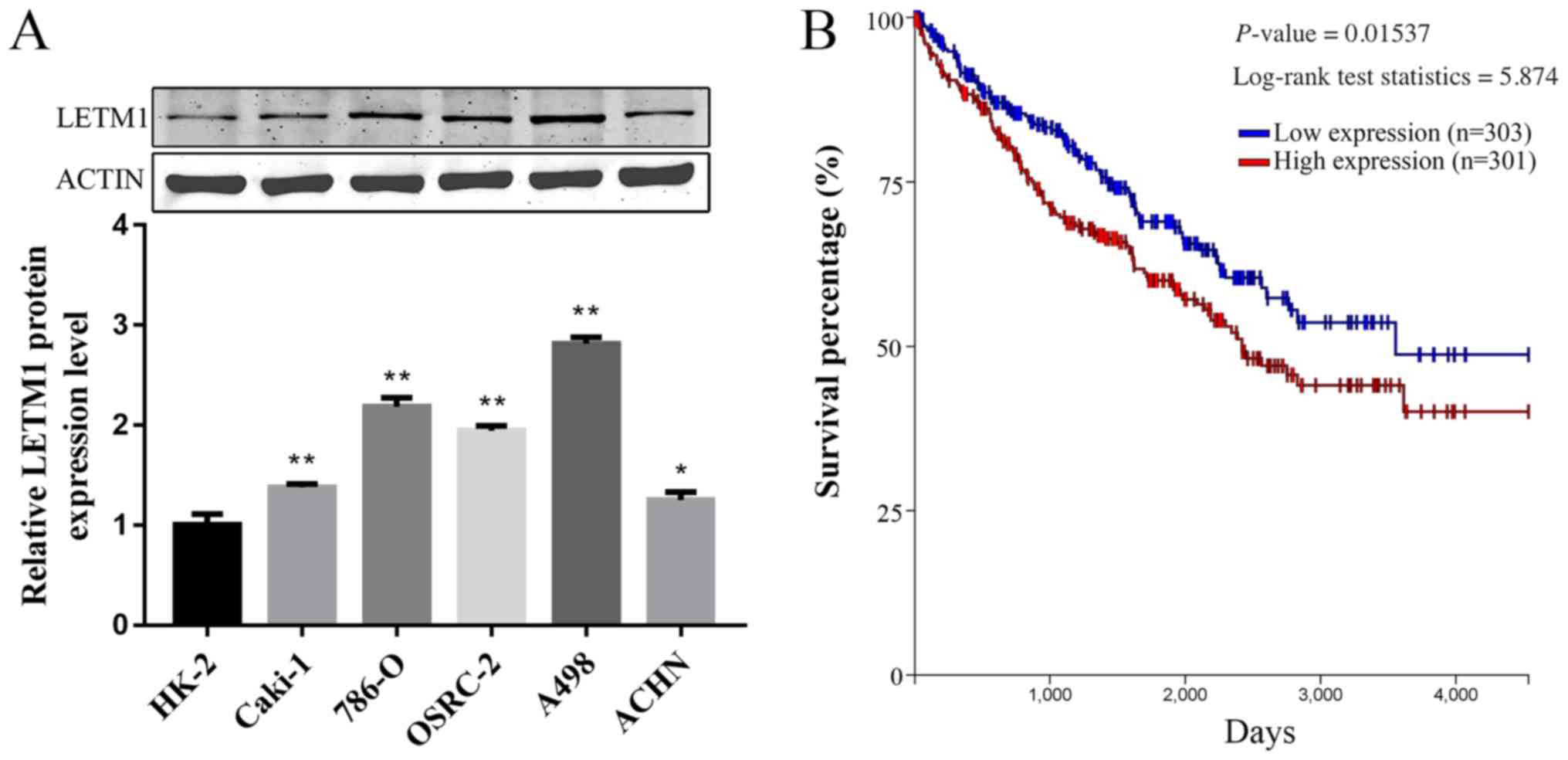
LETM1 is upregulated in RCC cell lines
and associated with poor prognosis
To explore the expression difference of LETM1
between RCC cell lines and human normal renal tubular epithelial
cell line HK-2, western blot is performed. As shown in Fig. 1A, the protein level of LETM1 were
significantly higher in RCC cell lines when compared with that in
the normal human normal renal tubular epithelial cell line HK-2
cells. We further examined whether LETM1 expression correlated with
outcome in RCC patients. Kaplan-Meier survival analysis and
log-rank tests was performed using TCGA database. The Kaplan-Meier
survival curve demonstrated that patients with high LETM1
expression had lower overall survival than those with low LETM1
expression (Fig. 1B). These results
indicated that LETM1 was upregulated in RCC cell lines, we assumed
that LETM1 might act as an oncogene in RCC.
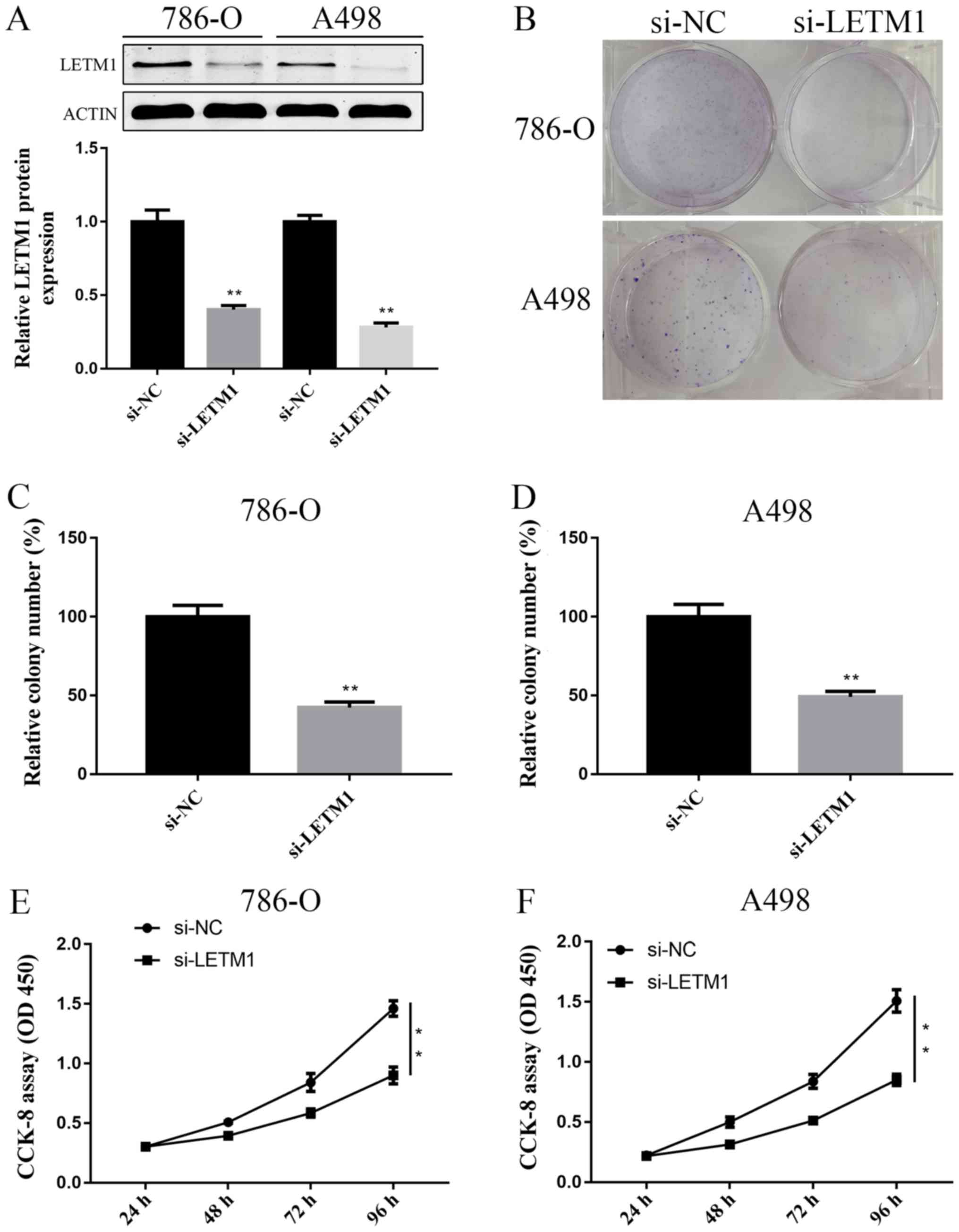
Knockdown of LETM1 inhibits RCC cell
proliferation
To further explore the role of LETM1 in RCC cells,
786-O and A498 cells were both transfected with si-LETM1 or si-NC
respectively, the expression of LETM1 at protein level were
detected at 72 h after transfection. We found that knockdown of
LETM1 by si-LETM1 significantly decreased the LETM1 protein
expression in both 786-O and A498 cells (Fig. 2A). Colony formation assay showed that
knockdown of LETM1 obviously decreased the proliferation of 786-O
and A498 cells, when compared with controls (Fig. 2B-D). Similar effects were observed in
the CCK-8 proliferation assay (Fig. 2E
and F). Taken together, knockdown of LETM1 markedly inhibited
786-O and A498 cell proliferation.
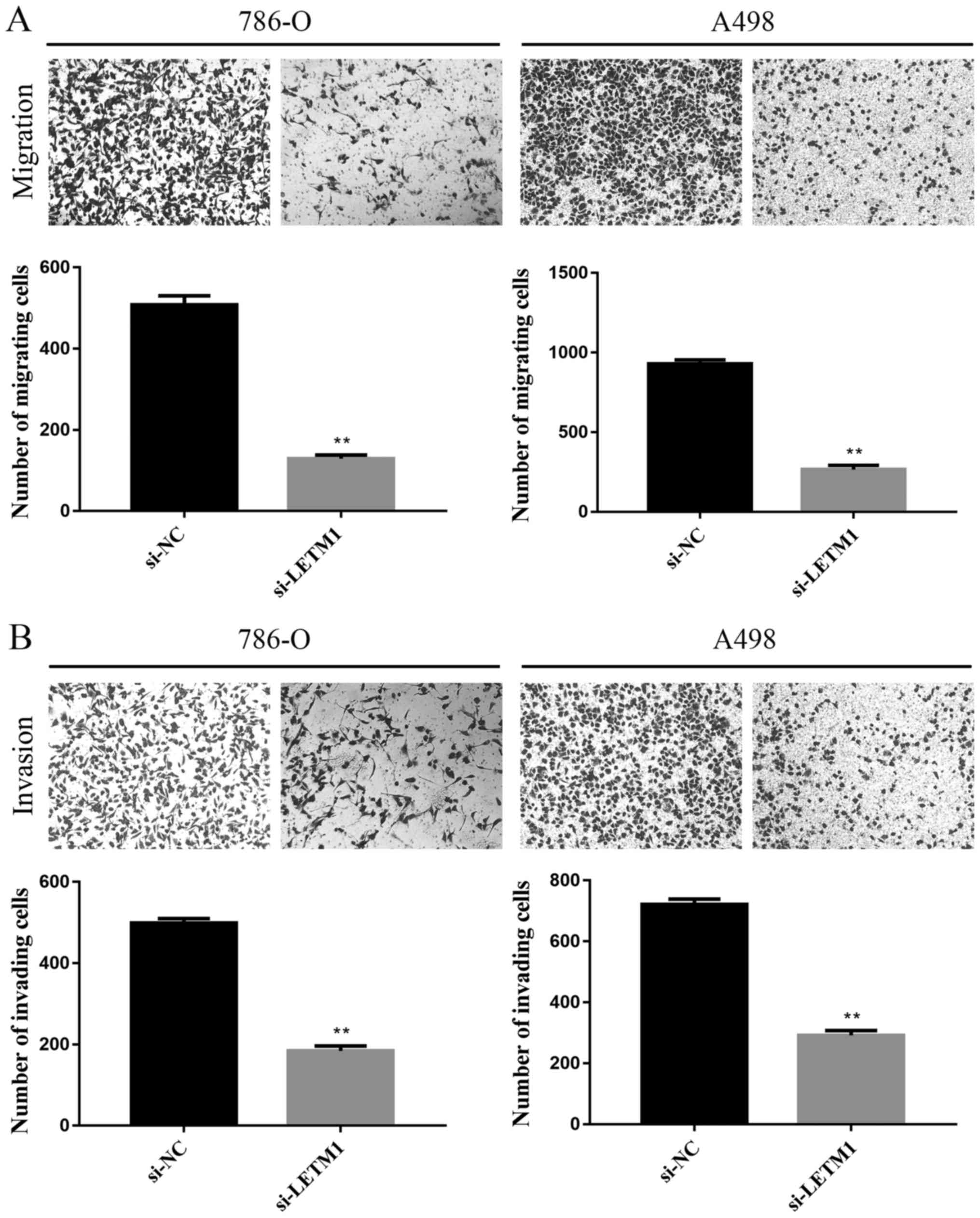
Knockdown of LETM1 inhibits RCC cell
migration and invasion
As shown in Fig. 3A,
the number of cells identified across the membrane in the negative
control groups were significantly higher than the number of cells
across the membrane in the si-LETM1 group by using transwell
chambers without Matrigel. The results showed that knockdown of
LETM1 markedly inhibited the migration in 786-O and A498 cells. In
addition, we explored the role of LETM1 on cell invasion of RCC
cells using transwell chambers with Matrigel. As showed Fig. 3B, knockdown of LETM1 significantly
suppressed the number of 786-O and A498 cells that invaded through
Matrigel as compared with the negative control groups.
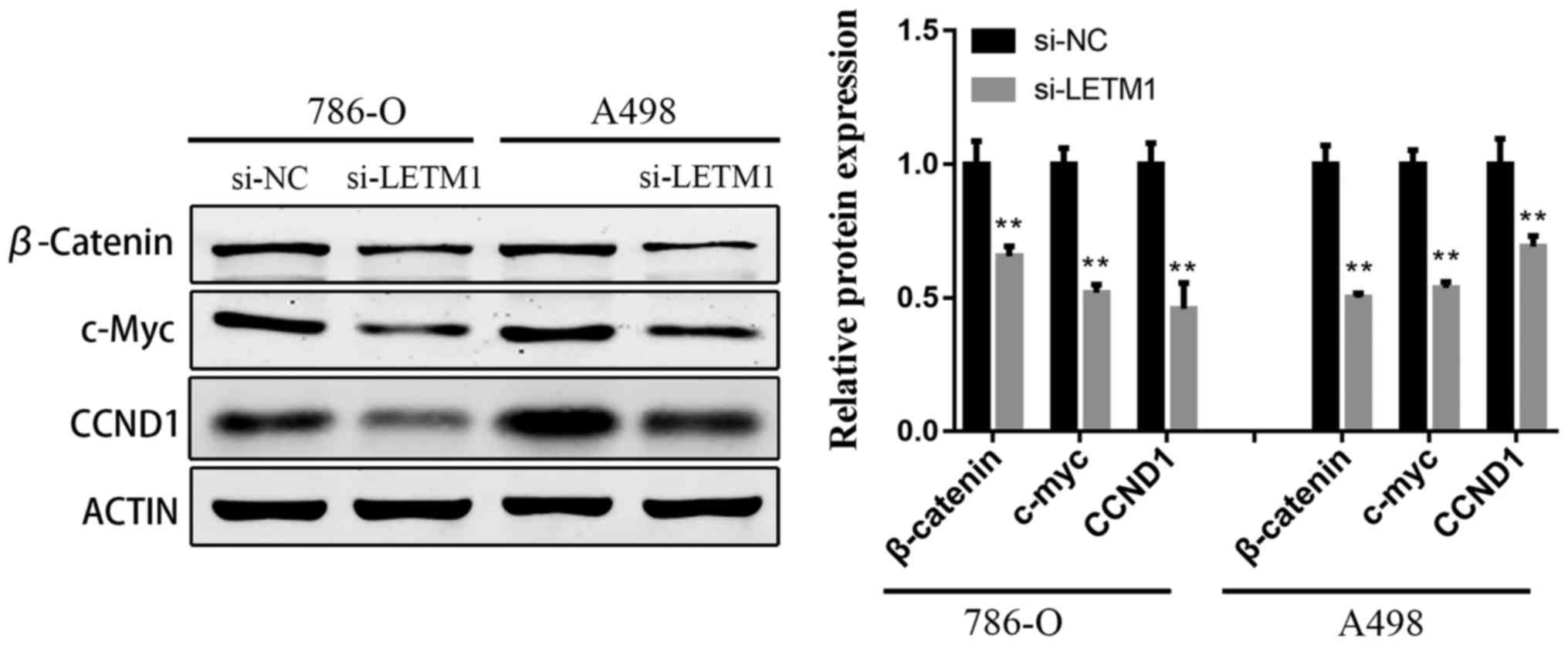
Knockdown of LETM1 inhibits the
activation of Wnt/β-catenin pathway
To explore the underlying molecular mechanism of
si-LETM1 inhibiting RCC cell proliferation and metastasis, the
protein levels of β-Catenin, Cyclin D1, and c-Myc were evaluated
using western blot in 786-O and A498 cells transfected with
si-LETM1. As illustrated in Fig. 4A and
B, knockdown of LETM1 significantly suppressed the protein
expression of β-Catenin, Cyclin D1, and c-Myc in RCC cells compared
with the negative control groups.
Discussion
RCC is a public health challenge worldwide as it is
difficult to be detected in early stages. Emerging evidences
indicate that investigating the underlying molecular mechanisms of
RCC pathogenesis, and identifying novel treatments and targets for
gene therapy are required to improve the prognosis of patients with
RCC. Although great progress in diagnosis and treatment has been
achieved in the past decades, several key factors and pathways have
been identified to be responsible for renal tumorigenesis such as
VHL, VEGF, NFκB, mTOR pathways (11–13).
However, once metastatic disease develops, the prognosis for
long-term survival of RCC patients is poor (14–16). In
the present study, we first demonstrated that LETM1 is up-regulated
in RCC cell lines, and suppression of LETM1 significantly
suppressed cell proliferation, migration and invasion.
LETM1was first reported to be deleted in nearly all
WHS patients (4). Recently, an
increasing number of studies have shown that LETM1 is highly
expressed in many kinds of human cancers and predicts poor
prognosis (7–10,17), this
is consist with our results that up-regulated LETM1 expression was
found in RCC cell lines and was related with poor prognosis of RCC
patients. As for the molecular function of LETM1, Piao et al
(6), found that LETM1 is
overexpressed in various human cancers, and they demonstrated that
metabolic alteration of cells may affect the progress of
tumorigenesis. Doonan et al (18) found that knockdown of LETM1 caused
accumulation of S-phase cells, and restoration of LETM1 could
reverse S-phase accumulation, which was consistent with our
previous study (8), indicating that
suppression of LETM1 inhibited cell proliferation possibly by
disrupting cell cycle distribution. However, Hwang et al
(17) reported that LETM1 may acts a
tumor suppressor in lung cancer by activation of AMPK activity and
inhibition of Akt activity. It is still unclear how LETM1 affect
tumorigenesis differ by tumor types. In this study, we demonstrated
that suppression of LETM1 significantly suppressed cell
proliferation, migration and invasion in vitro. However,
more research is warranted in order to test the in vivo role
of LETM1 in the tumor growth in nude mice.
Numerous studies revealed that the Wnt/β-Catenin
pathway is an important signaling pathway involved in the malignant
progression of various tumors (19–22).
Activated β-Catenin promoting transcription of target genes. Some
target genes, including Cyclin D1 and c-Myc, are involved in cell
proliferation and differentiation, cell migration, and tumor
formation (23,24). To the best of our knowledge, this is
the first study that infers the relationship between LETM1 and
Wnt/β-Catenin pathway in RCC. In our study, we found that knockdown
of LETM1 inhibited the expression of β-Catenin, Cyclin D1, and
c-Myc in 786-O and A498 cells. These findings strongly suggest that
LETM1 played an important role in tumor progression of RCC through
the activation of Wnt/β-Catenin signaling pathway.
In conclusion, the present study revealed that
knockdown of LETM1 suppresses proliferation, migration and invasion
of 786-O and A498 cells perhaps via suppressing the Wnt/β-Catenin
signaling pathway. Therefore, suppression of LETM1 may be a
promising agent for treating RCC.
Acknowledgements
Not applicable.
Funding
This study was supported by research grants from the
National Natural Science Foundation of China (grant no.
81671446).
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX, BH and YX conceived and designed the
experiments. JX and BH performed the experiments. SL, XZ and TX
conducted the statistical analysis. JX and BH wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shuch B, Amin A, Armstrong AJ, Eble JN,
Ficarra V, Lopez-Beltran A, Martignoni G, Rini BI and Kutikov A:
Understanding pathologic variants of renal cell carcinoma:
Distilling therapeutic opportunities from biologic complexity. Eur
Urol. 67:85–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Capitanio U and Montorsi F: Renal cancer:
Lancet. 387:894–906. 2016.
|
|
4
|
Endele S, Fuhry M, Pak SJ, Zabel BU and
Winterpacht A: LETM1, a novel gene encoding a putative EF-hand
Ca(2+)-binding protein, flanks the Wolf-Hirschhorn syndrome (WHS)
critical region and is deleted in most WHS patients. Genomics.
60:218–225. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nowikovsky K, Froschauer EM, Zsurka G,
Samaj J, Reipert S, Kolisek M, Wiesenberger G and Schweyen RJ: The
LETM1/YOL027 gene family encodes a factor of the mitochondrial K+
homeostasis with a potential role in the Wolf-Hirschhorn syndrome.
J Biol Chem. 279:30307–30315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Piao L, Li Y, Kim SJ, Byun HS, Huang SM,
Hwang SK, Yang KJ, Park KA, Won M, Hong J, et al: Association of
LETM1 and MRPL36 contributes to the regulation of mitochondrial ATP
production and necrotic cell death. Cancer Res. 69:3397–3404. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen L, Yang Y, Liu S, Piao L, Zhang Y,
Lin Z and Li Z: High expression of leucine zipper-EF-hand
containing transmembrane protein 1 predicts poor prognosis in head
and neck squamous cell carcinoma. Biomed Res Int.
2014:8503162014.PubMed/NCBI
|
|
8
|
Huang B, Zhang J, Zhang X, Huang C, Hu G,
Li S, Xie T, Liu M and Xu Y: Suppression of LETM1 by siRNA inhibits
cell proliferation and invasion of bladder cancer cells. Oncol Rep.
38:2935–2940. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li N, Zheng Y, Xuan C, Lin Z, Piao L and
Liu S: LETM1 overexpression is correlated with the clinical
features and survival outcome of breast cancer. Int J Clin Exp
Pathol. 8:12893–12900. 2015.PubMed/NCBI
|
|
10
|
Wang CA, Liu Q, Chen Y, Liu S, Xu J, Cui
X, Zhang Y and Piao L: Clinical implication of leucine zipper/EF
hand-containing transmembrane-1 overexpression in the prognosis of
triple-negative breast cancer. Exp Mol Pathol. 98:254–259. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koul H, Huh JS, Rove KO, Crompton L, Koul
S, Meacham RB and Kim FJ: Molecular aspects of renal cell
carcinoma: A review. Am J Cancer Res. 1:240–254. 2011.PubMed/NCBI
|
|
13
|
Rini BI: Vascular endothelial growth
factor-targeted therapy in metastatic renal cell carcinoma. Cancer.
115 10 Suppl:S2306–S2312. 2009. View Article : Google Scholar
|
|
14
|
Motzer RJ, Bander NH and Nanus DM:
Renal-cell carcinoma. N Engl J Med. 335:865–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Figlin RA: Renal cell carcinoma:
Management of advanced disease. J Urol. 161:381–367. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lanigan D: Prognostic factors in renal
cell carcinoma. Br J Urol. 75:565–571. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang SK, Piao L, Lim HT, Minai-Tehrani A,
Yu KN, Ha YC, Chae CH, Lee KH, Beck GR, Park J and Cho MH:
Suppression of lung tumorigenesis by leucine zipper/EF
hand-containing transmembrane-1. PLoS One. 5:pii: e125352010.
View Article : Google Scholar
|
|
18
|
Doonan PJ, Chandramoorthy HC, Hoffman NE,
Zhang X, Cárdenas C, Shanmughapriya S, Rajan S, Vallem S, Chen X,
Foskett JK, et al: LETM1-dependent mitochondrial Ca2+ flux
modulates cellular bioenergetics and proliferation. FASEB J.
28:4936–4949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cojocaru E, Lozneanu L, Giuşcă SE, Căruntu
ID and Danciu M: Renal carcinogenesis-insights into signaling
pathways. Rom J Morphol Embryol. 56:15–19. 2015.PubMed/NCBI
|
|
20
|
Wang X, Lu X, Geng Z, Yang G and Shi Y:
LncRNA PTCSC3/miR-574-5p governs cell proliferation and migration
of papillary thyroid carcinoma via Wnt/β-catenin signaling. J Cell
Biochem. 118:4745–4752. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuan H, Yu S, Cui Y, Men C, Yang D, Gao Z,
Zhu Z and Wu J: Knockdown of mediator subunit Med19 suppresses
bladder cancer cell proliferation and migration by downregulating
Wnt/β-catenin signalling pathway. J Cell Mol Med. 21:3254–3263.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rahmani F, Avan A, Hashemy SI and
Hassanian SM: Role of Wnt/β-catenin signaling regulatory microRNAs
in the pathogenesis of colorectal cancer. J Cell Physiol.
233:811–817. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Polakis P: The many ways of Wnt in cancer.
Curr Opin Genet Dev. 17:45–51. 2007. View Article : Google Scholar : PubMed/NCBI
|